Abstract
The relationship between the environment and population has been of concern for centuries and climate change is making this an even more pressing area of study. In poor rural areas declining environmental conditions may elicit changes in family related behaviors. This paper explores this relationship in rural Nepal looking specifically at how plant density, species richness, and plant diversity are related to women’s fertility limitation behavior. Taking advantage of a unique data set with detailed micro-level environmental measures and individual fertility behavior I link geographically weighted measures of flora at one point in time to women’s later contraceptive use as a way to examine this complex relationship. I find a significant, positive relationship between plant density, species richness, and plant diversity and the timing of contraceptive use. Women in poor environmental conditions are less likely to terminate childbearing, or do so later, and therefore more likely to have larger families.
Sociologists have been concerned with the relationship between the environment and population processes for centuries and climate change is leading to increased interest and call for scholarship in this area (Dunlap 2010; Molnar 2010). The bulk of social science research in this area has focused on the effects of population processes on the environment, on the health effects of the environment on people, or on the effects of land use, as opposed to environmental health or quality, on population processes (e.g. Carr, Lopez, and Bilsborrow 2009; Clay and Johnson 1992; Cole and Neumayer 2004; Ghimire and Helter 2007; de Sherbinin et al. 2007; Pebley 1998; Thompson and Jones 1999; Yabiku 2006; Yu and Liu 2007). Some recent research has addressed the complex and reciprocal nature of this relationship (Bhattacharya and Innes 2008; Hummell et al 2012; Liu, Dietz, Carpenter, Alberti et al. 2007; Liu, Dietz, Carpenter, Folke et al. 2007), but even this work has rarely explicitly looked at the effects of environmental health or quality on family formation (for notable exceptions see Aggarwal, Netanyahu, and Romano 2001, Biddlecom, Axinn, and Barber 2005, and de Sherbinin et al. 2008). This paper addresses this gap in the literature by examining the relationship between micro-level measures of environmental quality (operationalized as plant density, species richness, and plant diversity) and individuals’ fertility limitation.
The environment-fertility connection is particularly salient to the many poor, rural communities where livelihoods are intricately connected to the natural environment and population pressures have been a growing concern (Dunlap 2010; Molnar 2010; York, Rosa, and Dietz 2003). In these places the natural environment is one crucial component of the social context that many sociological theories predict influence family behaviors (e.g. Bulatao and Lee 1983; Thornton and Lin 1994)—“the land ethic simply enlarges the boundaries of the community to include soils, waters, plants, and animals, or collectively: the land (Leopold 1966: 239).” At the same time, many of these locations are also important environmental zones because they contain large forests and/or important watersheds.
I explore this relationship in an ecological hotspot, Nepal (Chaudhary 1998, 2000; Myers 1988). Given its unique and sensitive environmental situation and the dramatic change in population processes that has occurred in recent years, Nepal is an ideal location for this investigation. I am able to use unique, micro-level measures of both the environment and individual behavior to provide evidence of how environmental conditions may influence that behavior. These findings are important both because of what they tell us about population processes, and, because of their reciprocal relationship, also about the future of the environment.
This paper makes four contributions to the literature. First, it presents a theoretical framework for understanding how environmental quality is related to individual level behaviors. This is an extension of existing frameworks both because it focuses specifically on fertility behavior as opposed to migration, health, or land use patterns and because it extends the discussion of mechanisms. The second major contribution of this paper is that it is a micro-level analysis of fertility behavior. Detailed, micro-level measures of the environment are not common and the ability to link them directly to individual behavior even less so. Much of the work that does look at individual behavior uses perceptions of the environment or focuses on migration behaviors (Carr 2005; Ghimire and Mohai 2005; MacDonald 1999; VanWey, Guedes, and D’Antona 2011). This paper links highly detailed, micro-level measures of both the environment and fertility. Third, the specific measures of environmental quality I use are unique. They are geographically weighted measures that incorporate environmental data from a wide area and are tied to each individual based on their geographic location. The unique measurement better captures the true or lived environment that people actually experience. Finally, this paper examines this important relationship between environmental quality and individual fertility behavior in an area of crucial importance to the global environment.
Linking the environment to fertility
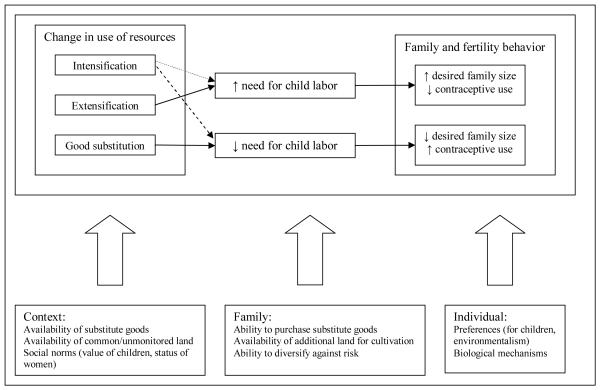
This section describes a theoretical framework for understanding how environmental quality is related to fertility behavior in poor, mainly agrarian settings. Figure 1 presents a diagram of this framework. A vast body of sociological research has provided evidence that community, or context, matters for family and fertility behaviors (e.g. Axinn and Yabiku 2001; South and Baumer 2000; Brewster 1994a, 1994b; Brewster, Billy, and Grady 1993). Most of that work has focused on the institutions in a certain place, for example, job availability or access to health services. Some research has considered how the physical environment or land use patterns are related to fertility. The natural environment is one more dimension of social context likely to influence fertility (Leopold 1966).
Figure 1.
Heuristic representation of theoretical framework for the effect of poor environmental quality on family and fertility behaviors. Pathways are not mutually exclusive and diversification involves simultaneous pathways operating. Dashed lines signify conflicting theoretical possibilities.
The multi-phasic response model can help identify some of the mechanisms through which poor environmental conditions may influence fertility behaviors (Billsborrow and Okoth-Ogendo 1992; Davis 1993). Multiple strategies exist for families to cope with poor environmental conditions and families may use more than one at any given time (Billsborrow and Okoth-Ogendo 1992; Davis 1993). Poor environmental conditions, conditions that may lead to low agricultural productivity or shortages in natural resources like fodder and fuel wood, may be related to contraceptive use because limiting family size is part of a family’s approach to coping with the environmental limitations. The potential set of responses available can reveal the specific theoretical mechanisms through which the environment influences fertility behavior. Which response(s) is(are) chosen depends at least partly on other contextual, family, and individual characteristics (bottom row of Figure 1).
Consider, first, mechanisms or responses that operate through direct consumption of natural resources. In poor environmental situations families can use resources differently (upper left box in Figure 1). They may use their existing resources more intensely than those in better environments (intensification) (Axinn, Barber, and Biddlecom 2010; Boserup 1965). Related to intensification is good substitution (Axinn et al. 2010; Simon 1990). When natural resources are not sufficient families may purchase consumer goods as a substitute (e.g. purchasing gasoline for fuel instead of collecting firewood).
Theoretically, the direction of the effect of intensification and substitution on fertility is unclear (represented by dashed lines in Figure 1). If families are using existing, non-labor, resources more intensely or substituting consumer goods for materials others could gather from their surroundings, fertility behaviors may be the same because the family would still be able to support that sized family. Or, if the family is farming their existing land more intensely by using more labor than families in better quality environments are using, they may have more children as a way to supply that labor. Of course, this option is only available to a certain point after which additional labor will not increase agricultural yield. On the other hand, if children are generally desired for their labor contributions, families who are buying consumer goods may have a lower demand for children and their labor and therefore be more likely to terminate fertility than families who can still rely on the natural environment. For example, children are often tasked with collecting firewood (Cain 1981, 1983). If the family chooses to purchase fuel instead of gathering firewood children have less of a use or benefit to the family. Empirical research supports the latter of these possible fertility behavior responses demonstrating that couples in areas where good substitution is likely (i.e. places with a more market oriented mode of production) have lower fertility and higher contraceptive use (Axinn and Yabiku 2001; Thornton and Fricke 1987; Thornton and Lin 1994).
Alternatively, families in areas with poor environmental quality may collect and use natural resources from a wider area (extensification) (Entwisle et al. 2005). This is typically done by cultivating additional land or harvesting from land that is farther away. Extensification is particularly common in areas where natural resources are gathered from common lands. If families elect it as a way of coping with poor environmental conditions we would expect fertility to be higher and fertility limiting behaviors to be less common because of the greater demand for child labor, compared to families who can rely on a smaller land area for their environmental needs. A study in Nepal showed that households facing a scarcity of environmental goods spent more time collecting those goods (Cooke 1998). This greater time could be related to higher fertility because families do not want to spread their existing labor resources more thinly.
Whether and how families use the environment are complicated questions and depend on factors at the contextual, family, and individual level. On the contextual level one should consider the type of land in question and what other community resources are present. Intensification may be an appropriate option when the poor environment in question is a family’s agricultural land and they can add fertilizers or otherwise alter their farming practices (in non-labor dependent ways). However, intensification and the substitution of consumer products for natural resources requires access to those consumer products (e.g. access to markets) and, at the family level, economic resources (i.e. the money to purchase chemical fertilizers), making this option untenable for most rural, poor, subsistence farmers.
Another macro level factor to consider is social norms, particularly those regarding the value of children. When children have low social status and are valued for their household labor contributions, extensification may be an appropriate strategy to cope with poor environmental conditions. In areas where resources are more scarce families need to invest more time in obtaining them (e.g. walking farther to collect fodder for animals and fuel wood). Because children are often the ones assigned to these household tasks the value of their labor is greater and families respond to their poor environmental situation with larger families and less contraceptive use to limit childbearing, as opposed to families in better quality areas (Cain 1981, 1983). This is referred to as the “vicious circle” argument because environmental conditions will continue to worsen in the face of this increased fertility (Aggarwal et al. 2001; Filmer and Pritchett 2002; O’Neill, MacKellar, and Lutz 2001).
However, extensification is only an option if there is additional land to be exploited or land is only loosely monitored or controlled so those resources that exist can be obtained in greater amounts. When families obtain natural resources for consumption from open access lands—that is, places where families do not absorb the full costs of producing the resources—then they will have less incentive to cope with a poor environment by land intensification and/or contraceptive use (Filmer and Pritchett 2002). In this case, they may choose extensification, larger families, and consequently less contraceptive use to limit childbearing.
When thinking about all of these factors in Nepal, extensification is particularly likely. Most people are subsistence farmers with little or no disposable income available to purchase substitutes for natural resources and children are typically engaged in the labor intensive household work such as collecting natural resources for firewood, water, and fodder.
Direct consumption of natural resources is not the only way poor environmental conditions may lead to different fertility and contraceptive use behaviors. As you can see in Figure 1 context, family, and individual characteristics may influence fertility behavior through many points in the process, not just via the box concerning resource use. If families are unable or uninterested in changing their use of natural resources poor environmental conditions may lead directly to contraceptive use. This is because families are generally less able to support many children when environmental conditions are poor so they may choose to stop having children.
There are several other mechanisms through which the natural environment may influence fertility related behaviors. First, biological mechanisms. Living in a poor quality environment is related to poorer health outcomes—certain diseases may be more prevalent and overall health may be lower, both leading to increased child mortality. Fertility tends to be high, and contraceptive use to limit fertility low, in the face of high child mortality (Davis 1945; Demeny 1968). Also, a healthy environment may be related to generally healthier people, and overall we see a connection between better health and lower fertility and greater fertility limitation. On the other hand, on an individual level, if you are sick you may be less able to raise your family, so a poorer environment would be related to lower family size and increased fertility limitation. These biological mechanisms may be at work whether the poor environmental conditions are the result of increased toxins from industrial pollutants, droughts or other natural disasters, excessive pesticide and chemical fertilizer use, or simple overuse.
Diversification against risk is a second potential mechanism linking poor environmental conditions to larger families and less contraceptive use (Cain 1981, 1983; Poffenberger 1980). Research in South Asia and South Africa indicates that in periods of high agricultural or economic risk, parents may choose to have more children because they lack other low-risk investment alternatives (Aggarwal et al. 2001; Cain 1981, 1983). Similarly, we may expect that families who are facing poor environmental conditions will desire larger families, and therefore be less likely to use contraception than families in better environments.
There may also be psychological mechanisms at work. Previous research has found that those who perceive that their environment has deteriorated are more likely to use contraception to limit family size than those who think their environment is doing well or has not changed (Ghimire and Mohai 2005). Other research has documented similar relationships between perceived neighborhood conditions and academic aspirations (Henry et al. 2008). This may be because those living in poor quality communities have less optimistic views of the future and consequently a lower desire to have children and higher desire to use contraception to limit childbearing (Dupere, Leventhal, and Vitaro 2012; Plunkett et al. 2007).
Finally, there may be an explicit environmentalism mechanism. The growing concern over environmental degradation has lead to explicit public policy discussions and public service campaigns, some of which directly or indirectly address family related issues. For example, a campaign may discuss how human activity, such as over harvesting for fuel wood, is diminishing the quality of the natural environment. People may then choose to limit their family size in an explicit attempt to minimize the negative consequences of their actions.
No discussion of the link between the environment and fertility would be complete without mentioning Malthus (1798). This perspective stresses the limits placed on human population growth by the finite supply of natural resources. It predicts that as human populations grow they will increase their use of, and therefore impact on, the environment for food and other resources. As the environment is depleted and pushed to its limits, hunger, disease, and poverty will result, causing populations to decrease until they are again in balance with the environment. That is, there is a positive relationship between environmental quality and fertility—as environmental quality decreases we expect fertility to decrease. Malthus’ theory does not incorporate conscious control of family size to avoid depleting environmental resources and therefore does not explicitly address how individuals may choose to limit childbearing. However, we can extend this logic and say that if we allow for the conscious control of fertility we would expect contraceptive use to be higher in areas with lower environmental quality.
This framework leaves us with multiple, at times competing, hypothesis. We expect to find that poor environmental quality is associated with efforts to limit fertility when good substitution, health issues that make raising a family challenging, strong non-family social support networks, or substantial environmental concern are present. On the other hand, we expect that poor environmental quality will be associated with little fertility limitation when extensification is a more tenable option (e.g. low status of children and additional land is available), there is high infant and child mortality due to the poor environment, and/or when the family is the most substantial form of social support. Given the many different scenarios or contingent characteristics that operate simultaneously at multiple levels, which specific mechanisms are at work is an empirical question that depends on the specific context in question and ultimately requires incredibly detailed data at the contextual, family, and individual data. The research presented here is not set up to identify which specific mechanism and processes may be at work. Rather, it is a first step in under standing the overall relationship between environmental quality and fertility behavior and looks at only the direction of this relationship. Of course, this can help narrow down the list of potential mechanisms, but it cannot clearly identify any one over another.
Other influences on fertility
As described, contextual factors other than the natural environment may influence fertility behavior. Land use and infrastructure are of particular concern in this area because they may influence both the quality of the environment and behavior (i.e. cause a spurious relationship). Places with more markets or vehicle traffic will likely have lower quality natural environments and lower fertility (Axinn and Ghimire 2011; Axinn and Yabiku 2001; Ghimire and Axinn 2010). Health services are another important community characteristic since those with more access to contraceptive methods are more likely to use them (Brauner-Otto et al. 2007; Entwisle et al. 1996). Which, if any, fertility behavior responses individuals and families have to poor environmental conditions likely depends on these other contextual resources.
Measuring the environment
There are at least two important questions to answer before analyzing the relationship between environmental quality and fertility behavior: 1) what aspect of the environment to measure, and 2) at what level do we conduct the analyses? Regarding the former, the research presented here focuses on plant life. The quality of plant life is an important dimension of the environment in Nepal both because individuals and families regularly harvest and use non-agricultural plants for fuel and fodder, and because plants are an important component of this once jungle covered landscape. Areas with healthy plant life are better able to support animal life and to withstand some human use.
The appropriate measure of environmental quality, even when considering one feature like flora, likely varies by setting and land use type. This varies between rural and urban communities, but also within each type of community. What is a healthy environment in a high mountain region may not be the same in a wetland. Assessing the quality of vast range land certainly requires different measurement than for a coast line. For example, when thinking specifically of plant life, high quality grazing land will generally contain many grass-type plants whereas high quality fuel providing areas may have fewer actual plants, but they will generally be larger organisms. In both cases, plant diversity is likely an important component—both because the different plants have different uses and because plant diversity can be a sign of overall stability of the environment (Bukovinszky et al. 2008; Loreau 2010; Thibaut and Connolly 2013).
Regarding the level of analysis, the research presented here is conducted at the microlevel—a rarity in empirical population and environment research. To date, much of the research on the interconnections between family or fertility and the environment has been conducted at the macro-level (global, national, regional, county) (e.g. Hunter 1998; Jiggins 1994; National Research Council 1993). Although that research is useful for describing overall trends and macro correlations, it does not tell the complete story (Jiggins 1994). Furthermore, it is incorrect to assume that a relationship found at one level of analysis will hold true at another level. Individuals and their families make decisions about their families, including how many children to have and when to stop childbearing (Axinn 1992; Becker 1981; Caldwell 1982). These are the decisions that add people to a household, a village, a district, and a region. Individuals and their families also make decisions about household-level productive organization and a host of related behaviors, including how to consume natural resources and the nature of agricultural production. These decisions about the physical environment and natural resources, such as where to graze animals and gather fodder, may depend on the quality of those resources and, as described above, can influence fertility behavior (Ives and Messerli 1989). As such, to fully understand the relationship between the environment and fertility we need to conduct analyses at the individual level.
Although it is clear investigating this relationship at the micro level is important, how to measure the environment is less clear. To make connections to individual behavior we are forced to sample specific areas and aspects of the environment but it is unlikely that people are influenced by only one sampled area. People are probably most influenced by the environment that is closest to them or that they use most often (Boardman et al. 2008; Downey 2006), but it is difficult, if not impossible, to determine how far away something must be in order to no longer influence individual behavior. This is even more complicated because of the physical connections between environmental areas that are far apart (i.e. toxins released into a river upstream will influence flora downstream) (Harrison 2011). Consequently, we must create measures that incorporate a wide area, but at the same time account for the fact that the environment closest to where a person lives and works has the strongest influence on individual behavior. Research on the effect of other contextual factors such as the availability of community resources has found that measures that incorporate as much data as possible while weighting them based on their geographic location best capture the contextual effects (Brauner-Otto 2012; Brauner-Otto, Axinn, and Ghimire 2007; Downey 2006). This means that we need to consider the geographic distribution of the people relative to the measures of the environment and the specific land type being measured when operationalizing concepts.
Setting
The above discussion does provide some Nepal specific information, but a more thorough discussion of the specific setting is warranted. Chitwan, Nepal is an ideal setting to study the relationship between environmental quality and fertility limitation for both environmental and demographic reasons. The Himalayas is one of the world’s most diverse ecological regions, hosting an amazing variety of flora and fauna and ranking 31st in the world in terms of biodiversity (Chaudhary 1998, 2000; Myers 1988; Zurick and Karan 1999). The Himalayas are also an important ecological zone within the South Asian region. They form the main watershed for the Ganges and Indus river basins, and land slides, soil erosion, and other environmental problems within the Himalayas have an important impact on the millions of people living in Northern India, Bangladesh, and Pakistan.
Chitwan is located in the Terai region of Nepal in the central foothills of the Himalayas and at the confluence of one of the three main drainage systems in the central Himalayas. In addition, Chitwan is surrounded on three sides by forest land, including the Royal Chitwan National Park (a wildlife reserve and UNESCO World Heritage property), and is within the top 25 global biodiversity hotspots (Myers et al. 2000). Plant diversity is a crucial component of the environment in this area but is currently threatened (Lehmkuhl 1994; Peet et al. 1999). For example, M. micrantha, a plant which is not usable or edible by native animals and people, has been identified in the area, crowding out native plants, making the area less diverse and less stable (Paudyal 2007; Poudel et al. 2005; Ellison et al. 2007).
Until the 1950s, Chitwan was covered with virgin jungle and thinly inhabited by indigenous ethnic groups. In the 1950s, the government began clearing parts of the jungle, implemented malaria eradication efforts, and instituted a resettlement plan leading to the in-migration of many different ethnic groups. Much of Chitwan today is fertile agricultural land. By the late 1970s, roughly two-thirds of this valley was cultivated and the first all-weather road was completed, linking Narayanghat (the main town in the study area) to India and eastern Nepalese cities. Subsequently, other major highways were constructed making Narayanghat the transportation hub for the entire country. This led to the rapid expansion of non-family organizations such as health services, wage labor, and markets. These organizations are not distributed evenly across the study area and it may be that families who have better access to markets and wage labor opportunities are better able to substitute consumer durables for family labor or use some other, non-fertility, market based strategy to cope with poor environmental conditions.
Even with these changes daily life is still heavily connected to the natural environment. Subsistence agriculture dominates and even shop owners and others employed in non-farm ventures typically have tight connections to the environment through livestock or water use. Women and children are tasked with gathering fuel and fodder.
As these structural and community level changes were happening, individual fertility behaviors were also changing. The Total Fertility Rate dropped from over 6 in the 1960s to approximately 4.6 by 2001 (Ghimire 2003; Suwal 2001; Tuladhar 1989). First births rapidly follow marriage, the average first birth interval is 18 months (Ghimire 2003), and contraception to limit fertility was virtually non-existent until the very recent past (Banister & Thapa 1981; Tuladhar 1989). The decrease in fertility that has occurred is largely attributed to increasing contraceptive use, particularly contraceptive use to stop childbearing, as opposed to delaying the first birth or spacing births as is commonly done in Western countries. Analyses of the same data used in this paper (described below) reveal that almost all women who had used a long-term contraceptive method said they wanted no more children and over 80 percent of women who first used a contraceptive method between 1996 and 2007 eventually used a longer-term method designed for terminating childbearing (Axinn and Yabiku 2001; Brauner-Otto 2013). Therefore, in this setting, the commonly used phrase in demography “later means fewer” holds true—contraceptive use corresponds with smaller families (Axinn and Yabiku 2001; Bongaarts and Feeney 1998).
Contraceptive methods are widely available throughout the study area. Health service providers, which include everything from full-service health clinics to pharmacies, all provide contraception for free or at very low cost. Information on contraceptive methods and family planning motivation literature is similarly widely available at health services and other public settings.
DATA AND METHODS
I use the Chitwan Valley Family Study (CVFS) to examine the relationship between plant density, species richness, and plant diversity and women’s fertility limitation in Chitwan, Nepal as an example of the effect of environmental quality on population processes. In 1996, the CVFS collected information from residents of a systematic sample of 151 neighborhoods in Western Chitwan Valley. Every resident between the ages of 15 and 59 in the sampled neighborhoods and their spouses were interviewed, at which point surveys and Life History Calendars were collected. Starting in February 1997, the CVFS began collecting monthly data on contraceptive use for all the individuals in the selected households. Ninety-five percent of respondents have been interviewed, yielding roughly 4,154 individuals aged 13-80 with both 1996 interviews and contraceptive method records.1 Information for these individuals has been collected on a monthly basis for 126 months.
Although the theoretical discussion above centered on families and households I follow the vast majority of research on fertility by focusing on women as the unit of analysis. Ultimately, individuals execute their behavior, even if the decisions are made by a larger unit such as the couple or the extended family. The empirical evidence from a small and growing body of research conceptualizing childbearing behavior at the couple level demonstrates that both husband’s and wives’ characteristics have separate, independent effects on the couples’ fertility and contraceptive use (Thomson 1997, Axinn and Barber 2001). Because wives characteristics maintain separate and independent effects, I follow the majority of the literature on childbearing transitions and focus on women only.2
I analyze data gathered from 930 women in the CVFS who were between the ages of 15 and 45 in 1996, had not previously used contraception by the time of the 1996 interview, and married and had given birth at some point in the data collection period.3 I restrict the sample to these younger, non-sterilized women because analysis of childbearing behavior is not relevant for women beyond childbearing age or who have been surgically sterilized. I also limit the sample to married women who have already had a child because, in this setting, premarital sex is extremely rare and given the strong preference for children it is unlikely that anyone would want to have no children and therefore terminate their childbearing before having any children.4
The CVFS also collected detailed accounts of neighborhood resources such as health services and markets. In this rural setting, a neighborhood defines a cluster of approximately 5 to 15 households—a group of individuals who have face-to-face contact daily.
Also in 1996, flora samples were collected and analyzed from multiple land plots in the study site. Plots were selected from a variety of types of area (forest and common land), under widely different management policies (guarded by military, under local user group management), with varying usefulness to the community members (tourist location not available for use, common location to gather fuel and fodder). Of those, 62 were collected in the Barandabhar Forest which runs along one of the three sides of the triangular shaped study area.5 These plots are arranged at 250 meter intervals along equally spaced (1km) transects that extend 1250 meters into the forest. Each plot consists of a series of overlapping sample areas. A tree plot measures 10 by 10 meters and defines the perimeter. One shrub plot is located at the center of each forest plot and measures 3 by 3 meters. Five grass plots, each measuring 1 by 1 meter, are selected based on a W-shaped profile. Flora teams counted the number of different tree, shrub, and grass species. See Dangol and Maharjan 2012 for a detailed discussion of the data collection.
The Barandabhar Forest has been protected as Buffer Zone forest since 1993. The buffer zone program started an awareness program on forest and biodiversity conservation and allowed the local community to use the forest resources up to three hundred meters from the boundary of the settlements in the Barandabhar Forest. There are four forest user’s groups that lie in the outer region of the forest in our study area. One group started their conservation works in 1996, the other three groups began later. The forest was handed over to the user groups after 2001 by the Office of Royal Chitwan National Park. As a result, the handful of neighborhoods bordering the forest had somewhat regulated use of the forest for some of the period covered in the data analyses. However, overall in Nepal user groups have not attempted to severely restrict use of the forest and generally residents are able to obtain as many resources as desired (Jones 2007; Straede and Treue 2006). Residents of other neighborhoods are likely gathering their resources from other areas that are similar to the Barandabhar Forest.
Measures
Fertility behavior: Contraceptive use
To measure fertility behavior I focus on women’s contraceptive use. I treat contraceptive use as a transition from not currently using contraceptives to using contraceptives and consider any method (e.g. own sterilization, spouse’s sterilization, Depo-Provera, IUDs, Norplant, oral contraceptive pills, condoms). When contraceptive methods first began to be widely used, Nepalese, like many other South Asian populations, generally used longer-term methods such as sterilization, Depo-Provera, IUDs, or Norplant and used these methods to stop childbearing rather than to space births (Axinn 1992; Axinn and Yabiku 2001). Although more temporary methods have become more common the vast majority of women who use temporary methods such as pills and condoms eventually go on to use a permanent method and discontinuation is low even for more temporary methods (Brauner-Otto 2013). As a result, I focus on use of any contraceptive method as the dependent variable. I create a time-varying, dichotomous variable equal to 1 the month the respondent first uses any contraceptive method and 0 in months prior. Just over 60 percent of women in this sample used contraception at some point between their first birth and 2006. Months when women are pregnant or have given birth are dropped from the analyses.
Environmental quality
There are many ways to operationalize environmental quality. As the above discussion reveals one should consider the specific land use and location of the area being measured. For this study I use plant density, species richness, and plant diversity to measure environmental quality. This is just one way of assessing the quality of the overall natural environmental conditions residents of Chitwan experience. The specific plots analyzed here are all located in forest areas making the measures of quality applicable across all plots. Because they are forest plots, measures of plant density, species richness, and plant diversity should all reveal key aspects of the environmental health.6 Density, the number of plants in a given area, may be an appropriate measure partly because it is something that is easily observable by people living in the area—a place that has many plants may appear to be of better quality to those individuals who see that area. When someone is looking for an area to harvest fuel wood or fodder from a forest area with many more trees growing it may seem like a better option than one with sparse plant life. But, there are aspects of the quality of plant life that may not appear to the naked eye of the non-scientist, and plant species richness can reveal that. For instance, forest areas that are being taken over by an invasive species may appear green and healthy, but are not. Furthermore, areas with more diversity are generally found to be more stable (Bukovinszky et al. 2008; Loreau 2010; Thibaut and Connolly 2013). I create three measures to capture plant density, species richness, and plant diversity.
The first measure is of density and is a simple count of the total number of plants present in 1sq meter. This measure was used in previous research using these data and was not related to family size preferences or childbearing (Biddlecom, Axinn, and Barber 2005). However, that study included a huge array of plot types (i.e. common grazing land and grasslands along with forests) and did not account for the specific geographic distribution of people and measurement plots. As such, I believe it is important to re-examine it here.
The second measure I created is a simple measure of species richness—the total number of species present. This measure does not account for the prevalence of species so if a species appears once in the area it is counted the same as a species with 200 plants.
These simple measures are just that, simple, and do not fully capture the quality of a specific area (Grunewald and Schubert 2007). An additional measure to look at is the Shannon-Weiner Diversity Index, also called the Shannon Diversity Index, which incorporates both plant density and species richness. This index is commonly used in ecological research to characterize species diversity and to measure the biodiversity in a community or specific area (c.f. Chiarucci et al. 1999; Mouillot et al. 2005; Patil and Taillie 1982; Sharma and Rawat 2008). It accounts for both density (abundance) and species richness (evenness) present in an area (Shannon and Wiener 1962). The specific formula for calculating the index is:
where pi, the relative abundance of each species, is ai/A; ai is the number of individuals of each species in the plot; A is the total number of individuals; and s is the number of species.
Environmental quality: geographically weighted
Creating micro-level measures of the environment poses some challenges because individuals do not experience the environment as a contained thing the way they experience a school or health clinic. When creating measures of built aspects of the environment it is fairly easy to pinpoint a specific location for that piece of the environment. The same cannot be said of the natural environment—it exists all around. For example, the quality of a flora plot at the edge of an individual’s neighborhood will have an obvious impact on their household behaviors—e.g. if there is little density they will have to travel farther to gather fuel. However, a flora plot on the far side of the study area from where an individual lives may also influence their behavior. Perhaps that location was important because of the specific type of flora present there. Or, the quality of that area may be a sign of the environmental quality for a larger area. To understand more about the spatial dimension of environmental effects, I create measures that incorporate information on all the flora plots capturing the wide spatial distribution of certain environmental characteristics. For all three measures of environmental quality I create geographically weighted measures which can be represented as:
where is the geo-weighted average of environmental quality for characteristic c (e.g., plant density) and neighborhood n. Fcf is the characteristic c in flora plot f, and Wfn is the weight for flora plot f and neighborhood n. Because previous research and the theoretical framework employed here predict that aspects of social context farther away will have less influence than those closer to the individual, I define Wfn as the log of the distance between flora plot f and the center of the neighborhood n. Distance was calculated “as the crow flies” using latitude and longitude coordinates for the neighborhood and the flora plot and is measured in meters. Because households in neighborhoods are clustered closely together the geographic location of the center of the neighborhood very closely approximates the geographic location for all households. The weighting ensures the closest plot is most influential with the influence for the other plots decreasing as distance increases. The summation is over 62 plots because that is the total number of plots sampled. Previous research has found that this geo-weighted approach best captures community context in this setting (Brauner-Otto 2012; Brauner-Otto et al. 2007).
Table 1 presents descriptive statistics for the environmental quality measures. Panel A shows the results at the level of the flora plot—these are the raw measures and are not geo-weighted. The mean number of plants in a 1 sq. meter plot was 161—although as many as 721 were found. As we would expect, the number of species present (the richness of each plot) was much less with the mean being fewer than 20 and the maximum being 30 species. Turning to the diversity index we see that at best these flora plots are only moderately diverse. To illustrate, if a plot with 30 species had a completely balanced ecosystem, the value of the Shannon-Weiner Diversity Index would be around 9. For these plots the maximum value for the Shannon-Weiner Diversity Index is 2.74 revealing there is room for improving the environment and increasing plant diversity in this area of Nepal. These findings are in line with previous ecological research in this region, implying that the Barandahbar Forest is similar to other surrounding forests (Lehmkuhl 1994; Peet et al. 1999). Although not shown, note that plots that are high in density were not necessarily high in richness or in diversity.
Table 1.
Descriptive Statistics, Environmental Quality Measures: Plant Density, Richness, and Diversity, Chitwan, Nepal.
| MEAN | STD | MIN | MAX | |
|---|---|---|---|---|
| Panel A. Flora Plot Level (N=62) | ||||
|
| ||||
| Simple density (num of plants per square meter) | 160.64 | 149.57 | 34.91 | 721.21 |
| Simple richness (num of species) | 18.95 | 5.12 | 7.00 | 30.00 |
| Shannon-Weiner Diversity Index | 2.00 | 0.35 | 1.09 | 2.74 |
|
| ||||
| Panel B. Individual Level, Geoweighted (N=930) | ||||
|
| ||||
| Geographically weighted simple density | 1072.79 | 57.60 | 982.77 | 1289.75 |
| Geographically weighted simple richness | 126.07 | 7.18 | 115.48 | 149.83 |
| Geographically weighted Shannon-Weiner Diversity Index |
13.31 | 0.76 | 12.19 | 15.80 |
Panel B presents the descriptive statistics for the geo-weighted measures at the individual level. The important thing to note here, and remember when interpreting the hazard models, is that the metric for the geo-weighted measures is very different from that of the simple flora plot measures seen in Panel A. The range naturally increases tremendously since I summed across all flora plots to create these measures.
Other measures of location
Because the natural environment is not the only aspect of one’s surroundings that may be related to fertility behavior, the quality of the natural environment is likely influenced by many other aspects of the community, and how people respond to given environmental situations may depend on other characteristics of their context it is important to control for these other features.
Because the flora plots are clustered along one side of the study area, relatively closer to the main town of Narayangat, we may be concerned that the environmental quality measures are merely reflecting proximity to the main town. I include a measure of the distance in meters between each respondent’s neighborhood and Narayangat.
Table 2 presents descriptive statistics for these and the other control measures used in the analyses. The distributions refer to the respondent’s last person-month contributed to the analysis. For women who used contraception this is the first month that they used the method; for women who did not use contraception this is the last month of data collection. The length of the study area is about 27 kilometers and, as you can see from the mean and standard deviation, people live throughout the entire region.
Table 2.
Descriptive Statistics, Measures of Location and Control Variables. Chitwan, Nepal.
| MEAN | STD | MIN | MAX | |
|---|---|---|---|---|
| Measures of location in study area | ||||
| Distance to Narayangat (meters) | 14387 | 6764 | 32 | 28485 |
| Index of community organizations within 5 min walk in 1995 | 1.32 | 1.27 | 0 | 4 |
| Controls | ||||
| Index of community organizations within an hours walk in childhood |
2.89 | 1.33 | 0 | 4 |
| Index of individual non-family experiences | 1.44 | 0.61 | 0 | 3 |
| Number of children born by 1996 | 1.68 | 2.12 | 0 | 10 |
| Parental characteristics | ||||
| Father ever worked for pay | 0.42 | 0 | 1 | |
| Number of children mother had | 5.81 | 2.44 | 1 | 19 |
| Parents every used contraception | 0.37 | 0 | 1 | |
| Ethnicity | ||||
| Upper caste Hindu (reference group) | 0.44 | 0 | 1 | |
| Lower caste Hindu | 0.09 | 0 | 1 | |
| Newar | 0.07 | 0 | 1 | |
| Hill Tibeto Burmese | 0.14 | 0 | 1 | |
| Terai Tibeto Burmese | 0.26 | 0 | 1 | |
| Birth cohort | ||||
| Born 1951-1960 (aged 36-45 at birthday in 1996) | 0.13 | 0 | 1 | |
| Born 1961-1970 (aged 26-35 at birthday in 1996) | 0.21 | 0 | 1 | |
| Born 1971-1975 (aged 21-25 at birthday in 1996) | 0.21 | 0 | 1 | |
| Born 1976-1981(aged 15-20 at birthday in 1996) (ref. group) | 0.45 | 0 | 1 |
N=930 women
Non-family organizations—industry, buildings, community development, the level of economic development, modernization—are related both to lower environmental quality (e.g. Kramer, Urquhart, and Schmitt 2009) and to increased contraceptive use (e.g. Axinn and Yabiku 2001; Brauner-Otto et al. 2007). Consequently, I include a measure for the presence of markets, employers, bus stops, and health services in the individuals’ neighborhood. Following previous research, I create an index of the number of these organizations within a five minute walk of the respondent’s neighborhood in 1995 (Axinn and Yabiku 2001). I created four dichotomous variables equal to 1 if that organization was available within a five minute walk and then summed them to create the index. I use information from the year 1995 because that is before both the flora and contraceptive use are measured. We see that these community organizations are still somewhat rare in this setting. The mean number of organizations available in a 5 minute walk was just over 1.
Controls
It may also be that characteristics of the individual or experiences she had influence her choice of neighborhood (and therefore the quality of the natural environment she is exposed to).
One’s childhood community may influence both your later life community and your family related behaviors so I also create an index measure of the respondent’s childhood community. I create four dichotomous variables equal to 1 if the respondent had a employer, market, bus stop, or health service within an hour’s walk of her neighborhood before she was 12 years old and 0 otherwise and then summed those variables to create the index (Axinn and Yabiku 2001; Brauner-Otto et al. 2007). Most respondents had at least 2 of these organizations within an hour’s walk.
Substantial bodies of literature provide evidence that education, work, living experiences, and receipt of health services all influence childbearing behaviors (e.g. Axinn and Yabiku 2001; Axinn and Barber 2001; Lloyd et al. 2000). I create a series of three dichotomous variables equal to 1 if the respondent had worked for pay outside the home, lived away from her family, or visited a health service by 1995 and 0 otherwise and then summed them to create an index of experiences.
Previous research has found that parental characteristics are important predictors of family related behaviors (Axinn and Thornton 1992; Barber 2001; Thornton and Camburn 1987). Consequently, I use dichotomous measures to control for father’s employment (ever had non-family employment before respondent’s age 12) and whether parents ever used a contraceptive. I also include a count measure of the respondent’s mother’s number of children ever born. Roughly 40 percent of women had fathers who had worked for pay outside the home. The mean number of siblings women had was just over 5 and thirty-seven percent of women’s parents had used contraception themselves, suggesting a setting of high fertility and moderate contraceptive use for the respondent’s parents’ generation.
Additionally, because ethnicity in Nepal is complex and likely related to an individual’s behaviors (Acharya and Bennet 1981; Bista 1972; Fricke 1994; Gurung 1980), I use dichotomous variables to control for five classifications of ethnicity: upper caste Hindu, low caste Hindu, Newar, hill Tibeto-Burmese, and terai Tibeto-Burmese. Upper caste Hindus are the most represented ethnic group in our sample, as they are in Nepal as a whole, and will be the reference group in the hazard model analysis below. Terai-Tibeto Burmese, the ethnic group which includes those indigenous to this area, represent a quarter of the sample. Hill Tibeto-Burmese, lower caste Hindus, and Newari women are less commonly found in this area.
Because the prevalence of contraceptive use has increased over time I also control for birth cohort. I create dichotomous variables for four birth cohorts: 1951-1960 (age 36-45 at birthday 1996), 1961-1970 (age 26–35 at birthday in 1996), 1971-1975 (age 21–25 at birthday in 1996), and after 1975 (age 20 or under at birthday in 1996). The youngest birth cohort is the reference group for all the analyses.7 The birth cohort distribution is not surprising given the sample restrictions. Only 13 percent of the women were over age 35 and the largest percent of the sample was less than 20 years old in 1996. Because we have 10 years of monthly contraceptive use data, women who were only 15 years old in 1996 would have been 25 at the end of the data collection period. Since mean age at marriage is around 20, with childbearing typically occurring shortly thereafter, this is plenty of time to observe the transition to using contraception (Ghimire and Axinn 2010).
Analytic Strategy
The breadth of the CVFS allows me to estimate complex models of the relationship between plant density, species richness, and plant diversity and contraceptive use. I treat contraceptive use as a transition occurring over time and use discrete-time event history techniques to estimate the models (Allison 1982). Person-months of exposure are the unit of analysis, and I consider women to be at risk of contraceptive use after they have their first child.8 For women who had a child before February 1997, I start the hazard in the first month of the prospective data collection. Otherwise, I start the hazard the month after the respondent gives birth. To parameterize the baseline hazard and to account for seasonal variation in contraceptive behavior I also include a series of dichotomous variables for each calendar month (11 dichotomous variables in the models with one exclude as the reference category) and 9 dichotomous variables for the year (with a tenth excluded as the reference category).9 These variables are included in all the models described below.
Because the individuals in the CVFS are clustered, with several individuals living in the same community, I estimate multilevel models to account for this data structure. Techniques for multilevel modeling are well developed and have been widely applied in fertility research (Entwisle, Casterline, and Sayed 1989; Mason et al. 1983; Raudenbush and Bryk 2002). I use the multilevel hazard analysis proposed in Barber et al. (2000) and estimate discrete-time hazard models with random neighborhood level effects (commonly called a random intercept model). Because the outcome in question has only one destination state and is measured as a dichotomous variable, logistic regression is an appropriate estimation technique (Allison 1982; Guilkey and Rindfuss 1987).
RESULTS
Table 3 presents the findings from regressions of the geo-weighted measures of flora health on the hazard of contraceptive use. The coefficients displayed are the multiplicative effects on the odds of contraceptive use (the odds ratios). An exponentiated coefficient greater than 1.000 represents a positive effect, less than 1.000 a negative effect, and equal to 1.000 no effect. Because the frequency of events, contraceptive use, in any one-month interval is quite small, the odds of contraceptive use are very similar to the rate, and I discuss the results in terms of rates.
Table 3.
Multilevel Hazard Models: Quality of the Environment in Barandabhar Forest and Contraceptive Use, Chitwan, Nepal
| Geographically weighted simple density (plants/sq. meter) |
Geographically weighted simple richness (num of species) |
Geographically weighted Shannon-Weiner Diversity Index |
|
|---|---|---|---|
|
| |||
| 1 | 2 | 3 | |
| Flora measure | 1.003 * (2.055) |
1.026 * (2.112) |
1.272 * (2.110) |
| Measures of location in study area |
|||
| Miles to Narayangat | 1.000 (1.034) |
1.000 (1.253) |
1.000 (1.235) |
| Index of community organizations within 5 min walk in 1995 |
1.043 (0.880) |
1.047 (0.956) |
1.046 (0.951) |
| Controlsa | |||
| Index of community organizations within an hours walk in childhood |
1.039 (0.894) |
1.038 (0.870) |
1.038 (0.871) |
| Index of individual non-family experiences |
1.131 + (1.537) |
1.135 + (1.582) |
1.134 + (1.577) |
| Number of children born by 1996 |
1.159 ** (2.917) |
1.157 ** (2.896) |
1.158 ** (2.898) |
| Parental characteristics | |||
| Father ever worked for pay | 1.044 (0.454) |
1.046 (0.479) |
1.046 (0.479) |
| Mother’s children ever born | 0.987 (−0.626) |
0.986 (−0.642) |
0.986 (−0.638) |
| Parents used contraception | 1.080 (0.752) |
1.081 (0.765) |
1.081 (0.763) |
| Ethnicityb | |||
| Low caste Hindu | 1.399 * (2.008) |
1.400 * (2.018) |
1.400 * (2.015) |
| Newar | 1.087 (0.401) |
1.087 (0.402) |
1.087 (0.403) |
| Hill Tibeto-Burmese | 1.345 * (1.899) |
1.340 * (1.880) |
1.340 * (1.882) |
| Terai Tibeto-Burmese | 0.899 (−0.747) |
0.883 (−0.867) |
0.884 (−0.860) |
| Birth Cohortc | |||
| Born 1951-1960 (age 36-45 at birthday in 1996) |
0.016 *** (−8.697) |
0.016 *** (−8.709) |
0.016 *** (−8.708) |
| Born 1961-1970 (age 26-35 at birthday in 1996) |
0.386 *** (−5.296) |
0.385 *** (−5.314) |
0.385 *** (−5.313) |
| Born 1971-1975 (age 21-25 at birthday in 1996) |
0.828 + (−1.392) |
0.821 + (−1.449) |
0.822 + (−1.445) |
|
| |||
| Pseudo-R2 | 0.080 | 0.080 | 0.080 |
| Log likelihood | −2741 | −2741 | −2741 |
P < .10;
P < .05;
P < .01;
P < .001 all two-tailed tests
N= 53,765 person-months. Model fit statistics obtained from single level models.
Includes constant term and dummies for calendar month and year and for first month of prospective data collection.
Reference group is Upper caste Hindu
Reference group is born between 1976-1981
Turning first to the flora measures, Model 1 shows the effect of the simple measure of density (the number of plants) on the hazard of contraceptive use. We see a positive and significant odds ratio, implying that when more plants are found contraceptive use is more likely. Conversely, when fewer plants are found (i.e. the environment is less healthy) contraceptive use is lower, implying that family size is higher. The odds ratio of 1.003 means that a 1 percent increase in the geo-weighted number of plants results in a 0.3% increase in the rate of contraceptive use.
This may seem like a small effect, but let us consider how these geo-weighted measures might change. Table 4 presents means for the geo-weighted environmental quality measures assuming various increases in plant density, species richness, and plant diversity. Panel A presents the actual values at the neighborhood level. Panel B shows the means for each environmental quality measure if you increase the value of the raw data by one standard deviation for the two specific plots with the lowest values. This minor improvement in the environment would lead to a 3% increase in the geo-weighted measure of the number of plants, or a .9% increase in the rate of contraception. By contrast, Panel C presents the means and percent change corresponding to a one standard deviation increase for all plots. This more widespread environmental change would correspond with a 93% increase in the geo-weighted number of plants and an over 30% increase in the rate of contraception. A more moderate change in the environment is presented in Panel D, raising the values for the lowest 15 plots by 25 percent. Under this scenario the geo-weighted number of plants would increase by 2 percent.
Table 4.
Understanding Changes in Geoweighted Measures of Plant Density, Richness, and Diversity, Chitwan, Nepal
| Mean across 151 neighborhoods |
% change in mean (compared to Panel A) |
% change in hazard of contraceptive use (based on Table 3) |
|
|---|---|---|---|
| Panel A. Actual Data | |||
| Density (geo-weighted) | 1080 .11 | ||
| Richness (geo-weighted) | 126.92 | ||
| Shannon-Weiner Diversity Index (geo- weighted) |
13.40 | ||
| Panel B. Raise Plots with 2 Lowest Values 1 Standard D eviation | |||
| Density (geo-weighted) | 1112.00 | 3% | 0.9% |
| Richness (geo-weighted) | 128.01 | 1% | 3% |
| Shannon-Weiner Diversity Index (geo- weighted) |
13.47 | 1% | 29% |
| Panel C. Raise All Plots 1 Standard Deviation | |||
| Density (geo-weighted) | 2085.26 | 93% | 32% |
| Richness (geo-weighted) | 160.43 | 26% | 105% |
| Shannon-Weiner Diversity Index (geo- weighted) |
15.74 | 18% | 10247% |
| Panel D. Raise Lowest 15 Plots by 25 percent | |||
| Density (geo-weighted) | 1099.05 | 2% | 0.6% |
| Richness (geo-weighted) | 131.82 | 4% | 12% |
| Shannon-Weiner Diversity Index (geo- weighted) |
14.03 | 5% | 263% |
Returning to Table 3 and looking at Model 2 we see that the simple species richness measure, the geo-weighted number of species present, was also positively and significantly related to the hazard of contraceptive use. Women with more exposure to areas with more species present were more likely to use contraception. The technical interpretation of this measure is that a 1 percent increase in the geo-weighted number of species is related to a 2.8% increase in the odds of using contraception.
Model 3 in Table 3 shows the results when the index of plant diversity is included and again we see a positive and significant effect. Women with more exposure to plots with more plant diversity have higher rates of contraceptive use. Specifically, a one percent increase in the Shannon-Weiner Diversity Index corresponds with a 29.4% increase in the rate of contraceptive use.
This increase is markedly more impactful than those of the simple measures of the number of plants and species. To further understand this, let us return to Table 4. Looking at Panel C in Table 4, for all three environmental quality measures we see that the same calculated change in the raw data influences the geo-weighted measure quite differently. While raising the number of plants in all plots by one standard deviation increases that geo-weighted measure by 93 percent, raising the Shannon-Weiner Diversity Index for all plots by one standard deviation yields only an 18 percent increase in the geo-weighted measure. This wide range in how the different geo-weighted measures of environmental quality are affected by changes in raw data demonstrate the importance in theorizing carefully about what are realistic environmental changes to expect. Because the diversity index incorporates both the number of plants and the number of species, yielding information on not just the number of items present but also of their balance with one another, increasing its value requires a more substantively meaningful and difficult to obtain improvement in the environment. So, increasing the number of plants across the region (Panel C for the simple density measure) and raising the Shannon-Weiner Diversity in the two least diverse plots (Panel B) may both be realistic environmental improvements to consider. These changes in the environment would both yield about a 30 percent increases in the rate of contraceptive use.
In sum, returning again to Table 3, we see that across measures of plant density, species richness, and plant diversity higher environmental quality is related to higher rates of contraceptive use. Or, said another way, women exposed to poorer quality environments are less likely to use contraception, suggesting that they will have larger families. These findings provide evidence that individuals respond to poor environmental conditions by decreasing their likelihood of terminating childbearing (e.g. by having larger families). Poor environmental conditions mean families need to spend more household resources, such as time, on gathering natural materials and have more children to provide that effort.
Turing briefly to the control measures in Table 3 we see that birth cohort, ethnicity, and previous childbearing were significant predictors of the hazard of contraceptive use in these final models. Having had more children, being of a lower caste/ethnic group, and being younger were associated with higher rates of contraceptive use. These findings are in line with previous research. The measures of location and manufactured resources (i.e. community organizations) were not statistically significant, in these models or by themselves, supporting the idea that the geo-weighted measures are not simply picking up a spurious relationship that could be explained by other location measures.
DISCUSSION AND CONCLUSION
This paper investigates an important, and rarely looked at dimension of the broad population and environment field—the effect of environmental quality on population processes. I explore this topic by examining the relationship between plant density, species richness, and plant diversity and individual’s contraceptive use in rural Nepal. By employing unique, geo-weighted measures of the environment I provide new information on this complex, macro-micro link. Furthermore, because current theories are unclear about the direction of the effect of poor environmental conditions on contraceptive use the analysis presented here has important theoretical implications.
I find that women living in poor environmental conditions are less likely to use contraception to terminate their fertility, a finding counter to more economic or neo-Mathusian theories. The analyses presented here provide evidence that women and their families cope with the challenges brought on from poor environmental conditions by ending their childbearing later and, by extension, likely having larger families. In settings like Chitwan, Nepal, where children often contribute to household functioning through activities such as gathering firewood and fodder, adding additional children allows households to invest more time and effort into obtaining these materials—a necessity when their availability is low. The findings presented here are in line with other research that investigates the effects of the environment on population processes (e.g. Biddlecom et al. 2005; Filmer and Pritchett 2002). Additionally, although these results themselves do not speak to the entire reciprocal process, in conjunction with previous research that documents the negative effect of population growth on environmental quality, they do provide some support for the vicious cycle argument.
This paper focuses specifically on the timing of fertility limitation, largely because that is the most relevant demographic behavior for this question in this setting. There are many other possible demographic responses such as migration and other fertility related behaviors. Families in good environmental areas could have the same desires regarding altering family size but achieve those goals via abortion (illegal and extremely rare in Nepal), sending children to live elsewhere, marrying later, or lowering coital frequency. In Nepal, none of these options are very popular, but that may not be the case in other settings.
Although I am not able to test specific mechanisms, this research provides support of extensification and diversification against risk as two possible ones through which individuals and families cope with poor environmental conditions. Most forested areas in Chitwan are common land or land managed by user groups specifically designed to allow resource extraction. As such, residents can continue to extract resources from these areas making extensification a likely option. Also, as just mentioned, children in Chitwan have historically been valued for their labor, another factor necessary for extensification to occur. Regarding diversification against risk, like many rural, agrarian settings, having more children to help cope with hardship is common.
This does not mean we can rule out other mechanisms. First, these analyses are not longitudinal and were not designed to test hypothesis. Rather, this paper focuses on establishing an overall relationship between environmental quality and fertility behavior. Future research should incorporate measures of the specific mechanisms to shed light on which ones dominate in various settings. Second, different mechanisms may be more or less important in different settings. Whether someone decides to start purchasing gas to use for cooking when the local forest is depleted depends on many factors such as the relative cost and accessibility of gas and any normative value of children. In Nepal, both of these are fairly high, likely tipping behavioral responses towards increased contraceptive use. This is probably not the case in other areas—but that is an empirical question to be addressed by future research.
The positive link between environmental quality and contraceptive use is particularly troubling in light of global climate change. Changes such as rising temperatures and the spread of invasive species will continue to place these forest areas under stress and we would expect plant density, species richness, and plant diversity to all be negatively impacted. The analyses presented here lead us to believe that a result of this decrease in environmental quality will be larger families. Of course, these analyses rely on cross-sectional measures of the environment and therefore cannot speak directly to the effect of a decrease in environmental quality on fertility behaviors. Future research should incorporate micro-level measures of changes in plant density, species richness, and plant diversity, preferably incorporating the presence of invasive species to better explore the consequences of climate change itself.
Policy discussions on the importance of preventing environmental degradation should be expanded to include this longer term population consequence. Environmental problems are not going away, and awareness of them appears to be prevalent throughout the globe. However, policies that only target changing the environment or changing individual behavior that is directly related to environmental outcomes may be insufficient. Without addressing both the link between environmental quality and population growth and issues related to family size and contraceptive availability policy makers may fail to make substantial inroads into the complex problem of declining environmental quality.
The specific measures used in this paper of both the independent concept, environmental quality, and dependent concept, fertility behavior, are innovative and important contributions to the literature. Plant density, species richness, and plant diversity are key components of the environment to consider. People use the specific plants that they encounter and can base their assessment of the state of the environment on the plants that they see. Additionally, people live their lives over a wide area, traveling several kilometers daily when tending livestock, working the fields, or using community services. By creating micro-level measures that incorporate the environmental quality of the entire study area, and not just one arbitrary area close to the individual, I am better able to capture the entire area that may influence behavior. My focus on the connection between plant density, species richness, and plant diversity and contraceptive use at the micro-level is not a substitute for more macro-level approaches. Studies using remote sensoring or satellite imagery data to measure plant abundance have great potential for adding to our knowledge regarding the population and environment relationship (e.g. Entwisle et al. 2008; Liverman et al. 1998). The direct, micro-level approach to measurement of plants used here constitutes an important complement to these other research efforts. In part, this is because remotely sensed flora data do not tell the whole story when land has multiple species and multiple layers of plant life. The lower down plants, those captured in the micro-level measures used here but not necessarily in satellite imagery, are those that may have a more immediate impact on population processes simply because individuals are more likely to use them, see them, and notice their condition than they are the tree tops.
The focus on contraceptive behavior is also an innovation of this paper. Much previous research into the effects of the environment looks at morbidity and mortality outcomes (i.e. how environmental toxins cause cancer or death). While obviously important questions, they are not the only important dimensions of the environment-population relationship. The environment likely influences a wide array of individual behaviors, although through different mechanisms. The additional sociological mechanisms at work in the environment-fertility relationship relate to the value of children and family norms, rather than the pure biological mechanisms driving mortality effects. This paper documents the overall relationship between the environment and fertility behavior. Future research should explore the specific mechanisms, such as how children and women spend their time, the use of consumer goods as substitutes for environmental resources, and attitudinal factors such as environmentalism.
Of course, these measures have their limitations. In addition to being cross-sectional, they do not reveal the specific plant species present, are only measured in areas on the periphery of the study area, and we cannot link individuals to the specific areas that they see, use, or interact with. Knowing the specific plant species would allow us to identify situations where invasive species are changing the landscape, something particular important in this setting where we know M. micrantha is crowding out native plants and is not useful as fodder or fuel (Paudyal 2007; Poudel et al. 2005; Ellison et al. 2007). By design, the research presented here only includes measures of environmental quality from flora plots that lie outside the study area boundaries. Individuals in the study do not live inside the forests, and since I focus specifically on forest areas there is no geographic overlap. Analysis that incorporates the environmental quality of areas that are more proximate to individuals can help reveal more information on the mechanisms through which the environment influences population processes. Similarly, we do not know which areas people see or use. Because some of the mechanisms through which environmental quality influences fertility behavior are based on use of the environmental resources it would be helpful to incorporate actual use patterns with environmental quality information.
Another important dimension of the research presented here is that the relationship between environmental quality and individual behavior is independent of other measures of the local community. Environmental conditions are intricately connected to the spread of non-family organizations (e.g. the construction of new buildings often entails the destruction of natural habitat) but research is typically not able to separate the effects of the environmental changes from those of these other aspects of modernization or development. By controlling for the presence of markets, employers, health services, and public transportation I demonstrate that the characteristics of the natural environment themselves are related to fertility behaviors, independently of how the rest of the physical environment has changed. These findings are in line with previous research in this setting looking at the independent effects of non-family organizations and land use (Ghimire and Axinn 2010). In substantive terms these results imply that for residents, a “better” neighborhood is one rich in both social support services and natural resources. This may not be surprising given the intimate connection between individuals’ daily lives and the natural environment in Nepal. Future research should consider similar questions in areas where this connection is not so close.
In closing, I wish to highlight the importance of considering the specific setting in question when investigating macro-micro linkages. How daily life is organized, the initial quality of the environment, and availability of consumer goods may condition the effect of the natural environment on individual behavior and influence the preferred operationalization of measures of the environment. The measures used here are the result of extensive previous fieldwork and other data analysis on the relationship between community characteristics and individual behavior (e.g. Axinn, Barber, Ghimire 1997, Brauner-Otto et al. 2007). As such, the measures and results presented in this paper should serve as guides for researchers exploring this and similar relationships in other settings. The operationalization and model construction may need to be adjusted to incorporate setting specific circumstances and characteristics.
Footnotes
Households that move out of the study area are tracked and interviewed.
Because I include male methods of contraception (discussed below) this analysis is essentially using couples as the unit of analysis but only controlling for wife’s characteristics. I do not have data on the husbands for women who married after 1996 so I cannot control for both the husband’s and wife’s characteristics. As an alternative, I could have used husband as the unit of analysis, but faced with controlling for either the husband’s or the wife’s characteristics I elected to follow the majority of the literature and include information on the wives.
This sample also excludes 19 women who were missing data on any of the variables included in these analyses.
Limiting the sample to women who had no previous contraceptive use raises the possibility of left censoring. However, when I reduce the sample to include only women under age 25 in 1996, for whom previous contraceptive use rates are extremely low, the substantive conclusions drawn from the analyses do not change. In fact, the estimated effects are even greater than those presented below.
Data were also collected from 54 forest plots in two other areas, the Royal Chitwan National Park and along the Narayani river. However, plots in these areas were spaced (i.e. sampled) differently from those in the Barandabhar Forest and are under very different management protocols—the Royal Chitwan National Park has been a UNESCO World Heritage Site since 1984 and is heavily guarded by mounted and armed guards. Because of these differences I only present the results from analyses using information from the Barandabhar Forest. I did estimate models using information from all forest plots and found similar results to those presented here.
In contrast, diversity or species richness is likely less relevant for grassland plots.
I control for birth cohort as opposed to age because cohort will also control for some of the dramatic social change that as occurred over the data collection period. I did estimate models that include age as a continuous variable and found virtually the same results.
Note, using person-months does not artificially inflate standard errors (Allison 1982, 1984; Petersen 1986, 1991)
Because the 1996 interviews were collected over a period of six months, but the prospective data collection began for everyone in February 1997, the “first month” of the prospective data collection could have been as long as 6 months for some respondents. I include a control for whether the person-month was this first, potentially long, month in all of the analysis.
This research was supported by Award Number R01HD032912 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. The content is solely the responsibility of the author and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health. I am thankful to Rebecca Schewe, Amanda Clay Powers, Dirgha Ghimire, and William Axinn for their help with this research and to all of the staff at the Institute for Social and Environmental Research in Chitwan, Nepal for their data collection efforts.
REFERENCES
- Acharya M, Bennett L. The Status of Women in Nepal. Part 9. II. Center for Economic Development and Administration; Tribhuvan University; Kathmandu, Nepal: 1981. The Rural Women of Nepal: An Aggregate Analysis and Summary of 8 Village Studies. [Google Scholar]
- Aggarwal Rimjhim, Netanyahu Sinaia, Romano Claudia. Access to Natural Resources and the Fertility Decision of Women: The Case of South Africa. Environment and Development Economics. 2001;6:209–236. [Google Scholar]
- Allison Paul D. Discrete-Time Methods for the Analysis of Event Histories. In: Leinhart S, editor. Sociological Methodology. 1982. pp. 61–98. [Google Scholar]
- — — — . Event History Analysis. Sage Publishing; Newbury Park, CA: 1984. [Google Scholar]
- Axinn William G. Family Organization and Fertility Limitation in Nepal. Demography. 1992;29(4):503–521. [PubMed] [Google Scholar]
- Axinn William G., Barber Jennifer S. Mass Education and Fertility Transition. American Sociological Review. 2001;66(4):481–505. [Google Scholar]
- Axinn William G., Barber Jennifer S., Biddlecom Anne E. Social Organization and the Transition From Direct to Indirect Consumption. Social Science Research. 2010;39(3):357–68. doi: 10.1016/j.ssresearch.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axinn William G., Ghimire Dirgha J. Social Organization, Population, and Land Use. American Journal of Sociology. 2011;117(1):209–258. doi: 10.1086/661072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axinn William G., Thornton Arland. The Influence of Parental Resources on the Timing of the Transition to Marriage. Social Science Research. 1992;21:261–85. [Google Scholar]
- Axinn William G., Yabiku Scott. Social Change, the Social Organization of Families, and Fertility Limitation. American Journal of Sociology. 2001;106(5):1219–1261. [Google Scholar]
- Banister Judith, Thapa Shyam. Papers of the East-West Pop Inst. The Population Dynamic of Nepal. 1981. #78.
- Barber Jennifer S. The Intergenerational Transmission of Age at First Birth Among Married and Unmarried Men and Women. Social Science Research. 2001;30(2):219–47. [Google Scholar]
- Barber Jennifer S., Murphy Susan A., Axinn William G., Maples Jerry. Discrete-Time Multilevel Hazard Analysis. Sociological Methodology. 2000;30:201–235. [Google Scholar]
- Becker Gary S. A Treaties on the Family. Harvard University Press; Cambridge, Mass.: 1981. [Google Scholar]
- Bhattacharya Haimanti, Innes Robert. Income and the Environment in Rural India: Is There a Poverty Trap? American Journal of Agricultural Economics. 2013;95(1):42–69. [Google Scholar]
- Biddlecom Ann E., Axinn William G., Barber Jennifer S. Environmental Effects on Family Size Preferences and Subsequent Reproductive Behavior in Nepal. Population and Environment. 2005;26(3):183–206. [Google Scholar]
- Bilsborrow RE, Okoth-Ogendo HWO. Population-driven changes in land use in developing countries. Ambio. 1992;21(1):37–45. [Google Scholar]
- Bista Dor B. People of Nepal. Ratna Pustak Bhandar; Kathmandu: 1972. [Google Scholar]
- Boardman Jason D., Downey Liam, Jackson James S., Merrill JB, Saint Onge Jarron M., Williams David R. Proximate Industrial Activity and Psychological Distress. Population and Environment. 2008;30:3–35. doi: 10.1007/s11111-008-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaarts John, Feeney Griffith. On the Quantum and Tempo of Fertility. Quantum et tempo du taux de fécondité. 1998;24(2):271–291. [Google Scholar]
- Boserup E. The Conditions of Agricultural Growth: The Economics of Agrarian Change Under Population Pressure. Aldine Press; Chicago: 1965. [Google Scholar]
- Brauner-Otto Sarah R. Schools, Their Spatial Distribution and Characteristics, and Fertility Limitation. Rural Sociology. 2012;77(3):321–354. doi: 10.1111/j.1549-0831.2012.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner-Otto Sarah R. Attitudes About Children and Fertility Limitation Behavior. Population Research and Policy Review. 2013;32(1):1–24. doi: 10.1007/s11113-012-9261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner-Otto Sarah R., Axinn William G., Ghimire Dirgha J. The Spread of Health Services and Fertility Transition. Demography. 2007;44(4):747–70. doi: 10.1353/dem.2007.0041. [DOI] [PubMed] [Google Scholar]
- Brewster Karin L. Neighborhood Context and the Transition to Sexual Activity among Young Black Women. Demography. 1994;31:603–614. [PubMed] [Google Scholar]
- Brewster Karin L. Race Differences in Sexual Activity Among Adolescent Women: The Role of Neighborhood Characteristics. American Sociological Review. 1994;59:408–424. [Google Scholar]
- Brewster Karin L., Billy John O. G., Grady William R. Social Context and Adolescent Behavior: The Impact of Community on the Transition to Sexual Activity. Social Forces. 1993;71:713–740. [Google Scholar]
- Bukovinszky T, van Veen FJF, Jongema Y, Dicke M. Direct and Indirect Effects of Resource Quality on Food Web Structure. Science. 2008;319(5864):804–807. doi: 10.1126/science.1148310. [DOI] [PubMed] [Google Scholar]
- Bulatao RA, Lee RD. Determinants of Fertility in Developing Countries. Academic Press; New York: 1983. [Google Scholar]
- Cain M. Risk and Insurance: Perspective on Fertility and Agrarian Change in India and Bangladesh. Population and Development Review. 1981;7(3):435–74. [Google Scholar]
- — — — Fertility As an Adjustment to Risk. Population and Development Review. 1983;9(4):688–702. [Google Scholar]
- Caldwell JC. Theory of Fertility Decline. Academic Press; London, England: 1982. [Google Scholar]
- Carr Edward R. Placing the Environment in Migration: Environment, Economy, and Power in Ghana’s Central Region. Environment and Planning A. 2005;37(5):925–946. [Google Scholar]
- Carr David L., Lopez Anna C., Bilsborrow Richard E. The Population, Agriculture, and Environment Nexus in Latin American: Country-Level Evidence From the Latter Half of the Twentieth Century. Population and Environment. 2009;30:222–46. [Google Scholar]
- Chaudhary Ram P. Biodiversity in Nepal: Status and Conservation. S. Devi; Saharanpur, Uttar Pradesh, India: 1998. [Google Scholar]
- Chaudhary Ram P. Forest Conservation and Environmental Management in Nepal: A Review. Biodiversity and Conservation. 2000;9:1235–1260. [Google Scholar]
- Chiarucci A, Wilson JB, Anderson BJ, De Dominicis V. Cover versus biomass as an estimate of species abundance: Does it make a difference to the conclusions? Journal of Vegetation Science. 1999;10(1):35–42. [Google Scholar]
- Clay Daniel C., Johnson Nan E. Size of Farm or Size of Family: Which Comes First? Population Studies. 1992;46(3):491–505. [Google Scholar]
- Cole Matthew A., Neumayer Eric. Examining the Impact of Demographic Factors on Air Pollution. Population and Environment. 2004;26(1):5–21. [Google Scholar]
- Cooke PA. Intrahousehold Labor Allocation Responses to Environmental Goods Scarcity: A Case Study From the Hills of Nepal. Economic Development and Cultural Change. 1998;46(4):807–30. [Google Scholar]
- Dangol Dharma R., Maharjan Keshav L. Spatial and Temporal Dynamics of Flora in Forest, Grassland and Common Land Ecosystems of Western Chitwan, Nepal. Journal of International Development and Cooperation. 2012;18(4):77–92. [PMC free article] [PubMed] [Google Scholar]
- Davis Kingsley. The World Demographic Transition. Annals of the American Academy of Political and Social Science. 1945;237:1–11. [Google Scholar]
- — — — The theory of change and response in modern demographic history. Population Index. 1963;29(4):345–66. [PubMed] [Google Scholar]
- Demeny P. Early fertility decline in Austria-Hungary: a lesson in demographic transition. Daedalus. 1968;97:502–522. [PubMed] [Google Scholar]
- de Sherbinin Alex, Carr David, Cassels Susan, Jiang Leiwen. Population and Environment. Annual Review of Environment and Resources. 2007;32:345–73. doi: 10.1146/annurev.energy.32.041306.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sherbinin Alex, VanWey Leah K., McSweeney Kendra, Aggarwal Rimjhim, Barbieri Alisson, Henry Sabine, Hunter Lori M., Twine Wayne, Walker Robert. Rural Household Demographics, Livelihoods, and the Environment. Global Environmental Change. 2008;18:38–53. doi: 10.1016/j.gloenvcha.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey Liam. Using Geographic Information Systems to Reconceptualize Spatial Relationships and Ecological Context. American Journal of Sociology. 2006;112(2):567–612. doi: 10.1086/506418?origin=JSTOR-pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap Riley E. Climate Change and Rural Sociology: Broadening the Research Agenda. Rural Sociology. 2010;75(1):17–27. [Google Scholar]
- Dupéré V, Leventhal T, Vitaro F. Neighborhood Processes, Self-Efficacy, and Adolescent Mental Health. Journal of Health and Social Behavior. 2012;53(2):183–198. doi: 10.1177/0022146512442676. [DOI] [PubMed] [Google Scholar]
- Ellison CA, Puzari KC, Kumar PS, Dev U, Sankaran KV, Rabindra RJ, Murphy ST. Sustainable control of Mikania micrantha – implementing a classical biological control strategy in India using the rust fungus Puccinia spegazzinii. Proceedings of the 7th international workshop on biological control and management of Chromolaena odorata and Mikania micrantha.2007. [Google Scholar]
- Entwisle Barbara, Casterline John, Sayed H. Villages as Contexts for Contraceptive Behavior in Rural Egypt. American Sociological Review. 1989;54:1019–1034. [Google Scholar]
- Entwisle Barbara, Rindfuss Ronald R., Guilkey David K., Chamratrithirong A, Curran SR, Sawangdee Y. Community and Contraceptive Choice in Rural Thailand: a Case Study of Nang Rong. Demography. 1996;33(1):1–11. [PubMed] [Google Scholar]
- Entwisle Barbara, Rindfuss Ronald R., Walsh Stephen J., Page Philip H. Population Growth and Its Spatial Distribution As Factors in the Deforestation of Nang Rong, Thailand. Geoforum. 2008;39:879–97. doi: 10.1016/j.geoforum.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwisle Barbara, Walsh Stephen J., Rindfuss Ronald R., VanWey Leah K. Population and Upland Crop Production in Nang Rong, Thailand. Population and Environment. 2005;26(6):449–70. [Google Scholar]
- Filmer Deon, Pritchett Lant H. Environemental Degredation and the Demand for Children: Searching for the Vicious Circle in Pakistan. Economic and Development Economics. 2002;7:123–46. [Google Scholar]
- Fricke Thomas E. Himalayan Households: Tamang Demography and Domestic Processes. Columbia University Press; New York: 1994. [Google Scholar]
- Ghimire Dirgha J. Unpublished Ph.D. dissertation. Department of Sociology, The University of Michigan; 2003. The Social Context of First Birth Timing in Nepal. [Google Scholar]
- Ghimire Dirgha J., Axinn William G. Community Context, Land Use, and First Birth. Rural Sociology. 2010;75(3):478–513. doi: 10.1111/j.1549-0831.2010.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghimire Dirgha J., Hoelter Lynette. Land Use and First Birth timing in an Agricultural Setting. Population and Environment. 2007;28:289–320. [Google Scholar]
- Ghimire Dirgha J., Mohai Paul. Environmentalism and Contraceptive Use: How People in Less Developed Settings Approach Environmental Issues. Population and Environment. 2005;27(1):29–61. [Google Scholar]
- Grunewald R, Schubert H. The Definition of a New Plant Diversity Index “H’Dune” for Assessing Human Damage on Coastal Dunes--Derived From the Shannon Index of Entropy H’. Ecological Indicators. 2007;7:1–21. [Google Scholar]
- Guilkey David K., Rindfuss Ronald R. Logistic Regression Multivariate Life Tables: A Communicable Approach. Sociological Methods and Research. 1987;16(2):276–300. [Google Scholar]
- Gurung Harka B. Vignettes of Nepal. Sajha Prakashan; Kathmandu: 1980. [Google Scholar]
- Harrison Jill Lindsey. Pesticide Drift and the Pursuit of Environmental Justice. The MIT Press; 2011. [Google Scholar]
- Henry Carolyn S., Merten Michael J., Plunkett Scott W., Sands Tovah. Neighborhood, Parenting, and Adolescent Factors and Academic Achievement in Latino Adolescents From Immigrant Families. Family Relations. 2008;57(5):579–590. [Google Scholar]
- Hummel Diana, Adamo Susana, de Sherbinin Alex, Murphy Laura, Aggarwal Rimjhim, Zulu Leo, Liu Jianguo, Knight Kyle. Online first.”Inter- and transdisciplinary approaches to population–environment research for sustainability aims: a review and appraisal. Population and Environment [Google Scholar]
- Hunter Lori M. The Association Between Environmental Risk and Internal Migration Flows. Population and Environment. 1998;19(3):247–77. [Google Scholar]
- Ives JD, Messerli B. The Himalayan Dilemma: Reconciling Development and Conservation. Routledge; London: 1989. [Google Scholar]
- Jones Samanatha. Tigers, trees and Tharu: An analysis of community forestry in the buffer zone of the Royal Chitwan National Park, Nepal. Geoforum. 2007;38:558–575. [Google Scholar]
- Jiggins Janice. Changing the Boundaries: Women Centered Perspective on Population and the Environment. Island Press; Washington, DC: 1994. [Google Scholar]
- Kramer Daniel B., Urquhard Gerald, Schmitt Kristen. Globalization and the Connection of Remote Communities: A Review of Household Effects and Their Biodiversity Implications. Ecological Economics. 2009;68:2897–909. [Google Scholar]
- Lehmkuhl John F. A classification of subtropical riverine grassland and forest in Chitwan National Park, Nepal. Vegetatio. 1994;111:29–43. [Google Scholar]
- Leopold Aldo. Sand County Almanac. Random House Digital, Inc.; 1986. [Google Scholar]
- Liu Jianguo, Dietz Thomas, Carpenter Stephen R., Alberti Marina, Folke Carl, Moran Emilio, Pell Alice N., Deadman Peter, Kratz Timothy, Lubchenco Jane, Ostrom Elinor, Ouyang Zhiyun, Provencher William, Redman Charles L., Schneider Stephen H., Taylor William W. Complexity of Coupled Human and Natural Systems. Science. 2007;317(5844):1513–1516. doi: 10.1126/science.1144004. [DOI] [PubMed] [Google Scholar]
- Liu Jianguo, Dietz Thomas, Carpenter Stephen R., Folke Carl, Alberti Marina, Redman Charles L., Schneider Stephen H., Ostrom Elinor, Pell Alice N., Lubchenco Jane, Taylor William W., Ouyang Zhiyun, Deadman Peter, Kratz Timothy, Provencher William. Coupled Human and Natural Systems. AMBIO: A Journal of the Human Environment. 2007;36(8):639–649. doi: 10.1579/0044-7447(2007)36[639:chans]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Liverman Diana L., Moran Emilio F., Rindfuss Ronald R., Stern Paul C. People and Pixels: Linking Remote Sensing and Social Science. National Academy Press; Washington, D.C.: 1998. [Google Scholar]
- Lloyd Cynthia B., Kaufman Carol E., Hewett Paul. The Spread of Primary Schooling in Sub-Saharan Africa: Implications for Fertility Change. Population and Development Review. 2000;26(3):483–515. doi: 10.1111/j.1728-4457.2000.00483.x. [DOI] [PubMed] [Google Scholar]
- Loreau Michel. Linking biodiversity and ecosystems: towards a unifying ecological theory. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1537):49–60. doi: 10.1098/rstb.2009.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald Kevin. An Evolutionary Perspective on Human Fertility. Population and Environment. 1999;21(2):223–46. [Google Scholar]
- Malthus Thomas. Population: The First Essay. The University of Michigan Press; Ann Arbor, MI: 1959. 1798. reprinted. [Google Scholar]
- Mason William M., Wong George Y., Entwisle Barbara. Contextual Analysis Through the Multilevel Linear Model. Sociological Methodology. 1983;14:72–103. [Google Scholar]
- Molnar Joseph J. Climate Change and Societal Response: Livelihoods, Communities, and the Environment. Rural Sociology. 2010;75(1):1–16. [Google Scholar]
- Mouillot D, Sylvain G, Aliaume C, Verlaque M, Belsher T, Troussellier M, Thang Do Chi. Ability of taxonomic diversity indices to discriminate coastal lagoon environments based on macrophyte communities. Ecological Indicators. 2005;7:1–21. [Google Scholar]
- Myers N. Threatened Biotas: “Hot Spots” in Tropical Forests. Environmentalist. 1988;8:187–208. doi: 10.1007/BF02240252. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity Hotspots for Conservation Priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- National Research Council . Population and Land Use in Developing Countries. National Academy Press; Washington, DC: 1993. [Google Scholar]
- O’Neill BC, MackKellar FL, Lutz W. Population and Climate Change. IIASA; Laxenburg, Austria: 2001. [Google Scholar]
- Patil GP, Taillie C. Diversity as a Concept and its Measurement. Journal of the American Statistical Association. 1982;77(379):548–561. [Google Scholar]
- Paudyal A. Unpublished Thesis. Tribhuvan University; Kathmandu: 2007. Buffer zone resources and community conservation: a case study of Piple buffer zone Village Development Committee, Chitwan National Park. [Google Scholar]
- Pebley Ann R. Demography and the Environment. Demography. 1998;35(4):377–89. [PubMed] [Google Scholar]
- Peet NB, Watkinson AR, Bell DJ, Kattel BJ. Plant diversity in the threatened sub-tropical grasslands of Nepal. Biological Conservation. 1999;88:193–206. [Google Scholar]
- Petersen Trond. Estimating Fully Parametric Hazard Rate Models With Time-Dependent Covariates: Use of Maximum Likelihood. Sociological Methods and Research. 1986;14:219–46. [Google Scholar]
- — — — The Statistical Analysis of Event Histories. Sociological Methods and Research. 1991;19(3):270–323. [Google Scholar]
- Plunkett SW, Abarca-Mortensen S, Behnke AO, Sands T. Neighborhood structural qualities, adolescents’ perceptions of neighborhoods, and latino youth development. Hispanic Journal of Behavioral Sciences. 2007;29(1):19–34. [Google Scholar]
- Poffenberger Mark. Patterns of Change in the Nepal Himalaya. Westview Press; Boulder, CO: 1980. [Google Scholar]
- Poudel A, Baral HS, Ellison C, Subedi K, Thomas S, Murphy S. Mikania micrantha weed invasion in Nepal. A summary report of the first national workshop for stakeholders; Kathmandu, Nepal. 25 November 2004; Himalayan Nature, IUCN-Nepal, and CAB International, UK; 2005. [Google Scholar]
- Raudenbush Stephen W., Bryk Anthony S. Hierarchical linear models: Applications and data analysis methods. Sage Publications; 2002. [Google Scholar]
- Shannon CE, Wiener W. The Mathematical Theories of Communities. University of Illinois Press; Urbana, IL: 1962. [Google Scholar]
- Sharma Ramesh C., Rawat Jitendra S. Monitoring of aquatic macroinvertebrates as bioindicator for assessing the health of wetlands: A case study in the Central Himalayas, India. Ecological Indicators. 2009;9:118–128. [Google Scholar]
- Simon Julian. Population Matters: People, Resources, Environment, and Immigration. Transaction; New Brunswick, NJ: 1990. [Google Scholar]
- South Scott J., Baumer Eric P. Deciphering Community and Race Effects on Adolescent Premarital Childbearing. Social Forces. 2000;78(4):1379–1408. [Google Scholar]
- Straede Steffen, Treue Thorsten. Beyond buffer zone protection: A comparative study of park and buffer zone products’ importance to villagers living inside Royal Chitwan National Park and to villagers living in its buffer zone. Journal of Environmental Management. 2006;78:251–267. doi: 10.1016/j.jenvman.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Suwal Juhee V. Socio-Cultural Dynamics of First Birth Intervals in Nepal. Contribution to Nepalese Studies. 2001;28(1):11–33. [Google Scholar]
- Thibaut Loïc M., Connolly Sean R. Understanding diversity–stability relationships: towards a unified model of portfolio effects. Ecology Letters. 2013;16(2):140–150. doi: 10.1111/ele.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Ken, Jones Allen. Human Population Density and Prediction of Local Plant Extinction in Britain. Conservation Biology. 1999;13(1):185–89. [Google Scholar]
- Thomson Elizabeth. Couple Childbearing Desires, Intentions, and Births. Demography. 1997;34(3):343–54. [PubMed] [Google Scholar]
- Thornton Arland, Camburn Donald. The Influence of the Family on Premarital Sexual Attitudes and Behavior. Demography. 1987;24:323–40. [PubMed] [Google Scholar]
- Thornton Arland, Fricke Tom. Social Change and the Family: Comparative Perspectives from the West, China, and South Asia. Sociological Forum. 1987;2:746–779. [Google Scholar]
- Thornton Arland, Lin Hui-Sheng. Social Change and the Family in Taiwan. University of Chicago Press; Chicago: 1994. [Google Scholar]
- Tuladhar Jayanti M. The Persistence of High Fertility in Nepal. Inter-India Publications; New Dehli: 1989. [Google Scholar]
- VanWey Leah K., Guedes Gilvan R., D’Antona Álvaro O. Out-migration and Land-use Change in Agricultural Frontiers: Insights from Altamira Settlement Project. Population and Environment. 2011 doi: 10.1007/s11111-011-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabiku Scott T. Land Use and Marriage Timing in Nepal. Population and Environment. 2006;27(5-6):445–461. [Google Scholar]
- York Richard, Rosa Eugene A., Dietz Thomas. Footprints on the Earth: The Environmental Consequences of Modernity. American Sociological Review. 2003;68(2):279–300. [Google Scholar]
- Yu Eunice, Liu Jianguo. Environmental Impacts of Divorce. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20629–34. doi: 10.1073/pnas.0707267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurick David, Karan PP. Himalaya: Life on the Edge of the World. Johns Hopkins; Baltimore, MD: 1999. [Google Scholar]