Abstract
Background:
Autologous serum skin test (ASST) is a simple in-vivo clinical test for the detection of basophil histamine releasing activity and to diagnose chronic autoimmune urticaria (CAU) among chronic spontaneous urticaria (CSU) patients. Diagnosing these patients is also important as they may need high doses of antihistamines and systemic corticosteroids during acute exacerbations.
Aims and Objectives:
The aim of this study is to study the prevalence of CAU among cases of CSU by using ASST.
Materials and Methods:
This was a cross-sectional study done among 48 patients presenting with CSU. Detailed history, physical examination and routine investigations were recorded for all patients. ASST was done on all the 48 patients.
Results:
Of the 48 patients included in the study, 20 patients (41.6%) were ASST positive, while the remaining 28 (58%) were ASST negative. The median duration of disease in both ASST positive and negative patients was 1 year. ASST positivity was higher (66.6%) among patients with a history of round shaped weals, though not statistically significant. ASST positivity was seen in 5 (71.4%) out of seven patients with systemic involvement, which was again not statistically significant.
Conclusion:
Our study did not show any significant difference between patients with and without antibodies regarding mean age and sex distribution, clinical morphology of individual weals, duration, severity, systemic symptoms, angioedema, atopy, and association with other autoimmune conditions.
Keywords: Autoimmune thyroiditis, autologous serum skin test, chronic autoimmune urticaria, chronic spontaneous urticaria, vitiligo
INTRODUCTION
The word urticaria is derived from the Latin word “urtica” which refers to the stinging nettle plant, now known to contain histamine. Approximately 15-20% of the population will have urticaria at least once during their lifetime.[1]
The majority of the patients with chronic urticaria have an unknown or idiopathic etiology. This is referred to as chronic idiopathic urticaria or more recently, as chronic spontaneous urticaria (CSU).[2] In recent years, 30-50% of patients with CSU have been shown to have an autoimmune basis. This autoimmune subgroup referred to as chronic autoimmune urticaria (CAU) has autoantibodies directed at the FcεR1α receptor located on mast cells and basophils or less commonly against immunoglobulin E (IgE).[3]
It is often not possible to distinguish CAU from those without autoantibodies either clinically or histologically.[4] The gold standard diagnostic test for detecting clinically relevant autoantibodies to FcεR1α is the functional in-vitro donor basophil histamine assay.[4] However, this bioassay is difficult to standardize because it requires fresh basophils from healthy donors and is time consuming. Other tests like Western blot, ELISA, flow cytometry may be useful for screening in the future, but need to be validated.[5]
Autologous serum skin test (ASST) is a simple in-vivo clinical test for the detection of basophil histamine releasing activity.[6] In 1986, Grattan et al. were the first to use ASST to differentiate autoimmune urticaria from CSU.[7] (The term Chronic idiopathic urticaria is now referred to as chronic spontaneous urticaria).[2] ASST has a sensitivity of approximately 70% and a specificity of 80%.[4] It may be used as a reasonably accurate predictive clinical test to indicate the presence of functional circulating autoantibodies.[8]
Various practical considerations influence the outcome of the test, including the use of antihistamines, immunosuppresants and antidepressants. In the majority of studies antihistamines were withdrawn at least 2-3 days prior to ASST and immunosuppressants were discontinued at least two months before testing.[5,6,8] leukotriene receptor antagonists probably do not need to be discontinued before testing, since the early phase of ASST response is thought to be histamine mediated. Patients with autoimmune urticaria may have long standing disease unresponsive to antihistamines. Diagnosing these patients is also important as they may need high doses of antihistamines and systemic corticosteroids during acute exacerbations and immunomodulatory drugs may have therapeutic benefit in them.[6]
The percentage of patients with CAU among CSU patients varies widely in different studies conducted both in India and abroad. A study by Kulthanan et al. from Thailand found the percentage of patients with autoimmunity to be 24.7%.[5] A study by Godse from Mumbai found the incidence of autoimmune urticaria to be 26.6% which is similar to reports from Western countries.[3] The few studies conducted in South India using ASST among patients with CSU show widely varying ASST positivity ranging from 34% to 50%.[6,8] Genetic factors are probably responsible for this variation. Therefore, we conducted a study using ASST as an indicator of CAU because of the varying ASST positivity as well as possible differences is management and course of the disease.
MATERIALS AND METHODS
This was a cross-sectional study, done between March 2011 and June 2012, after obtaining approval from the Institutional Ethics Committee. During the study period, 48 patients with CSU who attended the Department of Dermatology were included in the study after obtaining informed consent. Patients who were <18 years of age, pregnant/lactating females, patients taking long-term corticosteroids and antidepressants were excluded from the study. All patients were asked to stop antihistamines 2 days before the test. A detailed history was taken and thorough physical examination was done for all the patients. Routine investigations (hemoglobin, total leucocyte count, differential leucocyte count, erythrocyte sedimentation rate, routine urine examination and random blood sugar examination) were done for all patients and recorded. Further tests such as liver and renal function tests, anti-nuclear antibody (ANA), thyroid stimulating hormone, antithyroid antibodies were done on those patients indicated by history and clinical examination. ASST was done on all the 48 patients.
Autologous serum skin test[8] - About 5 ml of venous blood was collected in a sterile vacutainer and allowed to clot at room temperature for 30 min. Serum was centrifuged at 2000 rpm for 15 min and 0.05 ml of autologous serum was injected intradermally, in uninvolved skin, using a 1 ml insulin syringe (30 gauge needle) into the right forearm 2 cm below the cubital fossa. Similarly, 0.05 ml of 0.9% sterile normal saline (negative control) was injected intradermally proximally into the left forearm, and at a distance of at least 5 cm, 0.05 ml of histamine (10 μg/ml) was injected distally into the left forearm as a positive control. A serum induced erythematous weal with a diameter of 1.5 mm more than the saline induced response within 30 min was taken as positive.
Statistical analysis was performed using Chi-square tests, Student's t-test, and Mann-Whitney U-test.
RESULTS
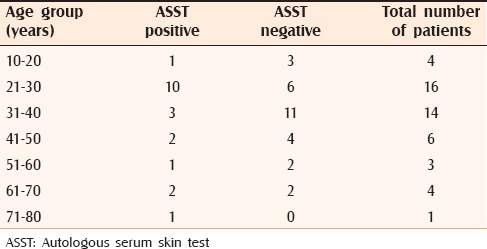
A total of 48 patients were included in the study. There were 20 (41.6%) males and 28 (58.3%) females. The male: female ratio was 1:1.4. The age range was 18 to 75 years with a mean age of 36.7 years (standard deviation [SD]: 15.1 years). As many as 36 out of 48 patients were found to be in the age group between 21 and 50 years. Age wise distribution of patients is described in Table 1. Total duration of the disease ranged from 2 months to 10 years with a median of 1 year (interquartile range [IQR]-0.5 years, 2 years).
Table 1.
Age distribution of ASST positive and negative individuals
After taking a detailed history 36 (75%) patients gave history of large weals (>3 cm in diameter) and the rest gave history of small weals. Classifying the patients on the basis of shape of weals, 20 (41.6%) of them gave history of linear weals and 15 (31.25%) of them gave history of round weals. Mixture of both these types was present in 13 (27.08%) patients.
The duration of weals ranged from 10 min to 6 h with a median of 1 h (IQR-0.5 h, 3 h). History of atopy was present in 21 (43.75%) of these patients. History of angioedema was present in 32 (66.6%) patients. History of systemic involvement was present in seven patients (14.58%). Three patients had associated autoimmune conditions like vitiligo and autoimmune thyroiditis. All four patients who had undergone ANA tests were found to be negative.
In this study, 20 patients (41.6%) were ASST positive [Figure 1] while the remaining 28 (58%) were ASST negative [Figure 2]. Females had a higher percentage (46.4%) of ASST positivity than males (35%), but this was not statistically significant (P = 0.43). Correlating ASST positivity with the age distribution of our patients it was found that the mean age of ASST positive patients was 36 years (SD: 17.3 years) and ASST negative patients was 37 years (SD: 13.4 years). Ten (62.5%) out of 16 patients in the third decade turned out to be ASST positive, a much higher percentage than all other groups. The median duration of disease in both ASST positive and negative patients was 1 year (IQR in ASST positive patients-0.5 years, 2 years and IQR in ASST negative patients-0.5 years, 2 years).
Figure 1.
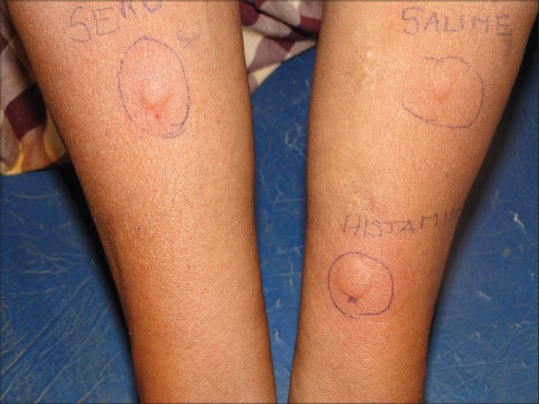
Autologous serum skin test positive
Figure 2.
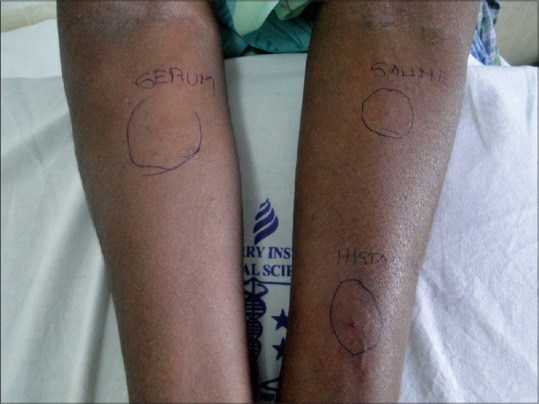
Autologous serum skin test negative
Autologous serum skin test positivity was low among patients with history of large weals. Fourteen out of 36 patients with large weals were found to be ASST positive (38.8%) and 6 out of 12 patients (50%) with small weals found to be ASST positive. ASST positivity was higher (66.6%) among patients with a history of round weals, though not statistically significant (P = 0.06) compared with patients with linear weals (30%) and patients with mixed morphology of weals (30.7%). The proportion of ASST negativity in patients present with linear weals was higher, but not statistically significant. Median duration of weals in ASST positive patients was 1 h (IQR-0.5 h, 2.87 h), whereas in ASST negative patients it was 0.5 h (0.25 h, 3 h).
Of the 21 atopic patients only 9 were found to be ASST positive (42.8%). 50% of the patients with angioedema were ASST positive. ASST positivity was seen in 5 (71.4%) out of seven patients with systemic involvement (such as nausea, abdominal pain, and vomiting), again not statistically significant (P = 0.084). All three patients with associated autoimmune conditions were found to be ASST positive.
DISCUSSION
Our study showed a female predominance, similar to previous reports.[6,8] In our study, ages ranged from 18 to 75 years with a mean age of 36.7 years. Other studies showed the mean age of presentation varying from 34 to 45 years[5,6,9] [Table 2]. The results of our study showed 36 out of 48 patients to be in the age group between 21 and 50 years similar to a study by George et al.[6] who reported 81 out of 100 patients in the above-mentioned age group.
Table 2.
Comparison of our study with previous studies
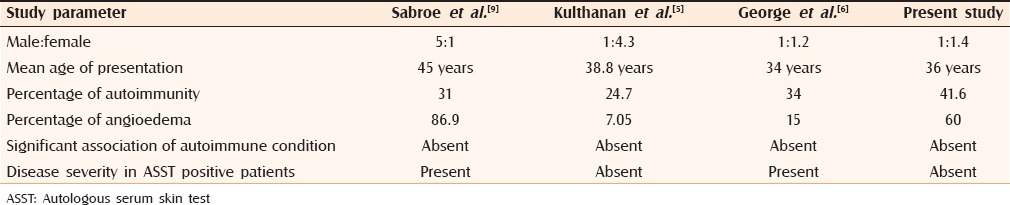
Various studies from abroad showed ASST positivity ranging from 24% to 67%[10,11] whereas studies from India by George et al.,[6] Krupa Shankar et al.,[8] Vohra et al.,[12] Godse[3] showed ASST positivity ranging from 26% to 51%. Our study showed ASST positivity of 41.6%.
No significant difference was found between ASST positive and negative patients regarding total duration and frequency of disease, matching results obtained by Nettis et al.[11] and Kulthanan et al.[5] Sabroe et al.[9] showed that both duration and frequency of disease was significantly more in ASST positive patients, whereas George et al. showed that patients with positive ASST had more frequent attacks.[6] As in many other studies[5,11,13] our study did not show any difference between ASST positive and negative patients regarding duration of weals. George et al.[6] however, found weals lasted significantly longer in ASST positive patients.
Our study showed no significant difference between ASST positive and ASST negative patients regarding mean age and sex distribution. Our study results were similar to Kulthanan et al.[5] where there was no correlation between disease severity and ASST positivity [Table 2].
There was no relationship between ASST positive and negative patients in terms of large weals (>3 cm in diameter) and small weals which was similar to other studies by Kulthanan et al.[5] and Caproni et al.[10] Seven out of 48 patients had systemic involvement and ASST positivity was seen in five out of those seven patients. A study from Sweden showed that ASST positive patients had significant systemic involvement such as gastrointestinal symptoms and flushing.[14] Other studies failed to reveal such associations.[5,6,10,11]
Regarding atopy, there was no significant difference between serum positive and serum negative groups. This was consistent with other studies.[5,11] It is expected that the atopic subjects would belong mostly to a less severe disease subgroup because it has been demonstrated that the atopic subject's trend toward high level of IgE can prevent the binding of anti-FcεR1α antibodies to the receptor, already saturated by immunoglobulin.
In our study, 66% of the patients presented with a history of angioedema and 50% of them were ASST positive. In other studies, there was no significant difference between patients with or without autoantibodies with regard to angioedema and ASST positivity ranged from 7% to 87%.[5,6,10]
Associated autoimmune conditions were found only in three patients, who were ASST positive. Autoimmune diseases such as thyroid disease, vitiligo, diabetes mellitus, pernicious anemia and rheumatoid arthritis were reported more commonly in autoimmune urticaria.[9,14] Although higher frequency of autoimmune diseases has been reported in patients with autoimmune urticaria by O’Donnell et al.,[15] we did not find any significant association between autoimmune diseases and ASST positive patients which was consistent with some other studies.[5,6,10,11]
The association of chronic urticaria with thyroid autoimmunity has been studied by Leznoff and Sussman.[16] They postulated that thyroid autoimmunity may play a role in pathogenesis of chronic urticaria and angioedema. We found abnormal thyroid function test (TFT) value and thyroid autoantibodies in two patients. In our study, TFT and thyroid autoantibodies were measured only if indicated, hence the significance of the finding cannot be commented upon.
Therefore, ASST positivity does not really imply a worse or prolonged clinical course. However, the possibility that a larger study may reach statistically significant results cannot be ruled out.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yadav S, Upadhyay A, Bajaj AK. Chronic urticaria: An overview. Indian J Dermatol. 2006;51:171–7. [Google Scholar]
- 2.Zuberbier T. Classification of urticaria. Indian J Dermatol. 2013;58:208–10. doi: 10.4103/0019-5154.110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godse KV. Autologous serum skin test in chronic idiopathic urticaria. Indian J Dermatol Venereol Leprol. 2004;70:283–4. [PubMed] [Google Scholar]
- 4.Goh CL, Tan KT. Chronic autoimmune urticaria: Where we stand? Indian J Dermatol. 2009;54:269–74. doi: 10.4103/0019-5154.55640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulthanan K, Jiamton S, Gorvanich T, Pinkaew S. Autologous serum skin test in chronic idiopathic urticaria: Prevalence, correlation and clinical implications. Asian Pac J Allergy Immunol. 2006;24:201–6. [PubMed] [Google Scholar]
- 6.George M, Balachandran C, Prabhu S. Chronic idiopathic urticaria: Comparison of clinical features with positive autologous serum skin test. Indian J Dermatol Venereol Leprol. 2008;74:105–8. doi: 10.4103/0378-6323.39690. [DOI] [PubMed] [Google Scholar]
- 7.Grattan CEH, Wallington TB, Warin RP, Kennedy CTC, Bradfield JW. A serological mediator in chronic idiopathic urticaria: A clinical, immunological and histological evaluation. Br J Dermatol. 1986;114:583–90. doi: 10.1111/j.1365-2133.1986.tb04065.x. [DOI] [PubMed] [Google Scholar]
- 8.Krupa Shankar DS, Ramnane M, Rajouria EA. Etiological approach to chronic urticaria. Indian J Dermatol. 2010;55:33–8. doi: 10.4103/0019-5154.60348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabroe RA, Seed PT, Francis DM, Barr RM, Black AK, Greaves MW. Chronic idiopathic urticaria: Comparison of the clinical features of patients with and without anti-FcepsilonRI or anti-IgE autoantibodies. J Am Acad Dermatol. 1999;40:443–50. doi: 10.1016/s0190-9622(99)70495-0. [DOI] [PubMed] [Google Scholar]
- 10.Caproni M, Volpi W, Giomi B, Cardinali C, Antiga E, Melaniw L, et al. Chronic idiopathic and chronic autoimmune urticaria: Clinical and immunopathological features of 68 subjects. Acta Derm Venereol. 2004;84:288–290. doi: 10.1080/00015550410026939. [DOI] [PubMed] [Google Scholar]
- 11.Nettis E, Dambra P, D’Oronzio L, Cavallo E, Loria MP, Fanelli M, et al. Reactivity to autologous serum skin test and clinical features in chronic idiopathic urticaria. Clin Exp Dermatol. 2002;27:29–31. doi: 10.1046/j.0307-6938.2001.00962.x. [DOI] [PubMed] [Google Scholar]
- 12.Vohra S, Sharma NL, Mahajan VK, Shanker V. Clinicoepidemiologic features of chronic urticaria in patients having positive versus negative autologous serum skin test: A study of 100 Indian patients. Indian J Dermatol Venereol Leprol. 2011;77:156–9. doi: 10.4103/0378-6323.77454. [DOI] [PubMed] [Google Scholar]
- 13.Kocatürk E, Kavala M, Kural E, Sarıgul S, Zındancı I. Autologous serum skin test vs autologous plasma skin test in patients with chronic urticaria: Evaluation of reproducibility, sensitivity and specificity and relationship with disease activity, quality of life and anti-thyroid antibodies. Eur J Dermatol. 2011;21:339–43. doi: 10.1684/ejd.2011.1294. [DOI] [PubMed] [Google Scholar]
- 14.Juhlin L. Recurrent urticaria: Clinical investigation of 330 patients. Br J Dermatol. 1981;104:369–81. doi: 10.1111/j.1365-2133.1981.tb15306.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell BF, Francis DM, Swana GT, Seed PT, Kobza Black A, Greaves MW. Thyroid autoimmunity in chronic urticaria. Br J Dermatol. 2005;153:331–5. doi: 10.1111/j.1365-2133.2005.06646.x. [DOI] [PubMed] [Google Scholar]
- 16.Leznoff A, Sussman GL. Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: A study of 90 patients. J Allergy Clin Immunol. 1989;84:66–71. doi: 10.1016/0091-6749(89)90180-2. [DOI] [PubMed] [Google Scholar]


