Abstract
Robotic surgery was initially developed to overcome problems faced during conventional laparoscopic surgeries and to perform telesurgery at distant locations. It has now established itself as the epitome of minimally invasive surgery (MIS). It is one of the most significant advances in MIS in recent years and is considered by many as a revolutionary technology, capable of influencing the future of surgery. After its introduction to urology, robotic surgery has redefined the management of urological malignancies. It promises to make difficult urological surgeries easier, safer and more acceptable to both the surgeon and the patient. Robotic surgery is slowly, but surely establishing itself in India. In this article, we provide an overview of the advantages, disadvantages, current status, and future applications of robotic surgery for urologic cancers in the context of the Indian scenario.
Keywords: Robot-assisted partial nephrectomy, robot-assisted radical cystectomy, robot-assisted radical prostatectomy, robot-assisted retroperitoneal lymph node dissection, robotic surgery, urologic oncology
EVOLUTION OF ROBOTIC SURGERY
International Organisation for Standardisation has defined an industrial robot as an automatically controlled, reprogrammable, multipurpose manipulator programmable in three or more axes.[1] Conforming to this definition, the first industrial robot was developed in 1937, and the first robotic patent was granted to George Devol in 1961.[2] Twenty two years later, the first surgical robot was developed in Vancouver, Canada in 1983 and 1-year later the first robotic surgery was performed at UBC Hospital, Vancouver.[3] Since then, robotic surgery has undergone a long evolution with the introduction of PROBOT (1992) to assist prostatic surgery at Guy's and St. Thomas’ Hospital, London, ROBODOC (1992) for assisting orthopaedic surgery;[4] and AESOP and the ZEUS robotic surgical systems (Computer Motion Inc., Santa Barbara, CA) for assisting gynaecological and cardiac surgeries.[5] The original telesurgery based robotic system was initially developed for assisting surgeries at the battlefield and other remote surgical areas. Based on this concept the da Vinci system (Intuitive Surgical Inc., Mountain View, CA) was first introduced in 1999 and was granted Food and Drug administration approval in July 2000 for laparoscopic surgeries.[6] Surgical robots have now evolved to become the epitome of minimally invasive surgery (MIS). Apart from the advantages of robotic surgery, market-driven forces and the patients’ choice for robotic surgery have also played a key role in the expansion of this technology.
ADVANTAGES OF ROBOTIC SURGICAL SYSTEM
The inception of surgical robots took place to overcome the hurdles of conventional laparoscopic technology and to develop distance monitored telesurgery. In a master-slave system, the surgeon operates while sitting comfortably on a master console thus making it ergonomically optimal and less stressful for the surgeon to operate. Vision is provided by a dual 3-chip camera, which gives a magnified three-dimensional (10-12X) vision and provides a better depth perception to the surgeon when compared to conventional laparoscopy. Robotic instruments are equipped with an “endowrist” technology, which puts the fulcrum right next to the tip of the instrument, thereby permitting fine, dexterous movements in multiple planes with a wide range of motion. Further, movements are scaled up to 3:1 and hence that large movements of the master controls are manifested as micro-movements of the robotic instruments. All these features of the surgical robot make it optimal for surgeries, which were once considered difficult or even impossible by conventional laparoscopy. Apart from these advantages, a surgical robot can also be used for telesurgery across the globe. One such example is operation Lindbergh performed on 7 September 2001, where a surgeon in New York performed a transatlantic cholecystectomy on a patient in Strasbourg, France over a dedicated fibre optic line.[7]
LIMITATIONS OF ROBOTIC SURGICAL SYSTEM
The main limitations of the surgical robot are its cost, need for specifically trained extra staff and lack of tactile sensations. The estimated cost of the da Vinci surgical system (Intuitive Surgical Inc., Sunnyvale, California, USA) is about $1.7 million with additional annual maintenance fees and a disposable supply cost of approximately $1500/case.[8] This heavy cost has made it out of reach for many institutions. Another concern regarding robotic surgery is its learning curve. Although it has been shown that robotic surgery can be learnt faster than conventional laparoscopy, there is a definite learning curve involved in the assimilation and optimal implementation of any robotic procedure. Depending on the variable being studied, it may take anywhere from 8 to 150 cases to reach a plateau on the learning curve.[9] A deficiency of structured training facilities in robotic surgery along with a lack of exposure to this modality during residency training in most institutions in this part of the world also adds to the hurdles in the expansion and safe implementation of robotic surgery in India.
ROBOTIC SURGERY FOR PROSTATE CANCER
The MIS benefits of robot-assisted radical prostatectomy (RARP) over conventional retro pubic radical prostatectomy (RRP) are well-documented in the literature. Many of the authors have reported significantly less blood loss during RARP,[10,11] even if it was performed by a non-robotic trained urologic oncologist assisted at the patient side by a trained robotic surgeon.[12] This can be attributed to better visualisation of the dorsal venous complex and the tamponade effect provided by the pneumoperitoneum. Contrary to most other laparoscopic or robotic procedures, there is conflicting evidence in support of RARP with regards to a decrease in postoperative pain.[13,14] This is attributed to the infraumblical midline muscle splitting incision used for RRP, which is generally less painful. With increasing experience, the length of hospital stay after prostatectomy has decreased drastically irrespective of the surgical modality used and mainly depends on the hospital policy. While few centres in the United States have a policy of equivalent stay and early discharge for both RRP and RARP,[15] others have reported shorter hospital stay after RARP compared with RRP.[16] As with other surgical procedures the mean operative duration during the initial phase of the learning curve is longer but once adequately experienced, the operating time of RARP decreases to <3 h.[17,18]
One of the most important concerns after radical prostatectomy is its functional outcome. Recovery of erectile function and urinary continence has improved over time due to a better understanding of the functional anatomy and refinement of surgical techniques. As surgical robots provide improved visualisation and dexterity as compared to open surgery, there is likely to be a better preservation of the constituents of the urinary sphincter complex, thus leading to superior continence rates over RRP. Many studies have demonstrated a faster recovery of urinary continence after RARP.[19] The impact of RARP on preservation of erectile function is difficult to predict due to variations in patient profiles, baseline erectile function, surgical approaches and definitions used for defining potency. There is, however, some evidence to support early recovery of erectile function with RARP when compared to RRP.[20] The oncological outcome of radical prostatectomy, irrespective of the surgical approach, is assessed by the incidence of positive surgical margins and rates of biochemical prostate-specific antigen recurrence. Studies have demonstrated that RARP and RRP and equivalent in this regard.[21,22]
ROBOTIC SURGERY FOR RENAL CANCER
After the success of robotics in radical prostatectomy, its use was explored for other urological malignancies. The benefits of laparoscopic radical nephrectomy, like shorter hospital stay, less pain and faster return to normal activities are well-established. Robotic surgery has not demonstrated any added advantage to conventional laparoscopic radical nephrectomy.[23] The significant negative impact of radical nephrectomy on renal function and overall survival is well documented.[24,25] This drawback of radical nephrectomy, along with an equivalent oncological outcome after partial nephrectomy[26] has led to increased indications and acceptance of a nephron-sparing approach. Many authors have reported an equivalence of laparoscopic and open techniques of partial nephrectomy in terms of survival and oncological outcomes.[27,28] Laparoscopic partial nephrectomy, however, is both technically and mentally challenging due to the pressure of performing a technically difficult surgery with intracorporeal suturing within a stipulated time to avoid ischaemic renal injury, while ensuring a good haemostasis. Surgical robots, when compared with conventional laparoscopic surgery, have better vision, stability and improved dexterity–qualities that can help in overcoming these hurdles. Thus, robotic surgery has a promising role in increasing the acceptability of partial nephrectomy. Many authors have reported the benefits of robot-assisted partial nephrectomy (RAPN) over conventional laparoscopy in terms of less bleeding, shorter warm ischemia time and a shorter operative time. In a retrospective review of 183 patients who underwent RAPN, Benway, et al. reported a mean total operative time of 210 min, mean ischemic time of 23.9 min and the mean estimated blood loss of 131.5 ml.[29] Another study that compared outcomes of RAPN with conventional laparoscopic partial nephrectomy reported less intra-operative blood loss (155 vs. 196 ml) and shorter warm ischemic times (19.7 vs. 28.4 min) in the RAPN group.[30] It is, however, more important to know the impact of RAPN on renal function preservation and oncological outcomes. In a systematic review of 8 studies comparing RAPN with open partial nephrectomy (OPN), RAPN had a lower overall complication rate and a shorter hospital stay as compared to OPN. There was no difference in total ischaemia time, post-surgery estimated glomerular filtration rate change and the rate of positive surgical margins between the two groups. However, the trend towards a higher rate of tumour recurrence and metastasis was observed in the OPN cohort.[31] These studies have established the feasibility, safety and advantages of RAPN over conventional laparoscopic partial nephrectomy.
ROBOTIC SURGERY FOR URINARY BLADDER CANCER
Radical cystectomy is the gold standard treatment for muscle invasive bladder cancer. The benefits of MIS have been extended to the management of this disease. Current data suggests that laparoscopic radical cystectomy (LRC) is associated with low morbidity and has comparable oncological outcomes when compared to the results of conventional open radical cystectomy (ORC).[32] Encouraged by outcomes of LRC, the feasibility of robot-assisted radical cystectomy (RARC) has been studied. These studies have reported advantages of RARC over ORC in terms of lesser blood loss, early recovery of bowel function and a shorter hospital stay.[33] Similarly, Cheung and associates, in their review of the literature, reported that most short term studies in their 2-3 years follow-up have reported a 70-90% overall survival with RARC.[34] These studies have shown a promising role of RARC in terms of feasibility, blood loss and oncological outcomes.
Pelvic lymph node dissection is an important part of radical cystectomy. It has vital implications towards staging the disease and planning adjuvant therapy, while having a potential therapeutic role in limited disease. Currently, lymph node yield is considered a surrogate for the adequacy of lymph node dissection.[35] There is clear evidence now to suggest that a robotic approach does not compromise the adequacy and completeness of lymph node dissection during radical cystectomy.[36,37] In a recent retrospective analysis of the international robotic cystectomy consortium database of 527 patients who underwent RARC at 15 institutions, the lymph node yield of RARC for advanced bladder cancer was similar to that of the open cystectomy series.[37] The learning curve of RARC is short and ranges from 16 to 20 cases.[38,39] For urinary diversion after robot-assisted cystectomy, the extracorporeal technique is the current preferred approach of most surgeons. However, studies have shown the feasibility of a complete robotic intracorporeal urinary diversion, with potential advantages like, lesser incisional pain, decreased bowel exposure, and the potential for decreased fluid imbalances.[40] Though the results of RARC are encouraging, most of these studies are small, single institution based case series, lacking long-term follow-up. There is clearly a need for more data in order to establish RARC at the epicentre of bladder cancer management.
ROBOTIC SURGERY FOR TESTICULAR CANCER
Published literature on laparoscopic retroperitoneal lymph node dissection (L-RPLND) suggests that it is a feasible and effective treatment option for low stage non-seminomatous germ cell testicular tumours (NSGCT) with its attendant MIS benefits.[41] Rassweiler et al. performed a systematic literature analysis of >800 patients treated with L-RPLND and found that lymph node dissection based on modified templates removed an average of 16 (5-36) lymph nodes. Compared to the open approach, L-RPLND did not differ in terms of relapse rates, percentage of patients receiving chemotherapy (29% vs. 31%) and rate of salvage surgery (1.2% vs. 1.5%).[42] However, L-RPLND is a technically difficult procedure requiring laparoscopic experience and skills. The surgical robot, equipped with a three-dimensional magnified vision and scaled movements of the instruments with an elimination of hand tremor, offers a great promise to overcome these hurdles. The initial data on robot-assisted RPLND (R-RPLND) is in the form of case reports[43] and small case series.[44] These studies have stated the feasibility of R-RPLND in low stage NSGCT. However, larger studies with long-term data are required to validate these results and establish R-RPLND at the forefront of NSGCT management.
ROBOTIC SURGERY FOR PENILE CANCER
Conventional radical inguinal lymph node dissection is associated with high local morbidity. Video endoscopic inguinal lymphadenectomy (VEIL) using traditional laparoscopic instruments has been reported to reduce the surgical morbidity without compromising oncologic outcomes.[45] Robotic-VEIL offers increased magnification, three-dimensional clarity, additional degrees of freedom and more precise and controlled dissection during surgery. Initial case reports have shown the feasibility of performing robotic-VEIL, with operating time ranging from 90 to 130 min for one side.[46,47] Sotelo et al. performed bilateral inguinal lymph node dissection in the same sitting and reported a total operative time of 360 min and mean blood loss of 100 ml.[48] These results point towards a promising role of robotic surgery in the management of inguinal lymph nodes, but need further long-term randomised studies for validation.
FUTURE ADVANCES IN ROBOTIC SURGERY
Currently, the da Vinci surgical system is an unchallenged leader in the $5 billion surgical robot market. Any new system, which wants to challenge the market stronghold of the da Vinci system has to tap into the drawbacks associated with it; by reducing the cost, shortening the learning curve, and introducing technical advancements like tactile sensation and reduction in the number of ports, etc. Titan Medical Inc., (Toronto, Ontario, Canada) is developing single port orifice robotic technology (SPORT™) robotic surgical system.[49] It's expected to be commercially available by 2015. It includes a single-port surgeon controlled robotic platform (with three-dimensional vision system and interactive instruments) and a surgeon workstation. This system is being developed with an aim to expand robotic surgery into areas that are currently underserviced, such as cholecystectomy, appendectomy and ENT procedures. With the advent of SPORT™ and other such robotic systems under development, the science and art of surgery may be at the cusp of a robotic revolution!
ROBOTIC SURGERY IN INDIA
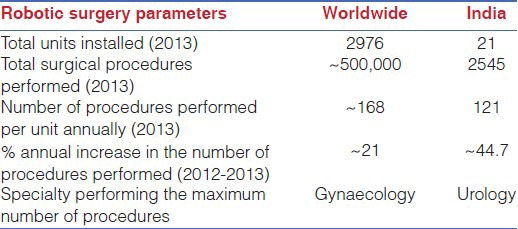
The robotic surgery market is dynamic and rapidly expanding worldwide, and India is not left untouched by it. Due to the increased cost of surgery, there was an initial apprehension about the success of the robotic surgical programs in India. Nelivigi, in his review article, discounted robotic surgery as just another “me too technology,” with no promising future in a developing country like India.[50] However, contrary to previous apprehensions, robotic surgery is now gaining increased acceptance in India as revealed by an annual 44% rise in the number of procedures performed in India in 2013 (in contrast to ~21% rise worldwide). As per the unpublished data sourced from Intuitive Surgical (Intuitive Surgical Inc., Mountain View, CA), 21 da Vinci surgical units had been installed in India at the time of preparation of this manuscript [Table 1]. Most of these (16/21), are installed in the private sector hospitals. There is an interesting contrast in the spectrum of robotic surgery performed worldwide and in India. While gynaecological surgeries remained the most commonly performed robotic surgeries worldwide, urological procedures dominated in India. As dedicated training facilities are created and a larger number of surgeons are trained in this craft, a further surge in robotic surgery can be expected in India in the very near future.
Table 1.
Robotic surgery in India
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.ISO Standard 8373: Manipulating Industrial Robots – Vocabulary. 1994. [Last retrieved on 2014 Apr 06]. Available from: http://www.iso.org/iso/catalogue_detail.htm?csnumber=15532 .
- 2.The First Industrial Robot. [Last retrieved on 2014 Apr 06]. Available from: http://www.historyofinformation.com/expanded.php?id=4071 .
- 3.Canada: Rogers Publishing Ltd; 1985. [Last retrieved on 2014 Apr 06]. Medical Post. Available from: http://en.wikipedia.org/wiki/Robotic_surgery . [Google Scholar]
- 4.Pransky J. ROBODOC – Surgical robot success story. Ind Rob. 1997;24:231–3. [Google Scholar]
- 5.Michelle M. Computer-Assisted Surgery: An Update. FDA Consumer magazine. [Last retrieved on 2014 Apr 06]. Available from: http://www.fda.gov/fdac/features/2005/405_computer.html .
- 6.da Vinci Si Surgical System. [Last retrieved on 2014 Apr 06]. Available from: http://www.intuitivesurgical.com/company/profile.html#sthash.FsxmQ5ZN.dpuf .
- 7.Brower V. The cutting edge in surgery. Telesurgery has been shown to be feasible – now it has to be made economically viable. EMBO Rep. 2002;3:300–1. doi: 10.1093/embo-reports/kvf083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Slow Rise of the Robot Surgeon. MIT Technology Review. [Last retrieved on 2014 Apr 06]. Available from: http://www.technologyreview.com/news/418141/the-slow-rise-of-the-robot-surgeon/
- 9.Bach C, Miernik A, Schonthaler M. Training in robotics: The learning curve and contemporary concepts in training. Arab J Urol. 2013;10:5–6. doi: 10.1016/j.aju.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: A systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–63. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Kordan Y, Barocas DA, Altamar HO, Clark PE, Chang SS, Davis R, et al. Comparison of transfusion requirements between open and robotic-assisted laparoscopic radical prostatectomy. BJU Int. 2010;106:1036–40. doi: 10.1111/j.1464-410X.2010.09233.x. [DOI] [PubMed] [Google Scholar]
- 12.Perer E, Lee DI, Ahlering T, Clayman RV. Robotic revelation: Laparoscopic radical prostatectomy by a nonlaparoscopic surgeon. J Am Coll Surg. 2003;197:693–6. doi: 10.1016/S1072-7515(03)00723-3. [DOI] [PubMed] [Google Scholar]
- 13.Webster TM, Herrell SD, Chang SS, Cookson MS, Baumgartner RG, Anderson LW, et al. Robotic assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy: A prospective assessment of postoperative pain. J Urol. 2005;174:912–4. doi: 10.1097/01.ju.0000169455.25510.ff. [DOI] [PubMed] [Google Scholar]
- 14.Tewari A, Srivasatava A, Menon M. Members of the VIP Team. A prospective comparison of radical retropubic and robot-assisted prostatectomy: Experience in one institution. BJU Int. 2003;92:205–10. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 15.Nelson B, Kaufman M, Broughton G, Cookson MS, Chang SS, Herrell SD, et al. Comparison of length of hospital stay between radical retropubic prostatectomy and robotic assisted laparoscopic prostatectomy. J Urol. 2007;177:929–31. doi: 10.1016/j.juro.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 16.Rocco B, Matei DV, Melegari S, Ospina JC, Mazzoleni F, Errico G, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: A matched-pair analysis. BJU Int. 2009;104:991–5. doi: 10.1111/j.1464-410X.2009.08532.x. [DOI] [PubMed] [Google Scholar]
- 17.Badani KK, Kaul S, Menon M. Evolution of robotic radical prostatectomy: Assessment after 2766 procedures. Cancer. 2007;110:1951–8. doi: 10.1002/cncr.23027. [DOI] [PubMed] [Google Scholar]
- 18.Patel VR, Palmer KJ, Coughlin G, Samavedi S. Robot-assisted laparoscopic radical prostatectomy: Perioperative outcomes of 1500 cases. J Endourol. 2008;22:2299–305. doi: 10.1089/end.2008.9711. [DOI] [PubMed] [Google Scholar]
- 19.Skolarus TA, Zhang Y, Hollenbeck BK. Robotic surgery in urologic oncology: Gathering the evidence. Expert Rev Pharmacoecon Outcomes Res. 2010;10:421–32. doi: 10.1586/erp.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Myers RP, Blute ML, et al. Radical prostatectomy for prostatic adenocarcinoma: A matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448–53. doi: 10.1111/j.1464-410X.2008.08012.x. [DOI] [PubMed] [Google Scholar]
- 21.Masterson TA, Cheng L, Boris RS, Koch MO. Open vs. robotic-assisted radical prostatectomy: A single surgeon and pathologist comparison of pathologic and oncologic outcomes. Urol Oncol. 2013;31:1043–8. doi: 10.1016/j.urolonc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Barocas DA, Salem S, Kordan Y, Herrell SD, Chang SS, Clark PE, et al. Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: Comparison of short-term biochemical recurrence-free survival. J Urol. 2010;183:990–6. doi: 10.1016/j.juro.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Boger M, Lucas SM, Popp SC, Gardner TA, Sundaram CP. Comparison of robot-assisted nephrectomy with laparoscopic and hand-assisted laparoscopic nephrectomy. JSLS. 2010;14:374–80. doi: 10.4293/108680810X12924466007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zini L, Perrotte P, Capitanio U, Jeldres C, Shariat SF, Antebi E, et al. Radical versus partial nephrectomy: Effect on overall and noncancer mortality. Cancer. 2009;115:1465–71. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 25.Touijer K, Jacqmin D, Kavoussi LR, Montorsi F, Patard JJ, Rogers CG, et al. The expanding role of partial nephrectomy: A critical analysis of indications, results, and complications. Eur Urol. 2010;57:214–22. doi: 10.1016/j.eururo.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–5. [PubMed] [Google Scholar]
- 27.Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–6. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 28.Marszalek M, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S. Laparoscopic and open partial nephrectomy: A matched-pair comparison of 200 patients. Eur Urol. 2009;55:1171–8. doi: 10.1016/j.eururo.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Benway BM, Bhayani SB, Rogers CG, Porter JR, Buffi NM, Figenshau RS, et al. Robot-assisted partial nephrectomy: An international experience. Eur Urol. 2010;57:815–20. doi: 10.1016/j.eururo.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: A multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866–72. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Li M, Liu B, Cai C, Ye H, Lv C, et al. Robotic versus open partial nephrectomy: A systematic review and meta-analysis. PLoS One. 2014;9:e94878. doi: 10.1371/journal.pone.0094878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haber GP, Gill IS. Laparoscopic radical cystectomy for cancer: Oncological outcomes at up to 5 years. BJU Int. 2007;100:137–42. doi: 10.1111/j.1464-410X.2007.06865.x. [DOI] [PubMed] [Google Scholar]
- 33.Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: Perioperative and pathologic results. Eur Urol. 2010;57:196–201. doi: 10.1016/j.eururo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Cheung G, Sahai A, Billia M, Dasgupta P, Khan MS. Recent advances in the diagnosis and treatment of bladder cancer. BMC Med. 2013;11:13. doi: 10.1186/1741-7015-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buscarini M, Josephson DY, Stein JP. Lymphadenectomy in bladder cancer: A review. Urol Int. 2007;79:191–9. doi: 10.1159/000107949. [DOI] [PubMed] [Google Scholar]
- 36.Richards KA, Hemal AK, Kader AK, Pettus JA. Robot assisted laparoscopic pelvic lymphadenectomy at the time of radical cystectomy rivals that of open surgery: Single institution report. Urology. 2010;76:1400–4. doi: 10.1016/j.urology.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Hellenthal NJ, Hussain A, Andrews PE, Carpentier P, Castle E, Dasgupta P, et al. Lymphadenectomy at the time of robot-assisted radical cystectomy: Results from the International Robotic Cystectomy Consortium. BJU Int. 2011;107:642–6. doi: 10.1111/j.1464-410X.2010.09473.x. [DOI] [PubMed] [Google Scholar]
- 38.Guru KA, Perlmutter AE, Butt ZM, Piacente P, Wilding GE, Tan W, et al. The learning curve for robot-assisted radical cystectomy. JSLS. 2009;13:509–14. doi: 10.4293/108680809X12589998404128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pruthi RS, Smith A, Wallen EM. Evaluating the learning curve for robot-assisted laparoscopic radical cystectomy. J Endourol. 2008;22:2469–74. doi: 10.1089/end.2008.0320. [DOI] [PubMed] [Google Scholar]
- 40.Pruthi RS, Nix J, McRackan D, Hickerson A, Nielsen ME, Raynor M, et al. Robotic-assisted laparoscopic intracorporeal urinary diversion. Eur Urol. 2010;57:1013–21. doi: 10.1016/j.eururo.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 41.Poulakis V, Skriapas K, de Vries R, Dillenburg W, Ferakis N, Witzsch U, et al. Quality of life after laparoscopic and open retroperitoneal lymph node dissection in clinical Stage I nonseminomatous germ cell tumor: A comparison study. Urology. 2006;68:154–60. doi: 10.1016/j.urology.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Rassweiler JJ, Scheitlin W, Heidenreich A, Laguna MP, Janetschek G. Laparoscopic retroperitoneal lymph node dissection: Does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur Urol. 2008;54:1004–15. doi: 10.1016/j.eururo.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 43.Dogra PN, Singh P, Saini AK, Regmi KS, Singh BG, Nayak B. Robot assisted laparoscopic retroperitoneal lymph node dissection in testicular tumor. Urol Ann. 2013;5:223–6. doi: 10.4103/0974-7796.120289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams SB, Lau CS, Josephson DY. Initial series of robot-assisted laparoscopic retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell testicular cancer. Eur Urol. 2011;60:1299–302. doi: 10.1016/j.eururo.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Sotelo R, Sánchez-Salas R, Carmona O, Garcia A, Mariano M, Neiva G, et al. Endoscopic lymphadenectomy for penile carcinoma. J Endourol. 2007;21:364–7. doi: 10.1089/end.2007.9971. [DOI] [PubMed] [Google Scholar]
- 46.Josephson DY, Jacobsohn KM, Link BA, Wilson TG. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology. 2009;73:167–70. doi: 10.1016/j.urology.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 47.Dogra PN, Saini AK, Singh P. Robotic-assisted inguinal lymph node dissection: A preliminary report. Indian J Urol. 2011;27:424–7. doi: 10.4103/0970-1591.85458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sotelo R, Cabrera M, Carmona O, de Andrade R, Martin O, Fernandez G. Robotic bilateral inguinal lymphadenectomy in penile cancer, development of a technique without robot repositioning: A case report. Ecancermedicalscience. 2013;7:356. doi: 10.3332/ecancer.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Titan Medical Inc; [Last retrieved on 2014 Apr 06]. Available from: http://www.titanmedicalinc.com/wpcontent/uploads/2014/03/Titan-Investor-Presentation-March-18-2014.pdf . [Google Scholar]
- 50.Nelivigi GG. Robotic surgery: India is not ready yet. Indian J Urol. 2007;23:240–4. doi: 10.4103/0970-1591.33443. [DOI] [PMC free article] [PubMed] [Google Scholar]


