Abstract
Introduction:
Histopathological features in retinoblastoma are considered high-risk factors (HRF) for tumor progression and metastasis, thus their presence becomes an indication for adjuvant chemotherapy. Present study was undertaken to evaluate the incidence of HRF in retinoblastoma and to correlate them with p53 expression.
Materials and Methods:
This was a retrospective study where 17 diagnosed cases of retinoblastoma were included. Cases were re-evaluated for various histomorphological parameters. Immuno-histochemical analysis was done with p53 antibody by Streptavidin biotin method.
Results:
The patients were in the age range of 1.5-50 years. Common histological features included necrosis (70.5%), calcification (64.7%), and retinal detachment (58.8%). Incidence of various morphological parameters was anterior chamber seeding (47.2%), ciliary body involvement (29.4%), iris involvement (29.4%), choroid involvement (58.8%), scleral invasion (29.4%), extrascleral invasion (11.8%), and optic nerve infiltration (23.5%). p53 expression was present in four cases out of 13 cases (30.7%) and showed a significant association with choroid invasion (P = 0.02).
Discussion:
The presence of HRF should alert the physician for a possible metastasis, and such patients should be kept on regular follow-up to detect an early recurrence. p53 expression, a known poor prognostic indicator, showed significant association with choroid invasion, however, no association was seen with other HRF.
Conclusion:
Histopathological HRF have significant therapeutic and prognostic implications. The incidence of HRF is higher in developing countries as patients present with a more advanced stage of disease. p53 expression is significantly associated with choroid invasion out of all HRF.
Keywords: High-risk factor, p53, retinoblastoma
Retinoblastoma is the most common primary ocular malignancy of childhood. Many studies have outlined various histopathological features as high-risk factors (HRF) for tumor progression, metastasis, and overall prognosis.[1,2,3,4,5] While enucleation is the treatment of choice in retinoblastoma; presence of HRF is an indication for adjuvant chemotherapy.[6] While most of the western studies cite variable, but lower incidence of HRF in retinoblastoma,[1,3,4,7] few Indian series published so far on the subject reported a higher incidence in comparison to their western counterpart.[8,9,10] Similar findings were also reported from other developing countries.[11,12] Recent studies have reported p53 expression in retinoblastoma ranging from 50% to 60%.[13,14] Present study was undertaken to throw light on the incidence of various HRF in retinoblastoma and to correlate them with p53 expression in the Indian scenario.
Materials and Methods
This was a retrospective study conducted in Department of Pathology, where 20 diagnosed cases of retinoblastoma reported on enucleated eyeball specimens over duration of 2.5 years, from January 2009 to July 2011, were included. At least three histological sections were examined; one including whole eyeball passing from pupil-optic nerve axis, second from tumor, and third from resected end of the optic nerve. Three cases had received preoperative chemotherapy (injection etoposide, injection carboplatin) and revealed moderate to marked chemotherapy-related changes in form of extensive areas of calcification, organizing inflammatory infiltrate, sheets of foamy histiocytes, small collections of cholesterol clefts, and focal foreign body giant cell reaction. These cases showed little or no viable tumor cells and thus were excluded from further study. Remaining 17 cases were re-analyzed and sub-classified according to various histomorphological parameters and prognostic indicators including necrosis, calcification, uveal invasion, scleral and extrascleral extension, anterior chamber seeding, optic nerve infiltration, and presence of retinal detachment [Figs. 1 and 2]. Involvement of the anterior chamber included invasion of anterior chamber angle, iris or ciliary body. Choroid involvement was labeled as massive if infiltration was more than 3 mm in diameter and/or with full thickness involvement. Optic nerve involvement was recorded as prelaminar, retrolaminar, and at the point of surgical resection. Immuno-histochemical analysis was done with p53 antibody by Streptavidin biotin method. Expression was considered positive if nuclear staining was present in more than 5/100 cells [Fig. 2d]. Clinical data reviewed in the study were limited to the lab requisition forms accompanying the specimens and no additional clinical details concerning follow-up, postoperative use of chemotherapy, recurrence, or overall survival were available.
Figure 1.
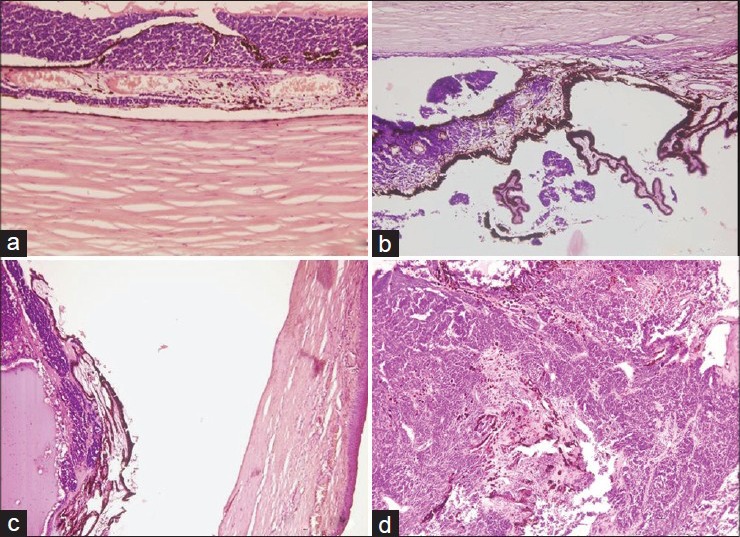
(a) Choroidal involvement (H and E, ×10). (b) Ciliary body involvement (H and E, ×10). (c) Tumor with iris infiltration and ant chamber involvement (H and E, ×4). (d) Iris infiltration and destruction (H and E, ×10)
Figure 2.
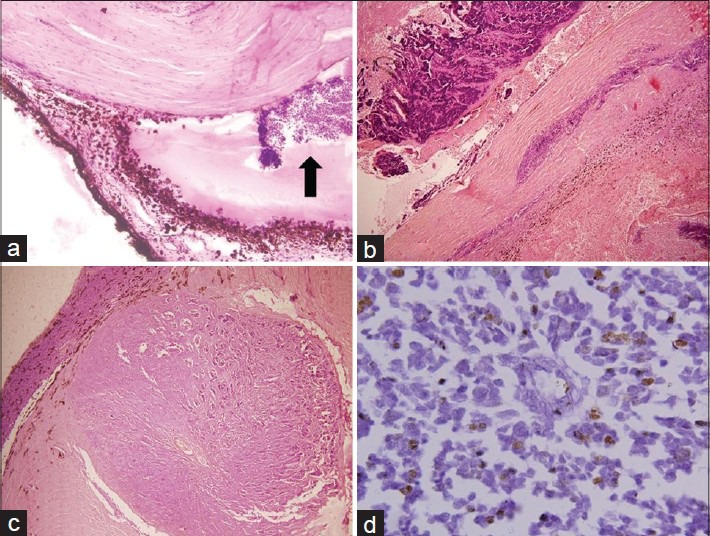
(a) Anterior chamber seeding (H and E, ×10). (b) Scleral and extrascleral involvement (H and E, ×4). (c) Retrolaminar optic nerve involvement (H and E, ×4). (d) p53 nuclear positivity on immunohistochemistry, ×60
Results
The patients were in the age range of 1.5-6 years with a single case of 50-year-old adult patient, the median being 3.5 years. No case of bilateral tumor was seen in the study. Of these patients, 11 (64.7%) were male, and 6 (35.3%) were female patients. Commonly encountered histological features included necrosis (70.5%), calcification (64.7%), and retinal detachment (58.8%). Incidence of various morphological parameters is recorded [Table 1]. Immunohistochemistry was performed for p53. Four cases were excluded due to the presence of extensive necrosis and scanty viable tissue. p53 expression was present in four cases out of 13 cases (30.7%). A nonparametric test for trend across groups (Fisher's exact test) was used to evaluate the significance of the relationship between HRF and p53 expression. Statistical analysis was performed using SPSS software (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.) p53 expression showed significant association with choroid invasion (P = 0.02). A significant association was not seen between p53 expression and presence of necrosis, ciliary body involvement, anterior chamber seeding, optic nerve involvement, iris involvement, scleral invasion, and extrascleral invasion [Table 2].
Table 1.
Incidence of morphological parameters in retinoblastoma
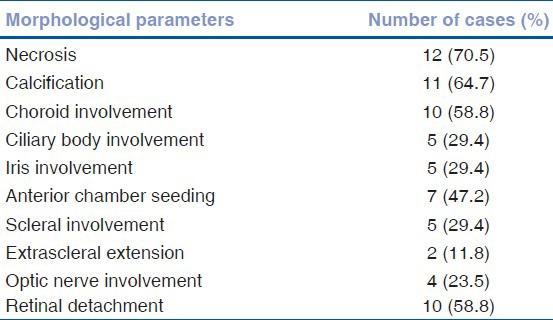
Table 2.
Correlation of various HRF with p53 positivity using Fisher's exact test

Discussion
Retinoblastoma being the most common primary ocular childhood malignancy deserves special attention. Specialized health care facilities, early detection, and use of adjuvant chemotherapy in selected patients have remarkably improved the survival in retinoblastoma over the years. Enucleation is the treatment of choice in localized tumor. Incidence of systemic metastasis is <10% with central nervous system, cranial bones, and long bones being the most favored sites.[15] Various histological factors associated with higher risk of metastasis have variously been reported as choroid involvement, extrascleral extension and optic nerve involvement.[1,2,3,4,5] Adjuvant chemotherapy is indicated in patients with metastatic disease, thus presence of HRF on histopathological examination of the surgical specimen becomes an indication for subsequent chemotherapy.[6] Incidence of HRF is reported to be higher in India and other developing countries.[8,9,10,11,12,16] Possible explanation being, patients coming to seek treatment at a more advanced stage of the disease, or probably a different biological behavior of the malignancy in different geographic locations.[9] In a study by Chantada et al.,[11] poor parental education, socioeconomic factors, and access to health care correlated significantly with late consultation, delayed diagnosis, and increased risk of extraocular disease. In a recent Indian study including 60 cases of retinoblastoma, the incidence of HRF was reported in 38 (64.4%) cases. p53 expression was observed in 21 (35%) of the tumors, however, there was no correlation in p53 and HRF statistically.[16]
We thus undertook this study to determine the frequency of HRF in our patients.
Incidence of various HRF in our study too, is much higher than what reported in western studies, and is more comparable to other studies from India and other developing nations. Present study, thus corroborates the previous claims of a higher incidence of HRF in such regions, though size of our series is small for a more confirmatory opinion. Importance of HRF cannot be undermined as it governs the use of adjuvant chemotherapy, which can considerably reduce the risk of future recurrence, thereby improving the disease-free and overall survival of such high-risk patients. The presence of HRF in the surgical tumor should alert the physician to do a thorough search for a possible metastasis by doing cerebrospinal fluid examination, bone marrow examination, and bone scan. Such patients should be kept on regular follow-up to detect an early recurrence and thus provide timely therapeutic intervention.
Molecular biology research has shown that p53, a tumor suppressor gene, plays an important role in DNA transcription, cell growth, proliferation, and DNA repair. p53 abnormalities can lead to the altered intracellular signal transduction pathways as well as loss of the regulation of cell growth, apoptosis, and DNA repair, which are responsible for carcinogenesis.[17] p53 expression is a known poor prognostic indicator for various tumors. Reported incidence of p53 expression in retinoblastoma is more than 50%.[13,14] p53 expression is related to a degree of tumor differentiation and proliferating cell nuclear antigen expression.[13] We tried to find out the incidence of p53 expression in our patients and to evaluate the correlation between HRF and p53 expression.
Conclusion
The incidence of HRF is higher in developing countries as patients present with a more advanced stage of disease. p53 expression is significantly associated with choroid invasion, but no association was found with other HRF. Histopathological HRF have significant therapeutic and prognostic implications. In such cases, further diagnostic work-up should be advised to rule out metastasis. The presence of HRF is an indication for chemotherapy. Patients with HRF should be kept on regular follow-up to detect early recurrences. This study signifies the need to include various HRF in the histopathological report of retinoblastoma to help the clinician in deciding about the use of chemotherapy and proper management of such patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kopelman JE, McLean IW, Rosenberg SH. Multivariate analysis of risk factors for metastasis in retinoblastoma treated by enucleation. Ophthalmology. 1987;94:371–7. doi: 10.1016/s0161-6420(87)33436-0. [DOI] [PubMed] [Google Scholar]
- 2.Khelfaoui F, Validire P, Auperin A, Quintana E, Michon J, Pacquement H, et al. Histopathologic risk factors in retinoblastoma: A retrospective study of 172 patients treated in a single institution. Cancer. 1996;77:1206–13. [PubMed] [Google Scholar]
- 3.Shields CL, Shields JA, Baez KA, Cater J, De Potter PV. Choroidal invasion of retinoblastoma: Metastatic potential and clinical risk factors. Br J Ophthalmol. 1993;77:544–8. doi: 10.1136/bjo.77.9.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields CL, Shields JA, Baez K, Cater JR, De Potter P. Optic nerve invasion of retinoblastoma. Metastatic potential and clinical risk factors. Cancer. 1994;73:692–8. doi: 10.1002/1097-0142(19940201)73:3<692::aid-cncr2820730331>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Hungerford J. Factors influencing metastasis in retinoblastoma. Br J Ophthalmol. 1993;77:541. doi: 10.1136/bjo.77.9.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honavar SG, Singh AD, Shields CL, Meadows AT, Demirci H, Cater J, et al. Postenucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–31. doi: 10.1001/archopht.120.7.923. [DOI] [PubMed] [Google Scholar]
- 7.Eagle RC., Jr High-risk features and tumor differentiation in retinoblastoma: A retrospective histopathologic study. Arch Pathol Lab Med. 2009;133:1203–9. doi: 10.5858/133.8.1203. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R, Vemuganti GK, Reddy VA, Honavar SG. Histopathologic risk factors in retinoblastoma in India. Arch Pathol Lab Med. 2009;133:1210–4. doi: 10.5858/133.8.1210. [DOI] [PubMed] [Google Scholar]
- 9.Biswas J, Das D, Krishnakumar S, Shanmugam MP. Histopathologic analysis of 232 eyes with retinoblastoma conducted in an Indian tertiary-care ophthalmic center. J Pediatr Ophthalmol Strabismus. 2003;40:265–7. doi: 10.3928/0191-3913-20030901-05. [DOI] [PubMed] [Google Scholar]
- 10.Kashyap S, Meel R, Pushker N, Sen S, Bakhshi S, Sreenivas V, et al. Clinical predictors of high risk histopathology in retinoblastoma. Pediatr Blood Cancer. 2012;58:356–61. doi: 10.1002/pbc.23239. [DOI] [PubMed] [Google Scholar]
- 11.Chantada GL, Dunkel IJ, de Dávila MT, Abramson DH. Retinoblastoma patients with high risk ocular pathological features: Who needs adjuvant therapy? Br J Ophthalmol. 2004;88:1069–73. doi: 10.1136/bjo.2003.037044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz K. Why look at retinoblastoma in developing countries as a potentially unique disease? Med Pediatr Oncol. 1997;29:373. [Google Scholar]
- 13.Yang X, Zhang Z, Zeng Q. A study of expression of p53, c-myc and PCNA in retinoblastoma. Zhonghua Yan Ke Za Zhi. 1999;35:252–4,14. [PubMed] [Google Scholar]
- 14.Schwimer CJ, Prayson RA. Clinicopathologic study of retinoblastoma including MIB-1, p53, and CD99 immunohistochemistry. Ann Diagn Pathol. 2001;5:148–54. doi: 10.1053/adpa.2001.25406. [DOI] [PubMed] [Google Scholar]
- 15.Karcioglu ZA, al-Mesfer SA, Abboud E, Jabak MH, Mullaney PB. Workup for metastatic retinoblastoma. A review of 261 patients. Ophthalmology. 1997;104:307–12. doi: 10.1016/s0161-6420(97)30319-4. [DOI] [PubMed] [Google Scholar]
- 16.Saxena P, Kashyap S, Bajaj MS, Pushker N, Ghose S. Expression of p53 and mdm2 in human retinoblastoma. J Clin Exp Ophthalmol. 2012;3:236. [Google Scholar]
- 17.Dong M, Ma G, Tu W, Guo KJ, Tian YL, Dong YT. Clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer. World J Gastroenterol. 2005;11:2162–5. doi: 10.3748/wjg.v11.i14.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]


