Abstract
Minimally invasive surgery for rectal cancer is now widely performed via the laparoscopic approach and has been validated in randomized controlled trials to be oncologically safe with better perioperative outcomes than open surgery including shorter length of stay, earlier return of bowel function, better cosmesis, and less analgesic requirement. Laparoscopic surgery, however, has inherent limitations due to two-dimensional vision, restricted instrument motion and a very long learning curve. Robotic surgery with its superb three-dimensional magnified optics, stable retraction platform and 7 degrees of freedom of instrument movement offers significant benefits during Total Mesorectal Excision (TME) including ease of operation, markedly lower conversion rates and better quality of the specimen in addition to shorter (steeper) learning curves. This review summarizes the current evidence for the adoption of robotic TME for rectal cancer with supporting data from the literature and from the authors’ own experience. All relevant articles from PubMed using the search terms listed below and published between 2000 and 2014 including randomized trials, meta-analyses, prospective studies, and retrospective reviews with substantial numbers were included.
Keywords: Laparoscopic surgery, rectal cancer, robotic surgery, total mesorectal excision
INTRODUCTION
Robotic total mesorectal excision (RTME) is a natural step forward in the evolution of the minimally invasive approach to rectal cancer extirpation. The evidence for the equivalence of laparoscopic total mesorectal excision (LTME) and the open approach for rectal cancer surgery essentially is based on the results of one multicenter randomized controlled trial, the MRC CLASICC trial which compared the open versus laparoscopic approach in colorectal cancers.[1,2] Subgroup analysis of this study revealed oncologic outcomes of LTME to be equal to that of open TME, which continues to remain the gold standard. However, the study did reveal a high conversion rate to open surgery of 34% and an increased radial margin positivity rate of 12% in the laparoscopic arm compared to 4% in the open arm though this did not translate into an increase in recurrence rate.
LTME is a difficult operation to master and has been associated with learning curves as high as 50 cases to achieve consistent results.[3] It is difficult to quantify the learning curve accurately due to the subjective nature of many studies as to what constitutes an acceptable outcome. Conversion rates decrease and stabilize by 150 cases and complication rates bottomed out at 200 cases in Kayano's study; if a low conversion rate is taken as the parameter to judge the learning curve, the laparoscopic curve could therefore be as high as 150 cases.[3] Total mesorectal excision (TME) as advocated by Heald is a technically demanding and unforgiving operation with a very low margin for error.[4] Positive margins usually herald a poor outcome and hence the need for an approach which retains the benefits of the minimally invasive approach while being easier to perform, reproduce and teach. Robotic surgery addresses many of the limitations of conventional laparoscopic surgery and provides magnified three-dimensional optics, surgeon controlled camera vision, three working arms allowing very stable retraction and unparalleled ergonomics due to 7 degrees of instrument motion. The result is an operation that satisfies all measures of oncologic adequacy while being easier to teach and associated with less fatigue for the surgeon. The evidence from ongoing randomized trials is awaited to provide concrete evidence for this approach.
Methods
All relevant articles from PubMed using the search terms, Rectal Cancer, Total Mesorectal excision, robotic surgery, laparoscopic surgery and open surgery published between 2000 and 2014 including randomized trials, meta-analyses, prospective studies and retrospective reviews with substantial numbers were retrieved and used to produce this review in addition to unpublished work from the authors’ institution.
Laparoscopic TME for Rectal Cancer: Current Status
To fully comprehend the role of robotic TME, the current evidence for laparoscopic TME must first be analysed. As alluded to earlier, the MRC CLASICC trial established the safety of this approach. There were however concerns raised due to a higher conversion rate and a trend towards a higher Circumferential Margin Positivity [CRM] rate in the laparoscopic arm [Table 1].[1] The CRM positivity however did not reach statistical significance and indeed did not worsen survival on long-term analysis. Conversion to open surgery however was not a benign event and was associated with a worse long term oncological outcome irrespective of surgeon experience indicating that conversion is a worse indicator of long term outcomes than CRM positivity in this study.[2] The COLOR II trial was specifically designed to look at outcomes of laparoscopic versus open surgery for rectal cancer and showed better short-term perioperative outcomes including less blood loss, earlier return of bowel function and shorter length of stay but with increased operative time.[5] Pathologic outcomes were similar but there was a 17% conversion rate and data on locoregional recurrence are awaited.
Table 1.
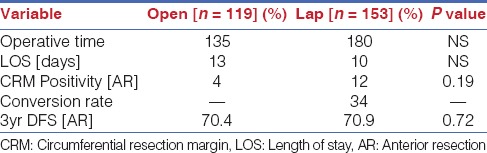
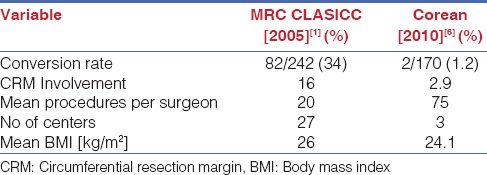
A more recent study, the COREAN (Comparison of Open versus laparoscopic surgery for mid and low rectal cancers After Neoadjuvant chemoradiotherapy) study from Korea compared open versus laparoscopic TME after neoadjuvant chemoradiation and showed equivalent outcomes [Table 2].[6] The conversion rates and CRM positivity were very low as compared to the CLASICC study. However it is important to note that the surgeons in the COREAN study were highly experienced with a median case load of 75 cases before the trial and that the trial was performed by only three highly trained surgeons as against a mean case load of only 20 colorectal operations in the MRC study and multiple participating centers. The implication is that the results of the MRC study are more applicable to the real world scenario and reflect what happens with surgeons with limited caseloads performing TME.
Table 2.
Comparison of MRC CLASICC trial and Corean trial data
Laparoscopic TME is also a difficult operation to master and teach. The average caseload to achieve mastery varies from 50-150 cases and may be difficult to achieve outside large tertiary referral centers.[3] Robotic surgery on the other hand has markedly shorter learning curves either non-existent or of the order of about 20 cases to achieve proficiency.[7,8] In the context of fellowship training this essentially implies the possibility of completing the learning curve before exit from the fellowship, which is highly desirable.
Literature Review of Robotic TME
Multiple large prospective studies and retrospective reviews have firmly established the safety and feasibility of robotic surgery for rectal cancer. A variety of approaches both totally robotic and hybrid procedures performing part of the operation laparoscopically have been used. In all patients however the total mesorectal excision was performed robotically which is the critical area we appraise in this paper.[9,10,11,12,13,14]
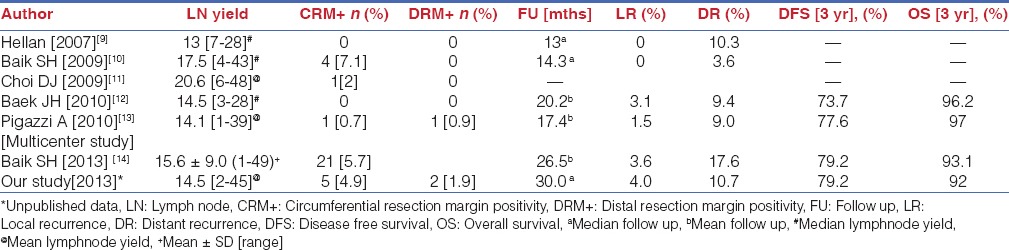
All the series’ are characterized by low conversion rates (0-4.9%), acceptable lymph node yield, low CRM and distal margin positivity rates. Local and systemic recurrences were also similar to the data from the MRC study. Long-term outcomes are now accruing and 3 year disease free survivals between 73.7% and 79.2% and overall survivals between 92% and 97% are reported [Tables 3 and 4].[9,10,11,12,13,14]
Table 3.
Perioperative outcomes of robotic TME for rectal cancer
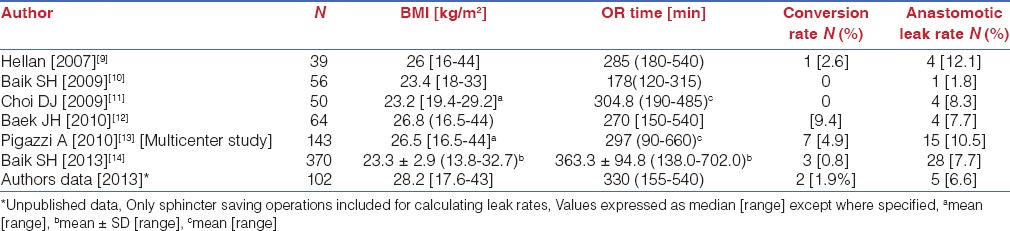
Table 4.
Short-term and long-term oncologic outcomes of robotic TME for rectal cancer
Comparison of Open Versus Robotic TME
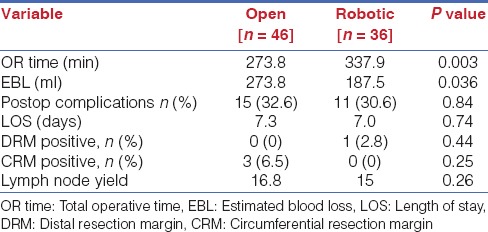
Multiple large prospective series’ have proven the safety and feasibility of RTME. Our own data as that of others show perioperative outcomes comparable to open surgery, with the caveat of decreased blood loss and increased operative time in the initial phases of the robotic approach. Short term and long term oncological outcomes are equivalent especially with reference to mesorectal grade, CRM positivity, lymph node yield and disease free and overall survival [Table 5].[15,16] A recent comparative study with long-term outcomes data however found a significant reduction in local recurrence rate and a higher, though not statistically significant long-term cancer specific survival in the RTME group.[17]
Table 5.
Comparison of open versus robotic TME[15]
Comparison of Laparoscopic versus Robotic TME
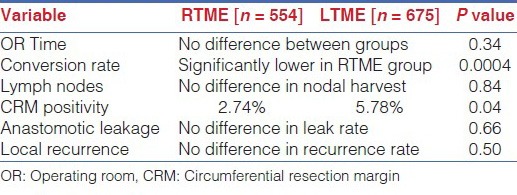
The only published randomized data from a pilot study comparing laparoscopic and robotic TME with 18 patients in each arm, found no difference in operative time, conversion rates or pathologic quality of the specimen.[18] A statistically significant shorter hospital stay was however found favouring the robotic arm [Standard laparoscopic arm, 8.7±1.3 days; robotic assisted arm 6.9 ±1.3 days; P<0.001]. There are a plethora of publications involving systematic reviews and case matched series, which show equivalent clinical and oncologic outcomes. The meta-analysis by Trastulli which focused on short term outcomes revealed a markedly lower conversion rate compared to open surgery in the Robotic arm [2% vs 7.5%, P=0.0007] with operative time, lymph node harvest, CRM positivity rate and anastomotic leak rates being similar [Table 6].[19] Another large meta-analysis by Xiong et al., showed statistically significant lower CRM positivity and conversion rates favouring the robotic approach with operative times and local recurrence rates remaining similar [Table 6].[20]
Table 6 a.
Meta-analysis of robotic versus laparoscopic TME for rectal cancer, short-term outcomes[19]

Table 6b.
Meta-analysis of robotic versus laparoscopic TME, short term and long-term outcomes [20]
Genitourinary Function After Robotic TME
An important aspect of Robotic TME is better visualization of the autonomic plexii in the pelvis, which translates into better preservation of genitourinary function as assessed by erectile dysfunction and voiding function.
The MRC CLASICC trial showed a trend towards increased sexual dysfunction in the laparoscopic arm in comparison to the open group.[1] Comparison of robotic and laparoscopic groups in the study by Kim et al.[21] showed decreased sexual desire and voiding function in both groups 1 month after surgery with more rapid and complete recovery of both parameters in the robotic group; possibly due to a more delicate operation with the robotic apparatus. Luca et al., found better preservation of voiding and sexual function with robotic TME in comparison with open and laparoscopic TME with complete recovery of both functions 1 year after surgery.[22]
For surgeons starting the learning process of minimally invasive proctectomy for rectal cancer, it appears reasonable to invest more time in learning the robotic approach as against the laparoscopic approach to attain the goal of curative radical rectal resection. However as we describe in detail below proficiency in laparoscopic techniques is still mandatory regardless of whether a purely robotic approach or a hybrid laparoscopic-robotic approach is used, to maximize the efficiency of the operation.
Technical Aspects of RTME
The three essential components of the operation namely:
Inferior mesenteric pedicle ligation and left colon mobilization.
Splenic flexure mobilization.
Total Mesorectal excision and anastomosis.
Can be performed using a purely robotic approach with multiple docking, moving the robot around the patient from the left shoulder to the left hip and then to the position between the legs or as a hybrid laparoscopic robotic approach which we propagate as it is more efficient and utilizes the robot for the more difficult part of the operation which is the pelvic component.
The completely robotic approach has also been described with a single position of the robot at the left hip, with use of two sets of port positions with two dockings, one set for steps 1 and 2 and one for step 3.[23] A purely robotic approach is more time consuming and does not provide any benefit over the hybrid approach.
Robotic TME in the Obese Patient
Obesity BMI≥ 30 Kg/m2 is a rapidly increasing epidemic in the western world with an estimated 35.7% of the American population being obese.[24] The age-adjusted prevalence of obesity was 35.5% (95% CI, 31.9-39.2%) among adult men and 35.8% (95% CI, 34.0-37.7%) among adult women in the 2009-2010 National Health and Nutrition Examination Survey (NHANES).[24]
Obesity is associated with several anatomic issues specific to colorectal surgery including:
Problems with exposure.
Fragile peritoneum.
Bulky and heavy omentum.
Heavy mesorectum with fragile mesorectal fascia.
Abundant presacral tissue.
A more vertical course of the levator muscles.
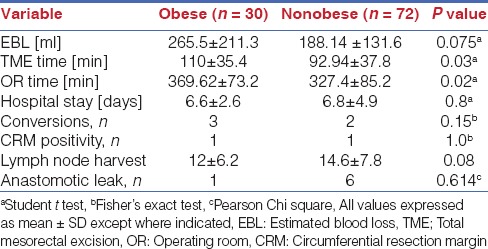
All of these make TME in the obese pelvis especially in the male patient with a low rectal cancer after preoperative radiation a difficult proposition. Obesity is a known risk factor for worse perioperative outcomes and morbidity including anastomotic leaks in open and laparoscopic rectal cancer surgery.[25] Recurrence rates and sphincter preservation rates are also inversely correlated with BMI in some studies.[26] Increasing BMI has also been identified as a risk factor for conversion from laparoscopy to open approach in rectal cancer surgery[27,28,29,30] [Table 7] and the conversion from laparoscopy to the open approach leads to longer operating times, increasing cost, higher perioperative complications and a longer hospital stay, besides the possibility of worse long term survival.[2] It is in this subgroup that robotic surgery shows its greatest promise and our experience shows that the robot levels the playing field and enables equivalent outcomes irrespective of BMI and regardless of whether the patient is male or female [Table 8].
Table 7.
Relation between BMI and conversion rates
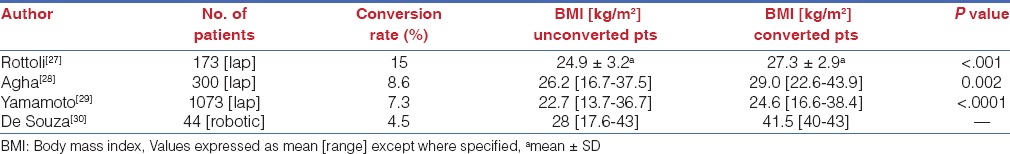
Table 8.
Comparison of outcomes of RTME obese versus nonobese patients
Robotic Cylindrical APR
The abdominoperineal resection is the only oncologically sound operation for patients with low rectal cancers with sphincter complex involvement or encroachment into the anal canal. The absence of mesorectum in the terminal portions of the rectum however make it difficult to achieve a wide cuff of soft tissue at this location and it is known that CRM positivity and iatrogenic perforation rates tend to be higher with a corresponding worse clinical outcome in this subgroup of patients when a standard resection is performed.[31] The technique of extralevator APR [eLAPR] is now the preferred approach to these tumors, but is difficult to perform using the open or laparoscopic technique because the wide transection of the levators is performed via the perineum and is essentially blind with respect to the planes already developed abdominally. Our technique of robotic cylindrical levator transection allows a controlled wide levator transection from above and completes the essential oncologic steps under vision.[32] Only the skin and ischioanal fat need to be transected perineally. This allows for a better quality of specimen and avoids CRM positivity and perforation and produces a cylindrical specimen.
Cost Issues with RTME
Robotic surgery is more expensive than laparoscopic or open surgery due to:
The fixed costs of purchase and maintenance which has to be amortized.
Consumables (drapes and instruments with limited lifespan).
Increased operative time.
Cost needs to be weighed against parameters like shorter length of stay and oncologic outcomes. Without robust randomized data however cost continues to remain an issue especially in systems where robotic surgery is paid on par with laparoscopic surgery. The additional cost is borne either by the hospital or the patient and does not make for a good economic model.
The da Vinci surgical system [Intuitive surgical Inc, Sunnyvale CA, USA] is the only surgical robot currently available for use. The lack of competition may be a factor keeping costs static and high today.
One modifiable factor, which can decrease this cost, is an increase in the annual caseload of robotic procedures, which reduces the amortized costs of the robot and the annual maintenance per procedure.[33] This can be achieved in 2 ways: By increasing the number of surgeons who are trained in the use of the robotic system and secondly by having a well trained perioperative nursing team. Having a consistent team of surgeons, perioperative nurses and scrub personnel also reduces setup times markedly as shown by Hanly et al., who demonstrated reduced setup times by 29.2% and 56.1% on the second and third robotic setups respectively.[34]
Operative Time and RTME
Operative time is a critical issue when studying outcomes of robotic rectal surgery because it decreases the number of procedures that can be performed and drives up the operating room costs. Operative times are related to some extent to the learning curves and with increasing surgeon and institution volumes the gulf between robotic and laparoscopic colorectal procedure times is steadily decreasing. D’Annibale et al., published their experience showing no difference in total operating times between laparoscopic and robotic groups, though patient preparation and operating room times were prolonged in the robotic group.[35] They found that the time added in robotic docking was balanced by faster, more accurate dissection due to use of the robot.
Standardization of the procedure, consistency of surgical-nursing teams, and incremental increase in surgeon experience and volumes all have the potential to decrease operative time.
Ongoing Trials of RTME
The ROLARR Trial [Robotic assisted versus laparoscopic assisted resection for rectal cancer] is an ongoing pan world multicenter prospective, controlled, unblended, parallel group superiority trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer, which aims to randomize 200 patients in each arm.[36] The primary outcome measure is rate of conversion to open surgery. The secondary outcome measures are pathological CRM positivity and 3-year local recurrence rates. This trial should provide conclusive evidence regarding the role of robotic assisted resection for rectal cancer.
CONCLUSION
In conclusion, Robotic total mesorectal excision has several benefits in the treatment of rectal cancer and should be part of the armamentarium of the experienced surgeon dealing with this disease. The intraoperative ergonomics are superb, facilitated by excellent vision and manoeuvrability in the narrow confined pelvic space and this translates into a more reproducible operation for the master surgeon and the trainee. While cost remains an issue, as with laparoscopy costs are expected to decrease with time especially in a multispecialty setup where multiple departments are using the robot. Operative time while still higher than the laparoscopic approach, rapidly decreases with experience and is likely to be less of an issue once advanced platforms that permit multiquadrant surgery without the need for redocking are more widely available. Short term and long-term oncological outcomes satisfy all measures of oncologic adequacy and the results of the ROLARR trial are awaited to clearly give a direction to this technique.
Footnotes
Source of Support: No financial support was received in the research and writing of this manuscript
Conflict of Interest: None of the authors have any conflict of interest.
REFERENCES
- 1.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet. 2005;365:1718–26. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 2.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–45. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 3.Kayano H, Okuda J, Tanaka K, Kondo K, Tanigawa N. Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc. 2011;25:2972–9. doi: 10.1007/s00464-011-1655-8. [DOI] [PubMed] [Google Scholar]
- 4.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–60. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 5.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Colorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–8. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 6.Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–45. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 7.Akmal Y, Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Robot-assisted total mesorectal excision: Is there a learning curve? Surg Endosc. 2012;26:2471–6. doi: 10.1007/s00464-012-2216-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim YW, Lee HM, Kim NK, Min BS, Lee KY. The learning curve for robot-assisted total mesorectal excision for rectal cancer. Surg Laparosc Endosc Percutan Tech. 2012;22:400–5. doi: 10.1097/SLE.0b013e3182622c2d. [DOI] [PubMed] [Google Scholar]
- 9.Hellan M, Anderson C, Ellenhorn JD, Paz B, Pigazzi A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol. 2007;14:3168–73. doi: 10.1245/s10434-007-9544-z. [DOI] [PubMed] [Google Scholar]
- 10.Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, et al. Robotic versus laparoscopic low anterior resection of rectal cancer: Short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16:1480–7. doi: 10.1245/s10434-009-0435-3. [DOI] [PubMed] [Google Scholar]
- 11.Choi DJ, Kim SH, Lee PJ, Kim J, Woo SU. Single-stage totally robotic dissection for rectal cancer surgery: Technique and short-term outcome in 50 consecutive patients. Dis Colon Rectum. 2009;52:1824–30. doi: 10.1007/DCR.0b013e3181b13536. [DOI] [PubMed] [Google Scholar]
- 12.Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg. 2010;251:882–6. doi: 10.1097/SLA.0b013e3181c79114. [DOI] [PubMed] [Google Scholar]
- 13.Pigazzi A, Luca F, Patriti A, Valvo M, Ceccarelli G, Casciola L, et al. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17:1614–20. doi: 10.1245/s10434-010-0909-3. [DOI] [PubMed] [Google Scholar]
- 14.Baik SH, Kim NK, Lim DR, Hur H, Min BS, Lee KY. Oncologic outcomes and perioperative clinicopathologic results after robot-assisted tumor-specific mesorectal excision for rectal cancer. Ann Surg Oncol. 2013;20:2625–32. doi: 10.1245/s10434-013-2895-8. [DOI] [PubMed] [Google Scholar]
- 15.deSouza AL, Prasad LM, Ricci J, Park JJ, Marecik SJ, Zimmern A, et al. A comparison of open and robotic total mesorectal excision for rectal adenocarcinoma. Dis Colon Rectum. 2011;54:275–82. doi: 10.1007/DCR.0b013e3182060152. [DOI] [PubMed] [Google Scholar]
- 16.Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: A comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25:240–8. doi: 10.1007/s00464-010-1166-z. [DOI] [PubMed] [Google Scholar]
- 17.Ghezzi TL, Luca F, Valvo M, Corleta OC, Zuccaro M, Cenciarelli S, et al. Robotic versus open total mesorectal excision for rectal cancer: Comparative study of short and long-term outcomes. Eur J Surg Oncol. 2014;40:1072–9. doi: 10.1016/j.ejso.2014.02.235. [DOI] [PubMed] [Google Scholar]
- 18.Baik SH, Ko YT, Kang CM, Lee WJ, Kim NK, Sohn SK, et al. Robotic tumor-specific mesorectal excision of rectal cancer: Short-term outcome of a pilot randomized trial. Surg Endosc. 2008;22:1601–8. doi: 10.1007/s00464-008-9752-z. [DOI] [PubMed] [Google Scholar]
- 19.Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, et al. Robotic resection compared with laparoscopic rectal resection for cancer: Systematic review and meta-analysis of short-term outcome. Colorectal Dis. 2012;14:e134–56. doi: 10.1111/j.1463-1318.2011.02907.x. [DOI] [PubMed] [Google Scholar]
- 20.Xiong B, Ma L, Zhang C, Cheng Y. Robotic versus laparoscopic total mesorectal excision for rectal cancer: A meta-analysis. J Surg Res. 2014;188:404–14. doi: 10.1016/j.jss.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: Laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19:2485–93. doi: 10.1245/s10434-012-2262-1. [DOI] [PubMed] [Google Scholar]
- 22.Luca F, Valvo M, Ghezzi TL, Zuccaro M, Cenciarelli S, Trovato C, et al. Impact of robotic surgery on sexual and urinary functions after fully robotic nerve-sparing total mesorectal excision for rectal cancer. Ann Surg. 2013;257:672–8. doi: 10.1097/SLA.0b013e318269d03b. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Kwak JM. Robotic total mesorectal excision: Operative technique and review of the literature. Tech Coloproctol. 2013;17(Suppl 1):S47–53. doi: 10.1007/s10151-012-0939-x. [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 25.Gendall KA, Raniga S, Kennedy R, Frizelle FA. The impact of obesity on outcome after major colorectal surgery. Dis Colon Rectum. 2007;50:2223–37. doi: 10.1007/s10350-007-9051-0. [DOI] [PubMed] [Google Scholar]
- 26.You JF, Tang R, Changchien CR, Chen JS, You YT, Chiang JM, et al. Effect of body mass index on the outcome of patients with rectal cancer receiving curative anterior resection: Disparity between the upper and lower rectum. Ann Surg. 2009;249:783–7. doi: 10.1097/SLA.0b013e3181a3e52b. [DOI] [PubMed] [Google Scholar]
- 27.Rottoli M, Bona S, Rosati R, Elmore U, Bianchi PP, Spinelli A, et al. Laparoscopic rectal resection for cancer: Effects of conversion on short term outcome and survival. Ann Surg Oncol. 2009;16:1279–86. doi: 10.1245/s10434-009-0398-4. [DOI] [PubMed] [Google Scholar]
- 28.Agha A, Furst A, Iesalnieks I, Fichtner-Feigl S, Ghali N, Krenz D, et al. Conversion rate in 300 laparoscopic rectal resections and its influence on morbidity and oncological outcome. Int J Colorectal Dis. 2008;23:409–17. doi: 10.1007/s00384-007-0425-5. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S, Fukunaga M, Miyajima N, Okuda J, Kopnishi F, Watanabe M. Impact of conversion on surgical outcomes after laparoscopic operation for rectal carcinoma: A retrospective study of 1073 patients. J Am Coll Surg. 2009;208:383–9. doi: 10.1016/j.jamcollsurg.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 30.deSouza AL, Prasad LM, Marecik SJ, Blumetti J, Park JJ, Zimmern A, et al. Total mesorectal excision for rectal cancer: The potential advantage of robotic assistance. Dis Colon Rectum. 2010;53:1611–7. doi: 10.1007/DCR.0b013e3181f22f1f. [DOI] [PubMed] [Google Scholar]
- 31.West NP, Finan PJ, Anderin C, Lindholm J, Holm T, Quirke P. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008;26:3517–22. doi: 10.1200/JCO.2007.14.5961. [DOI] [PubMed] [Google Scholar]
- 32.Marecik SJ, Zawadzki M, Desouza AL, Park JJ, Abcarian H, Prasad LM. Robotic cylindrical abdominoperineal resection with transabdominal levator transection. Dis Colon Rectum. 2011;54:1320–5. doi: 10.1097/DCR.0b013e31822720a2. [DOI] [PubMed] [Google Scholar]
- 33.Nayeemuddin M, Daley SC, Ellsworth P. Modifiable factors to decrease the cost of robotic-assisted procedures. AORN J. 2013;98:343–52. doi: 10.1016/j.aorn.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Hanly EJ, Marohn MR, Bachman SL, Talamini MA, Hacker SO, Howard RS, et al. Multiservice laparoscopic surgical training using the daVinci Surgical System. Am J Surg. 2004;187:309–15. doi: 10.1016/j.amjsurg.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 35.D’Annibale A, Morpurgo E, Fiscon V, Trevisan P, Sovernigo G, Orsini C, et al. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum. 2004;47:2162–8. doi: 10.1007/s10350-004-0711-z. [DOI] [PubMed] [Google Scholar]
- 36.Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis. 2012 Feb;27:233–41. doi: 10.1007/s00384-011-1313-6. [DOI] [PubMed] [Google Scholar]


