Abstract
Objective
To perform a systematic review and meta-analysis that quantitatively tests and summarizes the hypothesis that depression results in elevated oxidative stress and lower antioxidant levels.
Methods
We performed a meta-analysis of studies that reported an association between depression and oxidative stress and/or antioxidant status markers. PubMed and EMBASE databases were searched for articles published from January 1980 through December 2012. A random-effects model, weighted by inverse variance, was performed to pool standard deviation (Cohen’s d) effect size estimates across studies for oxidative stress and antioxidant status measures, separately.
Results
Twenty-three studies with 4980 participants were included in the meta-analysis. Depression was most commonly measured using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria. A Cohen’s d effect size of 0.55 (95% confidence interval = 0.47–0.63) was found for the association between depression and oxidative stress, indicating a roughly 0.55 of 1-standard-deviation increase in oxidative stress among individuals with depression compared with those without depression. The results of the studies displayed significant heterogeneity (I2 = 80.0%, p < .001). A statistically significant effect was also observed for the association between depression and antioxidant status markers (Cohen’s d = −0.24, 95% confidence interval = −0.33 to −0.15).
Conclusions
This meta-analysis observed an association between depression and oxidative stress and antioxidant status across many different studies. Differences in measures of depression and markers of oxidative stress and antioxidant status markers could account for the observed heterogeneity. These findings suggest that well-established associations between depression and poor heath outcomes may be mediated by high oxidative stress.
Keywords: depression, oxidative stress, meta-analysis, review
INTRODUCTION
Individuals who experience more depression exhibit greater functional limitations, morbidity, and premature mortality than do individuals without depression. Research in the last 20 years has shown that the relationship between depression and its central nervous system influences the body’s physiological processes (1). Most of this research has been on the cardiovascular and endocrine systems. An increase in cortisol levels, activated by the hypothalamic-pituitary-adrenal axis, is one well-accepted explanation for the relationship between psychological distress, such as depression, and physical disease (1), including cardiovascular diseases and diabetes (2).
Oxidative stress is the imbalance between oxidative stress and antioxidant defenses, which leads to oxidized proteins, lipids, and nucleic acids. Higher oxidative stress is indicative of greater reactive oxygen species circulating the body. Antioxidants counteract the damaging effects of oxidative stress. Lower antioxidant status indicates fewer circulating antioxidant molecules to offset the oxidation of molecules. High oxidative stress or low antioxidant status are increasingly established in the biology of poor health outcomes and progression of a wide range of diseases. Previous research has shown that high oxidative stress is associated with the onset and progression of diabetes (3), atherosclerosis (4), and mortality (5). Oxidative stress is a candidate pathway that may link depression to physiological changes.
An association between depression and oxidative stress has been reported, although findings are inconsistent due to heterogeneous clinical populations, small sample sizes, and the use of varying measures of oxidative stress or antioxidant status (6). Published reviews in this subject area have systematically reviewed the literature and provide explanations for possible biological mechanisms (7–9), but have not quantitatively assessed the literature by performing a meta-analysis. Our goals in this article are to summarize this growing body of literature and evaluate the current state of evidence both qualitatively and quantitatively through the use of a meta-analysis.
METHODS
Search Strategy
A literature search of observational studies examining the association between depression and oxidative stress was performed. The search was conducted for articles published from January 1980 through December 2012 using PubMed and EMBASE electronic databases. These databases were searched without language or subject restrictions. Controlled vocabulary (MeSH and emtree) and keywords were used in the search strategy. Searches involved several combinations of terms, including “depression” OR “depressive symptoms” OR “emotional depression” AND “protein carbonyls” OR “MDA” OR “glutathione” OR antioxidant markers, for example, “carotenoids” OR “SOD.” A complete list of the search terms used for each database is provided in Appendix A, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A94. A bibliography search of included studies was done to identify additional studies.
Study Selection
Only observational human studies that examined the association between depression and oxidative stress and antioxidant status markers were included. Studies were excluded if they did not have a valid depression or oxidative stress (or antioxidant status) measure. All articles retrieved by our search strategy were imported and merged into RefWorks. Two coauthors (P.P. and S.L.S.) independently reviewed 2032 titles and abstracts for inclusion and classified the abstracts as “include” or “exclude.” Any disagreements in the abstracts review were adjudicated by consensus. Titles and abstracts categorized as exclude by both of the reviewers were excluded, and the reason for exclusion was documented. Agreement between the reviewers’ decisions on inclusion/exclusion of articles was assessed using Cohen’s k statistic. The k statistic for title and abstract inclusion/exclusion was 0.56, indicating moderate interrater agreement. Studies that were included based on title and abstract review underwent full-text review (n = 70). Forty-seven studies were excluded after full-text review for the following reasons: no measure of oxidative stress (n = 11), no measure of depression (n = 4), no comparison group (n = 4), insufficient data (n = 15), nonhuman study (n = 2), review article (n = 8), and studies reporting on the same study population (n = 3). The study selection process resulted in 23 articles that met study inclusion criteria (Fig. 1).
Figure 1.
Flow diagram of study selection process.
Data Extraction
Two coauthors (P.P. and L.J.S.) independently abstracted all included articles into a standardized excel spreadsheet. Disagreements or uncertainties were adjudicated by consensus. From each article, we abstracted a) characteristics of the study population, including sample size and age; b) measures of oxidative stress or antioxidant status, including how it was measured and in what fluid it was measured; and c) measures of depression. A complete list of the data abstraction fields is included in Appendix B, Supplemental Digital Content 2, http://links.lww.com/PSYMED/A95.
Statistical Analysis
Measures of depression and oxidative stress varied across studies. Comparable with other meta-analyses (10), a Cohen’s d effect size was calculated for each study to allow for comparability across studies because of varied outcome measures. In all included studies, depression had already been dichotomized as either nondepressed or depressed based on either clinical criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (11–26) or other epidemiologic measures with specified cutoffs (27–33).
For studies with multiple oxidative stress and antioxidant status measures, an effect size estimate was first computed for each individual oxidative stress and antioxidant status measure. This was calculated by taking the difference in the mean change in oxidative stress or antioxidant status measure between the nondepressed and depressed groups and dividing by the pooled standard deviation. These individual effect size estimates were then pooled to obtain a Cohen’s d effect size estimate within each study for oxidative stress and anti-oxidant status, separately. A positive effect size estimate indicates either high oxidative stress or high antioxidant status. A negative effect size estimate indicates either low oxidative stress or low antioxidant status. A random-effects meta-analysis, weighted by inverse variance, was performed to pool the standard deviation (Cohen’s d) effect size estimates across studies for oxidative stress and antioxidant status measures, separately. A test for heterogeneity was performed to determine the presence of statistical heterogeneity or variation between the estimates of association for included studies. The presence of statistical heterogeneity provided justification for a random effects approach to the meta-analysis. All analyses were conducted using STATA 11.0 (STATA Corp, College Station, TX).
Quality Assessment of Reported Studies
Following guidelines from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement, we used the following items to assess study quality (34): clear description of participant eligibility and, for case-control studies, case ascertainment based on psychometric methods and comparable control selection; measurement of depression using reliable instruments; clear description of oxidative stress measurement; clear description of how depression and oxidative stress variables were handled in analyses; and control for potential confounders, such as excessive adiposity, recent infections or medication use, and presence of chronic disease or neurologic disorder, either by exclusion criteria or statistical adjustment.
RESULTS
Characteristics and Quality of Studies on Depression and Oxidative Stress
Sample Characteristics
Twenty-three published studies were included in this meta-analysis (Table 1). A total of 4980 participants were recruited from community and clinical populations (Table 1). Studies were conducted in 10 countries: Turkey (11,12,16,19,21–24,27), India (15,18,25), Japan (17,31), United States (26,29), China (32,33), Canada (13), Netherlands (30), Poland (14), Greece (28), and Belgium (20). Most studies had a small sample size (n < 100), with the exception of one population-based study (29) and five community-based or clinical populations (13,18,22,26,30). Two studies were conducted in adults 60 years and older (28,30). Most studies included more than 50% women (11–14,16,18–20,22–27,29,31,32). All studies conducted in countries outside the United States and one conducted in the United States did not report on the distributions of race/ethnicity. Of the studies that did report on race/ethnicity, study samples were predominantly white (13,26).
TABLE 1.
Characteristics of Clinical Studies Examining the Association Between Depression and Oxidative Stress, by Year of Publication
| Study | Country | Depression Measure |
Oxidative Stress Markers |
Antioxidant Status Markers | Depressed/ Nondepressed (n/n)a |
Depressed, Mean Age (y) |
Nondepressed, Mean Age (y) |
Depressed, % Female |
Nondepressed, % Female |
|---|---|---|---|---|---|---|---|---|---|
| Selek et al. (23) | Turkey | DSM-IV criteria | TOS | TAS | 21/40 | 35.5 | 35.5 | 57.1 | 57.5 |
| Ghodake et al. (15) | India | DSM-IV criteria | MDA, NO | SOD, vitamin E, vitamin C, TAC | 30/30 | 32.2 | 32.2 | NA | NA |
| Bal et al. (27) | Turkey | Hamilton Depression Rating Scale | MDA | Vitamin E | 42/38 | 44.1 | 44.0 | 88.8 | 81.2 |
| Kotan et al. (19) | Turkey | DSM-IV criteria | — | Uric acid, albumin, bilirubin, GPx, vitamin A, vitamin C, vitamin E | 50/44 | 33.1 | 33.2 | 78.0 | 77.3 |
| Yager et al. (26) | USA | DSM-IV criteria | F2a-isoprostanes | — | 73/72 | 28.4 | 28.8 | 82.4 | 79.7 |
| Maes et al. (20) | Belgium | DSM-IV criteria | OxLDL, peroxides | — | 54/37 | 43.5 | 43.6 | 57.4 | 83.8 |
| Kobrosly and van Wijngaarden (29) | USA | PHQ-9 | GGT | Vitamin C, bilirubin, uric acid | 82/3080 | NA | NA | 64.6 | 49.3 |
| Wei et al. 2009 (32) | China | Hamilton Depression Rating Scale | MDA, NO, 8-OHdG | T-AOC, CAT, SOD | 52/30 | 56.0 | NA | 65.5 | NA |
| Kupper et al. (30) | Netherlands | Beck Depression Inventory | 8-OHdG, XO | — | 38/72 | 67.5 | 65.9 | 34.2 | 25.0 |
| Galecki et al. (14) | Poland | DSM-IV criteria | MDA | SOD, catalase, GPx | 50/30 | 36.7 | 32.1 | 56.0 | 53.3 |
| Cumurcu et al. (12) | Turkey | DSM-IV criteria | TOS | T-AOC | 57/40 | 35.5 | 35.2 | 80.7 | 82.5 |
| Selek et al. (24) | Turkey | DSM-IV criteria | NO | SOD | 29/30 | 32.8 | 29.7 | 48.3 | 53.3 |
| Dimopoulos et al. (28) | Greece | Geriatric Depression Scale | 8-iso-PGF2α | — | 33/33 | 65.8 | 65.4 | 50.0 | 50.0 |
| Sarandol et al. (22) | Turkey | DSM-IV criteria | — | GPx, TAC, vitamin E, vitamin C, carotenoids, uric acid, albumin, bilirubin | 96/54 | 40.0 | 37.0 | 75.0 | 74.1 |
| Herken et al. (16) | Turkey | DSM-IV criteria | XO, NO | SOD | 36/20 | NA | NA | 52.8 | 45.0 |
| Zhou et al. (33) | China | Zung Self-Rating Depression Scale | MDA, NO, ROS | T-AOC, SOD | 44/20 | NA | NA | NA | NA |
| Forlenza and Miller (13) | USA | DSM-IV criteria | 8-OHdG | — | 84/85 | 28.7 | 28.9 | 81.0 | 81.2 |
| Irie et al. (17) | Japan | DSM-IV criteria | 8-OHdG | — | 30/60 | 49.4 | 48.1 | 33.3 | 33.3 |
| Tsuboi et al. (31) | Japan | CES-D | LOOH | — | 27/32 | NA | NA | 100.0 | 100.0 |
| Ozcan et al. (21) | Turkey | DSM-IV criteria | MDA, NO | SOD | 30/21 | 39.8 | 39.8 | 46.7 | 46.7 |
| Khanzode et al. (18) | India | DSM-IV criteria | MDA | SOD, ascorbic acid | 62/40 | 43.8 | 40.9 | 45.0 | 54.8 |
| Srivastava et al. (25) | India | DSM-IV criteria | — | SOD, catalase, GPx | 26/43 | 34.0 | 36.2 | 33.3 | 16.7 |
| Bilici et al. (11) | Turkey | DSM-IV criteria | MDA | GPx, GR | 12/32 | 40.4 | 42.1 | 66.7 | 50.0 |
DSM-IV = Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; TOS = total oxidative species; TAS = total antioxidant status; MDA = malondialdehyde; NO = nitric oxide; SOD = superoxide dismutase; T-AOC/TAC = total antioxidant capacity; NA = data not available; oxLDL = oxidized low-density lipoproteins; PHQ-9 = Patient Health Questionnaire; GGT = g-glutamyltransferase; 8-OHdG = 8-oxo-2′-deoxyguanosine; XO = xanthine oxidase; 8-iso-PGF2α = plasma 8-iso-prostaglandin F2α; ROS = reactive oxygen species; CES-D = Center for Epidemiologic Depression Scale; LOOH = lipid hydroperoxide; GPx = glutathione peroxidase; GR = glutathione reductase.
Numbers subject to change based on participants with available oxidative stress or antioxidant status marker data.
Study Design Characteristics
Current literature on the associations between depression and oxidative stress is primarily cross sectional. Few prospective studies have examined the effect of depression treatments on health outcomes, but only baseline data were used to calculate effect sizes. Age- and sex-matched healthy controls were included in most studies, with the exception of nine (20,23,24,27–32). Although unadjusted data were used to create comparable effect sizes estimates across studies for this meta-analysis, potentially confounding factors were dealt with through statistical adjustment in seven studies (13,17,26,29–31,33).
Depression Assessment Characteristics
Sixteen studies assessed depression using the DSM-IV criteria (11–26), two used the Hamilton Depression Rating Scale (27,32), one used the Center for Epidemiologic Studies Depression Scale (31), one used the Patient Health Questionaire-9 (29), one used the Beck Depression Inventory (30), one used the Geriatric Depression Scale (28), and one used a reliable but nonstandardized measure of depression called the Zung Self-Rating Depression Scale (33). Of the 16 studies assessing depression using the DSM-IV criteria, 7 additionally used the Hamilton Depression Rating Scale to assess severity of depressive symptoms (11,13,14,16,18,22,26).
Oxidative Stress Assessment Characteristics
The heterogeneity in oxidative stress and antioxidant status markers reported is shown in Table 1. A total of 12 measures of oxidative stress were described in these studies. The most frequently reported oxidative stress markers include malondialdehyde and nitric oxide (NO). Oxidative stress markers were most frequently collected from serum or plasma. The heterogeneity in measurement of antioxidant status markers was even greater. Investigators most commonly examined superoxide dismutase and glutathione peroxidase in analyses. Antioxidant status markers were most frequently collected from serum or plasma. Two studies collected their oxidative stress and antioxidant status markers from erythrocytes (14,21).
Study Results and Meta-Analyses
Meta-Analysis: Overall
Heterogeneity in oxidative stress and antioxidant status markers reported provided justification for calculating study-specific effect sizes based on the means and pooled standard deviations to determine an overall Cohen’s d effect size and the use of a random effects meta-analysis to pool effect size estimates. The pooled effect size estimates are presented separately for oxidative stress and antioxidant status markers collected. A Cohen’s d effect size estimate of 0.50 would suggest that depression was associated with a roughly ½ of a 1-standard-deviation increase in oxidative stress.
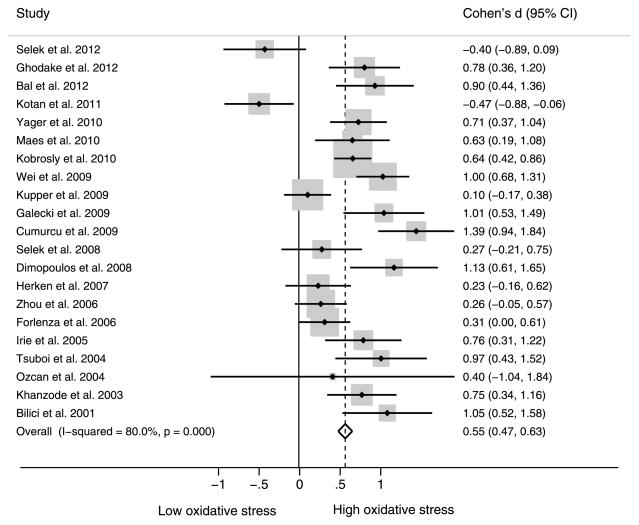
Figure 2 shows the forest plot of effect sizes when observing the associations between depression and oxidative stress measures. Among the 23 studies, 21 included a measure of oxidative stress and two reported on antioxidant measures only (22,25). The 21 studies yielded a pooled effect size (Cohen’s d) of 0.55 (95% confidence interval [CI] = 0.47–0.63). This value can be interpreted as a roughly 0.55 of 1-standard-deviation increase in oxidative stress among individuals with depression compared with those without depression. The test for heterogeneity yielded a significant I2 of 80.0% (p < .001).
Figure 2.
Cohen’s d (95% CI) for depression and oxidative stress. CI = confidence interval.
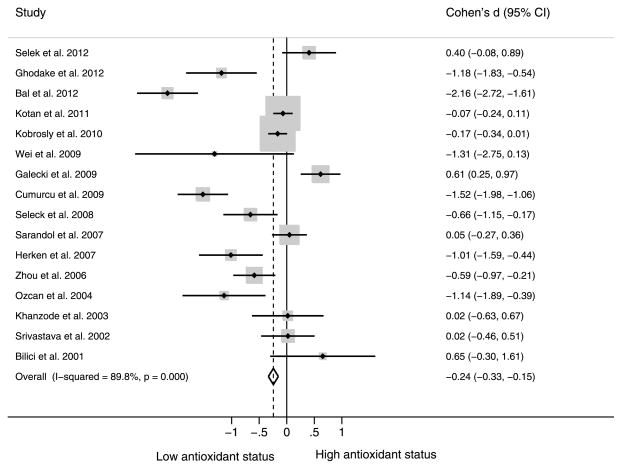
Figure 3 depicts the forest plot of effect sizes when observing the associations between depression and antioxidant status markers. Among the 23 studies, 16 had a measure of antioxidant status. The 16 studies yielded a statistically significant pooled effect size (Cohen’s d) of −0.24 (95% CI = −0.33 to −0.15). This value can be interpreted as a roughly 0.24 of 1-standard deviation decrease in antioxidants among individuals with compared with those without depression. The test for heterogeneity yielded a significant I2 of 89.8% (p < .001).
Figure 3.
Cohen’s d (95% CI) for depression and antioxidants. CI = confidence interval.
Quality Assessment of Reported Studies
Using guidelines from the STROBE statement, we created a checklist to assess the quality of included studies (Table 2). Among the 23 included studies, 5 met all the criteria for quality assessment (13,17,26,29,30). The methods/sources of participant selection were not clearly defined in two studies (11,31). With the exception of three studies, all others provided an adequate explanation of their assessment of depression (11,16,31). All studies provided a clear description of how depression and oxidative stress measures were handled in analyses. Although unadjusted analyses were used to create comparable effect sizes across studies in this meta-analysis, potentially confounding factors were dealt with through statistical adjustment in only seven studies (13,17,26,29–31,33).
TABLE 2.
Quality Assessment Based on Guidelines From the STROBE Statement
| Study | Clear Description of Participant Eligibility and Sources/Methods of Participant Selection | Clearly Defined Exposure Ascertainment (Depression) | Clear Defined Outcome Ascertainment (Oxidative Stress) | Clear Description of How Depression and Oxidative Stress Were Handled in Analyses | Control for Potential Confounders by Exclusion or Statistical Adjustment |
|---|---|---|---|---|---|
| Selek et al. (23) | ✓ | ✓ | ✓ | ✓ | |
| Ghodake et al. (15) | ✓ | ✓ | ✓ | ✓ | |
| Bal et al. (27) | ✓ | ✓ | ✓ | ✓ | |
| Kotan et al. (19) | ✓ | ✓ | ✓ | ✓ | |
| Yager et al. (26) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Maes et al. (20) | ✓ | ✓ | ✓ | ✓ | |
| Kobrosly and van Wijngaarden (29) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Wei et al. (32) | ✓ | ✓ | ✓ | ✓ | |
| Kupper et al. (30) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Galecki et al. (14) | ✓ | ✓ | ✓ | ✓ | |
| Cumurcu et al. (12) | ✓ | ✓ | ✓ | ✓ | |
| Selek et al. (24) | ✓ | ✓ | ✓ | ✓ | |
| Dimopoulos et al. (28) | ✓ | ✓ | ✓ | ✓ | |
| Sarandol et al. (22) | ✓ | ✓ | ✓ | ✓ | |
| Herken et al. (16) | ✓ | ✓ | ✓ | ||
| Zhou et al. (33) | ✓ | ✓ | ✓ | ✓ | |
| Forlenza and Miller (13) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Irie et al. (17) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tsuboi et al. (31) | ✓ | ✓ | ✓ | ||
| Ozcan et al. (21) | ✓ | ✓ | ✓ | ✓ | |
| Khanzode et al. (18) | ✓ | ✓ | ✓ | ✓ | |
| Srivastava et al. (25) | ✓ | ✓ | ✓ | ✓ | |
| Bilici et al. (11) | ✓ | ✓ |
STROBE = Strengthening the Reporting of Observational Studies in Epidemiology.
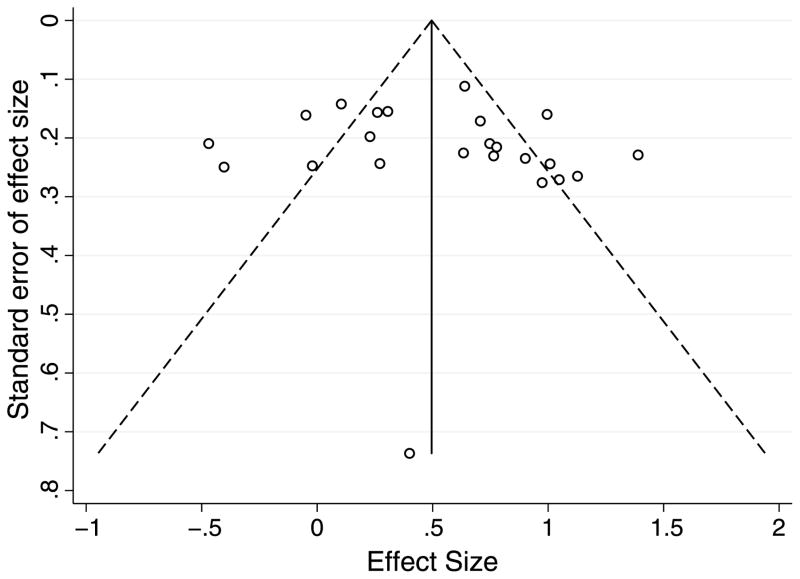
A funnel plot of the study effect sizes and associated standard errors was created to check for possible publication bias (Fig. 4). Asymmetry is evident from visual inspection of the funnel plot. One limitation of our study is the inability to capture unpublished studies. Null association studies are at a higher risk for being unpublished, but for most of the studies included in this meta-analysis, the oxidative stress markers were secondary outcomes and would not have influenced a decision not to publish.
Figure 4.
Funnel plot for publication bias.
DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis of the literature on the associations between depression and oxidative stress. In this pooled analysis of 23 studies that met our inclusion criteria, depression was associated with increased oxidative stress and lower antioxidant status. This finding suggests a possible increase in oxidative stress marker levels or decrease in antioxidant status with greater depressive symptoms. Although the variability was greater in oxidative stress measures, the effect size relating high oxidative stress to depression was stronger than the effect size relating low antioxidant status to depression. These findings suggest a possibly greater impact of depression on oxidative stress than anti-oxidant status levels, necessitating interventions for targeting depression and subsequent elevations in oxidative stress.
Most studies included in this meta-analysis resulted in positive and significant effect estimates for the association between depression and oxidative stress (11–18,20–24,26–28,30–33), whereas a few did not (19,25,29). The statistical test for heterogeneity was highly significant, suggesting that the treatment effect estimates are more variable between studies than would have been expected by chance alone. The studies varied widely in measures of both depression and of oxidative stress. Among the 23 studies, 7 different depression measures and 12 different oxidative stress markers were reported. Some of the oxidative stress measures analyzed exhibited stronger associations with depression than others (e.g., malondialdehyde and 8-oxo-2′-deoxyguanosine). The selection of study participants was dissimilar between the studies, with several performed in clinical populations and a few conducted using healthy volunteers from the community. A clinical population may have included patients with mild, moderate, or severe depression. This is important to consider when comparing results across these studies because the degree of depressive symptoms may impact the level of an oxidative stress or antioxidant status markers present in the body.
Limitations
The results of this meta-analysis are subject to limitations. The heterogeneity among included studies should be considered when interpreting the results. The sample sizes ranged from 30 to 3000 consisting of individuals from 10 different countries. The strength of study-specific effect sizes is highly dependent on the total sample size. Only 5 studies met all STROBE criteria at once, but 15 met all but one. In addition, the level of depression and coping mechanisms for depression may differ between individuals of differing cultural backgrounds. Seven different measurements of depression were used. Measurement of oxidative stress markers also varied widely with 12 different measures used. That an association between depression and oxidative stress was found even with a variety of methods and samples is also a strength because it is a more robust finding and not likely caused by consistent measurement artifact.
Strengths
Strengths of the study include that it was across many populations, sick and well, and of differing cultural backgrounds. A further strength is the use of the Cohen’s d statistical measure of effect size that allows us to pool the data from all available studies resulting in increased power for statistical analyses. Although there is significant heterogeneity between the studies, the use of Cohen’s d and random effects meta-analysis accounts for heterogeneity and ensures more valid estimates.
Future Research
As noted in the results, most of the literature on depression and oxidative stress is cross sectional. The literature would be strengthened by prospective research to assess whether depression is predictive of oxidative stress. It is possible that there are bidirectional causal associations between depressive symptoms and oxidative stress (9,35). Also, feedback mechanisms between psychological stress, such as depression, and oxidative stress might together contribute to adverse health and cognitive outcomes and accelerated aging (7,8). Prospective research is needed to tease this out.
Much of the literature was with whites and other ethnic majorities. As in the United States and other countries, where there is higher psychological stress including depression, in ethnic minorities, it would be useful to have more literature on these associations in ethnic minorities.
CONCLUSIONS
In this pooled analysis of 23 studies, depression was associated with increased oxidative stress and lower antioxidant status. Findings from this analysis suggest that oxidative stress may be a mediating factor in the associations between depression and poor health outcomes. Further research needs to be conducted in this area, including prospective studies with larger sample sizes, consistency in standardized measures of depression, and biologically stable measures of oxidative stress.
Supplementary Material
Acknowledgments
Source of Funding: P. Palta is supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (Grant No. T32 DK062707, a pre-doctoral training fellowship).
Glossary
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.psychosomaticmedicine.org).
Conflicts of Interest: For the remaining authors, none were declared. The authors report no conflicts of interest.
References
- 1.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–97. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 4.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 5.Semba RD, Ferrucci L, Sun K, Walston J, Varadhan R, Guralnik JM, Fried LP. Oxidative stress is associated with greater mortality in older women living in the community. J Am Geriatr Soc. 2007;55:1421–5. doi: 10.1111/j.1532-5415.2007.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szanton SL, Rifkind JM, Mohanty JG, Miller ER, Thorpe RJ, Nagababu E, Epel ES, Zonderman AB, Evans MK. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int J Behav Med. 2011;19:489. doi: 10.1007/s12529-011-9188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel TM, Pulschen D, Thome J. The role of oxidative stress in depressive disorder. Curr Pharm Des. 2012;18:5891. doi: 10.2174/138161212803523554. [DOI] [PubMed] [Google Scholar]
- 8.Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuroendocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- 9.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68:261–75. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Miller ER, Juraschek SP, Appel LJ, Madala M, Anderson CA, Bleys J, Guallar E. The effect of n-3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: meta-analysis of clinical trials. Am J Clin Nutr. 2009;89:1937–45. doi: 10.3945/ajcn.2008.26867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 12.Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci. 2009;63:639–45. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 13.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- 14.Galecki P, Szemraj J, Bienkiewicz M, Florkowski A, Galecka E. Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol Rep. 2009;61:436–47. doi: 10.1016/s1734-1140(09)70084-2. [DOI] [PubMed] [Google Scholar]
- 15.Ghodake SR, Suryakar AN, Kulhalli PM, Padalkar RK, Shaikh AK. A study of oxidative stress and influence of antioxidant vitamins supplementation in patients with major depression. Curr Neurobiol. 2012;3:107–11. [Google Scholar]
- 16.Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, Kap O, Yumru M, Savas HA, Akyol O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38:247–52. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Irie M, Miyata M, Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res. 2005;39:553–60. doi: 10.1016/j.jpsychires.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8:365–70. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 19.Kotan VO, Sarandol E, Kirhan E, Ozkaya G, Kirli S. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: a 24-week follow-up study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1284–90. doi: 10.1016/j.pnpbp.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery disease. J Affect Disord. 2010;125:287–94. doi: 10.1016/j.jad.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term anti-depressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 23.Selek S, Dalkilic A, Kaya MC, Savas HA, Bez Y, Celik H, et al. The relationship of oxidative metabolism to treatment response in major depression: a biological basis for treatment duration. Neurology, Psychiatry Brain Res. 2012;18:15–8. [Google Scholar]
- 24.Selek S, Savas HA, Gergerlioglu HS, Bulbul F, Uz E, Yumru M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord. 2008;107:89–94. doi: 10.1016/j.jad.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava N, Barthwal MK, Dalal PK, Agarwal AK, Nag D, Seth PK, Srimal RC, Dikshit M. A study on nitric oxide, beta-adrenergic receptors and antioxidant status in the polymorphonuclear leukocytes from the patients of depression. J Affect Disord. 2002;72:45–52. doi: 10.1016/s0165-0327(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 26.Yager S, Forlenza MJ, Miller GE. Depression and oxidative damage to lipids. Psychoneuroendocrinology. 2010;35:1356–62. doi: 10.1016/j.psyneuen.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Bal N, Acar ST, Yazici A, Yazici K, Tamer L. Altered levels of malondialdehyde and vitamin E in major depressive disorder and generalized anxiety disorder. J Psychol Neurol Sci. 2012;25:206–11. [Google Scholar]
- 28.Dimopoulos N, Piperi C, Psarra V, Lea RW, Kalofoutis A. Increased plasma levels of 8-iso-PGF2alpha and IL-6 in an elderly population with depression. Psychiatry Res. 2008;161:59–66. doi: 10.1016/j.psychres.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Kobrosly R, van Wijngaarden E. Associations between immunologic, inflammatory, and oxidative stress markers with severity of depressive symptoms: an analysis of the 2005–2006 National Health and Nutrition Examination Survey. Neurotoxicology. 2010;31:126–33. doi: 10.1016/j.neuro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Kupper N, Gidron Y, Winter J, Denollet J. Association between type D personality, depression, and oxidative stress in patients with chronic heart failure. Psychosom Med. 2009;71:973–80. doi: 10.1097/PSY.0b013e3181bee6dc. [DOI] [PubMed] [Google Scholar]
- 31.Tsuboi H, Shimoi K, Kinae N, Oguni I, Hori R, Kobayashi F. Depressive symptoms are independently correlated with lipid peroxidation in a female population: comparison with vitamins and carotenoids. J Psychosom Res. 2004;56:53–8. doi: 10.1016/S0022-3999(03)00567-1. [DOI] [PubMed] [Google Scholar]
- 32.Wei YC, Zhou FL, He DL, Bai JR, Hui LY, Wang XY, Nan KJ. The level of oxidative stress and the expression of genes involved in DNA-damage signaling pathways in depressive patients with colorectal carcinoma. J Psychosom Res. 2009;66:259–66. doi: 10.1016/j.jpsychores.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhou F, Zhang W, Wei Y, Zhou D, Su Z, Meng X, Hui L, Tian W. The changes of oxidative stress and human 8-hydroxyguanine glycosylase1 gene expression in depressive patients with acute leukemia. Leuk Res. 2007;31:387–93. doi: 10.1016/j.leukres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–4. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 35.Moretti M, Colla A, de Oliveira Balen G, dos Santos DB, Budni D, de Freitas AE, Farina M, Severo Rodrigues AL. Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J Psychiatr Res. 2012;46:331–40. doi: 10.1016/j.jpsychires.2011.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.