Abstract
INTRODUCTION:
Even today, open lobectomy involves significant morbidity. Video-assisted thoracic surgery (VATS) lobectomy results in lesser blood loss, pain, and hospital stay compared to lobectomy by thoracotomy. Despite being an excellent procedure in expert hands, VATS lobectomy is associated with a longer learning curve because of its inherent basic limitations. The da Vinci surgical system was developed essentially to overcome these limitations. In this study, we report our initial experience with robotic pulmonary resections using the Completely Portal approach with four arms. To the best of our knowledge this is the first series of robotic lobectomy reported from India.
MATERIAL AND METHODS:
Data on patient characteristics, operative details, complications, and postoperative recovery were collected in a prospective manner for patients who underwent Robotic Lung resection at our institution between March 2012 and April 2014 for various indications including both benign and malignant cases.
RESULTS:
Between March 2012 to April 2014, a total of 13 patients were taken up for Robotic Lobectomy with a median age of 57 years. The median operative time was 210 min with a blood loss of 33 ml. R0 clearance was achieved in all patients with malignant disease. The median lymph node yield in nine patients with malignant disease was 19 (range 11-40). There was one intra-operative complication and two postoperative complications. The median hospital stay was 7 days with median duration to chest tube removal being 3 days.
CONCLUSION:
Robotic lobectomy is feasible and safe. It appears to be oncologically sound surgical treatment for early-stage lung cancer. Comparable benefits over VATS needs to be further evaluated by long-term studies.
Keywords: Lobectomy, lung cancer, robot assisted thoracic surgery, robot, robotic lobectomy
INTRODUCTION
Lung resection morbidity and mortality had been high during the initial development of thoracic surgery. With greater knowledge of the anatomy and superior surgical, anaesthetic, and postoperative care, the mortality of the procedure has decreased but even today thoracotomy involves significant morbidity and leads to a relatively poorer quality of life in the postoperative period. Video-assisted thoracic surgery (VATS) lobectomy evolved in the early nineties and has become an efficient procedure that results in lesser blood loss, pain, and hospital stay compared to lobectomy by thoracotomy.[1] Majority of the thoracic surgeon from across the globe have accepted VATS lobectomy as the standard of care for early-stage lung cancer.[2] Numerous reports suggest toward an equivalent or some times better oncologic and long-term out comes for patients treated by VATS lobectomy compared with open thoracotomy.[3,4,5,6,7]
Current standards define minimally invasive lobectomy as an anatomic resection of the lobe with individual ligation of the vein, artery, and bronchus using video-thoracoscopic assistance and utilizing an incision not more than 5-7 cm with no rib-spreading. Management of the lymph nodes is controversial, but resection and pathological analysis of 11 to 16 nodes including two to three different mediastinal stations and the hilum appear to provide sufficient staging information for patients with lung cancer.[8]
Despite being an excellent procedure in expert hands, VATS lobectomy is associated with a longer learning curve because of certain basic limitations. This is because the surgeon has to work with long rigid instruments essentially in a counterintuitive manner with a 2-D vision and relatively poor ergonomics. The lack of manoeuvrability, especially in limited and poorly accessible areas makes fine dissection and dealing with large and fragile pulmonary vessels really demanding. These concerns about the VATS approach make it difficult to adopt.[9,10] Recent reviews have shown that over 70% of stage I lung cancers are still being performed by open technique.[11] The reported benefits of the length of stay for VATS lobectomy and nodal upstaging (being used as a marker of oncologic adequacy) in clinical stage I lung cancers are different when high volume VATS surgeons are compared with larger database like the STS database.[11,12,13] These findings suggest that surgeons are still struggling with the VATS platform and the outcomes claimed by expert VATS groups may not be replicated uniformly across the general thoracic surgical community.
The introduction of binocular 3-D vision together with the endo-wrist technology leading to intuitive instrument movements in the da Vinci surgical system essentially helped overcome these limitations and allowed the surgeon to virtually perform the lobectomy with his hands without the morbidity of a thoracotomy. It allows for a precise dissection with improved surgeon ergonomics. In our country, there are very few centres that offer VATS Lobectomy. Even at these centres, the technical difficulty in safely and adequately addressing the hilar structures and the mediastinal lymph nodes result in a relatively higher rate of conversion to open thoracotomy. The use of robotic assistance may reduce conversions and simplify minimally invasive thoracic surgery.
In this study, we report our initial experience with robotic pulmonary resection using the Completely Portal Robotic Lobectomy using four arms (CPRL-4) as popularized by Cerfolio.[14] To the best of our knowledge this is the first series of robotic lung resection reported from India.
MATERIALS AND METHODS
During March 2012 to April 2014, 13 patients underwent minimally invasive lobectomy using robotic assistance. The indications for lobectomy were nonsmall cell lung cancer in seven patients, bronchiectasis in one patient, metastatic lung lesion in one patient, aspergilloma in one patient, a neuro endocrine tumour in one patient, and a pulmonary hamartoma in one patient. The preoperative diagnosis together with the final histopathology is listed in Table 1.
Table 1.
Preoperative and histopathological diagnoses of patients who underwent robotic lobectomy
Robotic lobectomy was defined as use of the da Vinci Surgical System (Si) during a VATS lobectomy for individual dissection, isolation, and ligation of the pulmonary hilar structures, as well as mediastinal lymph node dissection. An informed written consent for robotic lobectomy was taken from all patients. Data on patient characteristics, operative details, complications, and postoperative recovery were collected in a prospective manner. We used the CPRL-4[14] approach in all our patients. Robotic lobectomy was offered to all patients who required lobectomy for various indications including both benign and malignant cases. All the cases included were otherwise good surgical candidates with normal pulmonary and cardiac function. Those with malignant lesions were proven to be mediastinal (N2) node negative. The patients who were not offered robotic lobectomy were those who had central tumors very close to the hilum and those with chest wall involvement.
Operative Technique
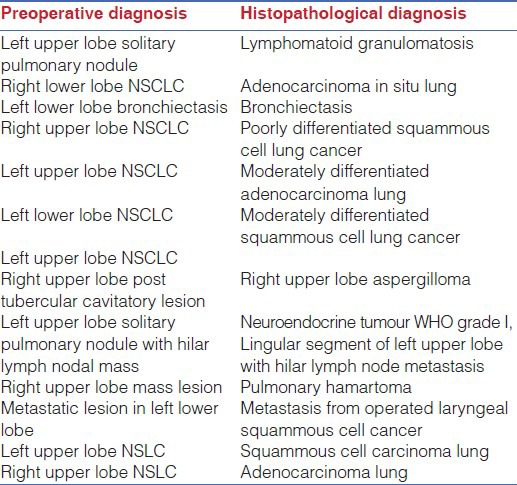
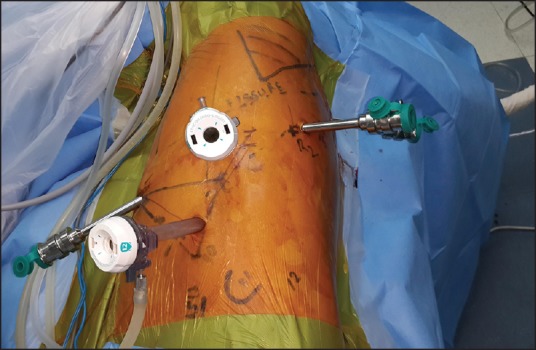
The completely portal robotic lobectomy using four robotic arms (CPRL-4) technique developed by Cerfolio RJ[14] has been described. The pleural space is entered using a 5-mm port inserted over the ninth rib into the eighth intercostal space approximately 20 cm away from the vertebral column. A 5 mm 30° telescope is used to explore the pleural cavity and to guide placement of other ports. This is followed by a paravertebral posterior intercostal block under visual guidance from third to eleventh intercostal space using 0.025% Bupivacaine. A 5 mm robotic port for robotic arm 3 is then placed 2 cm from the vertebral column under direct vision in the same inter costal space. Another 8 mm port for robotic arm 2 is placed approximately 10 cm from posterior port. It is very important to maintain this 10 cm distance between the third robotic arm and the adjacent arm, either robotic arm 1 and 2, to prevent external arm collisions. Two additional ports for robotic arm 1 and assistant port are then placed in a way that an isosceles triangle is formed between the camera port, assistant port and port for robotic arm 1 with apex at the assistant port [Figure 1]. The robotic cart was subsequently docked from the cranial side at an angle of 15° from the anterior side [Figure 2]. In our experience this port placement suffices for all the lobes whether upper or lower: Placing the ports in the space above eighth, as we did in the first few cases of upper lobectomies, caused problems in division of the inferior pulmonary ligament. Moreover, placing the camera port as far inferiorly as possible, taking care of the triangulation between the camera, the anterior and the assistant port, allows for a more panoramic view which helps in division of incomplete fissures.
Figure 1.
Triangulation between camera, assistant port and arm 1
Figure 2.
The patient cart is docked from the cranial end at an angle
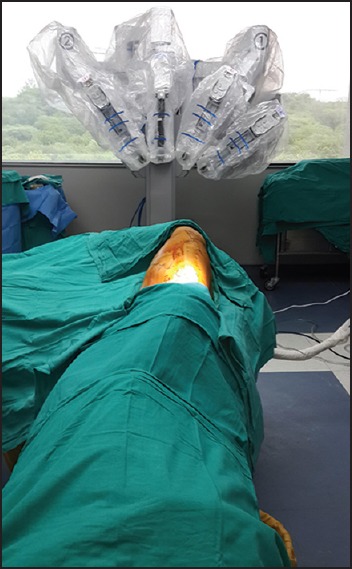
Further dissection is then carried out with robotic assistance and complete anatomical lobectomy is performed with individual division of the lobar vessels and the bronchus [Figure 3]. Endoscopic linear stapler with vascular loads was used for most vessels, although smaller vessels were sometimes divided between clips. A complete mediastinal lymph node dissection is then carried out with the robot. The lobectomy specimen is then placed in tough endobag and extracted from the assistant port by enlarging the incision to 3-4 cms.
Figure 3.
Intraoperative view of an anatomical left lower lobectomy. (a) The superior segmental branch to left lower lobe being dissected and looped. (b) Basilar Trunk to left lower lobe dissected and being looped. (c) Inferior Pulmonary vein dissected. (d) Left lower lobe bronchus being ready for division
RESULTS
Between March 2012 to April 2014, a total of 13 patients were taken up for Robotic Lobectomy. The patient characteristics are listed in Table 2. We performed five Left upper Lobectomies, three Right Upper Lobectomies, three Left Lower Lobectomies, one Right Lower Loebctomy and 1 Bilobectomy (Right Lower and Middle Lobes). The median operative time was 210 min (range 150-300 min). The median blood loss was 33 ml (range 25-150 ml). There was one conversion early in our experience while doing a right upper lobectomy. The procedure required conversion because of completely fused horizontal fissure and very close proximity of the lesion (NSCLC) to the fissure. In order to ensure a complete oncological resection the procedure was converted to a mini-thoracotomy for completing the procedure. Mediastinal Lymph Node dissection was done with robotic assistance in nine patients who had malignant disease. The median lymph node yield in these nine patients was 19 (range 11-40). Intraoperative complication occurred in one patient in the form of a mild injury to a branch of superior pulmonary vein during a left upper lobectomy for lung cancer. The bleeding was controlled with application of a vascular clamp through the assistant port and then dissecting the injured vein proximal to the injury site. We were able to apply an endoscopic linear stapler proximal to the injured area and divide the vein thus controlling the bleeding. Postoperative complications occurred in two patients. One of them developed atrial fibrillation on the second postoperative day. The other patient developed a prolonged air leak lasting for 2 weeks. The median hospital stay was 5 days. All patients had a single no 24 Fr chest drain placed except in one patient where two drains were used. The median duration to chest tube removal was 3 days (range 2-14 days).
Table 2.
Patient characteristics and perioperative results (n = 13)

DISCUSSION
In modern surgical practice, every effort is made to give due importance to patient's postoperative comfort and quality of life. Minimally Invasive Lobectomy for benign as well as malignant lesions with no compromise in terms of oncologic adequacy offers patients relatively reduced postoperative pain, shorter length of hospital stay with quicker recovery and significantly improved quality of life when compared to open procedures.[15,16,17,18,19,20] However, there are certain limitations of VATS which limits its widespread use. Lack of depth perception due to two-dimensional visualisation and counter-intuitive movements due to long rigid instruments fixed to the thoracic wall, offers poor operative ergonomics leading to longer learning curves making many thoracic surgeons relatively uncomfortable in handling the fragile pulmonary vessels.
The da Vinci Surgical System has now made it possible to overcome many of these disadvantages, without compromising oncologic adequacy or patient safety. Robotic surgery for thoracic disease is therefore likely to become widespread, provided the high costs of the robotic systems can be significantly reduced.
Our experience reported in this study reveals that robotic lobectomy is feasible and safe for a variety of indications including primary lung cancers. We had one complication in a cohort of 13 patients wherein there was a minor superior pulmonary vein injury, but we were able to manage the complication safely without conversion. Only one case in our series had to be electively converted to thoracotomy early in our experience with robotic approach, due to completely fused fissures and close proximity of the tumour with the fissure, and that too was not an emergency conversion. The median operative time in our series was 210 min, this is similar to 217 reported by Guilia Veronesi et al.[20] 218 min reported by Park, Flores, and Rusch[21] in 34 published cases and the 240 min reported by Gharagozloo, Margolis, and Tempesta.[22]
In our experience, we found the use of the fourth arm as a major advantage compared with the three-arm technique. It allows the console surgeon to retract the lung directly from the console; allowing adequate exposure just the way he wants. It also allows the assistant surgeon at the table to use the accessory port more efficiently while assisting in the operation.
In our series, we considered robotic approach for any patient with indication for lobectomy, who would otherwise be fit candidates for open surgical resection. There is a general perception amongst thoracic surgeons in our country that majority of our patients are not good candidates for minimally invasive surgery of the chest because of the fear of adhesions due to wide spread tuberculosis. One of the patients in our series was a case of posttubercular cavitatory lesion in right upper lobe with Aspergilloma. Moderately dense pleural symphysis was noted in this case. However, after an initial creation of enough space for port placement, we were able to complete the lobectomy using robotic assistance. We found that adhesiolysis was in fact made easier using robotic assistance. We want to emphasize the fact that most of the patients requiring chest surgery in India may be suitable candidates for minimally invasive approach and at the least warrant a thoracoscopic examination to assess feasibility of doing the procedure thoracoscopically.
One of our patients had persistent postoperative air leak. She was a case of Cushing's syndrome due to ectopic ACTH secreting tumour in left upper lobe with hilar nodal metastasis. Nine of our 13 patients were operated with a preoperative diagnosis of malignancy and all these nine patients underwent a complete robotic mediastinal lymph node dissection. The median lymph node yield was 19 ranging from 11-40. We were able to achieve a R0 resection in all these nine patients. The no of lymph nodes resected compares well with the lymph node yield reported by Veronesi et al.,[20] who reported a median lymph node yield of 17.5. As the experience with robotic dissection increases, we believe the lymph node yield will be higher. The median duration of stay in our report is 5 days. We believe it can be reduced further by liberal use of sealants and fibrin glue which may reduce the air leak and hence the duration of chest tube drainage.
An important consideration, particularly in our country is the cost involved. The use of robotic system involves an initial cost (between 1.5-2.5 million US Dollars). In addition to the initial cost there is a recurring cost of instruments and drapes together with annual maintenance charges. One can only use the instruments 10-20 times depending on the type of instrument, that is, whether of 5 or 8 mm diameter. In our hospital, the initial cost of the surgical system was absorbed by the hospital and therefore does not get transferred to patient cost. When compared with VATS Lobectomy Robotic Lobectomy costs an approximate INR 75000-100000 (1250-1650 USD) more. Probably with more extensive usage of the Robotic Surgical System the cost may be further brought down. According to a retrospective review by Shaun A Deen[23] et al., comparing open vs VATS vs Robotic Lung Resection VATS is the least expensive surgical approach. They concluded that robotic cases must be shorter in operative time or reduce supply costs, or both, to be competitive. Lessening operating time, eradicating unnecessary laboratory work, and minimizing intensive care unit stays will help decrease direct hospital costs.
The current literature shows that robotic surgery is feasible with safety and oncological adequacy. It gives equivalent if not inferior long-term results when compared to VATS and thoracotomy. R0 resection can be achieved in patients with cancer, even in patients with relatively larger tumours.[24] We strongly feel that the quality of lymph node dissection is far superior in robotic operations than VATS.
Despite all the obvious advantages of the robotic system, its limitations should also be borne in mind, which include loss of haptic feedback, limited platform availability, high initial and recurring cost and requirement of specially trained team.
CONCLUSION
Robotic lobectomy is a feasible and safe. It appears to be oncologically sound surgical treatment for early-stage lung cancer. There appears no doubt that it offers certain advantages to the surgeon, but whether it translates into cost effective benefits to the patients, needs to be further evaluated by long term studies. In conclusion we can say that robotic-assisted lung resection is here to stay, but long-term survival studies particularly in terms of long-term survival in cancer patients have to be carried out to prove its, as of now, perceived benefits over VATS. Moreover, the enthusiasm to adopt the surgical robot into one's thoracic surgical practice should be adequately backed up by proper training, careful patient selection, and a team-based robotic program development.
ACKNOWLEDGEMENT
We acknowledge the contribution of Dr. Robert James Cerfolio (Professor of Surgery and Chief of the Section of Thoracic Surgery at the University of Alabama at Birmingham) who performed three of the 13 cases in our series, as a part of a collaborative workshop organised at our institution.
Footnotes
Source of Support: None
Conflict of Interest: None.
REFERENCES
- 1.Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: A propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–78. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Cao C, Tian DH, Wolak K, Oparka J, He J, Dunning J, et al. Cross-sectional Survey on Lobectomy Approach (X-SOLA) Chest. 2014;146:292–8. doi: 10.1378/chest.13-1075. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi M, Yaginuma H, Yonechi A, Kanno R, Ohishi A, Suzuki H, et al. Long-term outcomes after video-assisted thoracic surgery (VATS)lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg. 2014;9:88. doi: 10.1186/1749-8090-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry MF, D’Amico TA, Onaitis MW, Kelsey CR. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg. 2014;98:197–202. doi: 10.1016/j.athoracsur.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao C, Zhu ZH, Yan TD, Wang Q, Jiang G, Liu L, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: A propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg. 2013;44:849–54. doi: 10.1093/ejcts/ezt406. [DOI] [PubMed] [Google Scholar]
- 6.Lee PC, Nasar A, Port JL, Paul S, Stiles B, Chiu YL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2013;96:951–60. doi: 10.1016/j.athoracsur.2013.04.104. [DOI] [PubMed] [Google Scholar]
- 7.Chen FF, Zhang D, Wang YL, Xiong B. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: A meta-analysis. Eur J Surg Oncol. 2013;39:957–63. doi: 10.1016/j.ejso.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Yan TD, Cao C, D’Amico TA, Demmy TL, He J, Hansen H, et al. International VATS Lobectomy Consensus Group.Video-assisted thoracoscopic surgery lobectomy at 20 years: A consensus statement. Eur J Cardiothorac Surg. 2014;45:633–9. doi: 10.1093/ejcts/ezt463. [DOI] [PubMed] [Google Scholar]
- 9.Yim AP. Video-assisted thoracic lung surgery: Is there a barrier to widespread adoption? Ann Thorac Surg. 2010;89:S2112–3. doi: 10.1016/j.athoracsur.2010.02.100. [DOI] [PubMed] [Google Scholar]
- 10.Rocco G, Internullo E, Cassivi SD, Van Raemdonck D, Ferguson MK. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin. 2008;18:235–47. doi: 10.1016/j.thorsurg.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Boffa DJ, Kosinski AS, Paul S, Mitchell JD, Onaitis M. Lymph Node Evaluation by Open or Video-Assisted Approaches in 11,500 Anatomic Lung Cancer Resections. Ann Thorac Surg. 2012;94:347–53. doi: 10.1016/j.athoracsur.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 12.Louie BE. Robotic lobectomy for non-small cell lung cancer. Indian J Surg Oncol. 2013;4:125–31. doi: 10.1007/s13193-013-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: Experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–5. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 14.Cerfolio RJ, Bryant AS, Skylizard L, Minnich DJ. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg. 2011;142:740–6. doi: 10.1016/j.jtcvs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Nakata M, Saeki H, Yokoyama N, Kurita A, Takiyama W, Takashima S. Pulmonary function after lobectomy: Video-assisted thoracic surgery vs.thoracotomy. Ann Thorac Surg. 2000;70:938–41. doi: 10.1016/s0003-4975(00)01513-7. [DOI] [PubMed] [Google Scholar]
- 16.Daniels LJ, Balderson SS, Onaitis MW, D’Amico TA. Thoracoscopic lobectomy: A safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg. 2002;74:860–4. doi: 10.1016/s0003-4975(02)03764-5. [DOI] [PubMed] [Google Scholar]
- 17.Li WW, Lee RL, Lee TW, Ng CS, Sihoe AD, Wan IY, et al. The impact of thoracic surgical access on early shoulder function: Video-assisted thoracic surgery vs.posterolateral thoracotomy. Eur J Cardiothorac Surg. 2003;23:390–6. doi: 10.1016/s1010-7940(02)00795-9. [DOI] [PubMed] [Google Scholar]
- 18.Ng CS, Wan S, Hui CW, Lee TW, Underwood MJ, Yim AP. Video-assisted thoracic surgery for early stage lung cancer-can short-term immunological advantages improve long-term survival? Ann Thorac Cardiovasc Surg. 2006;12:308–12. [PubMed] [Google Scholar]
- 19.Roviaro G, Varoli F, Vergani C, Nucca O, Maciocco M, Grignani F. Long-term survival after video thoracoscopic lobectomy for stage I lung cancer. Chest. 2004;126:725–32. doi: 10.1378/chest.126.3.725. [DOI] [PubMed] [Google Scholar]
- 20.Veronesi G, Galetta D, Maisonneuve P, Melfi F, Schmid RA, Borri A, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg. 2010;140:19–25. doi: 10.1016/j.jtcvs.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: Technique and initial results. J Thorac Cardiovasc Surg. 2006;131:54–9. doi: 10.1016/j.jtcvs.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg. 2008;85:1880–6. doi: 10.1016/j.athoracsur.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 23.Deen SA, Wilson JL, Wilshire CL, Vallières E, Farivar AS, Aye RW, et al. Defining the cost of care for lobectomy and segmentectomy: A comparisonof open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg. 2014;97:1000–7. doi: 10.1016/j.athoracsur.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin. 2014;24:151–6. doi: 10.1016/j.thorsurg.2014.02.006. [DOI] [PubMed] [Google Scholar]


