Abstract
Bullous pemphigoid (BP) is a blistering disorder due to autoantibodies to the epidermal basement membrane zone. The triggering factor could be localized damage to the skin by physical or chemical agents. We report a case of a 68-year-old woman with a three year history of oral lesions of BP following radiotherapy for carcinoma of the hypopharynx, and a three month history of BP over lymphedematous sites on the right hand and right lower limb. Localized BP induced by radiotherapy or lymphedema is rare; both factors working simultaneously in the same patient is even rarer.
Keywords: Bullous pemphigoid, lymphedema, radiotherapy
INTRODUCTION
Bullous pemphigoid (BP) is an immunobullous disorder due to autoantibodies against the hemidesmosomes of the basal keratinocytes. At present date, 31 reports of localized BP induced by radiotherapy[1] and two of localized BP over areas of lymphedema[2,3] have been described in the international literature. We herein describe a patient with localized BP lesions sharply limited to the radiotherapy site on the oral mucosa, and to lymphedema over the hand and lower limb. To the best of our knowledge, this is the first report of localized BP induced by radiotherapy and lymphedema occurring simultaneously in the same patient.
CASE REPORT
A 68-year-old woman presented with recurrent episodes of mildly painful, grouped hemorrhagic bullae on the right hand, right leg, and ankle of three months duration, and painful oral ulcers of three years duration. She had been diagnosed with poorly differentiated squamous cell carcinoma of the hypopharynx four years earlier (T2 N1 M0), and had been treated with radiotherapy in a dose of 60 Gy for 26 days, with complete resolution of the lesion. In addition, she had filarial lymphedema of the right hand and the right lower limb, diagnosed 40 years earlier, and treated with diethylcarbamazine citrate. Her subsequent history was uneventful, except for rare episodes of cellulitis of the lymphedematous leg that subsided quickly with appropriate treatment.
Physical examination at the time of hospital admission revealed bilateral inguinal lymphadenopathy. There was non-pitting edema of the dorsum of the right hand and right lower limb. Multiple hemorrhagic bullae and erosions, with a few vesicles, were present on the dorsum of the right hand and fingers, and the anterior aspect of the right leg and ankle [Figures 1 and 2]. All the lesions were sharply localized to the areas of lymphedema. There was a well defined erosion 1.2 × 0.7 cm in the oral cavity at the junction of the hard and soft palate [Figure 3]. There was no erythema at the base of the lesions. No other areas were involved.
Figure 1.
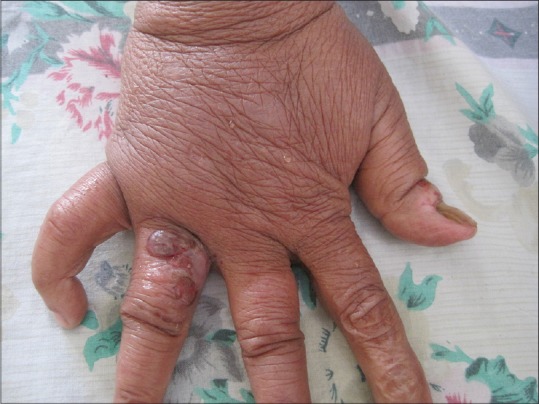
Bullae on the lymphedematous hand
Figure 2.
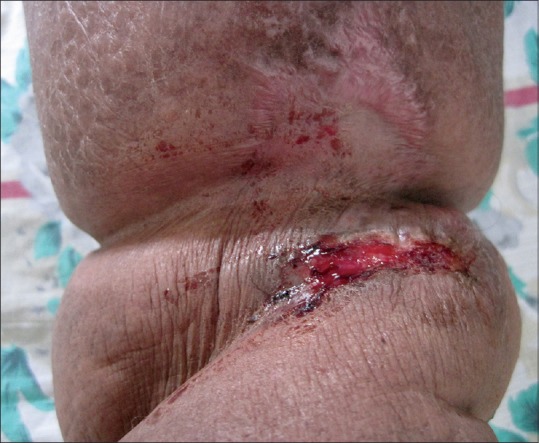
Bullae on the leg with lymphedema
Figure 3.
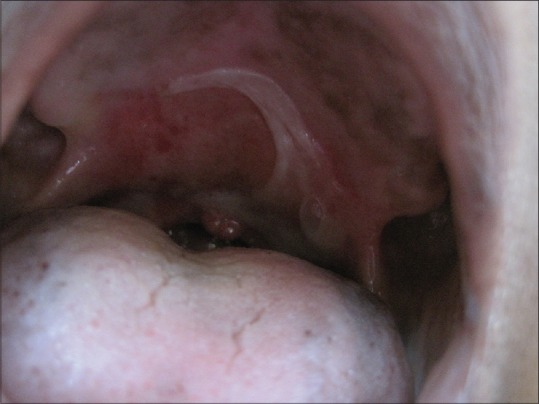
Lesions of bullous pemphigoid on the palate at the site of radiotherapy
Results of laboratory investigations including total hemogram, blood sugar levels, liver and renal function tests, human immunodeficiency virus antibody test, chest radiograph and urinalysis were all within normal limits. A Tzanck smear from fresh oral and cutaneous vesicles revealed only a few neutrophils.
A skin biopsy drawn from an intact vesicle showed a subepidermal plane of cleavage with a perivascular lymphocytic infiltrate. A direct immunofluorescence (DIF) study on a perilesional biopsy demonstrated linear deposits of immunoglobulin G (IgG) and C3 along the dermoepidermal junction. The blood vessels also showed C3 (2+) deposits. A repeat DIF study using salt split skin revealed complete separation of the epidermis from the dermis, with linear staining of the basement membrane zone with IgG and C3. The band was seen mainly on the epidermal side of the split (roof pattern), diagnostic of BP. The patient was advised an oral biopsy by the dental surgeon which she declined. An otolaryngology consultation showed no evidence of local recurrence of the carcinoma in the hypopharynx.
The patient was started on 1 mg of intravenous dexamethasone along with 100 mg of dapsone daily and topical fluticasone ointment thrice a day. Topical treatment for the oral lesions was not given as the patient had difficulty in applying medication over the posterior part of the palate. Within a week, significant healing of the lesions over both sites occurred, and the steroid was tapered to an oral dose of prednisolone 15 mg/day with continuation of dapsone. After discharge, oral steroid was gradually tapered and stopped over a period of six months, when no further breakthrough lesions were noted. After a year, the dose of dapsone was gradually reduced to 50 mg/day and stopped after a further period of six months. The skin lesions healed with perifollicular pigmentation,[4] simultaneously with the oral ulcers, with no relapse on follow up [Figure 4].
Figure 4.
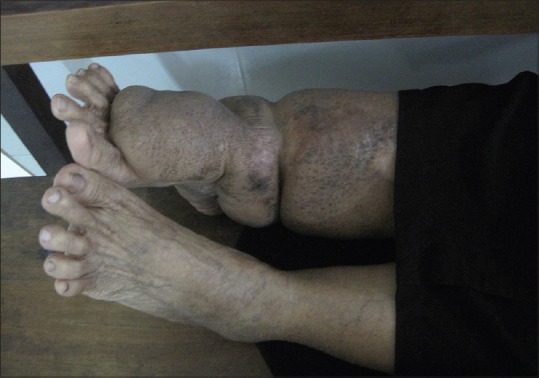
Complete resolution of the lesions with perifollicular pigmentation
DISCUSSION
A few cases of BP occurring at sites of lymphedema have been reported.[2,3] The lymphedema may be primary, or acquired as a result of trauma or other causes. Slow circulation of the lymphatic vessels, increased capillary permeability with preferential localization of antibodies in the area, and potential cleavage of the epidermal junction due to increased hydrostatic pressure have all been postulated as possible causes.[2] The lesions of BP may be localized to the lymphedematous sites, or may be associated with generalized lesions. Paradoxically, generalized BP sparing an area of post-surgical lymphedema has also been reported, indicating that an unexplained mechanism may be involved.[5] However, linear binding of anti-IgG and anti-C3 autoantibodies was observed in the spared area, which may be suggestive of subclinical involvement.
With regard to BP induced by radiotherapy, a larger number of reports are available, mostly for carcinoma of the breast, and less frequently cancers of the thorax, cervix, vulva, squamous cell carcinoma and non-Hodgkins lymphoma. Generalized BP, BP strictly confined to the irradiated area, as well as pre-existing BP exacerbated by irradiation have all been reported.[6,7,8,9] Radiotherapy may lead to structural alteration of the basement membrane zone and auto-antibody induction. In addition, matrix metallopeptidase-9 and vascular endothelial growth factor levels may also have an association with radiotherapy treatment.[6] Radiotherapy paradoxically acting as a cure for BP has also been reported in one case, probably due to remission of the underlying mycosis fungoides.[6]
Our case is unique in that the BP occurred exclusively over the oral mucosa which had undergone radiation four years previously, as well as on hand and lower limb on lymphedema of separate etiology, of 40 years duration. Another unusual feature was that the oral lesions were of three years duration, and appeared one year after radiotherapy, while the lesions on the limbs were of only three months duration, although the lymphedema was of 40 years duration. This suggests that there is no predictable time interval between the precipitating stimulus and the appearance of BP in a predisposed individual.
Though mucosal BP of the vulva after radiotherapy has been reported,[7] there have been no previous reports of radiotherapy-induced BP on the oral mucosa.
A single case was reported of localized cicatricial pemphigoid which appeared nine years after radiotherapy for breast carcinoma and was preferentially localized to the lymphedematous site over the upper limb; cicatricial pemphigoid was assumed to be solely due to the lymphedema, and not due to the radiotherapy, because of the long interval between the radiotherapy and the onset of pemphigoid.[10] We believe that ours is the only case where BP was separately induced by radiotherapy and lymphedema in the same patient at nearby time points. It was noteworthy that the bullous lesions did not involve any other area at any time in the course of the disease. Immunological alterations occurring as a result of damage to the skin may be the triggering factors for autoantibody production and activation of lymphocytes in these situations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Heyes C, Lau D, Yazdabadi A, Hamilton C, Poon E. Radiation induced bullous pemphigoid: Case report and review of the literature. Austral-Asian J Cancer. 2013;12:165–7. [Google Scholar]
- 2.Perez A, Clements SE, Benton E, Robson A, Bhogal B, Stefanato CM, et al. Localized bullous pemphigoid in a patient with primary lymphoedema tarda. Clin Exp Dermatol. 2009;34:e931–3. doi: 10.1111/j.1365-2230.2009.03722.x. [DOI] [PubMed] [Google Scholar]
- 3.Ruocco E, Aurilia A, Simonetti G, Cozzani E, Baroni A, Argenziano G. Bullous pemphigoid: Three atypical cases. Acta Derm Venereol. 2002;82:222–3. doi: 10.1080/00015550260132613. [DOI] [PubMed] [Google Scholar]
- 4.Kumaresan M, Srinivas CR. Perifollicular pigmentation in bullous pemphigoid: A diagnostic sign. Indian Dermatol Online J. 2011;2:36–7. doi: 10.4103/2229-5178.79862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roguedas AM, Crespel E, Kupfer I, De Saint Martin L, Misery L, Sassolas B. Bullous pemphigoid sparing acquired lymphedema. Ann Dermatol Venereol. 2006;133:250–2. doi: 10.1016/s0151-9638(06)70890-0. [DOI] [PubMed] [Google Scholar]
- 6.Mul VE, van Geest AJ, Pijls-Johannesma MC, Theys J, Verschueren TA, Jager JJ, et al. Radiation-induced bullous pemphigoid: A systematic review of an unusual radiation side effect. Radiother Oncol. 2007;82:5–9. doi: 10.1016/j.radonc.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Srifi N, Benomar S, Zaghba N, Qasmi S, Senouci K, Elgueddari B, et al. Generalized bullous pemphigoid induced by radiotherapy. Ann Dermatol Venereol. 2011;138:311–4. doi: 10.1016/j.annder.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Leconte-Boulard C, Dompmartin A, Verneuil L, Thomine E, Joly P, Rogerie MJ, et al. Localized bullous pemphigoid following radiotherapy. Ann Dermatol Venereol. 2000;127:70–2. [PubMed] [Google Scholar]
- 9.Isohashi F, Konishi K, Umegaki N, Tanei T, Koizumi M, Yoshioka Y. A case of bullous pemphigoid exacerbated by irradiation after breast conservative radiotherapy. Jpn J Clin Oncol. 2011;41:811–3. doi: 10.1093/jjco/hyr049. [DOI] [PubMed] [Google Scholar]
- 10.Callens A, Vaillant L, Machet MC, Arbeille B, Machet L, De Muret A, et al. Localized atypical pemphigoid on lymphoedema following radiotherapy. Acta Derm Venereol. 1993;73:461–4. doi: 10.2340/0001555573461464. [DOI] [PubMed] [Google Scholar]


