Abstract
Impaired uterine invasion by extravillous trophoblast (EVT) in early gestation is implicated in the genesis of preeclampsia, a potentially lethal malady of human pregnancy. However, reasons for EVT dysfunction remain unclear due to virtual inaccessibility of early placental and uterine tissues from women who develop preeclampsia, and the absence of animal models in which the disease spontaneously occurs. Consequently, the possibility that deficient or defective maturation of the endometrium (“decidualization”) may compromise EVT invasion in preeclampsia remains unexplored. Using a bioinformatics approach, we tested this hypothesis identifying 396 differentially expressed genes (DEG) in chorionic villous samples (CVS) from women at ~11.5 gestational weeks who developed severe preeclampsia symptoms 6-months later compared to CVS from normal pregnancies. A large number, 154 or 40%, overlapped with DEG associated with various stages of normal endometrial maturation before and after implantation as identified by other microarray datasets (p=4.7×10−14). 116 of the 154 DEG or 75%, overlapped with DEG associated with normal decidualization in the absence of EVT, i.e., late-secretory endometrium and endometrium from tubal ectopic pregnancy (p=4.2×10−9). Finally, 112 of these 154 DEG or 73% changed in the opposite direction in microarray datasets related to normal endometrial maturation (p=0.01) including 16 DEG up-regulated in decidual (relative to peripheral blood) Natural Killer cells that were down-regulated in CVS from women who developed preeclampsia (p<0.0001). Taken together, these results suggest that insufficient or defective maturation of endometrium and decidual Natural Killer cells during the secretory phase and early pregnancy preceded the development of preeclampsia.
Keywords: pregnancy, decidualization, endometrial cycle, natural killer cell, trophoblast
Introduction
Preeclampsia (PE), a multi-organ disease affecting 3–5% of human pregnancies, is associated with significant maternal, fetal and neonatal, morbidity and mortality 1–4. In addition, PE increases the risk of lifelong cardiovascular and metabolic disease for both mother and offspring 5–7. Clear understanding of PE etiology is lacking, which hampers identification of early predictive biomarkers and development of specific treatment and prophylactic measures.
Though knowledge of PE pathogenesis has markedly improved over the past decade, etiology remains less certain, and largely due to formidable investigative challenges, has been only infrequently addressed, e.g.,8. It is widely believed that insufficient extravillous trophoblast (EVT) invasion of uterine spiral arteries starting in early pregnancy is a causal factor 9. Consequently, there has been considerable investigation of the cellular and molecular mechanisms of EVT in this biological event. In contrast, little attention has been given to the uterine niche in which EVT invade. Perhaps the “soil”, rather than or in addition to the “seed” is aberrant in women destined to develop preeclampsia 10,11.
A major stumbling block to finding etiological factors in PE is that the disease is thought to begin in early pregnancy related to inadequate EVT invasion (vide supra). Accordingly, etiology is widely separated in time from the onset of disease symptoms, which does not occur until late pregnancy 12. Presently, we are not certain about who will develop PE due to lack of predictive biomarkers 13, although these are being actively pursued in numerous labs using discovery-based approaches. This ignorance precludes identification of women for prospective exploration of disease etiology in early pregnancy. Nevertheless, even if we knew who would develop PE, we cannot readily obtain the relevant tissue in which to investigate potential causes, i.e., first trimester placenta and decidua. Nor can we study third trimester placentas and basal plate decidua, and necessarily gain insight into disease etiology, because one cannot necessarily discern cause from effect at this late stage. Finally, preeclampsia is considered to be a disorder peculiar to human pregnancy, which makes investigation of etiological factors in animal models potentially problematic 14.
In an attempt to overcome these challenging hurtles we undertook a unique discovery-based approach to study the etiology of preeclampsia 11,15. By whole genome gene expression profiling of a collection of surplus chorionic villous sampling (CVS) tissue obtained for prenatal genetic screening, we unexpectedly noted putative decidualization marker genes, IGFBP1, PAEP or glycodelin and PRL to be down-regulated in women who developed PE 6-months later. Decidualization is a process of endometrial maturation that begins in the secretory phase of the menstrual cycle (pre-decidualization) and continues after conception and implantation. An important part of this biological process is the enrichment of decidual Natural Killer (dNK) cells starting in the secretory endometrium 16. In essence, pre-decidualization and decidualization are a biological continuum in preparation of the “soil” for the “seed” (EVT and conceptus) 17. Dysregulated endometrial maturation is emerging as an important precursor of recurrent pregnancy loss and infertility 18,19. By analogy, we asked if insufficient or defective endometrial maturation might also contribute to the pathogenesis of preeclampsia.
The objective of the present work was to employ a bioinformatics approach 20 to rigorously test the hypothesis that preeclampsia is antedated by disturbances in endometrial maturation before and after implantation. In turn, accumulating evidence links impaired decidualization, as well as deficient dNK cell number and/or function to compromised extravillous trophoblast invasion, spiral artery remodeling and placentation 10,21–23. We reasoned that, if genes up-regulated in the endometrium and dNK cells during the biological processes of (pre-) decidualization 24–28 are down-regulated in CVS from women destined to develop PE 11, then this would provide critical missing, prospective evidence needed to underpin the concept of “endometrial antecedents of preeclampsia”.
Methods
We re-analyzed publically available microarray datasets, in order to determine differentially expressed genes, which increase expression in late secretory endometrium (pre-decidualization) and during endometrial maturation after implantation (decidualization), the latter in the presence or absence of extravillous trophoblast. In addition, we investigated DEG up-regulated in decidual relative to peripheral blood NK cells by re-analyzing other microarray datasets. These up-regulated DEG were then compared to DEG down-regulated in CVS obtained at ~11.5 gestational weeks from 4 women who developed severe, late onset preeclampsia 6-months later matched to 8 women with normal pregnancy (Results and Table S1). This overall approach was chosen because our hypothesis was that genes which increased expression during the process of normal endometrial maturation before and after implantation will be decreased in the endometrium of women destined to develop preeclampsia (detailed Materials and Methods are presented in the Data Supplement).
Results
Differentially expressed genes between chorionic villous samples obtained from preeclamptic and normal pregnant women
CVS obtained at ~11.5 gestational weeks from 4 women who developed preeclampsia 6-months later were matched to CVS from 8 women with normal pregnancy 11. Each of the 4 CVS specimens from women who developed preeclampsia was matched for parity, gestation age at CVS within 3 days and race with 2 unaffected control specimens 11. In addition to fetal chorion, CVS invariably contains maternal tissue that derives mainly from adherent decidual basal plate with another potential source being placental septae projecting upward from the basal toward the chorionic plate containing an admixture of decidual and uterine NK cells, and EVT 29,30. Because surplus CVS was frozen within 10 min of extraction in this study, they were not cleaned of maternal decidual tissue 11. The presence of decidua in the CVS is corroborated by the transcriptomics as revealed in this work being consistent with the molecular signature of decidua (see below). Women with preeclampsia met criteria for severe disease 4,31, and all delivered >34 weeks. There were no co-morbidities except the women with preeclampsia tended to have higher BMI (Table S1). RNA integrity was evaluated on an Agilent Bioanalyzer (RIN ≥ 6.0; Agilent, Santa Clara, CA, 11). Casting a wide net, we established differentially expressed genes (DEG) by t-test (p<0.05), fold change (FC) and J5 analysis (Data Supplement). There was a total of 396 DEG between CVS obtained from women with preeclampsia compared to CVS from women with normal pregnancy outcome of which 201 were up-regulated and 195 down-regulated in preeclampsia (see Table S2 for gene lists).
Differentially expressed genes down-regulated in CVS from preeclamptic women are up-regulated during normal endometrial maturation in the late-secretory phase and early pregnancy
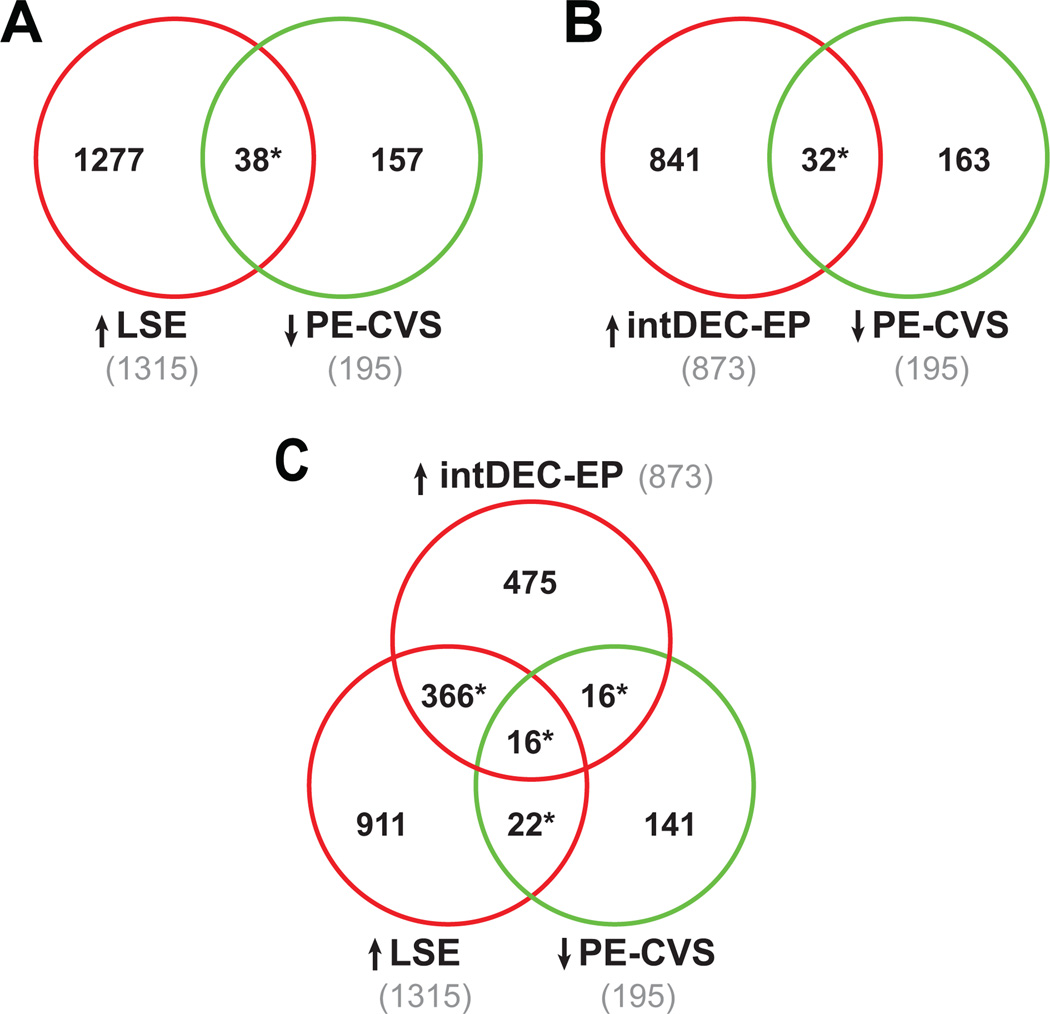
We analyzed gene expression in normal endometrium from different phases of the menstrual cycle (GSE4888 28 and GSE6364 24) to identify the cluster of co-expressed genes strongly increasing expression in the endometrium throughout the menstrual cycle and peaking in the late-secretory endometrium (LSE). There was a significant overlap of 38 genes between the LSE cluster of 1315 up-regulated differentially expressed genes and the 195 down-regulated DEG in CVS from preeclamptic compared to normal pregnant women (PE-CVS; p<0.0001 by Pearson’s chi-square test; Figure 1A, Table S3A).
Figure 1. Clusters of genes induced during pre-decidualization and decidualization in ectopic pregnancy are down-regulated in CVS from preeclamptic women.
The Venn Diagrams show significant overlap (*P<0.0001 by Pearson’s chi-square test) between DEG down-regulated in CVS from PE women (PE-CVS; relative to CVS from women with normal pregnancy) and DEG up-regulated in: (A) late-secretory endometrium (LSE; relative to proliferative endometrium; 38 DEG, Table S3A) and (B) EP endometrium with intermediate-decidualization (intDEC) changes (relative to EP endometrium without decidualization changes), which lacks extravillous trophoblast (32 DEG, Table S3B). In (C), there is significant overlap (*P<0.0001) between DEG down-regulated in PE-CVS and DEG up-regulated in LSE and EP endometrium with intermediate-decidualized changes (16 DEG, Table S3C).
Gene expression in endometrium from tubal ectopic pregnancy (EP) showing intermediate-decidualization morphology on H&E stained sections as described by Duncan and coworkers was first compared to gene expression in non-decidualized endometrium obtained from women with EP (E-MTAB-680 25). The up-regulated differentially regulated genes in this decidualized endometrium (873 DEG) were then compared to the down-regulated DEG in CVS from preeclamptic compared to unaffected control women. There was significant overlap of 32 genes (p<0.0001; Figure 1B, Table S3B). There was also a large overlap of 382 differentially regulated genes increasing in LSE with DEG up-regulated in intermediate-decidualized endometrium from EP (p<0.0001). Of these, 16 significantly overlapped with the DEG down-regulated in CVS from preeclamptic women (p<0.0001; Figure 1C, Table S3C). These results suggest an impairment of endometrial maturation in late-secretory phase and early pregnancy in the women who developed preeclampsia. This impairment is independent of extravillous trophoblast, because they are absent from late-secretory and ectopic pregnancy endometrium (the latter verified by histology and cytokeratin immunohistochemistry 25).
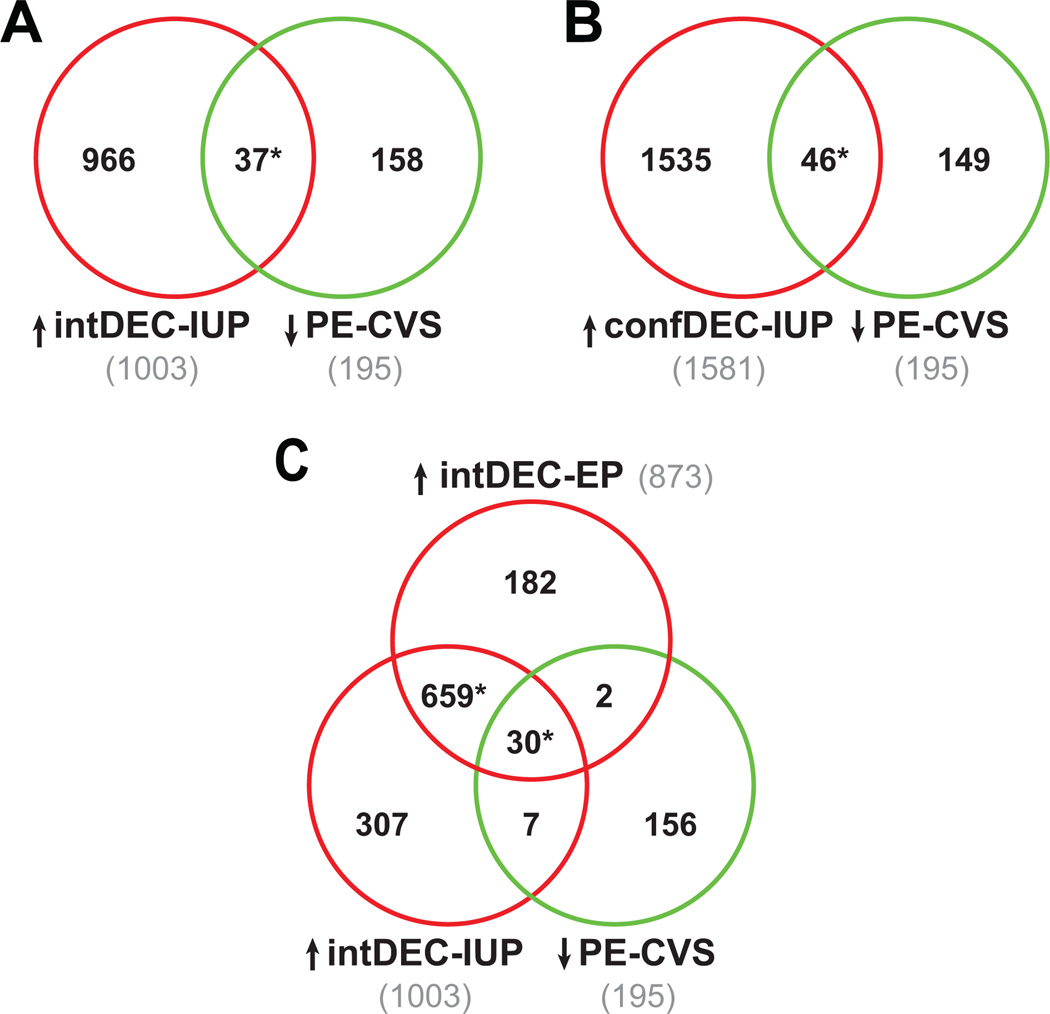
Gene expression in both intermediate- and confluent-decidualized endometrium from women with intrauterine pregnancy (IUP) was initially compared to gene expression in non-decidualized endometrium (E-MTAB-680 25). The up-regulated DEG in IUP endometrium with intermediate- (1003 DEG) and confluent- (1581 DEG) decidualized changes were next compared to the 195 down-regulated DEG in CVS from preeclamptic compared to normal pregnant women. Thirty-seven and 46 DEG up-regulated in intermediate- and confluent-decidualized endometrium from IUP, respectively, overlapped with DEG down-regulated in PE-CVS (both p<0.0001; Figure 2A and B, Table S4A and S4B, respectively).
Figure 2. Clusters of genes induced during decidualization in intrauterine and ectopic pregnancy are down-regulated in CVS from preeclamptic women.
The Venn Diagrams show significant overlap (*P<0.0001 by Pearson’s chi-square test) between DEG down-regulated in PE-CVS relative to CVS from women with normal pregnancy and DEG up-regulated in: (A) intermediate-decidualized endometrium (intDEC; 37 DEG, Table S4A) and (B) confluent-decidualized endometrium (confDEC; 46 DEG, Table S4B) both from IUP (relative to EP endometrium without decidualization changes) and containing extravillous trophoblast. In (C), there are 32 DEG in common between DEG down-regulated in PE-CVS and DEG up-regulated in EP endometrium with intermediate-decidualized changes and without EVT (also see Figure 1B), and 37 DEG in common between DEG down-regulated in PE-CVS and DEG up-regulated in IUP endometrium with intermediate-decidualized changes (EVT present). The majority of these DEG, in turn, are overlapping (30 DEG, Table S4C; *p<0.0001) suggesting minimal EVT contribution to the overlap.
Because the decidua from intrauterine, but not ectopic pregnancy was populated by extravillous trophoblast, we were able to estimate the potential EVT contribution to the overlap of differentially expressed genes down-regulated in PE-CVS and up-regulated in ectopic and intrauterine pregnancy endometrium matched for the extent of decidualization (“intermediate”). There was large overlap of 689 DEG up-regulated in intermediate-decidualized endometrium from EP and IUP (relative to non-decidualized endometrium; p<0.0001, Figure 2C). As illustrated in Fig. 2C, 30 of these 689 differentially expressed genes overlapped significantly with DEG down-regulated in CVS from preeclamptic relative to normal pregnant women (p<0.0001, Table S4C). The majority of overlapping DEG between those up-regulated in intermediate-decidualized endometrium from intrauterine and ectopic pregnancies and down-regulated in PE-CVS were the same genes (30 of 37 for intermediate-decidualized endometrium from IUP and 30 of 32 for intermediate- decidualized endometrium from EP). These results reinforce the notion of impaired endometrial maturation during early pregnancy in the decidua from ectopic pregnancy did not contain extravillous trophoblast 25.
Differentially expressed genes down-regulated in CVS from preeclamptic women are not up-regulated in decidualized endometrial stromal cells by trophoblast conditioned medium (TrCM)
There was no significant overlap of endometrial genes increasing in expression after treatment of decidualized stromal cells in culture with TrCM with DEG down-regulated in CVS from women who developed preeclampsia compared to women with normal pregnancy outcome, with only 4 in common (Figure S1, p=0.5). These results further support the idea that impaired decidualization in the women who developed preeclampsia may be mostly independent of trophoblast influence. See Results in the Supplemental Data and Figure S1 for details.
Confluence of overlapping genes
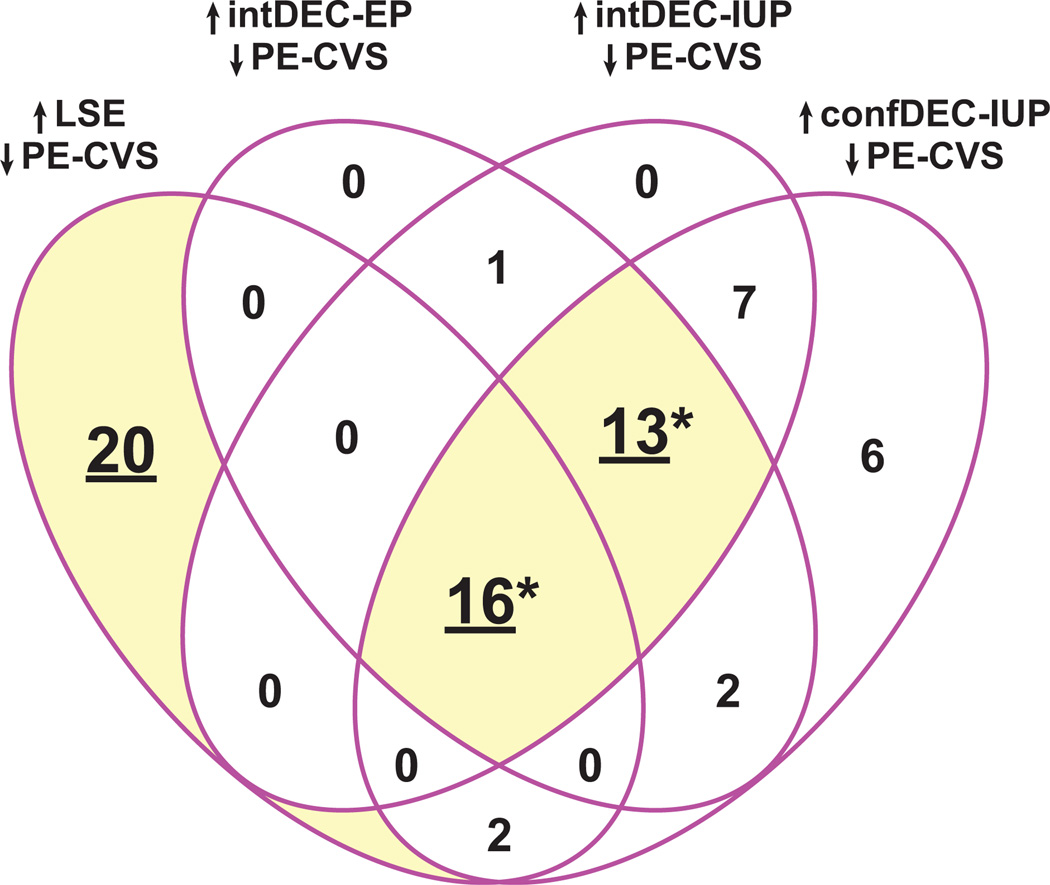
We next investigated the confluence of differentially expressed genes down-regulated in CVS from women with preeclampsia relative to normal pregnancy and up-regulated in late-secretory endometrium, intermediate-decidualized endometrium from intrauterine and ectopic pregnancies, as well as confluent-decidualized endometrium from IUP. As portrayed by the Venn Diagram in Figure 3, there were 20 down-regulated DEG in PE-CVS, which were up-regulated in LSE but not in intermediate- or confluent-decidualized endometrium; 13 DEG down-regulated in PE-CVS and up-regulated in intermediate- and confluent-decidualized endometrium, but not in LSE (p<0.0001), and 16 DEG down-regulated in PE-CVS and up-regulated in all datasets related to endometrial maturation (p<0.0001). Individual differentially expressed genes are presented in Table S5 and mean expression values are illustrated in Figure 4.
Figure 3. Confluence of gene clusters induced during (pre-) decidualization is down-regulated in CVS from preeclamptic women.
There are 20 DEG down-regulated in CVS from PE women (PE-CVS; relative to CVS from women with normal pregnancy) uniquely up-regulated in late-secretory endometrium (LSE, relative to proliferative endometrium). There is also significant overlap (*p<0.0001 by Pearson’s chi-square test) between DEG down-regulated in PE-CVS and DEG up-regulated in EP and IUP endometrium both with intermediate-decidualized (intDEC) changes, and IUP endometrium with confluent-decidualized (confDEC) changes, but not LSE (13 DEG); and in all 4 of the datasets related to different degrees of endometrial maturation (16 DEG). See Table S5 for individual genes and Figure 4 for average expression levels of these genes.
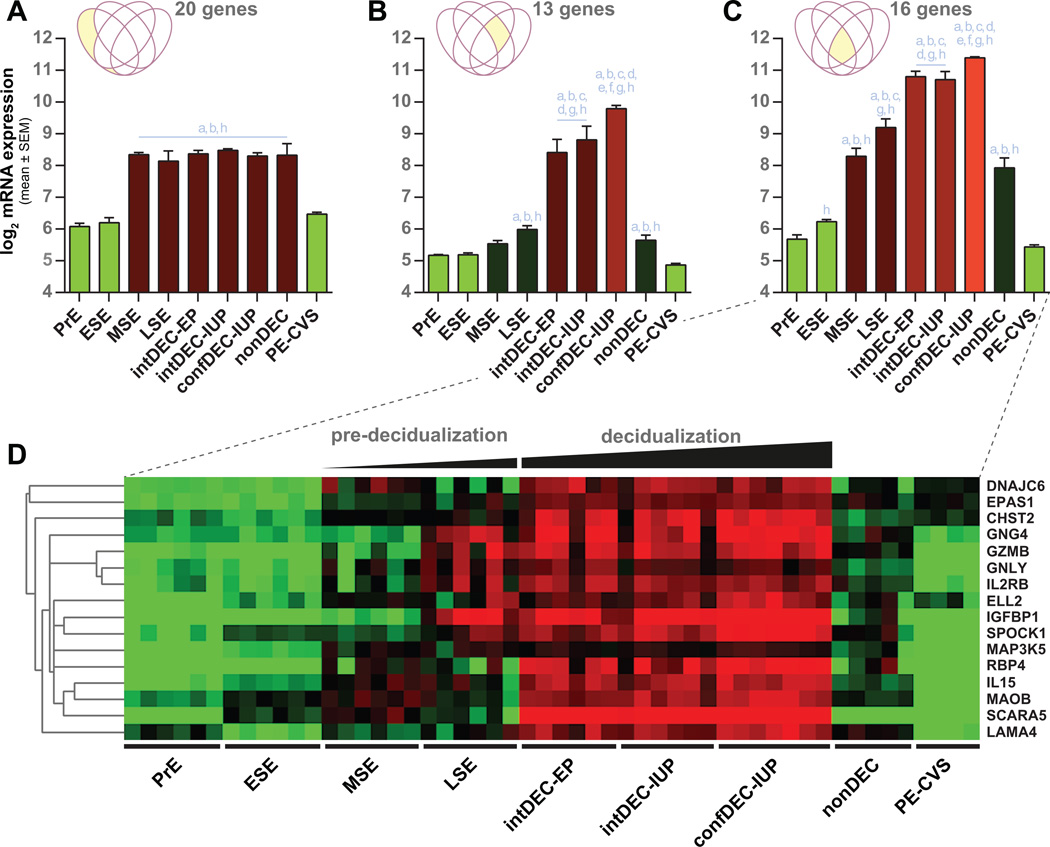
Figure 4. Average gene expression levels (log base 2) in endometrium from different stages of endometrial maturation and CVS from preeclamptic women.
(A) Average expression of 20 DEG down-regulated in CVS obtained from women who developed PE (PE-CVS; relative to CVS from women with normal pregnancy) and up-regulated in mid- and late-secretory endometrium (MSE and LSE, respectively; relative to proliferative endometrium, PrE). (B) Average expression of 13 DEG down-regulated in PE-CVS and up-regulated in IUP and EP endometrium with intermediate-decidualized (intDEC) changes, and IUP endometrium with confluent-decidualized (confDEC) changes, but not LSE. (C) Average expression for 16 DEG down-regulated in PE-CVS and upregulated in in all 4 of the datasets related to different degrees of endometrial maturation. (D) Heat map corresponding to Figure 4C. The individual DEG in Figure 4A, B and C are listed in Table S5. EP, ectopic pregnancy; IUP, intrauterine pregnancy; nonDEC, non-decidualized endometrium from EP; ESE, early-secretory endometrium. Significantly different (p<0.05) from: a, PrE; b, ESE; c, MSE; d, LSE; e, intDEC-EP; f, intDEC-IUP; g, nonDEC; h, PE-CVS.
Figure 4 depicts log2 mean expression values for the differentially expressed genes down-regulated in CVS from preeclamptic compared to unaffected control women and up-regulated in various states of normal endometrial maturation (refer to Figure 3). Twenty differentially expressed genes were identified as uniquely up-regulated in LSE and down-regulated in PE-CVS; therefore, their average expression did not further increase with decidualization in early pregnancy (Figure 4A). Average gene expression of these 20 DEG was significantly less in PE-CVS than in mid-secretory and LSE (p<0.05), and comparable to proliferative and early-secretory endometrium. The 13 differentially expressed genes down-regulated in PE-CVS and uniquely up-regulated in intermediate- and confluent-decidualized endometrium, only increased slightly in LSE (by definition), and mostly rose during decidualization in early pregnancy (Figure 4B). Average gene expression of these 13 DEG was markedly less in PE-CVS than in intermediate- and confluent-decidualized endometrium (p<0.05). Finally, the 16 differentially expressed genes down-regulated in PE-CVS and up-regulated in LSE, and intermediate- and confluent-decidualized endometrium increased expression beginning in mid-secretory endometrium and progressively rose thereafter (Figure 4C). Again, average gene expression of these 16 DEG was markedly less in PE-CVS compared to intermediate- and confluent-decidualized endometrium (p<0.05). The heat map shown in Figure 4D corresponds with the bar graph in Figure 4C. These observations reveal that endometrial maturation was not only impaired in early pregnancy, but also during the secretory phase in the women who developed preeclampsia.
Differentially expressed genes up-regulated in decidual NK cells are down-regulated in CVS from preeclamptic women
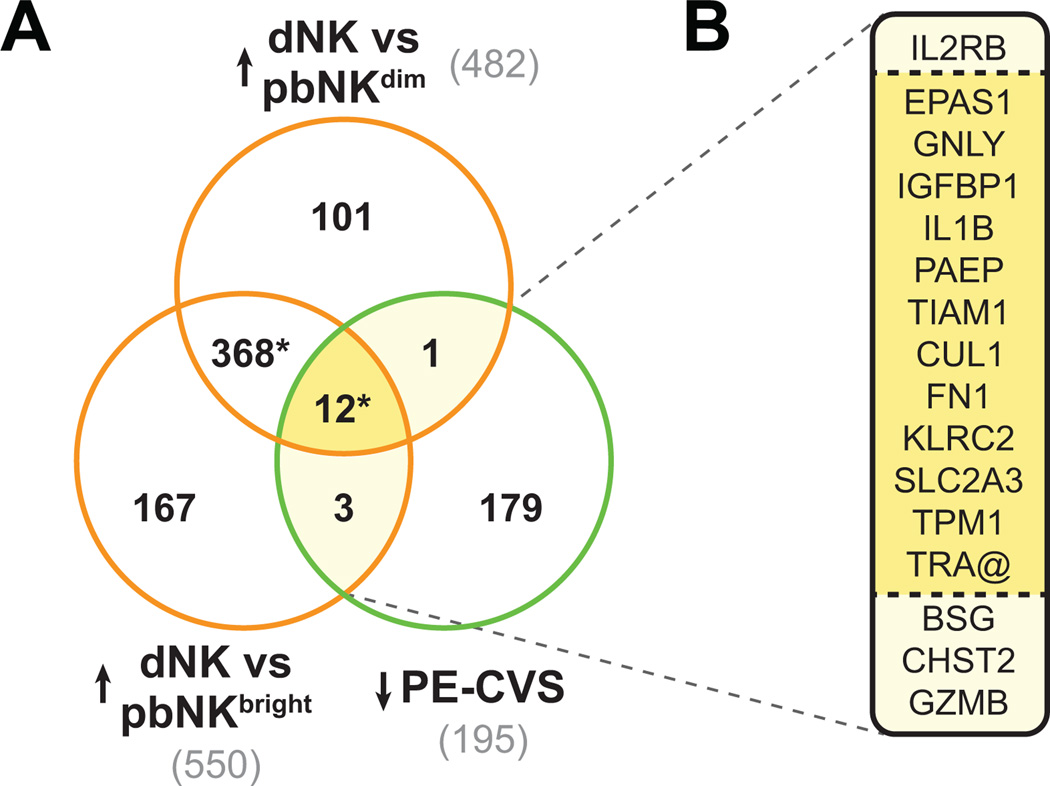
Sixteen DEG up-regulated in dNK relative to CD56dim and CD56bright pbNK cells were down-regulated in CVS from women who developed preeclampsia relative to women who experienced a normal pregnancy (p<0.0001; Figure 5). (See comprehensive Results, Figure S2, and Table S6 in the Data Supplement.)
Fig. 5. Clusters of genes induced in decidual Natural Killer cells are down-regulated in CVS from preeclamptic women.
(A) The Venn diagram shows significant overlap (*P<0.0001 by Pearson’s chi-square test) between DEG down-regulated in PE-CVS relative to CVS from women with normal pregnancy and DEG up-regulated in decidual Natural Killer cells (dNK) relative to peripheral blood CD56dim or CD56bright NK cells. The official symbols of the overlapping genes are listed in panel (B).
Systematic literature search
Because the biological process of “decidualization” is not available in public bioinformatic databases for pathway analysis, we conducted a systematic and comprehensive literature search of all 195 differentially expressed genes down-regulated in CVS from preeclampsia relative to normal pregnancy. Thirty-one were previously associated with decidualization/decidua in the literature. Of these 31 DEG,18 were in common with the 67 DEG identified by the bioinformatics approach. (See Figure S3, Table S2B, Table S5 and comprehensive Results in the Data Supplement.)
Discussion
In the present work, we asked whether expression of genes down-regulated in early placenta (chorionic villous samples) obtained from women who developed preeclampsia symptoms 6-months later significantly overlaps with expression of genes up-regulated during the normal biological processes of pre-decidualization in the late-secretory endometrium (LSE), during decidualization after implantation, and in isolated decidual NK cells (relative to peripheral blood NK cells). If so, then deficient pre-decidualization, decidualization and dNK cell number and/or function in women destined to develop preeclampsia may be instrumental in disease etiology. The overall methodology was to capitalize on unique genomics datasets in the public domain including our own from first trimester placental tissue of women who developed preeclampsia, and to analyze these datasets using a bioinformatics approach, in order to shed light on possible cause(s) of preeclampsia.
With regards to our own microarray dataset 11 we deliberately cast a wide net and identified 396 total up- and down-regulated differentially expressed genes in CVS from women who developed preeclampsia compared to those with a normal pregnancy (Table S2). Remarkably, 154 or 40% of these 396 DEG significantly overlapped with DEG associated with various stages of normal endometrial maturation before and after implantation 24–28. Second, at least 75% or 116 of these 154 DEG significantly overlapped with differentially expressed genes associated with normal endometrial maturation in the absence of extravillous trophoblast, i.e., late-secretory endometrium and decidualized endometrium from ectopic tubal pregnancy. Finally, 73% or 112 of the 154 DEG either up- or down-regulated in CVS from PE women changed in the opposite direction in the microarray datasets related to normal endometrial maturation. These findings implicate impairment of pre-decidualization and decidualization in the women who developed preeclampsia. Because the overlap of differential expressed genes in CVS from preeclamptic relative to normal pregnant women with DEG linked to normal endometrial maturation was mostly preserved regardless of the presence or absence of extravillous trophoblast, the results further imply that impaired endometrial maturation may be a primary event. This conclusion is underscored by the observation that differentially expressed genes uniquely up-regulated during normal endometrial maturation in the late-secretory phase were significantly down-regulated in CVS from women who developed preeclampsia relative to normal pregnancy suggesting that impairment of decidualization actually began before implantation (Figure 4).
As discussed earlier, we primarily focused on those differentially expressed genes down-regulated in CVS from preeclampsia relative to normal pregnancy, which were up-regulated during the biological process of pre-decidualization in the late-secretory phase (GSE4888 28 and GSE6364 24) and decidualization after implantation in women with ectopic pregnancy (E-MTAB-680 25) (Figure 1). Notably, 54 of the 195 DEG down-regulated in CVS from preeclamptic women were up-regulated in LSE or decidualized endometrium from ectopic pregnancy (Figure 1C). These results bolster the notion that there is impairment of endometrial maturation in the late-secretory phase and during early pregnancy in the women destined to develop preeclampsia. Included among the genes with diminished expression in decidua of CVS from the women who developed PE are those classically associated with the biological process of decidualization in the literature including IGFBP1, PAEP or glycodelin, and PRL (Tables S3 A–C, S5, and Fig. S3). The results also point to a primary defect in pre-decidualization and decidualization rather than in extravillous trophoblast, because EVT are lacking altogether in the late-secretory phase and EVT were absent from decidualized endometrium from ectopic pregnancy 25.
Further inspection of the microarray analyses from decidualization in early pregnancy revealed more than 1000 genes each up-regulated in endometrium morphologically characterized as being intermediate- or confluent-decidualized from intrauterine pregnancy compared to non-decidualized endometrium. Thirty-seven and 46 of these up-regulated differentially expressed genes, respectively, overlapped significantly with the 195 DEG down-regulated in PE-CVS (Figure 2A and B, and Tables S4A and B). Of further note, the vast majority of DEG up-regulated in intermediate-decidualized endometrium from ectopic pregnancy and down-regulated in CVS from preeclamptic compared to normal pregnant women (32 DEG), and those up-regulated in intermediate-decidualized endometrium from intrauterine pregnancy and down-regulated in CVS from PE women (37 DEG) were themselves overlapping (30 DEG; Figure 2C and Table S4C). These observations suggest that there may have been little, if any, contribution of extravillous trophoblast to the overlap of DEG down-regulated in PE-CVS and up-regulated in intrauterine and ectopic pregnancy endometrium matched for the degree of decidualization (intermediate), because the vast majority of DEG down-regulated in PE-CVS were up-regulated in intermediate-decidualized endometrium regardless of the presence (IUP) or absence (EP) of EVT.
To further scrutinize the potential contribution of EVT to impaired decidualization in preeclampsia, we compared differentially expressed genes down-regulated in CVS from preeclamptic relative to normal pregnant women with DEG up-regulated in endometrial stromal cells decidualized in culture after exposure to trophoblast conditioned medium (TrCM; GSE5809 32). There was only a non-significant overlap of 4 genes (see Results in the Supplemental Data and Figure S1). This finding is supportive of a minimal EVT contribution to the overlap observed between DEG down-regulated in CVS from preeclamptic women and DEG up-regulated in either intermediate- or confluent-decidualized endometrium from intrauterine pregnancy (Figures 2C and S1, respectively), which is consistent with the concept that there may have been a primary defect of endometrial maturation in the women destined to develop preeclampsia. In fact, there were 47 DEG uniquely down-regulated in PE-CVS which were up-regulated in intermediate or confluent decidualized endometrium from intrauterine pregnancy; however, the majority, 34 or 72% were also increased in late secretory endometrium or decidualized endometrium from ectopic pregnancy without extravillous trophoblast (Figure 3).
We examined the confluence of differentially expressed genes down-regulated in CVS from preeclamptic relative to normal pregnant women with DEG up-regulated in LSE, intermediate-decidualized endometrium from intrauterine and ectopic pregnancies, as well as confluent-decidualized endometrium from IUP (i.e., intersection of all 4 datasets; Figure 3 and Table S5). By definition, mean expression value of 20 DEG down-regulated in PE-CVS and uniquely up-regulated in secretory relative to proliferative endometrium was significantly increased in mid-secretory endometrium, maintained in LSE, but not further increased during decidualization in early pregnancy (Figure 4A). Of note, the mean expression for these 20 DEG was significantly lower in CVS from preeclamptic women compared to mid- and late-secretory endometrium by ~5-fold, and comparable to proliferative and early-secretory endometrium. Taken together, this analysis reinforces the idea that impairment of endometrial maturation in the women destined to develop preeclampsia may actually have begun before pregnancy in the secretory phase, a rather startling possibility that we are currently investigating further.
Mean expression of the 13 differentially expressed genes down-regulated in CVS from preeclamptic women and up-regulated in intermediate-decidualized endometrium from ectopic and intrauterine pregnancies and in confluent-decidualized endometrium from IUP, but not LSE is shown in Figure 4B. Once again, the mean expression level in CVS from preeclamptic women was markedly reduced relative to intermediate- and confluent-decidualized endometrium by ~15-fold. These results provide further evidence that, in addition to a defect in pre-decidualization (vide supra) there was also impairment of decidualization during early pregnancy in the women who developed preeclampsia.
Finally, a core set of 16 overlapping differentially expressed genes was down-regulated in CVS from preeclamptic compared to normal pregnant women, and up-regulated in LSE, intermediate-decidualized endometrium from ectopic and intrauterine pregnancies, and confluent-decidualized endometrium from IUP (Figure 4C and 4D). Relative to proliferative endometrium, the average expression level of the 16 DEG increased progressively beginning with the mid-secretory phase peaking in confluent-decidualized endometrium from IUP. It is noteworthy that mean gene expression in CVS of preeclamptic women was lower than mid- and late-secretory endometrium, and dramatically so compared to intermediate- and confluent-decidualized endometrium, the latter by ~50-fold. On balance, these data present a composite picture of Figure 4A and B underscoring the notion that both pre-decidualization and decidualization were compromised in the women who developed preeclampsia.
Because differentially expressed genes known to be involved in dNK function emerged from the aforementioned analyses (e.g., IL15, IL2RB), we explored the overlap of DEG up-regulated in isolated dNK (relative to CD56dim or CD56bright pbNK) cells and down-regulated in CVS from women who developed preeclampsia relative to women with normal pregnancy outcome (Figures 5 and S2). Our bioinformatics analysis implicates deficient dNK cell number and/or function in the women who developed preeclampsia, because 16 DEG up-regulated in dNK were down-regulated in PE-CVS. This finding is reassuring, because dNK cells are an important component of the biological process of (pre-) decidualization (see review 16).
In addition to the evidence provided by bioinformatics approaches linking the differentially expressed genes down-regulated in CVS from women who developed preeclampsia to deficient endometrial maturation, we pursued a different tack to marshal further evidence associating these down-regulated DEG to inadequate pre-decidualization and decidualization. To this end, we found that 31 of the 195 down-regulated DEG had been previously linked to decidualization/decidua in the literature (Figure S3, Table S2B and Table S5). Thus, the systematic literature search further strengthens the argument that endometrial maturation is inadequate in the women destined to develop preeclampsia.
Aberrant endometrial maturation has been previously linked to infertility 19 and recurrent pregnancy loss 18. Women with polycystic ovary syndrome (PCOS) suffer infertility and increased miscarriage rates. One potential explanation is that PCOS is associated with defective endometrial function possibly mediated through insulin resistance, hyperinsulinemia and androgen excess 33. Dehydroepisandrosterone, which is elevated in PCOS, has been shown to inhibit the pentose phosphate pathway, thereby impairing decidualization 34. By analogy, one might predict that defective (pre-) decidualization could also predispose to other adverse pregnancy outcomes including placental syndromes such as preeclampsia. In fact, women with PCOS and insulin resistance are at increased risk of developing preeclampsia 35,36. However, direct evidence to support this linkage is more difficult to obtain in the case of preeclampsia, because we do not know in early pregnancy who will develop disease, first trimester chorionic and decidual tissue is not easily accessible for investigation, and molecular interrogation of delivered placenta, basal plate decidua and cells derived thereof, cannot discern between cause or consequence of the disease. The latter is highlighted by further bioinformatics analysis (data not shown), which revealed only 5 differentially expressed genes in common between the 396 DEG in CVS from preeclamptic relative to normal pregnant women and 457 DEG in decidua basalis of third trimester placentas obtained from PE compared to NP women (E-TABM-682 37).
Nevertheless, recent evidence is consistent with the concept of endometrial antecedents of preeclampsia. First, women with high uterine vascular resistance as determined by ultrasound before elective termination in early pregnancy, demonstrated impairment of dNK cell function considered to be critical for spiral artery remodeling. However, pregnancy termination obviously precluded knowledge of pregnancy outcome in this study. Second, trophoblast isolated from placentas of severe preeclamptic women demonstrated increased expression of SEMA3B, a cytokine that impairs trophoblast invasion in vitro. After 48 hours in culture, SEMA3B expression spontaneously returned to normal levels suggesting that the in vivo milieu was responsible for the elevation 38. Though consistent with the idea that defective decidualization may perturb EVT SEMA3B expression during early pregnancy in women destined to develop PE, once again, this interpretation is problematic because trophoblast were isolated from delivered placentas. As such, it is not possible to discern whether the reported findings caused or resulted from the disease. Last, women with endometriosis may be at increased risk for PE 39, though not all agree 40,41.
Potential Study Limitations
The validity of our conclusions mainly rests on the reliability of the original investigations, which generated the DNA microarray datasets analyzed herein. Whenever possible, we built in redundancy or overlap by incorporating more than one dataset, e.g., GSE4888 28 and GSE6364 24 for endometrial gene expression in the menstrual cycle. Similarly, we tested the potential contribution of extravillous trophoblast utilizing 3 approaches: (i) analysis of LSE obviously devoid of EVT (GSE4888 28 and GSE6364 24), (ii) comparison of endometrium from intrauterine and ectopic pregnancies matched for the degree of decidualization with and without EVT influence, respectively (E-MTAB-680 25), and (iii) incubation of endometrial stromal cells decidualized in culture with trophoblast conditioned medium (TrCM) [GSE5809 (GEO database) or E-GEOD-5809 (EMBL-EBI database) 32].
To our knowledge, our genome-wide gene expression study on chorionic villous sampling of women who developed preeclampsia is the only one available in the public domain (GSE12767 11). Surplus villi were snap frozen within 10 min of extraction from women undergoing CVS for prenatal genetic screening. The tremendous labor and time involved in CVS collection, as well as the rigorous inclusion criteria impacted our sample size; however, this potential limitation may be at least partially offset by the validation methods of class predictions through cross validation (LOOCV), and by the rigorous statistical methods specifically designed to mitigate the potential limitation of small sample size frequently encountered in microarray studies by scarcity of tissue employed 42 (Supplemental Materials and Methods).
Other potential limitations to the study of CVS are detailed in the original publication 11. However, to date CVS is the only approach to obtain first trimester chorionic tissue and decidua in women with known pregnancy outcome. Therefore, the specimens for this study provided a rare glimpse into the transcriptomics of early placenta in women who developed severe preeclampsia. Moreover, CVS is dramatically decreasing in the US because of the emerging practice of non-invasive prenatal screening.
We cannot exclude the possibility of less decidua in CVS from the preeclamptic compared to normal pregnant women as an etiological factor in the disease, i.e., a quantitative rather than qualitative difference or both; conceivably, this deficiency could also compromise placentation. An assumption built into this work is that extravillous trophoblast invasion and spiral artery remodeling depend on normal decidualization and dNK function (vide supra). Although, this linkage is not proven, there is growing evidence supporting the concept 10,21–23. Moreover, because histiotrophic nutrition of the placenta and fetus in early pregnancy depends on healthy, optimally decidualized endometrial gland epithelium 43, it seems possible that this physiological process could also have been compromised in the women who developed preeclampsia. As a final cautionary note, preeclampsia is likely to be a disease of heterogenous etiology; consequently, the evidence revealed herein of inadequate or defective (pre-) decidualization may only pertain to a subset of women who develop the disease.
Perspectives
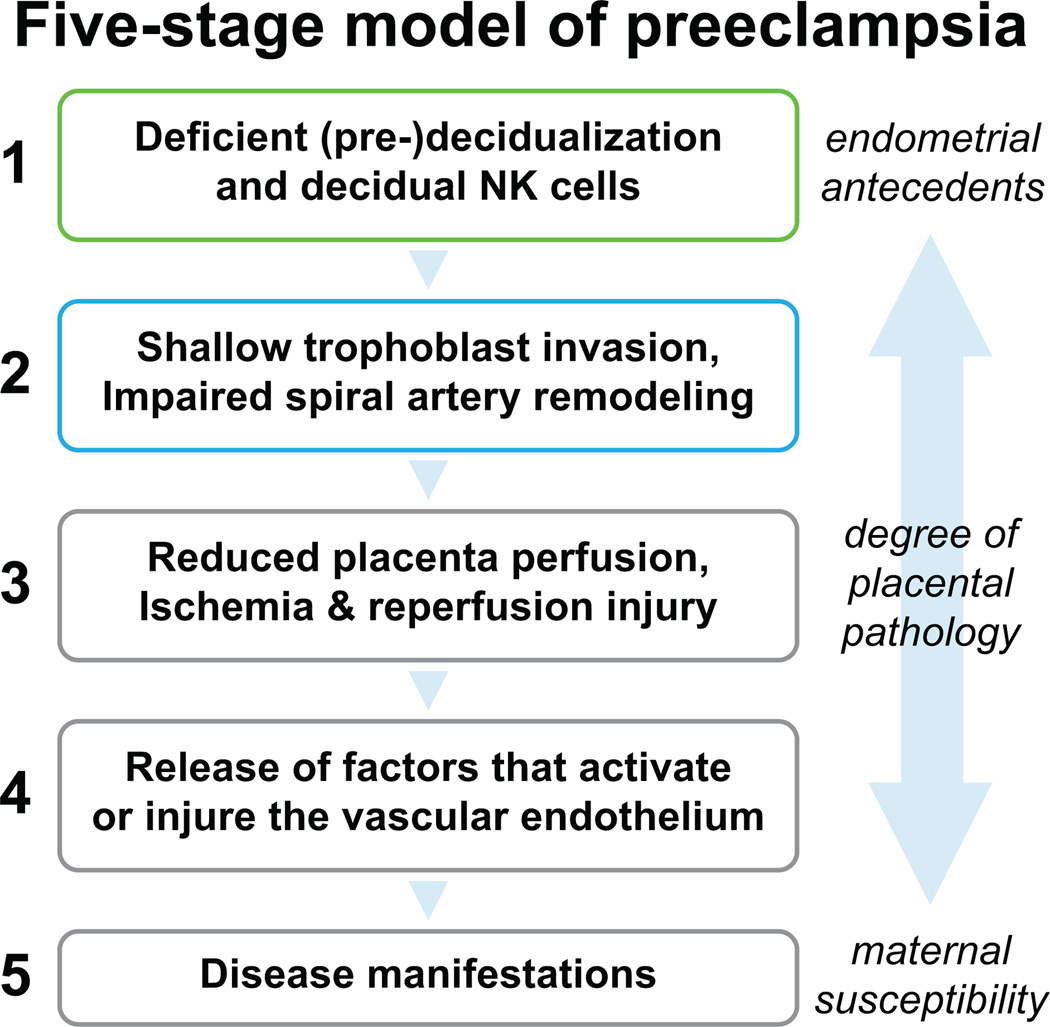
A bioinformatics approach implicates deficient or defective (pre-) decidualization and decidual NK cells in the late-secretory phase and early pregnancy in women who developed severe preeclampsia (Figure 6). Maternal constitutional factors such as PCOS, obesity, diabetes, poor cardiovascular health etcetera, may compromise endometrial maturation before and during early pregnancy, thereby predisposing to preeclampsia. Conceivably, aberrant endometrial gene expression could inform targeted investigation and discovery of protein biomarkers in blood, urine or uterine fluid for women at increased risk for preeclampsia even before conception. Ultimately designing interventions that improve endometrial maturation to facilitate normal placentation and reduce preeclampsia risk might be a logical therapeutic course of action. At the very least, the present study should motivate further inquiry into the concept that deficiency or defects in (pre-) decidualization and uterine NK cells antedate preeclampsia.
Figure 6. Five-stage model of placental preeclampsia.
Based on a bioinformatics approach, the findings of this study raise the possibility that impaired endometrial maturation and deficient decidual NK cell number and/or function in the secretory phase (pre-decidualization) and during early pregnancy (decidualization) precede the development of preeclampsia. As (pre-) decidualization and associated decidual NK cell function are emerging as important players in successful placentation, perturbation of these biological processes may contribute to the etiology of preeclampsia at least in a subset of women who develop the disease. Preeclampsia may arise in some women with little or no endometrial or placental pathology.
Supplementary Material
Novelty and Significance.
What Is New?
Using a bioinformatics approach, comparison of whole genome expression profiles of early placentas from women who developed severe preeclampsia in the context of other microarrays in the public domain related to normal endometrial maturation or decidualization revealed insufficient or defective decidualization during the secretory phase and early pregnancy in the women who developed preeclampsia.
Further evidence that genes related to uterine Natural Killer cells were also adversely affected corroborates the concept of impaired endometrial maturation as a cause of preeclampsia.
What Is Relevant?
By implicating impaired decidualization as an etiological factor in women who developed preeclampsia, this work opens up new avenues for investigation of the specific endometrial molecular defects and underlying causes, targeted biomarkers, and prophylactic or therapeutic measures to improve or repair endometrial maturation, thereby reducing preeclampsia risk.
Summary
Capitalizing on rare, early placental tissues, a bioinformatics analysis identified impairment of endometrial maturation and decidual Natural Killer cells in the secretory phase and during early pregnancy in women who developed preeclampsia.
Acknowledgments
We gratefully acknowledge Dr. Paulien H. Wiersma, Medical Information specialist of the University Library Utrecht for her expert advice and assistance in the systematic literature search, and Professor Jack L. Strominger and Dr. Hernan Kopcow, from Harvard University, for providing the microarray data on peripheral blood and decidual Natural Killer cells. We are also grateful to Sandra A. Founds, Yvette P. Conley and James Lyons-Weiler for their earlier collaboration on the initial bioinformatics and PCR validations of the CVS microarray data.
Funding: This work was supported by NIH PO1 HD065647 (KPC).
Glossary
Abbreviations used in this manuscript
- confDEC
Confluent-decidualized endometrium
- CVS
Chorionic villous samples
- DEG
Differentially expressed genes
- dNK
Decidual Natural Killer cells
- eNK
Endometrial Natural Killer cells
- EP
Ectopic pregnancy
- ESE
Early-secretory endometrium
- EVT
Extravillous trophoblast
- FC
Fold change
- FDR
False discovery rate
- IGFBP1
Insulin-like growth factor binding protein 1
- intDEC
Intermediate-decidualization
- IUP
Intrauterine pregnancy
- LOOCV
Leave-one-out cross validation
- LSE
Late-secretory endometrium
- MSE
Mid-secretory endometrium
- NK
Natural Killer cells
- nonDEC
Non-decidualized endometrium
- PAEP
Progestagen-associated endometrial protein
- pbNK
Peripheral blood Natural Killer cells
- PCOS
Polycystic ovary syndrome
- PE
Preeclampsia
- PrE
Proliferative endometrium
- PRL
Prolactin
- TrCM
Trophoblast conditioned medium
Footnotes
Author contributions: MBR directed and executed the bioinformatics, and wrote the manuscript; EPU performed the systematic literature search, directed the bioinformatics, wrote the manuscript and made the figures; AJ and WAH provided the clinical liaison for obtaining the CVS in the original study (Placenta 30:15-24, 2009) and approved of the manuscript; KPC conceived overall concept and design of the study, established collaborations with the co-authors, directed the bioinformatics, and wrote the manuscript.
Competing interests: None. Portions were published in abstract form: Reproductive Sci. 21:395A, 2014.
Contributor Information
Maria B. Rabaglino, Email: brabaglino@ufl.edu.
Kirk P. Conrad, Email: kpconrad@ufl.edu.
References
- 1.Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365. doi: 10.1155/2011/214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duley L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br Med Bull. 2003;67:161–176. doi: 10.1093/bmb/ldg005. [DOI] [PubMed] [Google Scholar]
- 3.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 7.Williams D. Long-term complications of preeclampsia. Semin Nephrol. 2011;31:111–122. doi: 10.1016/j.semnephrol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Hiby SE, Walker JJ, O'shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 10.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 11.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 13.Masoura S, Kalogiannidis IA, Gitas G, Goutsioulis A, Koiou E, Athanasiadis A, Vavatsi N. Biomarkers in pre-eclampsia: a novel approach to early detection of the disease. J Obstet Gynaecol. 2012;32:609–616. doi: 10.3109/01443615.2012.709290. [DOI] [PubMed] [Google Scholar]
- 14.Granger GPGEM, Roberts JM. In: Chesley’s Hypertensive Disorders in Pregnancy. Taylor RNCFG, Roberts JM, Lindheimer MD, editors. Amsterdam: Elsevier-Academic Press; 2014. [Google Scholar]
- 15.Founds SA, Terhorst LA, Conrad KP, Hogge WA, Jeyabalan A, Conley YP. Gene expression in first trimester preeclampsia placenta. Biol Res Nurs. 2011;13:134–139. doi: 10.1177/1099800410385448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6:28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- 17.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 18.Krieg SA, Fan X, Hong Y, Sang QX, Giaccia A, Westphal LM, Lathi RB, Krieg AJ, Nayak NR. Global alteration in gene expression profiles of deciduas from women with idiopathic recurrent pregnancy loss. Mol Hum Reprod. 2012;18:442–450. doi: 10.1093/molehr/gas017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matteo M, Serviddio G, Massenzio F, Scillitani G, Castellana L, Picca G, Sanguedolce F, Cignarelli M, Altomare E, Bufo P, Greco P, Liso A. Reduced percentage of natural killer cells associated with impaired cytokine network in the secretory endometrium of infertile women with polycystic ovary syndrome. Fertil Steril. 2010;94:2222–2227. doi: 10.1016/j.fertnstert.2010.01.049. 2227.e1-3. [DOI] [PubMed] [Google Scholar]
- 20.Kohl P, Crampin EJ, Quinn TA, Noble D. Systems biology: an approach. Clin Pharmacol Ther. 2010;88:25–33. doi: 10.1038/clpt.2010.92. [DOI] [PubMed] [Google Scholar]
- 21.Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1998;19:241–252. doi: 10.1016/s0143-4004(98)90055-8. [DOI] [PubMed] [Google Scholar]
- 22.Wallace AE, Host AJ, Whitley GS, Cartwright JE. Decidual natural killer cell interactions with trophoblasts are impaired in pregnancies at increased risk of preeclampsia. Am J Pathol. 2013;183:1853–1861. doi: 10.1016/j.ajpath.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 25.Duncan WC, Shaw JL, Burgess S, McDonald SE, Critchley HO, Horne AW. Ectopic pregnancy as a model to identify endometrial genes and signaling pathways important in decidualization and regulated by local trophoblast. PLoS One. 2011;6:e23595. doi: 10.1371/journal.pone.0023595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopcow HD, Eriksson M, Mselle TF, Damrauer SM, Wira CR, Sentman CL, Strominger JL. Human decidual NK cells from gravid uteri and NK cells from cycling endometrium are distinct NK cell subsets. Placenta. 2010;31:334–338. doi: 10.1016/j.placenta.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 29.Benirschke KPK. In: Pathology of the Human Placenta. 4th edition. Benirschke K, Kaufmann P, editors. New York: Springer-Verlag; 2000. pp. 226–228. [Google Scholar]
- 30.Haynes MK, Flanagan MT, Perussia B, Jackson LG, Smith JB. Isolation of decidual lymphocytes from chorionic villus samples: Phenotypic analysis and growth in vitro. American Journal of Reproductive Immunology. 1995;33:190–199. doi: 10.1111/j.1600-0897.1995.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 31.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 32.Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, Giudice LC. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- 33.Giudice LC. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20:235–244. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Frolova AI, O'Neill K, Moley KH. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol Endocrinol. 2011;25:1444–1455. doi: 10.1210/me.2011-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 36.Wolf M, Sandler L, Munoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: a prospective study. J Clin Endocrinol Metab. 2002;87:1563–1568. doi: 10.1210/jcem.87.4.8405. [DOI] [PubMed] [Google Scholar]
- 37.Løset M, Mundal SB, Johnson MP, Fenstad MH, Freed KA, Lian IA, Eide IP, Bjørge L, Blangero J, Moses EK. A transcriptional profile of the decidua in preeclampsia. American journal of obstetrics and gynecology. 2011;204:84. doi: 10.1016/j.ajog.2010.08.043. e1-84.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Gormley MJ, Hunkapiller NM, Kapidzic M, Stolyarov Y, Feng V, Nishida M, Drake PM, Bianco K, Wang F, McMaster MT, Fisher SJ. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J Clin Invest. 2013;123:2862–2872. doi: 10.1172/JCI66966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brosens IA, De Sutter P, Hamerlynck T, Imeraj L, Yao Z, Cloke B, Brosens JJ, Dhont M. Endometriosis is associated with a decreased risk of pre-eclampsia. Hum Reprod. 2007;22:1725–1729. doi: 10.1093/humrep/dem072. [DOI] [PubMed] [Google Scholar]
- 40.Hadfield RM, Lain SJ, Raynes-Greenow CH, Morris JM, Roberts CL. Is there an association between endometriosis and the risk of pre-eclampsia? A population based study. Hum Reprod. 2009;24:2348–2352. doi: 10.1093/humrep/dep123. [DOI] [PubMed] [Google Scholar]
- 41.Stephansson O, Kieler H, Granath F, Falconer H. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum Reprod. 2009;24:2341–2347. doi: 10.1093/humrep/dep186. [DOI] [PubMed] [Google Scholar]
- 42.Grant GR, Manduchi E, Stoeckert CJJ. Analysis and management of microarray gene expression data. Curr Protoc Mol Biol. 2007 doi: 10.1002/0471142727.mb1906s77. Chapter 19: Unit 19.6. [DOI] [PubMed] [Google Scholar]
- 43.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002;87:2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.