Abstract
Objective
We examined the association between urinary aMT6s levels (six-sulfatoxymelatonin (aMT6s) is a primary urinary metabolite of melatonin) and shift work estimated by job exposure matrix (JEM) in healthy participants of the Shanghai Women's Health Study.
Methods
Creatinine-adjusted aMT6s levels were measured in urine samples from 300 women and related to JEM shift work categories.
Results
Adjusted geometric means of aMT6s levels (95% confidence intervals (CIs)) from urine samples collected before 8:00 a.m. were lower among persons holding nighttime shift work jobs. The adjusted aMT6s levels (ng/mg creatinine) were 8.36 (4.47, 15.6), 6.37 (3.53, 11.5), 6.20 (3.33, 11.5), 3.81 (2.02, 7.19), and 3.70 (1.92, 7.11) from the lowest shift work (never held a shift work job) to the highest (current job likely involved all-night shift work) JEM scores (Ptrend = 0.05).
Conclusions
Our results indicate that nightshift work JEM scores were significantly and inversely associated with aMT6s levels in early morning spot urine samples.
Keywords: Nightshift work, JEM, melatonine, Chinese women, SWHS
Introduction
Nightshift work has been associated with both lower melatonin levels and an increased cancer risk in epidemiological studies (1-3). The International Agency for Research on Cancer has classified shift work involving disruption of circadian rhythms as a probable cause of human cancer based on sufficient animal evidence but limited epidemiologic data (4). The investigation of the role of nightshift work in relation to cancer risk in human populations is hampered by the lack of shift work information available in most epidemiologic studies. Only limited studies have collected data on shift work (5-11), while other studies have relied on methods such as the job exposure matrix (JEM) to estimate nightshift work from lifetime occupational history or occupation data reported to census (12-15). Although JEM has been used extensively for evaluation of a wide variety of occupational exposures in relation to disease risks, its use in studies of nightshift work has been relatively limited. Reasons for the dearth of JEM-based shift work studies are unclear, but may be partly due to the difficulties in using JEM to estimate nightshift work and concern for the potentially sizable misclassification that may occur (16-17). A decreased melatonin level during periods of nightshift work is a hypothesized mechanism through which nightshift work is linked to cancer risk (18). Melatonin is secreted by the pineal gland during the dark phase of the light-dark cycle in a 24-hour daily rhythm. In a typical night time sleep cycle, melatonin reaches its peak level during the early morning hours (around 2:00-3:00 a.m.), and is quickly metabolized during day time (19-20). Six-sulfatoxymelatonin (aMT6s) is a primary urinary metabolite of melatonin (21). Previous studies have found that nightshift work or light exposure at night could reduce secretion of melatonin and was associated with lower levels of urinary aMT6s (21-29).
Epidemiologic studies of the association between urinary melatonin and shift work mainly measured aMT6s in 24-hour or morning first-void urine samples (20, 24, 27, 30). However, collection of 24-hour or morning first-void urine samples is challenging for most large prospective cohort studies. Day time spot urine samples, however, have been collected in a few cohorts at the time of baseline interview and these could be used in shift work investigations. An additional limitation is that not all cohort studies have detailed shift work questionnaire. Many, however, have collected an occupational history (1-3). If future studies could rely on a shift work JEM and spot urine samples for assessment of melatonin levels, it would expand the opportunities for examining adverse health risk in relation to nightshift work. The present pilot study is conducted to examine the association between nightshift work JEM scores and aMT6s levels tested in spot urine samples in healthy Chinese women participating in the population-based Shanghai Women's Health Study (SWHS) cohort.
Materials and Methods
The SWHS study has been described in detail elsewhere (31). Between March 1997 and May 2000, all eligible women aged 40-70 years residing permanently in seven communities of urban Shanghai were invited to participate, and 74,942 (93%) of them responded. At baseline, in-person interviews were conducted to obtain information on demographic background, history of tobacco smoking and alcohol use, leisure time physical activities, height and weight history, family history of cancer, reproductive factors, and a lifetime occupational history that included job title, factory name (industry), job descriptions, and years starting and ending the jobs. Over 88% of participants provided a spot urine sample at baseline. The samples were kept cold and processed within 6 hours of collection for long-term storage at −-70°C. During the second follow-up four years later, a self-reported nightshift work history was elicited. A written informed consent was obtained from all study participants. The study was approved by the Institutional Review Boards of all participating study centers in China and the United States.
A nightshift job exposure matrix (JEM) was developed by an industrial hygienist (S.X.) familiar with local industrial conditions, based on data in the lifetime occupational history reported by all cohort participants at baseline. The JEM categorized all jobs into never, low, median, and high likelihood of exposure to nightshift work (32). In the present study, we modified the JEM categories to classify the current (at baseline) nightshift work status as: 1 = never held a job involving nightshift; 2 = did not have a current nightshift job at baseline (i.e., at the time of urine collection) but had one or more jobs involving shift work in the past; 3 = current job involving occasional nightshift work (e.g., reporters and army personnel); 4 = current job likely involving part of the nightshift or being on call (e.g., physicians and bakers); and 5 = current job involving all-night shifts without sleep (e.g., nurses and certain production workers).
For the pilot study, only healthy women employed at the time of urine collection were included. Women were stratified and randomly selected within each stratum to provide equal numbers at each level of JEM score and at 4 time segments of sample collection (before 8:00 a.m., 8:01 a.m. – 10:00 a.m., 10:01 a.m. – 12:00 noon, and after 12:00 noon). A total of 300 women with urine samples were included.
Urinary aMT6s was assayed at the Johns Hopkins University Bayview Medical Center Core Laboratory using 6-sulfatoxymelatonin ELISA assay kit (ALPCO Cat# 01-EK-M6S, Windham, NH). For comparison, we also assayed urinary levels of cortisol, a major mediator of the neuroendocrine response to stress that may be influenced by circadian rhythm (26-28). Urinary free cortisol concentration was measured by using APLCO cortisol urine ELISA assay kit (ALPCO Cat# 33-10110, Windham, NH). All urinary measurements were adjusted for creatinine (nanograms per milligram creatinine, ng/mg Cr). Since the distributions of measurements for both aMT6s and cortisol levels were not quite normal, we transformed them into logarithmic values for statistical analysis. After excluding subjects with outlier values (defined as >3 times the standard deviation from the logarithmic average), 296 subjects remained in all data analyses.
Data analyses were conducted using the SAS MIXED procedure, by setting aMT6s as a response variable, to fit linear regression models to calculate estimated means of aMT6s by nightshift work JEM levels, sample collection time, and other covariates, including age at sample collection (age 40-49, 50-59, and 50-70 years), education levels (college and above, high school, middle school, and elementary school or less), body mass index (BMI) (quartile: ≤21.1, >21.1 -≤22.9, >22.9 - ≤25.4, and >25.4 weight (kg) per height squared (m2), alcohol consumption (yes, no), menopausal status (pre- vs. post-menopausal), number of live births (0-1 and 2+ children), and creatinine-adjusted urinary cortisol level (quartile: ≤136.5, >136.5 - ≤197.9, >197.9 - ≤297.1, and >297.1 ng/mg Cr). Additional adjustment in multivariate models for other factors potentially associated with aMT6s level, such as smoking, tea drinking, family history of cancer, ever took medicine in past 24 hours, self-reported nightshift work in lifetime, and oral contraceptive use did not change the associations between urinary aMT6s or cortisol levels and nightshift work JEM scores. These factors therefore were not included in the final models. All means were back-transformed to present results in the original scale of nanograms aMT6s or cortisol per milligram creatinine (geometric means).
Results
Of the 296 women in this study, the median age was 45 years and over 47% had high school education or above. The median BMI was 23.4. Only 2.7% ever drank alcohol and 1.7% ever smoked cigarettes. Almost all women had at least a live birth (97.6%), and 50 (16.9%) had two or more live births. Less than one quarter (23.3%) were post-menopausal at enrollment.
The overall geometric mean level of aMT6s was 4.07 ng/mg Cr. The mean was highest (8.32 ng/mg Cr) in urine samples collected between 7:00-8:00 a.m., and consistently decreased in samples collected later in the day (Table 1). The levels were 4.54, 3.57, and 2.06 ng/mg Cr in urine samples collected at 8:01-10:00 a.m., 10:01 a.m. -12:00 noon, and after 12:00 noon, respectively. A similar pattern was observed for urinary levels of cortisol, with the overall average of 167.1 and the highest level (201.2 ng/mg Cr) in samples collected between 7:00-8:00 a.m. and lowest level (127.7 ng/mg Cr) in samples collected in the afternoon.
Table 1.
Observed Creatinine-adjsuted Geometric Means of 6-sulfatoxymelatonin (aMT6s) and Cortisol levels (ng/mg Cr) by 4 Time Segments of Urine Sample Collection (hour) in 296 Urine Samples, SWHS, Shanghai, China
| aMT6s (ng/mg Cr) | Cortisol (ng/mg Cr) | ||||
|---|---|---|---|---|---|
| Sample collection time | N | Geometric mean | SD | Geometric mean | SD |
| All time* | 296 | 4.07 | 2.38 | 167.1 | 1.64 |
| 8:01 - 10:00 | 75 | 4.54 | 2.12 | 191.7 | 1.55 |
| 10:01 - 12:00 | 74 | 3.57 | 2.10 | 158.2 | 1.46 |
| 12:01 - 19:00 | 74 | 2.06 | 1.62 | 127.7 | 1.56 |
Arithmetic means were 6.30 (SD=7.86) ng/mg Cr for aMT6s and 189.3 (SD=104.6) ng/mg Cr for cortisol.
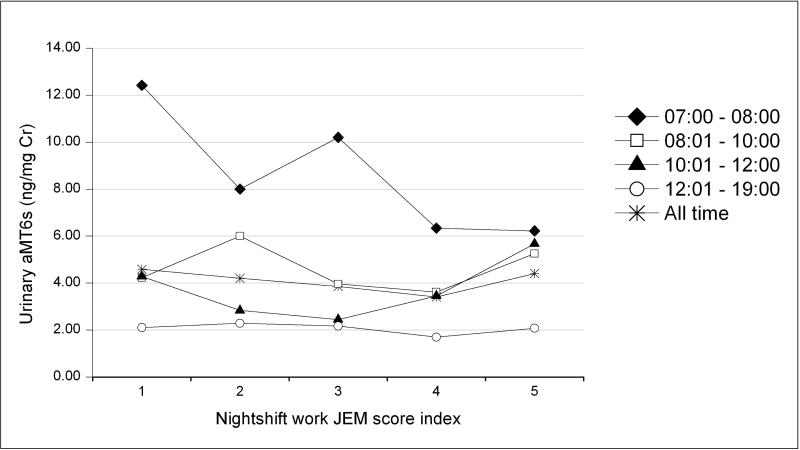
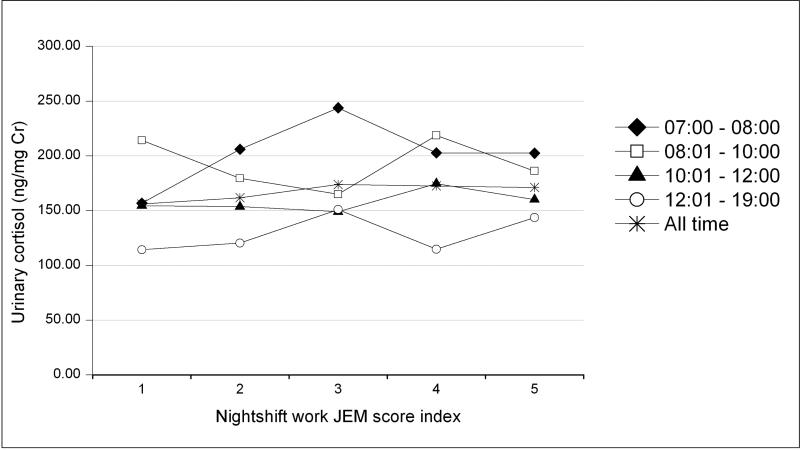
Figure 1a shows the association between urinary aMT6s levels and nightshift JEM scores by the four different time segments of urine sample collection. The inverse trends of aMT6s by nightshift work JEM scores was only seen in the early morning urine samples. No associations between urinary aMT6s and nightshift work JEM scores were observed in urine samples collected at later times of the day or in all urine samples. In contrast, urinary cortisol levels were not associated with JEM scores in samples collected at any time during the day, including the early morning urine samples (Figure 1b).
Figure 1a.
Observed creatinine-adjusted geometric mean 6-sulfatoxymelatonin (aMT6s) levels (ng/mg creatinine) by nightshift JEM score index, SWHS, Shanghai, China.
(Nightshift JEM score index: 1 = never had a nightshift work job in lifetime, 2 = currently did not have a nightshift work job but had one or more in the past, 3 = current job had a nightshift JEM score of 1, 4 = current job had a nightshift JEM score of 2, through 5 = current job had a nightshift JEM score of 3) by different sample collection time (7:00-8:00 h, 8:01-10:00 h, 10:01-12:00 h, 12:01-19:00h, and all time period) )
Figure 1b.
Observed creatinine-adjusted geometric mean cortisol levels (ng/mg creatinine) by nightshift JEM score index, SWHS, Shanghai, China
(Nightshift JEM score index: 1 = never had a nightshift work job in lifetime, 2 = currently did not have a nightshift work job but had one or more in the past, 3 = current job had a nightshift JEM score of 1, 4 = current job had a nightshift JEM score of 2, through 5 = current job had a nightshift JEM score of 3) by different sample collection time (7:00-8:00 h, 8:01-10:00 h, 10:01-12:00 h, 12:01-19:00h, and all time period))
We conducted further analysis restricting to samples collected during 7:00 to 8:00 a.m. In multivariate models, we examined whether aMT6s levels varied by lifestyle and reproductive factors (Table 2). Urinary aMT6s levels tended to be higher among women under age 45 years, who had at least some college education, did not consume alcohol, had only one or no live birth, or were post-menopausal at the time of urine donation, although the differences were not statistically significant. The aMT6s level was lowest among women in the highest quartile of BMI (>25.4) or with below median level of urinary cortisol.
Table 2.
Estimated (Adjusted) Geometric Means and 95% Confidence Intervals of Urinary 6-Sulfatoxymelatonin (aMT6s) by Lifestyle Factors in the Early Morning Urine Samples, SWHS, Shanghai, China*
| Lifestyle Factors | N | Geometric mean (ng/mg Cr) | 95% CI† |
|---|---|---|---|
| Age at Sample Collection(yr) | |||
| 40-<45 | 41 | 6.32 | (3.47, 11.51) |
| 45-70 | 32 | 5.55 | (3.35, 9.20) |
| P for difference | 0.57 | ||
| Education | |||
| 1 = College + | 17 | 7.49 | (4.16, 13.50) |
| 2 = High School | 24 | 4.78 | (2.54, 9.00) |
| 3 = Middle School & Less | 32 | 5.80 | (3.31, 10.16) |
| P for trend | 0.33 | ||
| BMI (Kg/M2)‡ | |||
| Q1 (<=21.1) | 19 | 6.45 | (3.42, 12.17) |
| Q2 (>21.1-<=22.9) | 18 | 6.13 | (3.14, 11.96) |
| Q3 (>22.9-<=25.4) | 17 | 8.35 | (4.74, 14.71) |
| Q4 (>25.4) | 19 | 3.73 | (2.06, 6.73) |
| P for trend | 0.06 | ||
| Ever alcohol consumption | |||
| Yes | 2 | 4.29 | (1.59, 11.59) |
| No | 71 | 8.18 | (5.57, 12.02) |
| P for difference | 0.25 | ||
| Number of live births | |||
| 0-1 | 62 | 6.95 | (3.75, 12.89) |
| 2+ | 11 | 5.05 | (2.62, 9.73) |
| P for difference | 0.41 | ||
| Menopausal status | |||
| Pre-menopause | 10 | 5.59 | (3.27, 9.56) |
| Post-menopause | 63 | 6.28 | (3.26, 12.10) |
| P for difference | 0.72 | ||
| Cortisol‡ | |||
| Q1 (<=136.5) | 18 | 9.16 | (5.04, 16.64) |
| Q2 (>136.5-<=197.9) | 19 | 6.21 | (3.46, 11.13) |
| Q3 (>197.9-<=297.1) | 18 | 4.12 | (2.19, 7.77) |
| Q4 (>297.1) | 18 | 5.26 | (2.79, 9.89) |
| P for trend | 0.05 |
Geometric mean of creatinine-adjusted 6-sulfatoxymelatonin (aMT6s) urinary concentration controlled for all covariates listed.
95% confidence interval.
Quartiles of BMI and cortisol levels were cut according to their distributions in the early morning urine samples
Urinary aMT6s levels from early morning urine collection decreased with increasing JEM scores (Table 3). The estimated aMT6s levels were reduced in all nightshift JEM categories in the fully adjusted models that included age, education, BMI, alcohol consumption, menopausal status, number of live births, and urinary cortisol levels. The adjusted aMT6s levels decreased from 8.36 ng/mg Cr (95% CI: 4.47, 15.6) in the lowest nightshift JEM score category (never held a job with shift work in lifetime) to 3.70 ng/mg Cr (95% CI: 1.92, 7.11) in the highest category (current job likely involved all-night shift work) (p for trend = 0.05).
Table 3.
Estimated Geometric Means and 95% Confidence Intervals of Urinary 6-Sulfatoxymelatonin (aMT6s) by Nightshift JEM Score Index in the Early Morning Urine Samples, SWHS, Shanghai, China
| Non-adjusted | Adjusted* | ||||
|---|---|---|---|---|---|
| Nightshift JEM score index† | N | Geometric Mean (ng/mg Cr) | 95% CI‡ | Geometric Mean (ng/mg Cr) | 95% CI‡ |
| 1 | 14 | 12.42 | (8.03, 19.22) | 8.36 | (4.47, 15.63) |
| 2 | 15 | 8.01 | (5.25, 12.20) | 6.37 | (3.53, 11.46) |
| 3 | 15 | 10.21 | (6.70, 15.56) | 6.20 | (3.33, 11.52) |
| 4 | 15 | 6.34 | (4.16, 9.67) | 3.81 | (2.02, 7.19) |
| 5 | 14 | 6.23 | (4.03, 9.63) | 3.70 | (1.92, 7.11) |
| P for trend | 0.11 | 0.05 | |||
Geometric mean of creatinine-adjusted 6-sulfatoxymelatonin (aMT6s) urinary concentration controlled for age (40-<50, 50-<60, and 60-70); education (college+, high school, and middle school and less), BMI (<=21.1, >21.1-<=22.9, >22.9-<=25.4, and >25.4), ever alcohol consumption (ever, never), menopausal status (pre- and post-menopausal), number of live births (0-1 and 2+ children), and urinary cortisol level (<=136.5, >136.5-<=197.9, >197.9-<=297.1, and >297.1 ng/mg Cr).
Nightshift JEM score index: 1 = never had a nightshift work job in lifetime, 2 = currently did not have a nightshift work job but had one or more in the past, 3 = current job had a nightshift JEM score of 1, 4 = current job had a nightshift JEM score of 2, through 5 = current job had a nightshift JEM score of 3.
95% confidence interval.
Discussion
In this cross-section of middle-aged working Chinese women, we found that aMT6s levels in spot urine samples collected in early morning consistently decreased with increasing likelihood of nightshift work as indicated by JEM score. In contrast, inverse associations between nightshift work and aMT6s levels were not observed in urine samples collected after 8 a.m.
Exposure to light at night has been documented to suppress the secretion of melatonin (19, 21-27). Nightshift work, a surrogate for exposure to light at night, has also been shown to lower urinary aMT6s levels among exposed workers (22-29). Our observation of an inverse association between nightshift work and aMT6s levels in early morning urine samples is consistent with previous work, and suggests that spot urine samples collected in early morning may be used for studies using aMT6s and shift work. Since melatonin is quickly metabolized throughout the course of a day (19-20), spot urine samples collected later in the day would not be suitable for evaluation of aMT6s levels as suggested by its lack of correlation with nightshift work in samples collected after 8:00 a.m. in our study.
The reliability of occupational exposure assessments based on JEMs has been questioned, since this approach depends largely on the subjective professional judgment of an industrial hygienist or occupational expert (33). Construction of a JEM for shift work maybe especially challenging. Our findings of an inverse association between nightshift work JEM and aMT6s levels in early morning urine samples, however, provides some validation of the JEM approach for the assessment of shift work in our study population. Although the shift work JEM developed for the SWHS must have considerable misclassification error, based on the correlation with urinary aMT6s it would appear to be useful for epidemiologic evaluations. The specificity of the associations of nightshift work with aMT6s, but not with urinary cortisol levels, also adds credibility to our finding. This lack of an association between urinary cortisol levels and nightshift JEM score in the SWHS pilot resembles findings among policemen where long-term nightshift work was also not associated with the cortisol response (34). Some previous studies, however, have found that nightshift work inversely reduced cortisol secretion (35-36). Since many epidemiologic studies did not collect information on shift work, but most collected lifetime occupational history, the ability to use nightshift JEMs for exposure characterization considerably broadens opportunities for further evaluation of health effects of shift work. However, it is unclear whether this approach, i.e., using JEM to assess shift work exposure and using spot urine collected in early morning to assess melatonin levels, would work as effectively in studies conducted in other settings. Individuals in Shanghai do not have as many lifetime jobs and may occur in other populations. Our results are encouraging, but additional pilot work may be necessary for studies conducted under different settings.
Our study was limited in that we were not able to verify the nightshift work JEM score directly because information on nightshift work pattern was not collected for each job in the participants’ occupational history. However, the JEM scoring was conducted by an experienced, local industrial hygienist who is familiar with the Shanghai work environment. Our study was also limited by the lack of sequential urine samples collected during different times of the day for the same individuals to examine whether the relative associations with JEM scores can be maintained using samples stratified by time segment of collection, despite the generally lower levels of aMT6s in samples collected later in the day than in samples collected before 8:00 a.m.
In conclusion, JEM scores of nightshift work as a surrogate for light exposure at night were significantly and inversely associated with urinary aMT6s levels in spot urine samples collected in early morning.
Acknowledgement
This study was supported by the US National Institutes of Health (grant R37 CA070867) and the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics (contract NO2-CP-11010-66). This publication was also made possible by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinical research/overview-translational.asp. The authors also thank Ying Ni at the Johns Hopkins University Bayview Medical Center Core Laboratory for laboratory analyses and David Check at the National Cancer Institute for development of figures.
Footnotes
Conflict of interest: none declared.
References
- 1.Kolstad HA. Nightshift work and risk of breast cancer and other cancers – a critical review of the epidemiologic evidence. Scand J Work Environ Health. 2008;34(1):5–22. doi: 10.5271/sjweh.1194. [DOI] [PubMed] [Google Scholar]
- 2.Wang XS, Armstrong MEG, Cairns BJ, Key TJ, Travis RC. Shift work and chornic disease: the epidemiological evidence. Occup Med. 2011;61:76–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, et al. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med. 2011;68:154–62. doi: 10.1136/oem.2009.053512. [DOI] [PubMed] [Google Scholar]
- 4.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–6. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 5.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 6.Davis S, Mirick D, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 7.Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17:108–11. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary ES, Schoenfeld ER, Stevens RG, Kabat GC, Henderson K, Grimson R, et al. Electromagnetic Fields and Breast Cancer on Long Island Study Group. Shift work, light at night, and breast cancer on Long Island, New York. Am J Epidemiol. 2006;164:358–66. doi: 10.1093/aje/kwj211. [DOI] [PubMed] [Google Scholar]
- 9.Lie JA, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control. 2006;17:39–44. doi: 10.1007/s10552-005-3639-2. [DOI] [PubMed] [Google Scholar]
- 10.Pesch B, Harth V, Rabstein S, Baisch C, Schiffermann M, Pallapies D, et al. Night work and breast cancer—results from the German GENICA study. Scand J Work Environ Health. 2010;36:134–41. doi: 10.5271/sjweh.2890. [DOI] [PubMed] [Google Scholar]
- 11.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–55. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 12.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–7. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female workers. Scand J Work Environ Health. 2007;35(5):336–43. doi: 10.5271/sjweh.1150. [DOI] [PubMed] [Google Scholar]
- 14.Lahti TA, Partonen T, Kyyrönen P, Kauppinen T, Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer. 2008;123:2148–51. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 15.Kauppinen T, Heikkilä P, Plato N, Woldbaek T, Lenvik K, Hansen J, et al. Construction of job-exposure matrices for the Nordic Occupational Cancer Study (NOCCA). Acta Oncol. 2009;18:1–11. doi: 10.1080/02841860902718747. [DOI] [PubMed] [Google Scholar]
- 16.Siemiatycki J, Day NE, Fabry J, Cooper JA. Discovering carcinogens in the occupational environment: A novel epidemiologic approach. J Natl Cancer Inst. 1981;66:217–25. [PubMed] [Google Scholar]
- 17.Coughlin S, Chiazze L. Job-Exposure matrices in epidemiologic research and medical surveillance. Occup Med. 1990;5(3):633–46. [PubMed] [Google Scholar]
- 18.Stevens RG, Davis S. The melatonin hypothesis: electric power and breast cancer. Environ Health Perspect. 1996;36:573–96. doi: 10.1289/ehp.96104s1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen AM, Garde AH, Hansen J. Diurnal urinary 6-sulfatoxiymelatonin levels among healthy Danish nurses during work and leisure time. Chronobiology Int. 2006;23(6):1203–15. doi: 10.1080/07420520601100955. [DOI] [PubMed] [Google Scholar]
- 20.Arendt J, Skene DJ. Melatonin as a chronobiotic. Clinical Review. 2005;9:25–39. doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Young IM, Leone RM, Francis P, Stovell P, Silman RE. Melatonin is metabolized to JV-acetyl serotonin and 6-hydroxymelatonin in man. J Clinical Endocrinology Metabolism. 1985;60:114–9. doi: 10.1210/jcem-60-1-114. [DOI] [PubMed] [Google Scholar]
- 22.Stevens RG, Rea MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control. 2001;12:279–87. doi: 10.1023/a:1011237000609. [DOI] [PubMed] [Google Scholar]
- 23.Schernhammer ES, Rosner B, Willet WC, Laden F, Colditz GA, Hankinson SE. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol Biomarkers Prev. 2004;13(6):936–43. [PubMed] [Google Scholar]
- 24.Borugian MJ, Gallagher RP, Friesen MC, Switzer TF, Aronson JK. Twenty-four-hour light exposure and melatonin levels among shift workers. J Occup Environ Med. 2005;47(12):1268–75. doi: 10.1097/01.jom.0000184855.87223.77. [DOI] [PubMed] [Google Scholar]
- 25.Grundy A, Sanchez M, Richardson H, Tranmer J, Boruqian M, Graham CH, et al. Light intensity exposure, sleep duration, physical activity, and biomarkers of melatonin among rotating shift nurses. Chronobiology Int. 2009;26(7):1445–61. doi: 10.3109/07420520903399987. [DOI] [PubMed] [Google Scholar]
- 26.Levallois P, Dumont M, Touitou Y, Gingras S, Mâsse B, Gauvin D, et al. Effects of electric and magnetic fields from high-power lines on female urinary excretion of 6-sulfatoxymelatonin. Am J Epidemiol. 2001;154(7):601–9. doi: 10.1093/aje/154.7.601. [DOI] [PubMed] [Google Scholar]
- 27.Cocco P, Cocco ME, Paghi L, Avataneo G, Salis A, Meloni M, et al. Urinary 6-sulfatoxymelatonin excretion in humans during domestic exposure to 50 herts electromagnetic fields. Neuroendocrinology Let. 2005;26(2):136–42. [PubMed] [Google Scholar]
- 28.Nagata C, Nagao Y, Yamamoto S, Shibuya C, Kashiki Y, Shimizu H. Light exposure at night, urinary 6-sulfatoxymelatonin, and serum estrogens and androgens in postmenopausal Japanese women. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1418–23. doi: 10.1158/1055-9965.EPI-07-0656. [DOI] [PubMed] [Google Scholar]
- 29.Grundy A, Tranmer J, Richardson H, Graham CH, Aronson JK. The influence of light at night exposure on melatonin levels among Canadian rotating shift nurses. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2404–12. doi: 10.1158/1055-9965.EPI-11-0427. [DOI] [PubMed] [Google Scholar]
- 30.Hsing AW, Meyer TE, Niwa S, Quraishi SM, Chu LW. Measuring serum melatonin in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2010;19(4):932–7. doi: 10.1158/1055-9965.EPI-10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, et al. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162(11):1123–31. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 32.Pronk A, Ji BT, Shu XO, Xue S, Yang G, Li HL, et al. Night-shift work and breast cancer risk in a cohort of Chinese women. Am J Epidemiol. 2010;171(9):953–9. doi: 10.1093/aje/kwq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kromhout H, Vermeulen R. Application of job-exposure matrices in studies of the general population: some clues to their performance. Eur Respir Rev. 2001;11:80–90. [Google Scholar]
- 34.Wirth M, Burch J, Violanti J, Burchfiel C, Fekedulegn D, Andrew M, et al. Shiftwork duration and the awakening cortisol response among police officers. Chronobiol Int. 2011;28(5):446–57. doi: 10.3109/07420528.2011.573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griefahn B, Robens S. The normalization of the cortisol awakening response and of the cortisol shift profile across consecutive night shifts – an experimental study. Psychoneuroendocrinology. 2010;35:1501–9. doi: 10.1016/j.psyneuen.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Kudielka BM, Buchtal J, Uhde A, Wüst S. Circadian cortisol profiles and psychological self-reports in shift workers with and without recent change in the shift rotation system. Biological Psychology. 2007;74(1):92–103. doi: 10.1016/j.biopsycho.2006.08.008. [DOI] [PubMed] [Google Scholar]