Abstract
B cells provide humoral protection against pathogens and promote cellular immunity through diverse nonclassical effector functions. To assess B cell function in promoting T cell homeostasis, mature B cells were either acutely or chronically depleted in mice using CD20 mAb. Acute B cell depletion in either 2- or 4-mo-old mice significantly reduced spleen and lymph node CD4+ and CD8+ T cell numbers, including naive, activated, and Foxp3+CD25+CD4+ regulatory T cell subsets. The numbers of IFN-γ– and TNF-α–producing T cells were also significantly reduced. Chronic B cell depletion for 6 mo in aged naive mice resulted in a 40–70% reduction in activated CD4+ and CD8+ T cell numbers and 20–50% reductions in IFN-γ–producing T cells. Therefore, B cells were necessary for maintaining naive CD4+ and CD8+ T cell homeostasis for subsequent optimal T cell expansion in young and old mice. To determine the significance of this finding, a week of B cell depletion in 4-mo-old mice was followed by acute viral infection with lymphocytic choriomeningitis virus Armstrong. Despite their expansion, activated and cytokine-producing CD4+ and CD8+ T cell numbers were still significantly reduced 1 wk later. Moreover, viral peptide-specific CD4+ and CD8+ T cell numbers and effector cell development were significantly reduced in mice lacking B cells, whereas lymphocytic choriomeningitis virus titers were dramatically increased. Thus, T cell function is maintained in B cell–depleted mice, but B cells are required for optimal CD4+ and CD8+ T cell homeostasis, activation, and effector development in vivo, particularly during responses to acute viral infection.
Blymphocytes are classically defined as the effector cells of humoral immunity that terminally differentiate into Ab-secreting plasma cells. However, B cells also contribute nonclassical functions during immunity, such as organizing lymphoid tissue organogenesis, positively and negatively regulating cellular immune responses, and modulating innate cell function (1). The nonclassical functions of B cells during cellular immune responses have received recent attention due to the clinical demonstration that therapeutic B cell depletion results in disease remission in multiple subsets of autoimmune patients (2). Even though patients undergoing B cell depletion therapies frequently remain B cell insufficient for 8–18 mo, their autoantibody titers may not decrease after treatment (2). Thus, B cells must contribute to auto-immune pathogenesis via mechanisms in addition to autoantibody production. However, the cellular effects of acute or chronic B cell depletion on the human or mouse immune systems remain inadequately characterized, particularly during cellular immune responses.
The effect of short-term and chronic B cell depletion on T cell homeostasis and immune responses to lymphocytic choriomeningitis virus (LCMV) infection was assessed in the current study using naive mice with intact immune systems and a potent mAb specific for mouse CD20 (3, 4). CD20 is a B cell–specific surface molecule that is first expressed during the late pre–B cell developmental stage and downregulated early during plasma cell differentiation. Thus, long-lived plasma cells are not depleted by CD20 mAb, and serum Ig levels remain stable after CD20 mAb-induced B cell depletion (5). CD20 mAb selectively depletes B cells in vivo by monocyte-mediated Ab-dependent cellular cytotoxicity/phagocytosis (3, 6, 7). More than 98% of mature B cells in the blood and primary lymphoid organs are depleted acutely following a single dose of CD20 mAb (MB20-11; 250 μg/mouse), with the effect lasting 6–8 wk (8). Under these experimental conditions, B cells were required for spleen and lymph node CD4+ and CD8+ T cell and Foxp3+CD25+CD4+ regulatory T cell (Treg) homeostasis in naive mice and for optimal T cell activation and numerical expansion following acute LCMV infection.
Materials and Methods
Mice, Abs, and immunotherapy
C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were housed in a specific pathogen-free facility. P14 mice with the LCMV gp33-H-2Db–specific TCR [B6.Cg-Tcratm1Mom Tg(TcrLCMV)327Sdz] (9) were from Taconic Farms (Hudson, NY). All studies were approved by the Animal Care and Use Committees of Duke University Medical Center, Emory University, and the Atlanta VA Medical Center and performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Prior to B cell depletion, mice used in LCMV studies were housed in isolator cages in a conventional animal facility.
To induce in vivo B cell depletion, sterile and endotoxin-free CD20 mAb (MB20-11, IgG2c; 250 μg) or isotype-matched control mAb were injected in 200 μl PBS as described (3). Mice aged 2 or 4 mo were injected once with control or CD20 mAb 14 d before analysis. For chronic B cell–depletion studies, mice were depleted of B cells by repeated injections with control or CD20 mAb once a month starting at 6 mo of age for 6 mo and analyzed 14 d following the final injection. For migration studies, 107 B cell–depleted splenocytes labeled with CellTracker Orange CMRA (Invitrogen Life Technologies, Carlsbad, CA) was injected through the lateral tail veins of mice that had received either control or CD20 mAb 7 d prior. Mice were then analyzed 2 d following cell transfer.
LCMV infection
Mice were infected i.p. with 2 × 105 PFU LCMV Armstrong 53b prepared as described (10). Mice were infected with LCMV 7 d after treatment with control or CD20 mAb and then analyzed 7 d postinfection. For LCMV-specific CD8+ T cell studies, P14 CD8+ T cells (1 × 105) were transferred through lateral tail veins 6 d after treatment with control or CD20 mAb. Mice were then infected with LCMV 1 d later and evaluated 7 d postinfection.
Cell preparation and immunofluorescence analysis
Single-cell leukocyte suspensions from spleens and peripheral lymph nodes (axillary, brachial, and inguinal) were generated by gentle dissection, and erythrocytes were hypotonically lysed. For multicolor immunofluorescence analysis, viable single-cell suspensions (1 × 106) were stained on ice using predetermined optimal concentrations of mAb in FACS buffer (2% FCS in PBS) for 30 min or with MHC class II tetramers for 2 h at 37°C. Cells were washed in PBS between all staining steps, and after the final wash, the cells were resuspended in PBS containing 1.5% paraformaldehyde and kept in the dark at 4°C until analysis. Dead cells were excluded from data analysis by staining with LIVE/DEAD Fixable Dead Cell Stain Kit (Invitrogen Life Technologies). Cells with the forward and side light scatter properties of lymphocytes were analyzed using an FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). Background staining was assessed using nonreactive, isotype-matched control mAbs (Caltag Laboratories, San Francisco, CA).
Treg visualization was carried out using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA) according to the manufacturer’s instructions. Intracellular cytokine staining was performed using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. Following the administration of control or CD20 mAb 14 d prior, spleen lymphocytes were isolated and depleted of B cells using CD19 mAb-coated magnetic beads (Invitrogen Life Technologies). The remaining non–B cells were cultured in vitro with plate-bound CD3ε (1 μg/ml) and CD28 (10 μg/ml) mAbs for 3.5 h in the presence of the secretion inhibitor brefeldin A (5 μg/ml; BD Biosciences) to identify T cells that were actively producing cytokines in vivo. The cells were stained for surface CD4 and CD8 and cytoplasmic IFN-γ and TNF-α expression and analyzed by flow cytometry. To quantify LCMV-specific T cell responses, T cells were stimulated with GP33–41 (CD8+ T cells) and GP61–80 (CD4+ T cells) peptides (10 μg/ml; AnaSpec, Fremont, CA) in the presence of brefeldin A for 5 h before cell-surface and intracellular cytokine staining.
FITC-, PE-, PE-Cy5-, allophycocyanin, or PE-Cy7–conjugated Abs for staining were as follows: CD4 (H129.19), CD8 (53-6.7), CD44 (IM7), IFN-γ (XMG1.2), Ly6C (AL-21), PSGL1 (2PH1), and TNF-α (MP6-XT22) mAbs were from BD Biosciences; B220 (RA36B2), CD25 (PC61), CD127 (A7R34), and killer cell lectin-like receptor G1 (KLRG1)/MAFA (2F1) were from BioLegend (San Diego, CA); L-selectin (CD62L; clone LAM1-116) mAb was as described (11); and Foxp3 (FJK-16s) mAb and functional-grade CD3ε (145-2C11) and CD28 (37.51) mAbs were from eBioscience. The MHC class I tetramers, DbGP33–41 (GP33), used to identify LCMV-specific CD8+ T cells were generated as described (12). The MHC class II tetramer I-AbGP66–77 (GP66; National Institutes of Health Tetramer Facility) was used to identify LCMV-specific CD4+ T cells.
Viral RNA quantification
RNA extracted from purified spleen lymphocytes was used to generate cDNA, with relative transcript levels determined by real-time quantitative RT-PCR of triplicate samples as described (13). Transcripts were amplified using gapdh- and LCMV glycoprotein-specific primers as described (13, 14). Cycle conditions were as follows: 1 denaturation step of 95°C for 2 min followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min. The specificity of RT-PCR products was confirmed by melting curve analysis. Viral RNA expression threshold values (2−ΔΔCt) were determined by normalizing viral RNA transcript levels to gapdh expression within both sample groups and then to one sample from a mouse treated with control mAb.
Statistical analysis
All data are shown as individual data points or as means ± SEM. The significance of differences between sample means was determined using the unpaired Student t test, with Welch’s correction for unequal variances where appropriate.
Results
Acute B cell depletion by CD20 mAb reduces CD4+ and CD8+ T cell numbers
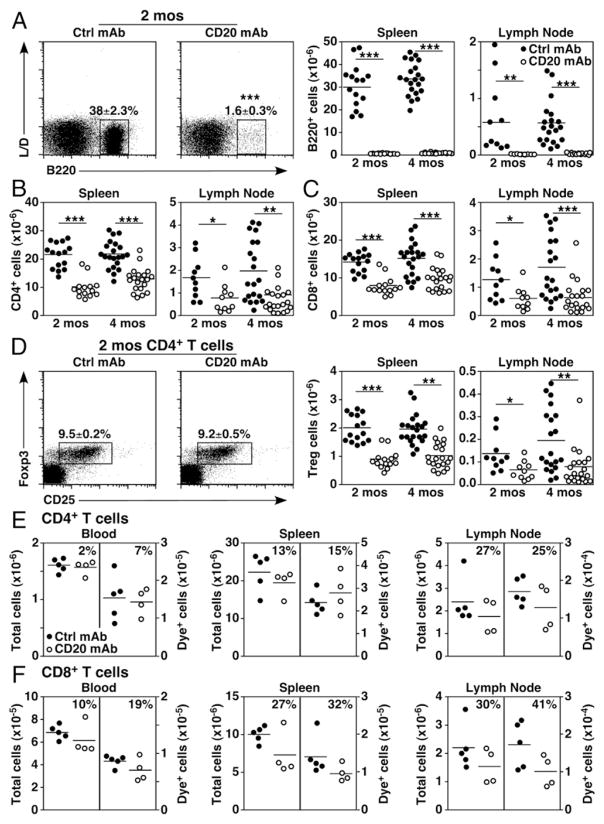
Studies examining the effects of CD20 mAb-induced B cell depletion on T cell numbers and function have generated variable findings (3, 15–20). Therefore, the effect of short-term B cell depletion on spleen and lymph node T cell frequencies and numbers was compared in littermate groups of 2- and 4-mo-old fully mature mice in which B and T cell numbers are stable. Tissue lymphocyte subsets were analyzed 14 d after a single dose of control or CD20 mAb treatment to optimally evaluate the effect of B cell depletion on T cell homeostasis. In all mice, spleen and lymph node B220+ B cell numbers were reduced by >99% in littermates given the MB20-11 CD20 mAb relative to control mAb (Fig. 1A). Consequently, the frequencies of CD4+ and CD8+ T cells among lymphocytes were increased. However, total spleen and lymph node CD4+ and CD8+ T cell numbers in 2-mo-old B cell–depleted mice were overlapping with control mAb-treated mice, but mean values were decreased by 44–53% (Fig. 1B, 1C), although variability was observed between individual mice and groups of littermate mice in four independent experiments. Mean CD4+ and CD8+ T cell numbers in 4-mo-old mice given CD20 mAb were similarly reduced by 35–40% and 54–62% in the spleen and lymph nodes, respectively, with a range of variability between mice and littermate groups between different experiments. Treg frequencies within spleens and lymph nodes did not change following B cell depletion, although total Treg cell numbers were significantly reduced (2 mo old, 55–57%; 4 mo old, 47–61%; Fig. 1D). The migration of adoptively transferred CD4+ and CD8+ T cells into blood, the spleen, and lymph nodes was not significantly affected by the absence of B cells, as transferred dye-labeled cells migrated normally into tissues (p > 0.05, Fig. 1E, 1F). However, when endogenous T cell numbers were reduced in CD20 mAb-treated mice, transferred T cell numbers were reduced similarly. Thus, B cell depletion significantly reduced overall CD4+ and CD8+ T cell and Treg homeostasis in 2- and 4-mo-old naive mice relative to control mAb-treated littermates.
FIGURE 1.
B cell depletion alters T cell homeostasis. (A–D) Naive 2- or 4-mo-old mice were treated with control (closed circles) or CD20 (open circles) mAb, with viable single spleen and lymph node lymphocytes assessed 14 d later by immunofluorescence staining with flow cytometry analysis. Representative panels show B220+ versus LIVE/DEAD spleen B cell staining (left panels) and absolute B cell numbers (right panels) (A), CD4+ T cell numbers (B), CD8+ T cell numbers (C), and CD25 versus intracellular Foxp3 staining for CD4+ T cells (left panels) and Foxp3+CD25+CD4+ Treg numbers (right panels) (D) following B cell depletion. Pooled results from four independent experiments are indicated in graphs (n = 10–21 mice/group with means indicated by horizontal bars). (E and F) CD4+ and CD8+ T cell migration is not profoundly impacted by B cell depletion. Dye-labeled B cell–depleted splenocytes were adoptively transferred into naive 2-mo-old mice given control or CD20 mAb 7 d earlier. Absolute numbers of total endogenous and adoptively transferred dye+ CD4+ (E) and CD8+ (F) T cells within the blood, spleen, and lymph nodes were assessed 2 d later by immunofluorescence staining with flow cytometry analysis. Percentage differences in T cell numbers for CD20 mAb-treated relative to control mAb-treated mice are shown, in which graphs show results from individual mice (n = 5 mice/group with means indicated by horizontal bars). (A–F) Mean cell frequencies (± SEM) within the indicated dot plot gates are shown. Significant differences between sample means are indicated: *p < 0.05, **p < 0.01, ***p < 0.001. Ctrl, control.
Acute B cell depletion reduces both naive and activated T cell numbers
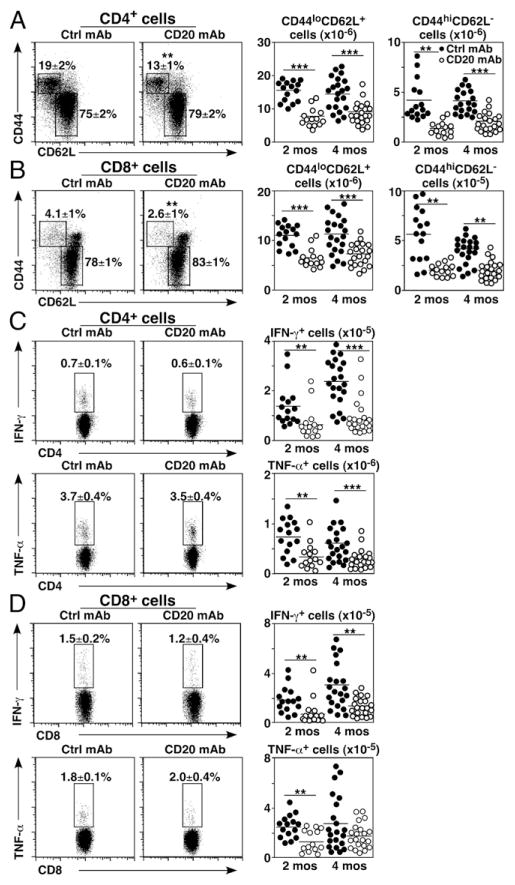
The effect of B cell depletion on spleen T cell subsets with naive CD44loCD62Lhi and activated CD44hiCD62Llo phenotypes was quantified relative to all CD4+ or CD8+ lymphocytes. The frequencies of activated CD4+ and CD8+ T cells were reduced by 30–38% in 2-mo-old mice treated with CD20 mAb in comparison with control mAb-treated littermates (Fig. 2A, 2B). The numbers of activated CD4+ and CD8+ T cells were reduced by 52–68% and 47–66% in 2- and 4-mo-old mice, respectively. B cell depletion reduced total naive CD4+ and CD8+ T cell numbers by 40–50% in 2-mo-old mice and by 35–37% in 4-mo-old mice. Thus, mean numbers of naive and activated CD4+ and CD8+ T cells were decreased in CD20 mAb-treated mice relative to littermate controls.
FIGURE 2.
B cell depletion alters naive and memory T cell homeostasis. Naive 2- or 4-mo-old mice were treated with control (closed circles) or CD20 mAb (open circles). Viable, single spleen lymphocytes were isolated 14 d later (A–D) and depleted of B cells using CD19 mAb-coated magnetic beads (C and D). The remaining non–B cells were cultured for 3.5 h with plate-bound CD3/CD28 mAbs before cell-surface CD4/CD8 labeling and intracellular cytokine staining with flow cytometry analysis. Representative panels show CD44 versus CD62L staining (left panels) and cell numbers (right panels) for CD4+ (A) and CD8+ (B) T cells. Representative panels show IFN-γ and TNF-α expression (left panels) and cell numbers (right panels) for CD4+ (C) and CD8+ (D) T cells. (A–D) Mean cell frequencies (± SEM) within the indicated dot plot gates are shown. Graphs show results from individual mice (n = 15–21 mice/group in three to four independent experiments) with means indicated by horizontal bars. Significant differences between sample means are indicated: **p < 0.01, ***p < 0.001. Ctrl, control.
As cytokine production is an integral component of the effector T cell immune response, the effects of B cell depletion on T cell cytokine production were quantified. IFN-γ– and TNF-α–expressing CD4+ and CD8+ T cell frequencies were not profoundly altered in either 2- or 4-mo-old mice after CD20 mAb treatment for 14 d (Fig. 2C, 2D). However, mean IFN-γ– and TNF-α–expressing CD4+ T cell numbers were reduced by 58–61% and 51–54%, respectively, in B cell–depleted 2- and 4-mo-old mice, whereas mean IFN-γ– and TNF-α–expressing CD8+ T cell numbers were reduced by 51–59% and 36–49%, respectively. Thus, B cell depletion significantly reduced the total numbers of cytokine-producing CD4+ and CD8+ T cells relative to control mAb-treated littermates.
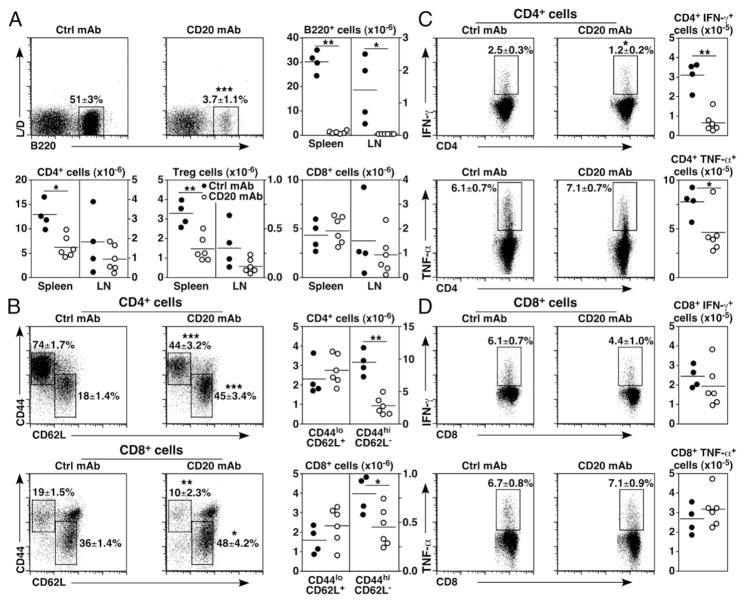
Chronic B cell depletion reduces activated CD4+ T cell numbers
Whether the effects of B cell depletion on T cell homeostasis observed in young mice were age-associated was assessed using naive 6-mo-old mice with fully mature immune systems that were then treated monthly with CD20 mAb for 6 mo. Spleen and lymph node B220+ B cell numbers were decreased by >98% after chronic B cell depletion (Fig. 3A). Spleen CD4+ T cell numbers were decreased by 51% in CD20 mAb-treated mice, including a 56% decrease in Treg cell numbers. Mean lymph node CD4+ T cell and Treg cell numbers were reduced by 48 and 63%, respectively. Spleen and lymph node CD8+ T cell numbers were affected less by chronic B cell depletion.
FIGURE 3.
Chronic B cell depletion alters T cell homeostasis and cytokine production. Naive 6-mo-old mice (n = 4–6 mice/group) were treated with control (closed circles) or CD20 mAb (open circles) monthly for 6 mo before spleen and lymph node (LN) lymphocyte isolation and analysis as in Figs. 1 and 2. (A) Chronic B cell depletion reduces CD4+ T cell and Treg cell numbers. Representative B220+ B cell depletion versus LIVE/DEAD cell staining, as well as B cell, CD4+, and CD8+ T cell, and Treg numbers are shown. (B) Chronic B cell depletion reduces CD4+ and CD8+ T cell activation. Representative panels show CD44 versus CD62L staining (left panels) for CD4+ and CD8+ T cells and cell numbers (right panels). (C and D) Chronic B cell depletion impairs IFN-γ and TNF-α expression by CD4+ and CD8+ T cells. Representative panels show IFN-γ and TNF-α expression (left panels) and cell numbers (right panels) for CD4+ (C) and CD8+ (D) T cells. (A–D) Mean cell frequencies (± SEM) within the indicated dot plot gates are shown. Graphs show results from individual mice (n = 4–6 mice/group) with means indicated by horizontal bars. Significant differences between sample means are indicated: *p < 0.05, **p < 0.01, ***p < 0.001. Ctrl, control.
Chronic B cell depletion significantly reduced activated CD44hi CD62Llo CD4+ and CD8+ T cell frequencies by 40 and 48%, respectively (Fig. 3B). Activated CD4+ and CD8+ T cell numbers were also decreased by 70 and 44%, respectively, whereas total CD8+ and naive CD44loCD62Lhi CD4+ T cell numbers did not change significantly with chronic B cell depletion. Consistent with these changes, mean IFN-γ–producing CD4+ T cell frequencies were decreased by 50% in B cell–depleted mice, but mean TNF-α–producing CD4+ T cell and IFN-γ– and TNF-α–producing CD8+ T cell frequencies were not changed significantly (Fig. 3C, 3D). Additionally, the numbers of IFN-γ– and TNF-α–producing CD4+ T cells were reduced by 70 and 40%, respectively, following B cell depletion, whereas the numbers of IFN-γ+ and TNF-α+ CD8+ T cells were unaffected. Thus, as occurred with acute B cell depletion, chronic B cell depletion in older mice significantly reduced the proportion and number of activated CD4+ and CD8+ T cells and only reduced cytokine-producing CD4+ T cell numbers relative to their control mAb-treated littermates.
B cell depletion impairs virus clearance in LCMV-infected mice
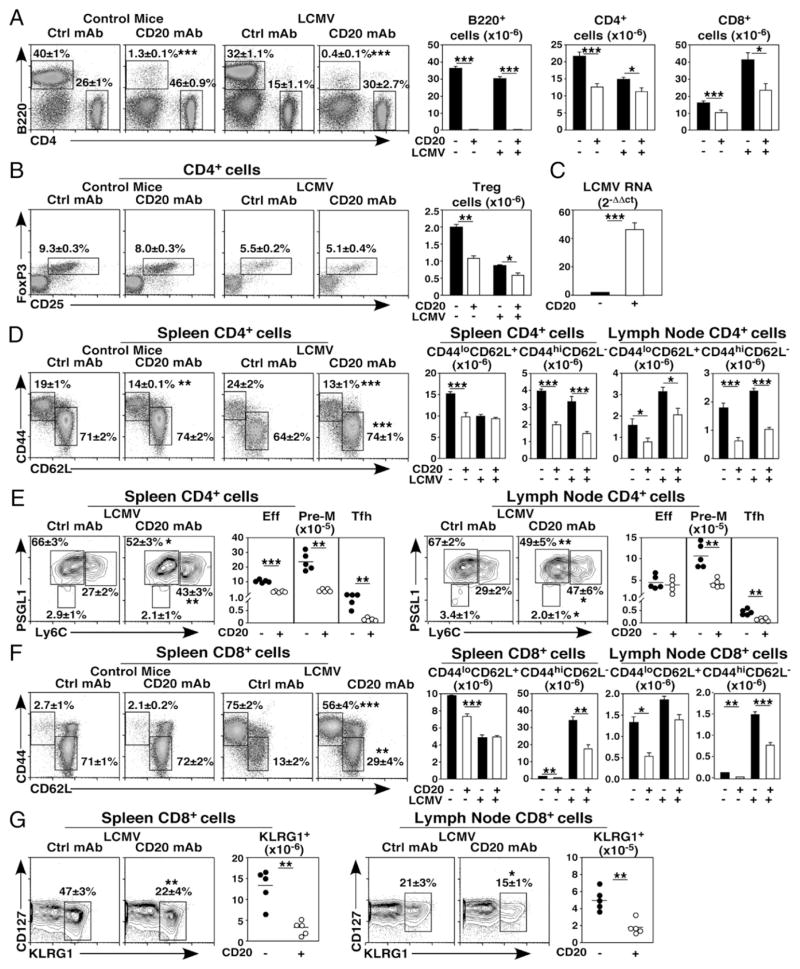
To understand whether reduced CD4+ and CD8+ T cell homeostasis in B cell–depleted mice affected cellular immune responses, 4-mo-old mice were given CD20 or control mAb 7 d before infection with LCMV Armstrong. Spleen CD4+ and CD8+ T cell numbers were assessed 7 d postinfection. In control mAb-treated mice, LCMV infection reduced mean CD4+ T cell numbers by 33% relative to uninfected mice (Fig. 4A). Prior CD20 mAb treatment significantly reduced CD4+ T cell numbers in virus-infected mice relative to infected control mAb-treated mice (23%). Treg numbers were comparably reduced during LCMV infection in B cell–depleted mice (30%, Fig. 4B). In contrast to CD4+ T cells, LCMV infection induced an ~3-fold expansion in CD8+ T cell numbers following control mAb treatment relative to naive mice. However, virus-induced CD8+ T cell expansion was profoundly inhibited following B cell depletion (42%, Fig. 4A). Thus, B cell depletion reduced total CD8+ T cell numbers generated in response to acute virus infection.
FIGURE 4.
B cell depletion impairs T cell expansion, memory conversion, and virus clearance during LCMV infection. Naive 4-mo-old mice were given control (closed bars) or CD20 mAb (open bars) 7 d prior to treatment with PBS or infection with LCMV. Splenocytes harvested 7 d later were assessed by immunofluorescence staining with flow cytometry analysis of viable, single lymphocytes. Representative panels show B220 versus CD4 staining (left panels) and B cell, CD4+, and CD8+ T cell numbers (right panels) (A) and CD25 versus intracellular Foxp3 staining for CD4+ T cells (left panels) and Treg numbers (right panels) (B). (C) B cell depletion reduces LCMV viral clearance. Spleens from control and CD20 mAb-treated mice infected with LCMV were harvested 7 d postinfection, with viral RNA levels quantified by RT-PCR. Bar graph values represent mean (± SEM) relative LCMV RNA expression levels (n = 4 mice/group in three independent experiments). (D–G) B cell depletion impairs LCMV-driven T cell activation and effector subset conversion. Representative panels show CD44 versus CD62L staining (left panels) for spleen CD4+ (D) and CD8+ T cells (F), with cell numbers for the spleen and lymph nodes shown (right panels). (E) Representative panels show PSGL1 versus Ly6C staining (left panels) for spleen and lymph node CD4+ cells, with activated CD4+ T cell conversion to effector (Eff; Ly6C+PSGL1+), prememory (Pre-M; Ly6C− PSGL1+), and T follicular helper (Tfh; Ly6C− PSGL1−) numbers shown (right panels). (G) Representative dot plots show CD127 versus KLRG1 staining (left panels) for spleen and lymph node CD8+ T cells, with KLRG1+ effector cell numbers shown (right panels). (A, B, D, and F) Graphs show mean (± SEM) numbers of the indicated cell types from mice treated with control or CD20 mAb (n = 21–26 mice/group in four to five independent experiments). (E and G) Graphs show results from individual mice (n = 5 mice/group), with means indicated by horizontal bars. (A–G) Mean (± SEM) cell frequencies within the indicated dot plot gates are shown. Significant differences between sample means are indicated: *p < 0.05, **p < 0.01, ***p < 0.001. Ctrl, control.
The effect of reduced T cell numbers in B cell–depleted mice on in vivo viral clearance was assessed by quantifying relative LCMV RNA levels as described (14). Spleen viral RNA levels were 48-fold higher in B cell–depleted mice relative to control mAb-treated mice 7 d postinfection (Fig. 4C). Accordingly, the numbers of activated CD44hiCD62L− CD4+ T cells were reduced by 54–60% in the spleen and lymph nodes, whereas naive CD4+ T cell numbers were less affected by B cell depletion following viral infection (Fig. 4D). Similarly, CD4+ effector (Ly6C+PSGL1+), prememory (Ly6C− PSGL1+), and T follicular helper (Ly6C− PSGL1−) cell numbers were reduced by 69–84% in the spleen, with comparable reductions in the numbers of prememory (62%) and T follicular helper (68%) CD4+ T cells within the lymph nodes of B cell–depleted mice infected with LCMV (Fig. 4E) (21). Although naive CD8+ T cell numbers were normal following virus infection in B cell–depleted mice, the numbers of activated CD44hiCD62L− spleen and lymph node CD8+ T cells were reduced by 51–52%, (Fig. 4F). CD8+ KLRG1+ effector T cell numbers were also reduced by 64–74% in the spleen and lymph nodes of B cell–depleted mice that were infected with LCMV (Fig. 4G) (21). Thus, B cells were needed for the optimal generation of CD4+ and CD8+ T cells with an activated phenotype and the expansion of effector cell subsets following infection.
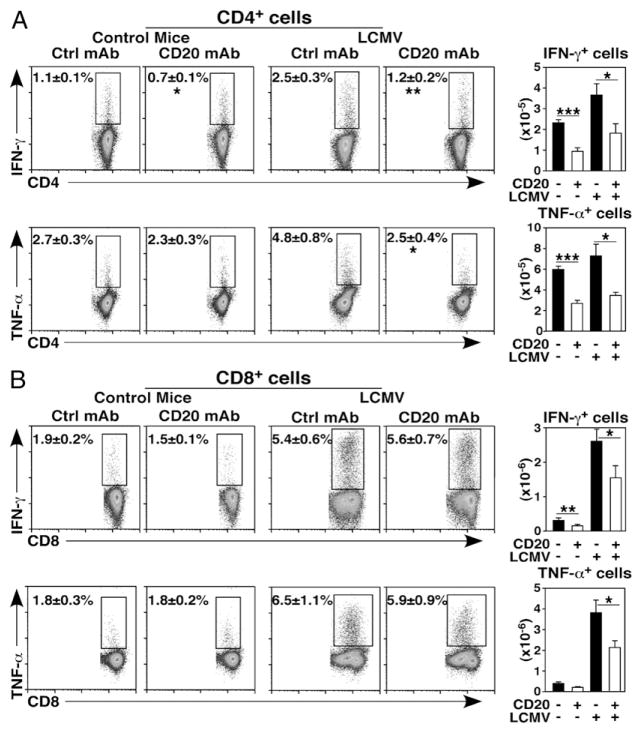
B cell depletion significantly reduced the frequencies (47–50%) and numbers (51–53%) of spleen IFN-γ– and TNF-α–expressing CD4+ T cells in infected mice (Fig. 5A). Although the relative frequencies of IFN-γ– and TNF-α–expressing CD8+ T cells were not altered in B cell–depleted and infected mice, IFN-γ– and TNF-α–expressing CD8+ T cell numbers were reduced by 35 and 44%, respectively (Fig. 5B). Thus, B cell depletion impaired CD4+ T cell cytokine production during immune responses to LCMV, but did not reduce the relative frequencies of CD8+ T cells producing cytokines despite their overall reduced numbers in B cell–depleted mice.
FIGURE 5.
B cell depletion impairs T cell IFN-γ and TNF-α production during LCMV infection. Naive 4-mo-old mice (n = 21–26 mice per group in four to five independent experiments) were given control (closed bars) or CD20 mAb (open bars) 7 d before infection with LCMV or treatment with PBS as in Fig. 4. Seven days postinfection, spleen lymphocytes were harvested, depleted of B cells with CD19 magnetic beads, and stimulated using plate-bound CD3/ CD28 mAbs as in Fig. 2. Intracellular cytokine production was assessed by immunofluorescence staining with flow cytometry analysis. Flow cytometry dot plots show representative CD4+ (A) or CD8+ (B) T cell cytokine staining (left panels), with mean (± SEM) cell frequencies shown for the indicated gates. Bar graphs represent mean (± SEM) numbers of the indicated cell types (right panels). Significant differences between sample means are indicated: *p < 0.05, **p < 0.01, ***p < 0.001. Ctrl, control.
B cell depletion impairs Ag-specific T cell responses
Consistent with reduced T cell numbers in B cell–depleted mice after LCMV infection, CD20 mAb treatment reduced mean spleen LCMV peptide-specific CD4+ (GP66+) and CD8+ (GP33+) T cell numbers by 67% (Fig. 6A). B cell depletion significantly reduced the numbers of activated GP66-specific CD44hiCD62L− CD4+ T cells (87–91%) and the numbers of effector (58–71%), pre-memory (90–94%), and T follicular helper (84–91%) subsets in the spleen and lymph nodes following LCMV infection (Fig. 6B). Virus-specific CD4+ and CD8+ T cell cytokine production was therefore assessed using B cell–depleted splenocytes from LCMV-infected mice that were restimulated with LCMV peptides in vitro for 5 h. B cell depletion reduced both virus-specific IFN-γ+ and TNF-α+ CD4+ T cell frequencies (56–62%) and numbers (72–75%) in infected mice (Fig. 6C). By contrast, the frequencies of GP33-specific CD8+ T cells expressing IFN-γ+ and TNF-α+ were not changed, but mean numbers of GP33-specific CD8+ T cells expressing IFN-γ+ and TNF-α+ were reduced (52–63%) in B cell–depleted LCMV-infected mice (Fig. 6D). Thus, B cell depletion reduced the numbers of Ag-specific CD4+ and CD8+ T cells responding to viral infection.
FIGURE 6.
B cell depletion impairs Ag-specific T cell function. (A–D) B cell depletion impairs virus peptide-specific CD4+ and CD8+ T cell function. Naive 4-mo-old mice were given control (closed bars) or CD20 mAb (open bars) 7 d before infection with LCMVor treatment with PBS as in Figs. 4 and 5. Spleen and lymph node lymphocytes were harvested 7 d postinfection. (A) B cell depletion reduces LCMV GP66 tetramer+ CD4+ T cell and GP33 tetramer+ CD8+ T cell numbers. Representative panels LCMV-specific tetramer staining (left panels) and cell numbers (right panels). (B) Representative panels show CD44 versus CD62L and PSGL1 versus Ly6C staining (top panels) for LCMV-specific GP66+ CD4+ T cells from the spleen and lymph nodes, with activated (CD44hiCD62Llo), effector (Ly6C+PSGL1+), prememory (Ly6C− PSGL1+), and T follicular helper (Tfh; Ly6C− PSGL1−) GP66+ CD4+ T cell numbers shown (bottom panels). B cell depletion reduces the frequencies and numbers of cytokine expressing LCMV-specific CD4+ (C) and CD8+ (D) T cells. Following B cell depletion using CD19 magnetic beads, T cells were stimulated with LCMV Armstrong GP61–80 or GP33–41 viral peptides for 5 h, with intracellular IFN-γ or TNF-α expression quantified by immunofluorescence staining. Flow cytometry dot plots show representative CD4 or CD8 expression versus cytokine staining. (E) B cell depletion impairs virus-specific CD8+ T cell effector development. Naive 4-mo-old mice were given control (closed bars) or CD20 mAb (open bars) 6 d before the adoptive transfer of naive TCR-transgenic CD8+ T cells that recognize GP33–41. Mice were infected with LCMV 1 d later and evaluated 7 d postinfection as in Figs. 4 and 5. Representative panels show CD44 versus CD62L and CD127 versus KLRG1 staining (top panels) for transferred LCMV-specific GP33+ CD8+ T cells from the spleen and lymph nodes, with numbers for total (GP33+), activated (CD44hiCD62L+), effector (KLRG1+), and IFN-γ+ cells shown (bottom panels). (A, C, and D) Graphs show mean (± SEM) numbers of the indicated cell types from mice treated with control or CD20 mAb (n = 21–26 mice/group in four to five independent experiments). (B and E) Graphs show results from individual mice (n = 5 mice/group), with means indicated by horizontal bars. (B–E) Mean (± SEM) cell frequencies within the indicated dot plot gates are shown. Significant differences between sample means are indicated: *p < 0.05, **p < 0.01, ***p < 0.001. Ctrl, control.
Impaired LCMV clearance following B cell depletion may occur as a direct consequence of reduced T cell numbers or may result from reduced CD8+ T cell function. To address this, naive TCR-transgenic CD8+ T cells that recognize the dominant LCMV epitope GP33–41 (22) were adoptively transferred into control or B cell–depleted mice prior to infection. B cells were not required for Ag-driven CD8+ T cell expansion, activation, or cytokine production in the spleen (Fig. 6E). However, effector KLRG1+ CD8+ T cell generation was reduced by 50% in the absence of B cells. In lymph nodes, B cell depletion significantly inhibited GP33+ CD8+ T cell expansion (63%), activation (67%), and KLRG1+ effector cell development (68%). Thus, B cell depletion impaired the induction of Ag-specific CD4+ and CD8+ T cells following viral infection predominantly by reducing total Ag-specific T cell num bers, although KLRG1+ effector cell generation was impaired.
Discussion
These collective studies demonstrate that B cells are required for spleen and lymph node T cell homeostasis and optimal CD4+ and CD8+ T cell responses during acute LCMV infection. Remarkably, short-term mature B cell depletion in both 2- and 4-mo-old mice significantly reduced both spleen and lymph node CD4+, CD8+, and Treg numbers (Fig. 1B–D). These reductions were most likely due to alterations in T cell homeostasis following B cell depletion, as no overt changes in T cell migration to peripheral organs were observed (Fig. 1E, 1F). In the absence of B cells, activated CD44hiCD62L− CD4+ and CD8+ T cell numbers in 2- and 4 mo-old mice were more dramatically reduced than naive CD44loCD62L+ CD4+ and CD8+ T cell numbers (Fig. 2A, 2B). As a consequence of fewer T cells in B cell–depleted mice, the number of effector CD4+ and CD8+ T cells producing IFN-γ and TNF-α was also significantly decreased (Fig. 2C, 2D). Importantly, B cell deficiency during acute LCMV infection led to significant reductions in CD4+ and CD8+ T cell numbers and the generation of activated, effector, and CD4+ cytokine-producing cells (Figs. 4, 5). Virus peptide-specific CD4+ and CD8+ T cell numbers and effector generation as well as CD4+ T cell activation and cytokine production were also dramatically reduced in mice lacking B cells (Fig. 6), whereas spleen LCMV titers were dramatically increased (Fig. 4C). Thus, B cells were essential for maintaining CD4+ and CD8+ T cell homeostasis and optimal T cell responses during viral infection.
B cell depletion in 2- and 4-mo-old mice had an overall negative effect on both CD4+ and CD8+ T cell homeostasis and function after only 2 wk (Figs. 1, 2). Chronic B cell depletion over 6 mo in year-old mice similarly resulted in a 50–70% reduction in activated CD4+ and cytokine-producing T cell numbers, but alterations in CD8+ T cell activation were less dramatic (Fig. 3). In complementary studies, acute B cell depletion in mice with otherwise intact immune systems impairs adaptive and autoreactive CD4+ T cell responses to Ag challenge, whereas CD8+ T cell reactivity is less affected (16, 23). B cell depletion also reduces the conversion of naive CD44loCD62L+ CD4+ T cells to an activated phenotype in response to Listeria challenge, whereas CD8+ T cell phenotypes are only modestly affected (16). A role for B cells in CD4+ T cell priming has also been demonstrated in mice given anti-IgM Ab since birth (24–27) and in genetically B cell–deficient (μMT) mice (28). Therapeutic B cell depletion in mice also reduces CD4+ and CD8+ T cell tumor immunity in the B16 melanoma model (20). Thereby, depending on mouse age, status of the immune system and magnitude of the challenge, CD4+ T cell function appears to be more B cell dependent than CD8+ T cell function.
Acute B cell depletion in mice before LCMV infection resulted in dramatically increased virus titers (Fig. 4C), which is in accordance with previous studies that demonstrated worsened but not protracted LCMV Armstrong infection in μMT mice (29, 30). Similarly, mice depleted of B cells since birth with anti-IgM serum do not develop fully protective T cell immunity to virus-induced tumors (31, 32). B cells promote optimal T cell activation and function following immunization (16, 33), during viral immunity (34–37) and in models of tumor immunity, autoimmunity, and graft rejection (19, 20, 32, 38–41). The influence of B cells on both CD4+ and CD8+ T cell activation thus reflects the diverse and multiple known molecular mechanisms through which B cells influence T cell immunity, including B cell contributions to Ag presentation, costimulatory molecule expression, and cytokine production (1). When the immune system develops in the complete absence of B cells in μMT mice, multiple immune system abnormalities have been identified. The absence of B cells has been shown to impair CD4+ T cell priming in some studies (25, 26, 33, 42–44), whereas others have reported that CD4+ T cell priming is not affected in μMT mice (45–49). Additionally, thymocyte and T cell numbers and repertoire are decreased significantly in μMT mice (30, 50). Also, because B cells help to organize lymphoid organ architecture, the spleens of μMT mice are smaller in size (30), exhibit significant defects within the spleen dendritic cells (DC) and T cell compartments (46, 51), lack follicular DC and marginal zone and metallophilic macrophages (52), have decreased chemokine expression (51, 52), and are deficient in Peyer’s patch organogenesis and follicular DC networks (52, 53). DC in μMT mice also skew immunity toward Th1 responses (46). B cell depletion after the establishment and functional maturation of lymphoid tissues as in the current studies may also affect nonlymphoid immune cell populations that influence LCMV clearance. Nonetheless, acute and chronic B cell depletion in the current studies appears to have primarily dampened T cell homeostasis and some cell-mediated immune responses in comparison with the more pleiotropic effects of con genital B cell deficiency or induced B cell depletion after birth.
There was a specific reduction in Ag-specific effector CD8+ cell development in the absence of B cells (Fig. 6E). Because CD4+ T cells are critical for CD8+ T cell responses to viral infection (54), the observed impact of B cell depletion on CD8+ T cell function may be due to B cell–induced alterations in CD4+ T cell expansion, function, and memory maintenance, as shown in this study and elsewhere (16, 23, 37). The absence of B cells may thereby also indirectly impact CD8+ T cell memory development, as memory CD8+ T cells derive from the responding Ag-specific effector CD8+ T cell pool (21), and there is a reduction in memory CD8+ T cell numbers following LCMV infection in the absence of B cells (30). The altered architecture of lymphoid tissues in B cell–depleted mice may also affect T cell responses. In either event, the delayed resolution of viral infection observed in this study may primarily result from reduced T cell homeostasis, resulting in lower numbers of activated and cytokine-producing CD4+ and CD8+ T cells responding to LCMV. Indeed, T cells derived from μMT mice are unable to develop memory responses sufficient to control persistent viral infection (29), and this effect is independent of B cell Ab production (44, 55). B cells are also required for optimal CD4+ T cell memory generation and recall responses upon secondary infection, a process dependent on B cell MHC class II expression (23, 37). Total and Ag-specific effector, prememory, and T follicular helper CD4+ T cell development was also significantly inhibited in the current study. Thus, both primary and secondary responses to infection are likely to be significantly affected by B cell depletion.
The importance of CD20 mAb-induced B cell depletion on CD4+, Treg, and CD8+ T cell frequencies or numbers in mice have yielded conflicting results, ranging from increased (18, 19) or unchanged (3, 15–17) to decreased (20, 23, 37). Variability between individual mice was also observed in the current studies, in which the numbers of Treg, CD4+, and CD8+ T cells remaining after CD20 or control mAb treatment overlap (Fig. 1B–D). In previous studies, differences between lymphocyte subsets among littermates, measurement of differences in T cell frequencies versus tissue numbers, variability between ages of mice being studied, differences in disease responses to treatments, inherent variability in lymph node sizes between littermates, and small sample groups are likely to have obscured differences between CD20 or control mAb-treated groups. The degree of B cell depletion due to the use of different CD20 mAbs and treatment regimens is also important, as changes in cell numbers and lymphoid tissue architecture are less pronounced when some B cells remain. For example, B cell depletion by CD20 mAb is less effective in NOD mice, due in part to FcγR deficiencies (41). CD20 mAb treatment significantly reduces the proliferative capacity of CD4+ and CD8+ T cells within tissues of NOD mice, but does not affect Treg, CD4+, or CD8+ T cell numbers or phenotypes. Others have also demonstrated impaired lymph node T cell activation in congenitally μMT NOD mice, suggesting a critical need for B cell costimulatory signals (56). The timing of evaluation following B cell depletion is also critical (3, 4), as the reductions in total CD4+ and CD8+ T cell numbers 1 wk following CD20 mAb treatment are less significant than those that were observed 2 wk after depletion (Fig. 1) (16). The global effects on T cell homeostasis induced by B cell depletion likely change with disease severity and also with age, as was observed for mice chronically depleted of B cells in this study. Nonetheless, reduced numbers of activated and effector cytokine-producing CD4+ T cells are most likely attributable to suboptimal activation in the absence of B cell Ag presentation in combination with normal T cell turnover (16, 20, 37, 39, 57).
B cell depletion reduces the pathogenesis of diverse autoimmune diseases in humans, in whom the reconstitution of B cells is often accompanied by disease recurrence (58, 59). A subset of human T cells has been reported to express CD20 (60–62), and a small subset of human T and NK cells has been reported to be depleted following CD20 mAb therapy in rheumatoid arthritis patients due to low level CD20 expression (59, 61). However, measurable cell-surface CD20 expression is widely considered to be B cell restricted in humans (63) as it is in mice (4, 6). CD20 mAb treatment in patients with pemphigus decreases autoreactive CD4+ T cell frequencies, whereas overall T cell numbers are unaffected (64). Rituximab reduces both B and T cell numbers in cerebrospinal fluid of multiple sclerosis patients (65). Treg frequencies are reported to increase following rituximab treatment in patients with lupus or mixed cryoglobulinemia vasculitis (66–68). However, Treg and CD4+ T cell numbers decreased in parallel following CD20 mAb treatment in the current mouse study (Fig. 1D). The complexities of studying humans, particularly in those with disease undergoing therapy, in combination with the difficulties in assessing the extent of tissue B cell depletion, may thereby obfuscate studies of T cell alterations in patients acutely or chronically depleted of B cells. Likewise, B cell depletion in mice does not result in overt in vivo monocyte activation nor does it measurably influence serum cytokines that could in turn modify T cell function (16). That B cells are also required for T cell homeostasis in naive mice and for T cell activation during immune responses to pathogens provides a potential mechanism by which B cell depletion delays and reduces the severity of T cell–mediated autoimmune diseases (15, 17–19, 41, 69–71), even though serum Ig levels are not significantly affected by B cell depletion (2, 5).
The effects of CD20 mAb treatment on T cell numbers in the current study appears to be a direct consequence of B cell depletion and the disruption of lymphoid tissue architecture (8), consequently impairing cellular immunity. Thereby, the current results suggest a model in which the therapeutic benefits of CD20 mAb treatment in autoimmune patients are, at least in part, due to dysregulated T cell homeostasis and impaired T cell activation, with the potential cost of rendering some patients more susceptible to infections, particularly when given in combination with immunosuppressive drugs (58). Indeed, diminished T cell numbers and altered responses may explain rare infections in lymphoma patients receiving rituximab with microorganisms generally associated with T cell immunosuppression such as JC-papovavirus, CMV, or parvovirus B19 (72). Consequently, the impact of efficient B cell depletion on T cell responses observed in the current study urges caution in the broad and prolonged application of B cell depletion therapies in the clinic.
Acknowledgments
This work was supported by National Institutes of Health Grant AI56363, Southeastern Regional Center of Excellence for Emerging Infections and Biodefense Grant U54 AI057157, and the Lymphoma Research Foundation. J.M.G. is supported by National Institute of Allergy and Infectious Diseases Grant R01-AI068952. M.N.W. is supported by the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development (5I01BX000105), National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR059364, and National Institutes of Health/National Institute on Aging Grant AG040013.
We thank Dr. Guglielmo Venturi for assistance with these experiments.
Abbreviations used in this article
- DC
dendritic cell
- KLRG1
killer cell lectin-like receptor G1
- LCMV
lymphocytic choriomeningitis virus
- μMT
B cell–deficient
- Treg
regulatory T cell
Footnotes
J.M.L., D.J.D., E.T.W., S.R.-P., M.N.W., and T.F.T. designed the research program; J.M.L., D.J.D., E.T.W., S.R.-P., and M.N.W. performed the experiments; M.T.H. and J.M.G. provided essential reagents and guidance; J.M.L., D.J.D., E.T.W., and T.F.T. analyzed the raw data; and J.M.L., D.J.D., E.T.W., M.T.H., J.M.G., S.R.-P., M.N.W., and T.F.T. collectively wrote the manuscript.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levesque MC, St Clair EW. B cell-directed therapies for auto-immune disease and correlates of disease response and relapse. J Allergy Clin Immunol. 2008;121:13–21. doi: 10.1016/j.jaci.2007.11.030. quiz 22–23. [DOI] [PubMed] [Google Scholar]
- 3.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, Bowen K, Steeber DA, Haas KM, Poe JC, Tedder TF. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 5.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 6.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 7.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med. 2006;203:743–753. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamaguchi Y, Uchida J, Cain DW, Venturi GM, Poe JC, Haas KM, Tedder TF. The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol. 2005;174:4389–4399. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 9.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steeber DA, Engel P, Miller AS, Sheetz MP, Tedder TF. Ligation of L-selectin through conserved regions within the lectin domain activates signal transduction pathways and integrin function in human, mouse, and rat leukocytes. J Immunol. 1997;159:952–963. [PubMed] [Google Scholar]
- 12.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJD, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods. 2008;333:167–179. doi: 10.1016/j.jim.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCausland MM, Crotty S. Quantitative PCR technique for detecting lymphocytic choriomeningitis virus in vivo. J Virol Methods. 2008;147:167–176. doi: 10.1016/j.jviromet.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz J-D, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, et al. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol. 2006;169:954–966. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 19.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misumi I, Whitmire JK. B cell depletion curtails CD4+ T cell memory and reduces protection against disseminating virus infection. J Immunol. 2014;192:1597–1608. doi: 10.4049/jimmunol.1302661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ron Y, De Baetselier P, Gordon J, Feldman M, Segal S. Defective induction of antigen-reactive proliferating T cells in B cell-deprived mice. Eur J Immunol. 1981;11:964–968. doi: 10.1002/eji.1830111203. [DOI] [PubMed] [Google Scholar]
- 25.Ron Y, Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987;138:2848–2856. [PubMed] [Google Scholar]
- 26.Janeway CA, Jr, Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138:1051–1055. [PubMed] [Google Scholar]
- 27.Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140:3773–3778. [PubMed] [Google Scholar]
- 28.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 29.Homann D, Tishon A, Berger DP, Weigle WO, von MG, Oldstone MB. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano MS, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon J, Holden HT, Segal S, Feldman M. Anti-tumor immunity in B-lymphocyte-deprived mice. III. Immunity to primary Moloney sarcoma virus-induced tumors. Int J Cancer. 1982;29:351–357. doi: 10.1002/ijc.2910290320. [DOI] [PubMed] [Google Scholar]
- 32.Schultz KR, Klarnet JP, Gieni RS, HayGlass KT, Greenberg PD. The role of B cells for in vivo T cell responses to a Friend virus-induced leukemia. Science. 1990;249:921–923. doi: 10.1126/science.2118273. [DOI] [PubMed] [Google Scholar]
- 33.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 34.Bergmann CC, Ramakrishna C, Kornacki M, Stohlman SA. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J Immunol. 2001;167:1575–1583. doi: 10.4049/jimmunol.167.3.1575. [DOI] [PubMed] [Google Scholar]
- 35.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 37.Mollo SB, Zajac AJ, Harrington LE. Temporal requirements for B cells in the establishment of CD4 T cell memory. J Immunol. 2013;191:6052–6059. doi: 10.4049/jimmunol.1302033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–2054. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill SK, Cao Y, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179:5109–5116. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 40.DiLillo DJ, Griffiths R, Seshan SV, Magro CM, Ruiz P, Coffman TM, Tedder TF. B lymphocytes differentially influence acute and chronic allograft rejection in mice. J Immunol. 2011;186:2643–2654. doi: 10.4049/jimmunol.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B lymphocyte depletion by CD20 mono-clonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc γ R effector functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, Zhao M. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 43.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 44.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 48.Lassila O, Vainio O, Matzinger P. Can B cells turn on virgin T cells? Nature. 1988;334:253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- 49.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.João C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. B cell-dependent TCR diversification. J Immunol. 2004;172:4709–4716. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 51.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crowley MT, Reilly CR, Lo D. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J Immunol. 1999;163:4894–4900. [PubMed] [Google Scholar]
- 53.Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 54.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cerny A, Sutter S, Bazin H, Hengartner H, Zinkernagel RM. Clearance of lymphocytic choriomeningitis virus in antibody- and B-cell-deprived mice. J Virol. 1988;62:1803–1807. doi: 10.1128/jvi.62.5.1803-1807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greeley SAW, Moore DJ, Noorchashm H, Noto LE, Rostami SY, Schlachterman A, Song HK, Koeberlein B, Barker CF, Naji A. Impaired activation of islet-reactive CD4 T cells in pancreatic lymph nodes of B cell-deficient nonobese diabetic mice. J Immunol. 2001;167:4351–4357. doi: 10.4049/jimmunol.167.8.4351. [DOI] [PubMed] [Google Scholar]
- 57.Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 59.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 60.Quintanilla-Martinez L, Preffer F, Rubin D, Ferry JA, Harris NL. CD20+ T-cell lymphoma. Neoplastic transformation of a normal T-cell subset. Am J Clin Pathol. 1994;102:483–489. doi: 10.1093/ajcp/102.4.483. [DOI] [PubMed] [Google Scholar]
- 61.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 62.Algino KM, Thomason RW, King DE, Montiel MM, Craig FE. CD20 (pan-B cell antigen) expression on bone marrow-derived T cells. Am J Clin Pathol. 1996;106:78–81. doi: 10.1093/ajcp/106.1.78. [DOI] [PubMed] [Google Scholar]
- 63.Zhou L-J, Tedder TF. CD20 workshop panel report. In: Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, Springer T, et al., editors. Leukocyte Typing V. White Cell Differentiation Antigens. Oxford University Press; Oxford: 1995. pp. 511–514. [Google Scholar]
- 64.Eming R, Nagel A, Wolff-Franke S, Podstawa E, Debus D, Hertl M. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. 2008;128:2850–2858. doi: 10.1038/jid.2008.172. [DOI] [PubMed] [Google Scholar]
- 65.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons JA. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 2006;180:63–70. doi: 10.1016/j.jneuroim.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vallerskog T, Gunnarsson I, Widhe M, Risselada A, Klareskog L, van Vollenhoven R, Malmström V, Trollmo C. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62–74. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 67.Vigna-Perez M, Hernández-Castro B, Paredes-Saharopulos O, Portales-Pérez D, Baranda L, Abud-Mendoza C, González-Amaro R. Clinical and immunological effects of Rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Arthritis Res Ther. 2006;8:R83–R91. doi: 10.1186/ar1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saadoun D, Rosenzwajg M, Landau D, Piette JC, Klatzmann D, Cacoub P. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111:5334–5341. doi: 10.1182/blood-2007-11-122713. [DOI] [PubMed] [Google Scholar]
- 69.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 70.Hamel K, Doodes P, Cao Y, Wang Y, Martinson J, Dunn R, Kehry MR, Farkas B, Finnegan A. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180:4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- 71.Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2:20–27. doi: 10.1038/ncprheum0042. [DOI] [PubMed] [Google Scholar]
- 72.Goldberg SL, Pecora AL, Alter RS, Kroll MS, Rowley SD, Waintraub SE, Imrit K, Preti RA. Unusual viral infections (progressive multifocal leukoencephalopathy and cytomegalovirus disease) after high-dose chemotherapy with autologous blood stem cell rescue and peri-transplantation rituximab. Blood. 2002;99:1486–1488. doi: 10.1182/blood.v99.4.1486. [DOI] [PubMed] [Google Scholar]