Abstract
Background:
Epidemiological data is limited for cutaneous adverse drug reactions (CADRs) in India. Most of the Indian studies have small sample size and are of limited duration.
Aims:
The aim of this study is to analyze CADRs with reference to the causative drugs and their clinical characteristics in Indian population.
Materials and Methods:
As per selection criteria, electronic databases were searched for publications describing CADRs from January-1995 to April-2013 by two independent investigators. Data of the causative drugs and clinical characteristics were extracted and summarized by absolute numbers, percentages, ranges, and means as presented by the authors. The subgroup analysis of causative drugs was performed for causality assessment, severe or nonsevere reactions and occurrence of common CADRs. Studies showing “definite” and “probable” categories of causality analysis were labeled as “definite and probable causality (DPC) studies”. The other included studies were labeled as “non-DPC studies”.
Results:
Of 8337 retrieved references, 18 prospective studies were selected for analysis. The pooled incidence was 9.22/1000 total among outpatient and inpatient cases. Commonly observed reactions were maculopapular rash (32.39%), fixed drug eruptions (FDEs) (20.13%), urticaria (17.49%) and Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) (6.84%). The major causative drug groups were antimicrobials (45.46%), nonsteroidal anti-inflammatory drugs (NSAIDs) (20.87%) and anti-epileptic drugs (14.57%). Commonly implicated drugs were sulfa (13.32%), β-lactams (8.96%) and carbamazepine (6.65%). High frequency of CADRs is observed with anti-epileptic drugs in DPC studies only. Carbamazepine, phenytoin and fluoroquinolones had higher severe to nonsevere cutaneous reaction ratio than other drugs. Antimicrobials were the main causative drugs for maculopapular rash, FDEs and SJS/TEN, and NSAIDs for the urticaria. The mortality for overall CADRs, SJS/TEN, and exfoliative dermatitis were 1.71%, 16.39%, and 3.57%, respectively. “Definitely preventable”, “probably preventable” and “not preventable” categories CADRs were 15.64%, 63.14%, and 34.64%, respectively.
Conclusion:
Antimicrobials, NSAIDs and antiepileptic are common causative agents of CADRs in India. Antiepileptic agents show high rates of severe cutaneous reactions.
Keywords: Anti-epileptic drugs, antimicrobials, causative drugs, cutaneous adverse drug reaction, nonsteroidal anti-inflammatory drugs, severe cutaneous reactions
INTRODUCTION
Cutaneous adverse drugs reactions (CADRs) are common among ADRs. They account for patients’ suffering, hospitalization and economic burden, and may sometimes be fatal. The common CADRs are skin rash, urticaria, fixed drug eruption (FDE), angioedema, and contact dermatitis. Serious CADRs endangering patient's life are Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS) and acute generalized exanthematous pustulosis (AGEP).[1,2] The common offending drugs are antimicrobials, nonsteroidal anti-inflammatory drugs (NSAIDs), anti-epileptic drugs and anti-gout agents.[3,4,5] The cutaneous reaction pattern and causative drugs may vary with prescribing habits and level of health care.[3,6]
Majority of CADRs are diagnosed clinically. Recognition of the offending drug enables early withdrawal and improved outcomes.[7] Observational studies are tools to know the pattern of reactions and causative drugs. Most Indian studies are of limited duration and have small sample sizes. Hence, a systematic review is required to generate the large scale Indian epidemiological data for CADRs.
MATERIALS AND METHODS
The publications describing CADRs in Indian population were searched. The key terms: “cutaneous adverse drug reaction”, OR “dermatological reaction”, OR “drug induced skin reaction” AND (“India” OR “Indian population”) were used. The search included the electronic databases - PubMed, MEDLINE, PubMed Central and Google Scholar. The bibliographies of relevant articles were also reviewed. Time period of the study was from January-1995 to April-2013. Only English language articles were considered. The protocol of the study was registered on PROSPERO for systematic review (CRD42013004386). Two reviewers independently searched for the studies. Disagreements were discussed and resolved by consensus. Title, abstract and if required full articles from the retrieved references were assessed as per inclusion and exclusion criteria.
Inclusion criteria
Studies on Indian population only
All cohort studies related to CADRs
All case series describing minimum 10 cases of CADRs
ADR studies with minimum 10 cases of cutaneous reactions
All age groups and clinical settings (outpatient and/or inpatient)
Causality analysis was performed or sufficient information about de-challenge and re-challenge was available.
Exclusion criteria
Studies other than Indian population
Retrospective studies
Studies on specific reactions only (e.g., SJS/TEN, etc.)
Studies focusing on intensive care or fatal or life threatening cases only
Studies with specific drug exposure only (e.g., anti-epileptics, NSAIDs, etc.)
No or insufficient information about causality analysis
“Doubtful”, “unlikely”, and/or “unclassifiable” type of reactions
Causative drugs or groups could not be identified
Case series or case reports with <10 CADR cases
Editorials, letter to editors, and review articles.
Review methods
From the included studies, data were collected for demography, clinical settings, prevalence, reaction type, causative drugs, incubation period, prodromal and clinical features, comorbid conditions, complications, mortality, causality, preventability, and severity. The quality of the included studies was assessed by “strengthening the reporting of observational studies in epidemiology (STROBE) statement”.[8,9]
Outcome analysis
a. Primary outcome variable: Causative drugs for the cutaneous reactions were considered as a primary outcome variables. Data of causative drugs were extracted from studies and summarized using absolute numbers and percentage.
Subgroup analysis: Three types of subgroups were analyzed for the causative drugs. First subgroup analysis was performed based on causality assessment. The included studies have used either WHO causality assessment method or Naranjo's algorithm or Krammer's algorithm. As per WHO causality assessment method, “certain”/“definite” category of ADR is based on-plausible time relationship to drug administration, known pharmacology of drug, absence of alternative explanation by underlying disease or concomitant drug (s), positive de-challenge and re-challenge. The “probable” category of ADR is based on all of the above criteria except re-challenge information is not required. The “possible” category of ADR is based on reasonable time sequence of administration of drug only; there could be alternative explanation by underlying disease or concomitant drugs; de-challenge or re-challenge information is lacking or unclear. The other WHO categories are “unlikely”, “unclassified” or “unclassifiable”. The Naranjo's algorithm uses 10 questions based on the information for previous conclusive reports of drug - ADR pair, temporal relationship, de-challenge, re-challenge, alternative explanation, plasma concentration of drug, dose and intensity of ADR relationship, past reactions with similar drugs and of confirmation of reaction with objective evidence. It scores every ADR from −4 to +13. Depending upon the score, ADR is considered as “definite” (>9), “probable” (5-8), “possible” (1-4) or “doubtful” (<1).[10] Krammer's algorithm scores the ADRs from −7 to +7 based on the information for the previous experience, alternative explanation, timing of events, drug levels, de-challenge, and re-challenge. As per this score, ADR is considered as “definite” (6-7), “probable” (4-5), “possible” (0-3) or “unlikely”.[11] Studies presenting the data in “definite” and/or “probable” categories by any of the causality assessment methods and excluded other category drug-ADR pair were considered as “definite and probable causality (DPC) studies”. Other studies were considered as non-DPC studies. The causative drugs from both these groups (DPC vs. non-DPC) were compared for the proportions by Chi-square test and their odds ratio (OR) were presented. Second type of subgroup analysis compared severe and nonsevere reactions. Exfoliative dermatitis, SJS/TEN, DRESS and AGEP were considered severe CADRs.[1,2] The other CADRs were considered nonsevere. The ratio of severe to nonsevere reactions was calculated for the causative drug. The statistical methods used were OR and Chi-square test for the above comparisons. Third type subgroup analysis extracted the causative drugs for the commonly observed CADRs wherever mentioned in the studies.
b. Secondary outcome variables: Data of secondary outcome variables was extracted and summarized using range, mean and proportion as described in the studies. Demographic data pertaining to the proportion of different age groups was compared by applying Chi-square test and male to female ratio. The incidence of CADRs was separately expressed per 1000 cases based on the setting of study - outpatient, inpatient or both. It was also pooled from all the studies to calculate overall incidence and was presented per 1000 patients. Data were presented in proportions for types of CADRs, site of cutaneous reactions, body surface area involvement, comorbidity, associated allergic conditions and complications. Chi-square test was used to compare severe and nonsevere CADRs with positive past history of cutaneous reactions. The mortality rate for various CADRs was calculated as a percentage with 95% confidence interval (CI); it was compared using one-way ANOVA followed by Tukey-Krammer multiple comparison test. The causality assessment data were presented as “certain”/“definite”, “probable” and “possible” categories; preventability assessment was presented as “definitely preventable”, “probably preventable” and “not preventable” reactions; and severity assessment was presented as “mild”, “moderate” and “severe” categories reactions as provided by authors of the studies.
c. SPSS software version 17.0 (SPSS Inc., Chicago, USA) was used for statistical analysis. P < 0.05 was considered to be significant.
RESULTS
Literature search
The search yielded 8337 references. References excluded as per criteria were 8276. Sixty-one references were fully evaluated and as per the criteria, 18 were included in analysis.[6,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] The selection tree for the review is presented as Figure 1.
Figure 1.
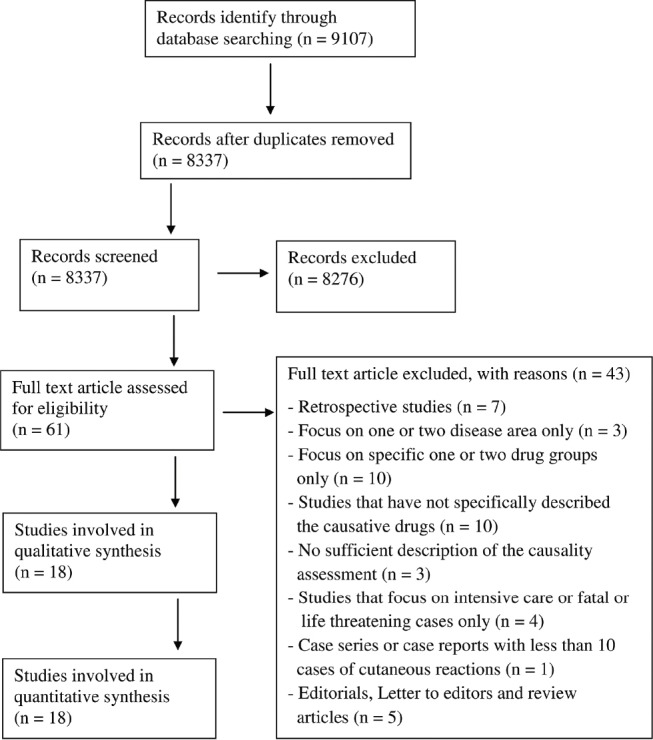
Study selection flow diagram
Characteristic and quality of the included studies
All included studies were case series. One study compared prospective and retrospective data for the reactions.[12] Only prospective data were used for analysis. No cohort study was found in the search. The total number of outpatient studies was 10;[12,13,14,15,16,18,19,22,23,27] inpatient studies numbered two;[21,23] and both types were five.[6,17,20,25,28] One study did not mention about the type of the clinical setting.[26] Ten studies used WHO causality definitions,[12,13,15,16,18,22,23,24,27,28] three used Naranjo's algorithm[14,17,19] and two used Krammer's algorithm[20,21] for causality analysis [Table 1]. Three studies did not mention the method of causality assessment.[6,25,26] However, selection of causative drugs was based on de-challenge and re-challenge, hence included for the analysis. All studies poorly adhered to the “STROBE statement” recommendations.[8,9]
Table 1.
Characteristics of the included studies
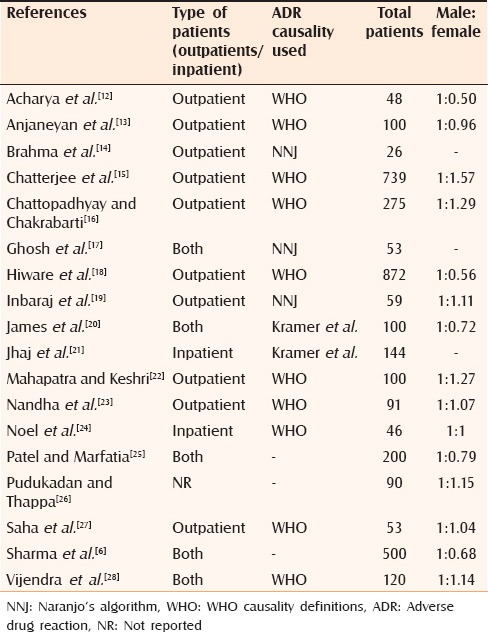
Characteristics of the patients
A total of 3671 cases of CADRs were reported in the included studies. The distribution of the patients as per age group 0-20, 21-39, 40-60 and >60 years was 18.84%, 54.42%, 18.78%, and 7.96%, respectively (P < 0.0001; Chi-square test). The youngest patient was a four-month child and the oldest was 82 years. Male to female ratio was 1:0.9.[6,12,13,15,16,18,19,20,22,23,24,25,26,27,28] The male patients were 52.49%.
Cutaneous adverse drug reactions
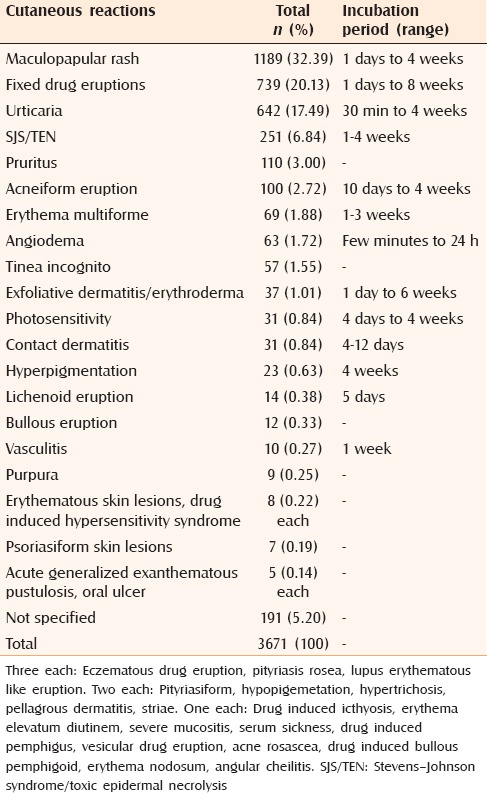
The incidence of CADRs for studies with inpatient settings was 82.59/1000 cases;[24] outpatient was 8.72/1000 cases;[12,13,14,15,16,22,27] and both types was 28.51/1000 cases,[17] respectively. The pooled incidence which was derived from all these nine studies was 9.22/1000 cases.[12,13,14,15,16,17,22,24,27] In all, 38 different types of CADRs were observed. Maculopapular rash (32.39%), FDEs (20.13%) and urticaria (17.49%) were the commonly reported CADRs. Severe CADRs constituted 8.17% in all. The most common severe CADR was SJS/TEN-6.84% [Table 2].
Table 2.
Frequency of distribution of cutaneous reactions and their incubation period
Causative drugs
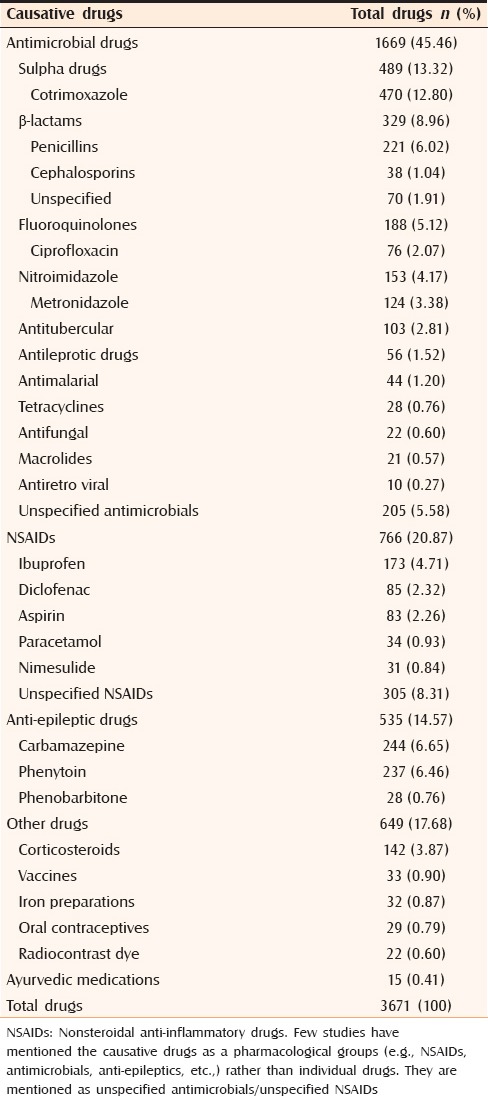
Total number of suspected drugs was 3671 (average one drug per case). The major suspect groups were antimicrobials (45.46%), NSAIDs (20.87%), antiepileptics (14.57%) and corticosteroids (3.87%). Total 60 antimicrobials, 16 NSAIDs, 7 anti-epileptics and 69 other drugs were involved. As shown in Table 3, commonly implicated drugs were sulfa (13.32%), β-lactams (8.96%), carbamazepine (6.65%), phenytoin (6.46%), fluoroquinolones (5.12%), ibuprofen (4.71%), nitroimidazole (4.17%), antituberculars (2.81%), topical betamethasone (2.34%), diclofenac (2.32%) and aspirin (2.26%).
Table 3.
Drugs causing cutaneous reactions
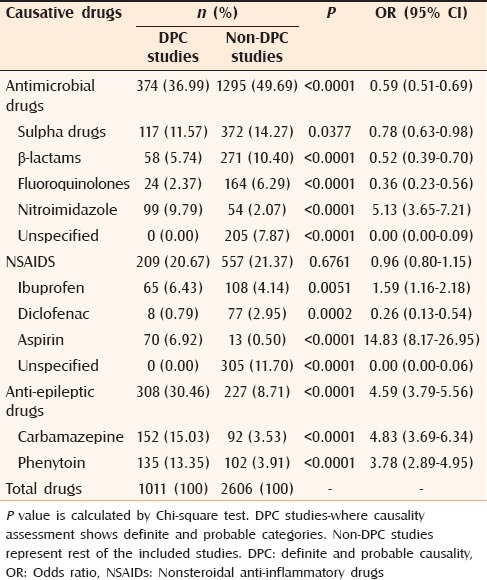
On subgroup analysis, DPC studies[14,15,20,22,24] and non-DPC studies showed a differing pattern for the common causative drugs. Antimicrobials caused most CADRs in both types of studies; however, they showed low frequency in DPC studies compared with non-DPC studies (36.99% vs. 49.69%). Of the implicated antimicrobials groups, nitroimidazoles showed a high frequency in DPC studies. The frequency of NSAIDs in DPC and non-DPC studies was comparable. However, ibuprofen and aspirin showed high frequency in DPC studies, and diclofenac and unspecified NSAIDs in non-DPC studies. DPC studies showed high frequency of CADRs with all anti-epileptics [Table 4].
Table 4.
Comparison of commonly observed causative drugs: DPC studies versus non-DPC Studies
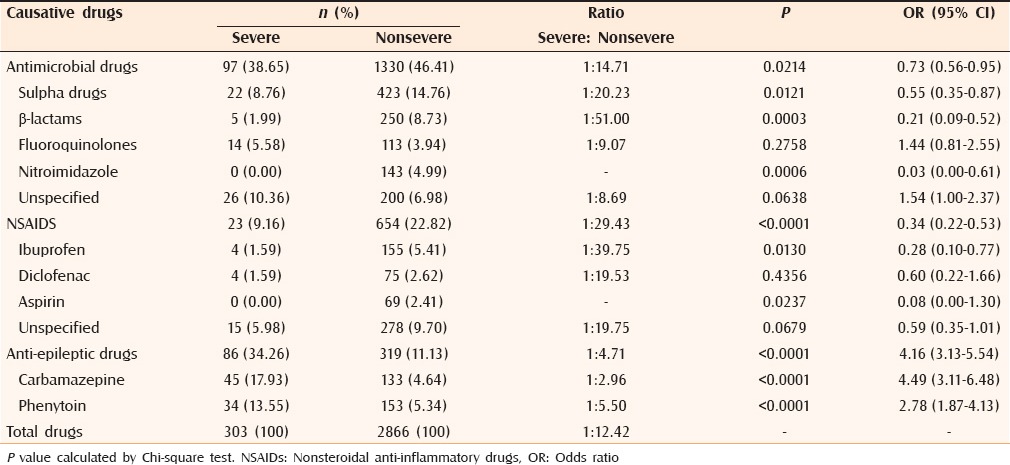
On subgroup analysis between severe and nonsevere CADRs,[6,12,13,14,15,16,17,18,20,21,24,25,26,27,28] carbamazepine and phenytoin were the common agents implicated in severe CADRs. Sulfa and β-lactam antibiotics were the common agents responsible for nonsevere CADRs. No severe CADRs were observed with nitroimidazoles and aspirin. β-lactams, ibuprofen and sulfa drugs had severe to nonsevere reactions in ratio of more than 1:20. With carbamazepine, phenytoin and fluoroquinolones this ratio was <1:10. Anti-epileptics had higher severe to nonsevere reaction ratio than antimicrobials and NSAIDs [Table 5].
Table 5.
Comparison of causative drugs: Severe versus nonsevere cutaneous reactions
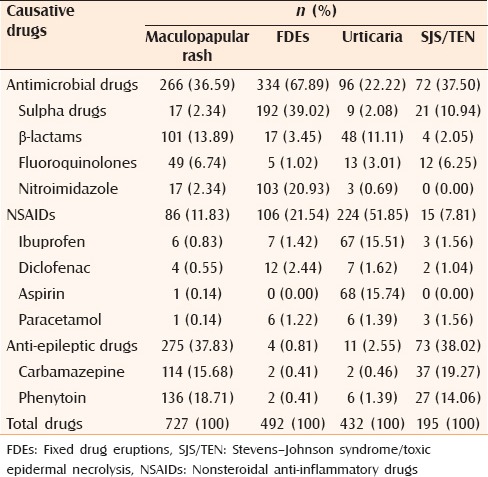
The commonly reported causative drugs for the maculopapular rash,[6,12,13,15,16,17,20,21,24,25,27] FDEs,[6,12,13,14,15,16,20,21,24,25,27] urticaria[6,12,13,14,15,16,20,21,24,25,27] and SJS/TEN[6,12,13,14,15,16,18,20,21,24,25,26,27,28] are presented in Table 6. Antimicrobials and anti-epileptics were common agents producing maculopapular rash and SJS/TEN. Antimicrobials and NSAIDs were main offending agents producing FDEs and urticaria, respectively.
Table 6.
Causative drugs for commonly observed cutaneous reactions
Oral medication produced 79.67% reactions.[13,18,28] Self medication was suspected in 12.13% (95% CI: 7.70, 16.55) patients.[18,27,28] Steroids (80.51%) and NSAIDs (12.71%) were common agents responsible for self medication related CADRs.
Incubation period, clinical features and comorbid conditions
It was possible to depict the range only for the incubation period [Table 2]. It varied from <1 h to 172 days.[6,12,13,16,20,23,24,25,26,27,28] Most common presenting complaints were symptomatic rash (51.94%), pruritus (31.29%) and blisters (9.68%). Most common sites of the lesions were the extremities (57.18%), face (24.12%) and trunk (19.78%).[13,19,26,28] Mucosal involvement was observed in 16.26% of patients. Percentage body surface area involvement <10, 10-30 and >30 were observed in 58.57%, 20.95%, and 20.48% cases, respectively.[26,28] Common comorbid conditions were diabetes mellitus (0.84%), HIV (0.65%), systemic lupus erythematosus (0.56%), and hypertension (0.28%).[6,16,18,22,26,27]
History of allergic disorders and previous experience of cutaneous adverse drug reactions
Commonly associated allergic conditions were positive family history (5.42%), seasonal/environmental allergy (5.05%), allergic rhinitis (3.61%), bronchial asthma (2.89%), and atopy (1.44%).[13,16,19,28] Past history of CADRs was present in 18.92% patients.[13,19,22,26,27,28] Severe (18.75%) and nonsevere (18.31%) cases were comparable for previous experience of CADRs (P = 0.9458; Chi-square test).[22,26] Previous experience of similar CADRs was present in 10.84% cases.[13,19,27,28]
Morbidity and mortality
Cutaneous adverse drug reactions required hospitalization in 11.39% cases.[14,16,27,28] Commonly observed complications were altered liver functions (3.90%), septicemia (2.54%) and acute renal failure (2.54%).[6,28] The mortality for overall CADRs, SJS/TEN, exfoliative dermatitis, erythema multiforme and maculopapular rashes was 1.71% (95% CI: 0.32, 3.10), 16.39% (95% CI: 0.89,31.89), 3.57% (95% CI: −4.14, 11.29), 0.13% (95% CI: −0.16, 0.42), and 0.45% (95% CI: −0.52, 1.41), respectively.[6,15,17,18,19,20,21,22,23,24,25,26,27,28] The mortality rate was significantly higher in SJS/TEN compared with overall CADRs, erythema multiforme and maculopapular rashes (P < 0.05; Tukey-Krammer multiple comparison test).
Assessment of cutaneous adverse drug reactions
Distribution of “certain”/“definite”, “probable” and “possible” categories were 34.25%, 58.59%, and 7.15%, respectively.[12,13,14,15,16,17,18,19,20,21,22,23,24,27,28] As per Schumock and Thorton preventability criteria “definitely preventable”, “probably preventable” and “not preventable” categories were 15.64%, 63.14%, and 34.64%, respectively.[12,14,17,19,22,28] Hartwig et al. scale and University of Virginia Health System Adverse Drug Reaction Reporting Program criteria for severity assessment were used in four[12,14,17,28] and three[13,19,26] studies respectively. Prevalence of “mild”, “moderate” and “severe” categories was 11.90%, 53.02%, and 35.08%, respectively.
DISCUSSION
In this study, CADRs in Indian population are systematically reviewed from selected published studies from January-1995 to April-2013. The literature reports increasing rate of the CADRs with age.[28,29] Campos-Fernández Mdel M et al. and Borch et al. have reported the mean age of patients to be above 50 years.[30,31] These findings are in contrast to this study where approximately 70% patients are less than 40 years of age. This coincides with high Indian population in this age group.[32] Female gender is considered a risk factor for CADRs.[29,30,31,33,34,35] Naldi et al. attributed this gender difference to the consumption of multiple drugs and high elderly population in females.[29] However, the reports are inconsistent - some studies have reported a predominance of males.[2,4,36,37] In this study, male preponderance (52.49%) matches with gender distribution of Indian population (52% male).[38] It seems that age and gender do not affect CADRs in Indian population.
The overall incidence of cutaneous reactions was 9.22/1000 cases. This is comparable to 8.6 from Malaysia[4] and 13.8 from Denmark.[31] It is higher than that from China (1.6).[2] Inpatients have high rates of CADRs as compared to outpatients that coincides with previous study.[29] Inpatients have severe ailments and are prescribed more number of drugs. High inpatient incidence (82.59/1000 cases) is reported in one study only.[24] In that numerator included patients of dermatology as well as transferred patients from other departments while the denominator was patients of dermatology wards rather than all the inpatients of the institute.
Maculopapular rash (32.39%) was the most commonly observed CADR that is consistent with previous studies.[2,3,4,5,34,36,39,40] Its frequency varies from 7.7% to 60.2% in studies abroad.[2,3,4,5,7,31,34,35,36,37,39,40] The second most common CADR was FDE (20.13%) in this study. This is high when compared with the European multicenter study (roughly 1 case/year/hospital).[41] Other Asian studies show a low incidence of FDE varying from 3.77% to 15.34%.[2,3,4,5,35,37,39] Urticaria (17.49%) was the third leading CADR in this study. Studies from abroad reported urticaria in a frequency of 4.7-48.1%.[2,3,4,5,31,34,36,39,37] SJS/TEN (6.84%) was the most common severe CADR, consistent with studies abroad.[2,3,4,34,39,37] Its total frequency of reporting varies from 2% to 33.8%.[2,3,4,34,35,36,37,39,40] A few studies observe DRESS as the most common severe CADR.[7,35,36]
In this study, antimicrobials are the major causative drugs of CADR that coincides with the reported literature.[2,3,4,7,29,31,35,36,39,40] Sulfa, β-lactam antibiotics, fluoroquinolones and nitroimidazoles are the common antimicrobials implicated. Most studies abroad observed cotrimoxazole[3,4,29,34] and the penicillin group[5,35,36,37,39] as common causative antimicrobials. Sulfonamides are implicated in those countries where they are commonly used.[36] The reported rates of cutaneous reactions to antimicrobials are: Cotrimoxazole (2.8-3.7%), aminopenicillins (1.2-8.0%), penicillins (1.1-4.4%), cephalosporins (1.0-4.8%), and fluoroquinolones (1.6%).[33] Exanthematic skin lesions are difficult to differentiate from CADRs. Drug exposure can cause CADR in association with infection. Ampicillin associated rashes in patients with infectious mononucleosis is an example.[29] One study reports 21% CADRs due to aminopenicillins.[36] However, authors could not rule out viral infection as a causative factor in one-third of the cases.
In the DPC versus non-DPC comparison, DPC studies showed low frequency of CADRs with antimicrobials (36.99% vs. 49.69%), sulfa drugs (11.57% vs. 14.27%), β-lactam antibiotics (5.74% vs. 10.40%), and fluoroquinolones (2.37% vs. 6.29%). This suggests that non-DPC studies are likely to overestimate CADRs to antimicrobials. This may be because of absence of re-challenge data as reported by Ramam et al.[42,43] Challenge testing is a reliable way of demonstrating spurious reactions and identifying a safe drug provided it is started from the drug least likely to cause reaction and is performed in a hospital setting under close supervision of the dermatologist.[42]
According to this study, antimicrobials caused all the common CADRs. β-lactams mainly cause rashes and urticaria; sulfas-FDEs and SJS/TEN; fluoroquinolones-rashes and SJS/TEN; and nitroimidazoles-FDEs. Nitroimidazoles mainly produce nonsevere reactions. Cross-reactivity for FDEs vary among nitroimidazoles and safe nitromidazole can be identified by oral provocation tests.[44] Patch testing provides inconsistent information for causative drugs on both affected and nonaffected skin.[41] β-lactams and sulfas produce more nonsevere than severe CADRs. One out of 50 β-lactam-induced CADRs and one out of 20 sulfa-induced CADRs are severe. They cause a wide-spectrum of reactions. In a clinical setting, it is difficult to identify which patient would develop what kind of reaction. Among the β-lactams, cross-reactivity is possible between penicillins, cephalosporins and carbapenems, but not with aztreonam.[45] Cross-reactivity between antibiotic and sulfa drugs is possible; however, it is unlikely for nonantibiotic sulfa drugs.[46] A systematic review of SJS/TEN in the Indian population reports fluoroquinolones and sulfa drugs as common causative antimicrobials.[47] One out of nine fluoroquinolone-related CADRs are severe. Clinicians should be cautious about cross-reactivity among fluoroquinolones keeping in mind their high frequency of severe reactions.[48,49] Slow acetylator phenotype or genotype predispose to sulfonamide-induced CADRs.[50] Indian population has a high frequency of the slow acetylator genotype.[51] This supports high frequency of sulfonamide-induced CADRs observed in this study.
In this study, NSAIDs accounted for all the commonly observed CADRs. They are the second most commonly implicated drugs.[4,31] CADR rate for NSAIDs ranges from 0.3% to 0.69%.[32] Ibuprofen, diclofenac and aspirin are the most common causative agents and produce few severe reactions. Considering their widespread use, the risk of severe CADRs seems minimal. One Indian study on NSAIDs reports CADRs (50.29%) as the most common ADR; ibuprofen (51.19%) and diclofenac (27.08%) were the commonly implicated drugs.[52] However, studies abroad observe mefenamic acid,[4,37] naproxen,[35] and paracetamol[5] as common agents.
In this study, NSAIDs as a group show no difference in CADRs frequency between DPC and non-DPC studies. Identification of NSAIDs as a causative agent is difficult because of their widespread over-the-counter (OTC) use and as co-prescription.[50] In accordance with the literature, aspirin and ibuprofen are most common NSAIDs causing urticaria.[53] Prevalence of urticaria is 0.1-0.3% among NSAID users. Urticaria occurs due to inhibition of prostaglandin synthesis, and the subsequently enhanced synthesis and release of cysteinyl leukotrienes. Other mechanism may be production of IgE antibodies to haptenated NSAID metabolites.[54]
Current study implicates carbamazepine and phenytoin as the common causative antiepileptics. That is consistent with other studies abroad.[3,4,35] High frequency of CADRs with antiepileptics was observed in DPC studies when compared with non-DPC studies. Co-prescriptions and comorbid conditions are less frequent with anti-epileptics than antimicrobials and NSAIDs. This makes causality assessment more precise.
According to this study, maculopapular rash and SJS/TEN are common with antiepileptics; Urticaria and FDEs are rare. Other studies from Asia show carbamazepine as most common offending drug for SJS/TEN.[3,4,47] Antiepileptics are also implicated with SJS/TEN in the western population. The EuroSCAR study in the European population suggests carbamazepine (relative risk [RR]: 33), phenytoin (RR: 26), phenobarbitone (RR: 17), and lamotrigine (RR >14) as important causative antiepileptics for SJS/TEN.[55] Antiepileptics show high severe to nonsevere case ratio compared with antimicrobials and NSAIDs. One of the possible reasons may be because of the pharmacogentic basis (HLA-B*1502 and HLA-A*3101 alleles) for carbamazepine induced-SJS/TEN. The association with HLA-B*1502 alleles with carbamazepine induced-SJS/TEN is detected in the Indian and other Asian populations, but not in Caucasians.[56] The presence of HLA-A*3101 is associated with carbamazepine-induced hypersensitivity reactions including SJS/TEN in patients of Northern European ancestry.[57] Cross-reactivity of carbamazepine is observed with phenytoin, oxcarbazepine, and lamotrigine.[58,59] Majority of the cutaneous reactions occur within six weeks of initiation of therapy with phenytoin or carbamazepine.[50] Caution is required during the initial period of therapy. This study reports allopurinol induced-SJS/TEN in a low frequency when compared with other Asian studies.[3,4,60] HLA-B*5801 is associated with severe CADRs with allopurinol in Korean, Han Chinese, and Thai descent.[61]
This study highlights the morbidity associated with CADRs. Almost one-third cases (35.08%) showed “severe” reactions on severity assessment scales and 11.39% cases required hospitalization. These figures match with those in the literature.[36,40] One of the common reasons for discontinuation of therapy is CADRs.[50] Cutaneous symptoms are most common in drug induced hypersensitivity reactions.[62,63] CADRs are main cause of mortality among dermatology inpatients.[40] Among the CADRs, SJS/TEN was the leading cause of mortality; this coincides with studies abroad.[3,7,40,64] Early withdrawal of the offending drug and treatment in a burns care unit improve survival.[7,65]
Of included reactions, 90% belong to “certain”/“definite” and “probable”/“likely” categories. There is possibility of personal bias because of disagreement in the causality assessment among the assessors.[66] No method is universally accepted.[67] Causality assessment methods applied by the Indian investigators were the commonly used ones. Studies with insufficient information about causality and studies with inclusion of “doubtful”, “unlikely” or “unclassified” categories were excluded to improve the quality of data.
Almost two-third of the reported reactions were preventable. This frequency is higher than western studies.[36,68] It requires cautious interpretation as preventability assessment is based on a small sample size (358) from six studies and the reasons for preventability were not specified. The modified Schumock and Thorton criteria is based on questionnaires.[69] ADR is considered as “definitely preventable,” if one or more of the following is present: History of allergy or previous reactions to the drug, inappropriate selection of drug in relation to diagnosis and characteristics of the patient, documentation of toxic serum drug concentration (or laboratory monitoring test), and the presence of a known treatment for the adverse drug reaction. ADR is considered “probably preventable”, if one or more of the following is present - lack of the required therapeutic drug monitoring or other necessary laboratory tests, involvement of drug interaction; involvement of poor compliance, and lack of preventive measures causing reaction. Otherwise ADR is considered as a “not preventable”. If we interpret the Schumock and Thorton scale of preventability[69] in the Indian set up, inappropriate prescribing, medication errors, self medication, OTC use and ignoring history of allergy or CADRs may be responsible factors. The frequency of past history of CADRs (18.92% vs. 20%) and that of similar CADRs (10.84% vs. 13.5%) were comparable in a previous study.[40] The availability of prescription drugs (including antimicrobials) without prescription is common in India.[70,71] Ignorance of the allergy to related drugs[36] and use of inappropriate medications[68] are also cited as common reasons for CADRs in western studies.
Major limitations of this study include possibility of heterogeneity in sample selection among the studies. However, only prospective studies with sufficient description of the causality assessment were included to improve the quality of the database. Only five included studies report causative drugs in the “definite” and/or “probable” categories. Ideally, only DPC studies along with re-challenge and de-challenge testing should have been included to reduce bias. There is the probability of overestimation of drug reactions due to lack of re-challenge data in non-DPC studies. Data from DPC studies was compared with non-DPC studies to demonstrate possible false-positive and false-negative frequency of the causative drugs. There is a need to describe suspected drugs as per causality categories to have evidence for drug-ADR pairing. As this systematic review is based on case-series only, control group, and denominator data are lacking. Hence, it is impossible to quantify the risk of CADRs associated with use of medication. Most included studies represent tertiary care teaching government hospitals. Antimicrobial prescription pattern differs in government and private health care set up in India.[72] Data on the clinical features, comorbid conditions, associated allergic conditions, past history of CADRs, complications, and hospitalization were based on a few studies only. The important parameters such as preventability and severity assessment were based on six and seven studies respectively.
CONCLUSION
The age and gender possibly do not affect CADRs. Maculopapular rash, FDEs, urticaria and SJS/TEN were the common CADRs. Frequency of FDE was higher compared with reports abroad. Sulfa drugs, β-lactams antibiotics, and carbamazepine were the most commonly implicated agents. Severe CADRs are common with carbamazepine and phenytoin. High mortality was observed with SJS/TEN than with other CADRs. Frequency of preventable CADRs appears high. There is a need for larger cohort studies considering the prescription pattern in India to confirm the findings of this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Roujeau JC, Allanore L, Liss Y, Mockenhaupt M. Severe cutaneous adverse reactions to drugs (SCAR): Definitions, diagnostic criteria, genetic predisposition. Dermatol Sin. 2009;27:203–9. [Google Scholar]
- 2.Li LF, Ma C. Epidemiological study of severe cutaneous adverse drug reactions in a city district of China. Clin Exp Dermatol. 2006;31:642–7. doi: 10.1111/j.1365-2230.2006.02185.x. [DOI] [PubMed] [Google Scholar]
- 3.Ding WY, Lee CK, Choon SE. Cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2010;49:834–41. doi: 10.1111/j.1365-4632.2010.04481.x. [DOI] [PubMed] [Google Scholar]
- 4.Choon SE, Lai NM. An epidemiological and clinical analysis of cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia. Indian J Dermatol Venereol Leprol. 2012;78:734–9. doi: 10.4103/0378-6323.102367. [DOI] [PubMed] [Google Scholar]
- 5.Zhong H, Zhou Z, Wang H, Niu J, Chen W, Song Z, et al. Prevalence of cutaneous adverse drug reactions in Southwest China: An 11-year retrospective survey on in-patients of a dermatology ward. Dermatitis. 2012;23:81–5. doi: 10.1097/DER.0b013e31823d1aae. [DOI] [PubMed] [Google Scholar]
- 6.Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: Clinical pattern and causative agents - A 6 year series from Chandigarh, India. J Postgrad Med. 2001;47:95–9. [PubMed] [Google Scholar]
- 7.Lee HY, Tay LK, Thirumoorthy T, Pang SM. Cutaneous adverse drug reactions in hospitalised patients. Singapore Med J. 2010;51:767–74. [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Epidemiology. 2007;18:805–35. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 10.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. JAMA. 1979;242:623–32. [PubMed] [Google Scholar]
- 12.Acharya T, Mehta D, Shah H, Dave J. Pharmacovigilance study of adverse cutaneous drug reactions in a tertiary care hospital. Natl J Physiol Pharm Pharmacol. 2013;3:75–81. [Google Scholar]
- 13.Anjaneyan G, Gupta R, Vora R. Clinical study of adverse cutaneous drug reactions at a rural based tertiary care centre in Gujarat. Natl J Physiol Pharm Pharmacol. 2013;3:129–36. [Google Scholar]
- 14.Brahma DK, Sangma KA, Lynrah KG, Marak MD, Wahlang JB, Bhattacharyya H, et al. Adversecutaneous drug reactions: A one year survey at dermatology outpatient clinic in a tertiary care hospital. Int J Pharm World Res. 2012;3:1–9. [Google Scholar]
- 15.Chatterjee S, Ghosh AP, Barbhuiya J, Dey SK. Adverse cutaneous drug reactions: A one year survey at a dermatology outpatient clinic of a tertiary care hospital. Indian J Pharmacol. 2006;38:429–31. [Google Scholar]
- 16.Chattopadhyay C, Chakrabarti N. A cross-sectional study of cutaneous drug reactions in a private dental college and government medical college in eastern India. Niger J Clin Pract. 2012;15:194–8. doi: 10.4103/1119-3077.97317. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Acharya LD, Rao PG. Study and evaluation of the various cutaneous adverse drug reactions in Kasturba Hospital, Manipal. Indian J Pharm Sci. 2006;68:212–5. [Google Scholar]
- 18.Hiware S, Shrivastava M, Mishra D, Mukhi J, Puppalwar G. Evaluation of cutaneous drug reactions in patients visiting out patient Departments of Indira Gandhi Government Medical College and Hospital (IGGMC and H), Nagpur. Indian J Dermatol. 2013;58:18–21. doi: 10.4103/0019-5154.105279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inbaraj SD, Muniappan M, Muthiah NS, Amutha A, Glory Josephine I, Rahman F. Pharmacovigilance of the cutaneous drug reactions in outpatients of dermatology department at a tertiary care hospital. J Clin Diagn Res. 2012;6:1688–91. doi: 10.7860/JCDR/2012/4809.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James J, Sushma M, Guido S, Elizabeth J. Cutaneous adverse drug reactions in a South Indian tertiary care center. Indian J Dermatol. 2005;50:17–21. [Google Scholar]
- 21.Jhaj R, Uppal R, Malhotra S, Bhargava VK. Cutaneous adverse reactions in in-patients in a tertiary care hospital. Indian J Dermatol Venereol Leprol. 1999;65:14–7. [PubMed] [Google Scholar]
- 22.Mahapatra S, Keshri PU. Adverse cutaneous drug reactions in a tertiary care center patients: A prospective analysis. J App Pharm Sci. 2012;2:96–8. [Google Scholar]
- 23.Nandha R, Gupta A, Hashmi A. Cutaneous adverse drug reactions in a tertiary care teaching hospital: A North Indian perspective. Int J Appl Basic Med Res. 2011;1:50–3. doi: 10.4103/2229-516X.81982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noel MV, Sushma M, Guido S. Cutaneous adverse drug reactions in hospitalized patients in a tertiary care center. Indian J Pharmacol. 2004;36:292–5. [Google Scholar]
- 25.Patel RM, Marfatia YS. Clinical study of cutaneous drug eruptions in 200 patients. Indian J Dermatol Venereol Leprol. 2008;74:430. doi: 10.4103/0378-6323.42883. [DOI] [PubMed] [Google Scholar]
- 26.Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India. Indian J Dermatol Venereol Leprol. 2004;70:20–4. [PubMed] [Google Scholar]
- 27.Saha A, Das NK, Hazra A, Gharami RC, Chowdhury SN, Datta PK. Cutaneous adverse drug reaction profile in a tertiary care out patient setting in eastern India. Indian J Pharmacol. 2012;44:792–7. doi: 10.4103/0253-7613.103304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijendra R, Pundarikaksha HP, Gopal MG, Girish K, Vasundara K, Jyothi R. A prospective study of cutaneous adverse drug reactions in a tertiary care hospital. Natl J Basic Med Sci. 2012;3:44–51. [Google Scholar]
- 29.Naldi L, Conforti A, Venegoni M, Troncon MG, Caputi A, Ghiotto E, et al. Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839–46. doi: 10.1046/j.1365-2125.1999.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos-Fernández Mdel M, Ponce-De-León-Rosales S, Archer-Dubon C, Orozco-Topete R. Incidence and risk factors for cutaneous adverse drug reactions in an intensive care unit. Rev Invest Clin. 2005;57:770–4. [PubMed] [Google Scholar]
- 31.Borch JE, Andersen KE, Bindslev-Jensen C. Cutaneous adverse drug reactions seen at a university hospital department of dermatology. Acta Derm Venereol. 2006;86:523–7. doi: 10.2340/00015555-0153. [DOI] [PubMed] [Google Scholar]
- 32.Census of India: Age structure and marital status. [Last accessed on 2013 Jul 18]. Available from: http://www.censusindia.gov.in/Census-And-You/age-structure-andmarital-status.aspx .
- 33.Bigby M. Rates of cutaneous reactions to drugs. Arch Dermatol. 2001;137:765–70. [PubMed] [Google Scholar]
- 34.Jelvehgari M, Azimi H, Montazam H. Prevalence of cutaneous drug eruption in hospitalized patients: A report from Sina Hospital of Tabriz. Iran J Dermatol. 2009;12:16–9. [Google Scholar]
- 35.Akpinar F, Dervis E. Drug eruptions: An 8-year study including 106 inpatients at a dermatology clinic in Turkey. Indian J Dermatol. 2012;57:194–8. doi: 10.4103/0019-5154.96191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiszenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand-Stocco C, Crickx B, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018–22. doi: 10.1111/j.1365-2133.2003.05584.x. [DOI] [PubMed] [Google Scholar]
- 37.Al-Raaie F, Banodkar DD. Epidemiological study of cutaneous adverse drug reactions in Oman. Oman Med J. 2008;23:21–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Census of India: Gender composition. [Last accessed on 2013 Jul 18]. Available from: http://www.censusindia.gov.in/Census-And-You/gender-composition.aspx .
- 39.Puavilai S, Choonhakarn C. Drug eruptions in Bangkok: A 1-year study at Ramathibodi Hospital. Int J Dermatol. 1998;37:747–51. doi: 10.1046/j.1365-4362.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 40.East-Innis AD, Thompson DS. Cutaneous drug reactions in patients admitted to the dermatology unit at the University Hospital of the West Indies, Kingston, Jamaica. West Indian Med J. 2009;58:227–30. [PubMed] [Google Scholar]
- 41.Brahimi N, Routier E, Raison-Peyron N, Tronquoy AF, Pouget-Jasson C, Amarger S, et al. A three-year-analysis of fixed drug eruptions in hospital settings in France. Eur J Dermatol. 2010;20:461–4. doi: 10.1684/ejd.2010.0980. [DOI] [PubMed] [Google Scholar]
- 42.Ramam M, Kumar U, Bhat R, Sharma VK. Oral drug provocation test to generate a list of safe drugs: Experience with 100 patients. Indian J Dermatol Venereol Leprol. 2012;78:595–8. doi: 10.4103/0378-6323.100563. [DOI] [PubMed] [Google Scholar]
- 43.Ramam M, Bhat R, Jindal S, Kumar U, Sharma VK, Sagar R, et al. Patient-reported multiple drug reactions: Clinical profile and results of challenge testing. Indian J Dermatol Venereol Leprol. 2010;76:382–6. doi: 10.4103/0378-6323.66587. [DOI] [PubMed] [Google Scholar]
- 44.Sanmukhani J, Shah V, Baxi S, Tripathi C. Fixed drug eruption with ornidazole having cross-sensitivity to secnidazole but not to other nitro-imidazole compounds: A case report. Br J Clin Pharmacol. 2010;69:703–4. doi: 10.1111/j.1365-2125.2010.03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James CW, Gurk-Turner C. Cross-reactivity of beta-lactam antibiotics. Proc Bayl Univ Med Cents. 2001;14:106–7. doi: 10.1080/08998280.2001.11927741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brackett CC. Sulfonamide allergy and cross-reactivity. Curr Allergy Asthma Rep. 2007;7:41–8. doi: 10.1007/s11882-007-0029-8. [DOI] [PubMed] [Google Scholar]
- 47.Patel TK, Barvaliya MJ, Sharma D, Tripathi C. A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol. 2013;79:389–98. doi: 10.4103/0378-6323.110749. [DOI] [PubMed] [Google Scholar]
- 48.Schmid DA, Depta JP, Pichler WJ. T cell-mediated hypersensitivity to quinolones: Mechanisms and cross-reactivity. Clin Exp Allergy. 2006;36:59–69. doi: 10.1111/j.1365-2222.2006.02402.x. [DOI] [PubMed] [Google Scholar]
- 49.Anovadiya AP, Barvaliya MJ, Patel TK, Tripathi CB. Cross sensitivity between ciprofloxacin and levofloxacin for an immediate hypersensitivity reaction. J Pharmacol Pharmacother. 2011;2:187–8. doi: 10.4103/0976-500X.83285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svensson CK, Cowen EW, Gaspari AA. Cutaneous drug reactions. Pharmacol Rev. 2001;53:357–79. [PubMed] [Google Scholar]
- 51.Singh N, Dubey S, Chinnaraj S, Golani A, Maitra A. Study of NAT2 gene polymorphisms in an Indian population: Association with plasma isoniazid concentration in a cohort of tuberculosis patients. Mol Diagn Ther. 2009;13:49–58. doi: 10.1007/BF03256314. [DOI] [PubMed] [Google Scholar]
- 52.Shrivastava MP, Chaudhari HV, Dakhale GN, Hiware SK, Solanke BP, Shinde A. Adverse drug reactions related to the use of non-steroidal anti-inflammatory drugs: Results of spontaneous reporting from central India. J Indian Med Assoc. 2013;111:99–102, 106. [PubMed] [Google Scholar]
- 53.Kasemsarn P, Kulthanan K, Tuchinda P, Dhana N, Jongjarearnprasert K. Cutaneous reactions to non-steroidal anti-inflammatory drugs. J Drugs Dermatol. 2011;10:1160–7. [PubMed] [Google Scholar]
- 54.Sánchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F. NSAID-induced urticaria and angioedema: A reappraisal of its clinical management. Am J Clin Dermatol. 2002;3:599–607. doi: 10.2165/00128071-200203090-00002. [DOI] [PubMed] [Google Scholar]
- 55.Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 56.Alfirevic A, Pirmohamed M. Drug induced hypersensitivity and the HLA complex. Pharmaceuticals. 2011;4:69–90. [Google Scholar]
- 57.McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiūtė D, Carrington M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang XQ, Lang SY, Shi XB, Tian HJ, Wang RF, Yang F. Cross-reactivity of skin rashes with current antiepileptic drugs in Chinese population. Seizure. 2010;19:562–6. doi: 10.1016/j.seizure.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010;11:349–56. doi: 10.2217/pgs.09.162. [DOI] [PubMed] [Google Scholar]
- 60.Yeung CK, Ma SY, Hon C, Peiris M, Chan HH. Aetiology in sixteen cases of toxic epidermal necrolysis and Stevens-Johnson syndrome admitted within eight months in a teaching hospital. Acta Derm Venereol. 2003;83:179–82. doi: 10.1080/00015550310007166. [DOI] [PubMed] [Google Scholar]
- 61.Dean L. Medical Genetics Summaries. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [Last accessed on 2013 Jul 18]. Allopurinol therapy and HLA-B*5801 genotype. 2013 Mar 26. Available from: http://www.ncbi.nlm.nih.gov/books/NBK127547/ [Google Scholar]
- 62.Chalabianloo F, Berstad A, Schjøtt J, Riedel B, Irgens A, Florvaag E. Clinical characteristics of patients with drug hypersensitivity in Norway: A single-centre study. Pharmacoepidemiol Drug Saf. 2011;20:506–13. doi: 10.1002/pds.2134. [DOI] [PubMed] [Google Scholar]
- 63.Lauritano EC, Novi A, Santoro MC, Casagranda I. Incidence, clinical features and management of acute allergic reactions: The experience of a single, Italian Emergency Department. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 1):39–44. [PubMed] [Google Scholar]
- 64.Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171–81. doi: 10.1016/j.clindermatol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323–7. doi: 10.1001/archderm.136.3.323. [DOI] [PubMed] [Google Scholar]
- 66.Arimone Y, Bégaud B, Miremont-Salamé G, Fourrier-Réglat A, Moore N, Molimard M, et al. Agreement of expert judgment in causality assessment of adverse drug reactions. Eur J Clin Pharmacol. 2005;61:169–73. doi: 10.1007/s00228-004-0869-2. [DOI] [PubMed] [Google Scholar]
- 67.Agbabiaka TB, Savović J, Ernst E. Methods for causality assessment of adverse drug reactions: A systematic review. Drug Saf. 2008;31:21–37. doi: 10.2165/00002018-200831010-00003. [DOI] [PubMed] [Google Scholar]
- 68.Dennehy CE, Kishi DT, Louie C. Drug-related illness in emergency department patients. Am J Health Syst Pharm. 1996;53:1422–6. doi: 10.1093/ajhp/53.12.1422. [DOI] [PubMed] [Google Scholar]
- 69.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 70.National Policy for Containment of Antimicrobial Resistance India. 2011. [Last accessed on 2013 Jul 18]. Available from: http://www.nicd.nic.in/ab-policy.pdf .
- 71.Bhanwra S. A study of non-prescription usage of antibiotics in the upper respiratory tract infections in the urban population. J Pharmacol Pharmacother. 2013;4:62–4. doi: 10.4103/0976-500X.107687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peripi SB, Thadepalli VG, Khagga M, Tripuraribhatla PK, Bharadwaj DK. Profile of antibiotic consumption, sensitivity and resistance in an urban area of Andhra Pradesh, India. Singapore Med J. 2012;53:268–72. [PubMed] [Google Scholar]


