Abstract
Rates of biodiversity loss are higher in freshwater ecosystems than in most terrestrial or marine ecosystems, making freshwater conservation a priority. However, prioritization methods are impeded by insufficient knowledge on the distribution and conservation status of freshwater taxa, particularly invertebrates. We evaluated the extinction risk of the world's 590 freshwater crayfish species using the IUCN Categories and Criteria and found 32% of all species are threatened with extinction. The level of extinction risk differed between families, with proportionally more threatened species in the Parastacidae and Astacidae than in the Cambaridae. Four described species were Extinct and 21% were assessed as Data Deficient. There was geographical variation in the dominant threats affecting the main centres of crayfish diversity. The majority of threatened US and Mexican species face threats associated with urban development, pollution, damming and water management. Conversely, the majority of Australian threatened species are affected by climate change, harvesting, agriculture and invasive species. Only a small proportion of crayfish are found within the boundaries of protected areas, suggesting that alternative means of long-term protection will be required. Our study highlights many of the significant challenges yet to come for freshwater biodiversity unless conservation planning shifts from a reactive to proactive approach.
Keywords: extinction risk, crayfish, IUCN Red List, threatened, freshwater biodiversity
1. Introduction
Freshwater ecosystems occupy less than 1% of the earth's surface, but support approximately 10% of the world's species and 30% of all vertebrates [1]. These systems provide a range of valuable services, including fisheries, domestic and commercial water supply, carbon sequestration and energy; however, a rapidly growing human population has increased the demand on freshwater resources leading to a freshwater biodiversity crisis [2]. While knowledge on the conservation status and distribution of freshwater taxa is disparate relative to terrestrial species [3], there is growing evidence that freshwater taxa (i.e. crabs, dragonflies, fish and molluscs) are at greater risk of extinction than terrestrial vertebrates (i.e. mammals, reptiles or birds) [3–9]. Given the disproportionately high biodiversity harboured in freshwater ecosystems, knowledge on the distribution and conservation status of freshwater species will be essential for monitoring targets set by the Convention on Biological Diversity [3]. For example, Target 6 aims to ensure that ‘all fish and invertebrate stocks and aquatic plants are managed and harvested sustainably by 2020’, Target 11 is to conserve 17% of inland water by 2020 and Target 12 requires that by 2020 ‘the extinction of known threatened species has been prevented and their conservation status, particularly of those most in decline, has been improved and sustained’ [10].
Limited resources available for conservation require practitioners to prioritize areas for action. Selection of priority areas requires knowledge on the distribution and conservation status of a globally representative sample of species. To date, global analyses of species diversity and patterns of threat have been biased towards terrestrial species, particularly vertebrates [11–13] producing the major tropical and subtropical hotspots described by Myers et al. [11]. However, there is growing evidence that vertebrates are a poor proxy for estimating invertebrate diversity [3,14,15], highlighting a need for improved knowledge on the distribution and status of invertebrate taxa.
Freshwater crayfish (Astacidea) exhibit a disjunct global distribution with the majority of species diversity restricted to temperate latitudes, and an absence of native species in continental Africa and the Indian subcontinent [16]. A number of hypotheses explaining crayfish distribution patterns have been proposed: competitive exclusion with the freshwater crabs that occupy a similar ecological niche [17–19]; unsuitable climatic conditions [17,19,20]; or the timing of the separation of Gondwana [16]. However, these hypotheses have been neither denied nor supported, and so an explanation for the absence of crayfish in Africa and India remains inconclusive.
The major crayfish diversity hotspots are split taxonomically into two superfamilies: Astacoidea and Parastacoidea [21]. Astacoidea is restricted to the Northern Hemisphere and comprises two families: Cambaridae, which is the largest crayfish family and native to North America (409 spp.) and East Asia (four spp.); and Astacidae, the smallest family, with native species in Europe (five spp.) and the USA and Canada (five spp.). Parastacoidea comprises only a single family, the Parastacidae, which is restricted to the Southern Hemisphere [15] with native species in Australasia (148 spp.), Madagascar (seven spp.) and South America (12 spp.).
Crayfish are found in a diversity of habitats, including: permanent and seasonal rivers, streams and lakes; freshwater caves and springs; and terrestrial burrows. Given their significant biomass in many freshwater systems [22], crayfish play a fundamental role in determining ecosystem structure and function [23], and are of significant economic importance, particularly in Madagascar, Europe, China and the US state of Louisiana [24–26]. However, in recent years, freshwater crayfish have been increasingly recognized as in need of ‘conservation attention’ [27,28]. Previous estimates suggest that 48% of North American crayfish species and 25% of all Australian species are threatened [27–29], and that extinction rates for crayfish may increase by more than an order of magnitude exceeding those of freshwater fishes and amphibians [8]. Heightened extinction risk in crayfish is often attributed to small range size and degradation of freshwater habitats [30]; however, even the wide-ranging European noble crayfish (Astacus astacus) has seen significant population declines since the arrival of crayfish plague (Aphanomyces astaci) [31].
Threats to crayfish are set to increase in both magnitude and extent. Consequently, there is an urgent need to better understand the extinction risk and patterns of threat in freshwater crayfish. In this study, we address these gaps by assessing the global extinction risk of all crayfish species described up to 2009, using the International Union for Conservation of Nature (IUCN) Red List of Threatened Species Categories and Criteria [32]. We report on patterns of extinction risk across families, analyse patterns of threat and data gaps, and make recommendations for conservation.
2. Methods
Species-specific data were collected on taxonomy, distribution, population trends, ecology, biology, threats and conservation measures for all 590 species of crayfish described up to 2009. Data were obtained from published and unpublished articles, government reports and personal communications. All species were evaluated against quantitative thresholds defined in the IUCN Red List Categories and Criteria [33] to assess extinction risk based on: A (past, present or future declining population), B (geographical range size, and fragmentation, decline or fluctuations), C (small population size and fragmentation, decline or fluctuations), D (very small population or very restricted distribution) and E (quantitative analysis of extinction risk). Based on the quantitative thresholds and available data, we assigned one of the eight IUCN Red List categories [32]: Extinct (EX), Extinct in the wild (EW), Critically Endangered (CR), Endangered (EN), Vulnerable (VU), Near Threatened (NT), Least Concern (LC) and Data Deficient (DD), of which CR, EN and VU are the threatened categories. Few invertebrate species have sufficient information on rates of population decline, so assessments under criterion A were based on presence/absence data over time, assuming equal abundance across the range and linear rates of decline. Following Darwall et al. [34], we mapped species distributions to river sub-basins as delineated by the HYDRO1k Elevation Derivative Database [35] using ArcGIS v. 9.3. Where existing distribution maps were available these were digitized, while others were created from georeferenced specimen collection records provided by species experts. We calculated species range either as: extent of occurrence (EOO), by computing a minimum convex polygon around all known, inferred and projected occurrences; or area of occupancy (AOO), by calculating the area of all known occupied sites. Species assessments and distribution maps were reviewed by a panel of experts in a workshop setting, and remotely by email. The majority of assessments (n = 573) were published on the IUCN Red List in 2010, with 17 assessments awaiting publication.
Following Hoffmann et al. [36], we estimated the proportion of threatened species as [(number of threatened)/(total − DD)], where ‘threatened’ is the number of species assessed VU, EN and CR, ‘total’ is the total number of species and DD is the number of species assessed as DD. This assumes that DD species show the same proportion of threatened species as better known species, and represents a mid-estimate of extinction risk for the group (see [31]). Threat levels have been reported this way in similar studies [6,13,36], representing the current consensus among conservation biologists about how the proportion of threatened species should be presented, while also accounting for the uncertainty introduced by DD species. We also calculated a lower estimate on the proportion of threatened species assuming that none of the DD species are threatened [(number of threatened)/total] and a high estimate assuming that all DD species are threatened [(number of threatened + DD)/total]. Extinction risk was summarized across all families and genera.
Identification of taxa that are more threatened than expected by chance can help prioritize conservation actions [37]. Using the methods described by Bielby et al. [38], we tested to see whether genera deviated from the expected level of threat. Chi-squared tests were used to test for significant departures from equal threat between genera, and binomial tests were used to find the smallest genus size necessary to detect a significant deviation from the observed proportion of threatened species. Genera represented by an insufficient number of species were excluded. A null frequency distribution of the number of threatened species was generated from 10 000 unconstrained randomizations, by randomly assigning Red List categories to all species, based on the frequency of occurrence of each category in the sample. The number of threatened species in the focal genera was counted and compared with the null frequency distribution. The null hypothesis (that extinction risk is taxonomically random) was rejected if this number fell in the 2.5% at either tail of the null frequency distribution.
Following Salafsky et al. [39], threats were categorized into: agriculture, logging, invasive species and disease, problematic native species, harvesting, urban development (i.e. commercial, domestic and industrial), energy production and mining, climate change and severe weather events, pollution, human disturbance (i.e. war and recreational activities), transportation infrastructure (i.e. roads, shipping lanes, railways) and water management/dams. Threats were summarized by geographical location only for threatened species.
We assessed the spatial congruence between threatened species richness and DD species richness in the major centres of diversity (i.e. Australia, Mexico and the USA). We defined centres of richness by selecting the top 10% species-rich river basins, with richness based on the absolute number of species, DD species and threatened species and compared congruence using Pearson's correlations. We accounted for spatial autocorrelation by implementing the method of Clifford et al. [40], which estimates effective degrees of freedom based on spatial autocorrelation in the data and applies a correction to the significance of the observed correlation. We also assessed the proportions of southeast US and Australian threatened species’ basins that intersect with protected areas (irrespective of the proportion of the basin area covered). Protected areas were selected using the IUCN Protected Areas Categories System [41], and included the following categories: strict nature reserve, wilderness area, national park, natural feature, habitat/species management area, protected landscape and protected area with sustainable use of natural resources. All statistical analyses were performed using the software package R v. 3.0.1 [42]. The critical value for α was set at 0.05.
3. Results
Nearly one-third of the world's crayfish species were assessed as threatened with extinction assuming that DD species are threatened in an equal proportion (32%: range 24–47%; table 1). Of the non-threatened species, 7% were assessed as NT and 47% as LC. Twenty-one per cent of all species were assessed as DD. Four species were assessed as EX; however of the 51 species assessed as CR, four were highlighted as possibly extinct. Of the EX species, two were previously found in Mexico (Cambarellus alvarezi and Cambarellus chihuahuae) and two in the USA, specifically Georgia (Procambarus angustatus) and California (Pacifastacus nigrescens). Of the possibly extinct species, two were known from Mexico (Procambarus paradoxus and Cambarellus areolatus), and one each from the US states of Alabama (Cambarus veitchorum) and Florida (Procambarus delicatus). All East Asian Cambaroides and South American Parastacidae (10 of 12 spp.) were assessed as DD. Only two of the seven species of Malagasy Astacoides were assessed as threatened, whereas the remaining species were assessed as DD (four of seven spp.) or LC (one of seven spp.).
Table 1.
Extinction risk summarized by family and genus. Figures for the proportion of threatened species represent the mid-estimate [(number of threatened)/(total−DD)], lower estimate [(number of threatened)/total] and high estimate [(number of threatened + DD)/total].
| taxa | native geographical locality | DD | LC | NT | VU | EN | CR | EX | total | proportion threatened (low estimate–high estimate) |
|---|---|---|---|---|---|---|---|---|---|---|
| Astacidae | 3 | 3 | 0 | 1 | 1 | 1 | 1 | 10 | 43% (30–60%) | |
| Astacus | Europe | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 50% (33–67%) |
| Austropotamobius | Europe | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 100% (50–100%) |
| Pacifastacus | USA, Canada | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 5 | 25% (20–40%) |
| Cambaridae | 91 | 221 | 26 | 20 | 33 | 19 | 3 | 413 | 22% (17–39%) | |
| Barbicambarus | USA | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0% (0–0%) |
| Bouchardina | USA | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 (0–100%) |
| Cambarellus | USA, Mexico | 3 | 8 | 1 | 0 | 1 | 2 | 2 | 17 | 21% (18–35%) |
| Cambaroides | East Asia | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0% (0–100%) |
| Cambarus | USA, Canada | 15 | 61 | 9 | 4 | 5 | 7 | 0 | 101 | 19% (16–31%) |
| Distocambarus | USA | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 5 | 100% (40–100%) |
| Fallicambarus | USA, Canada | 2 | 8 | 5 | 1 | 1 | 1 | 0 | 18 | 19% (17–28%) |
| Faxonella | USA | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 4 | 0% (0–0%) |
| Hobbseus | USA | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 7 | 75% (43–86%) |
| Orconectes | USA, Canada, Mexico | 9 | 62 | 3 | 10 | 4 | 1 | 0 | 89 | 19% (17–27%) |
| Procambarus | USA, Mexico, Cuba, Belize, Guatemala, Honduras | 51 | 77 | 6 | 3 | 19 | 8 | 1 | 165 | 26% (18–49%) |
| Troglocambarus | USA | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0% (0–0%) |
| Parastacidae | 31 | 50 | 14 | 12 | 33 | 27 | 0 | 167 | 53% (43–62%) | |
| Astacoides | Madagascar | 4 | 1 | 0 | 0 | 2 | 0 | 0 | 7 | 67% (29–86%) |
| Astacopsis | Australia | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 33% (33–33%) |
| Cherax | Australia, New Guinea | 9 | 12 | 6 | 2 | 7 | 3 | 0 | 39 | 40% (31%–54%) |
| Engaeus | Australia | 5 | 17 | 3 | 3 | 3 | 4 | 0 | 35 | 33% (29–43%) |
| Engaewa | Australia | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 5 | 60% (60–60%) |
| Euastacus | Australia | 1 | 8 | 1 | 5 | 17 | 17 | 0 | 49 | 81% (80–82%) |
| Geocharax | Australia | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 50% (50–50%) |
| Gramastacus | Australia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0% (0–0%) |
| Ombrastacoides | Australia | 2 | 4 | 2 | 1 | 0 | 2 | 0 | 11 | 33% (27–45%) |
| Paranephrops | New Zealand | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0% (0–0%) |
| Parastacus | South America | 6 | 1 | 1 | 0 | 0 | 0 | 0 | 8 | 0% (0–75%) |
| Samastacus | South America | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0% (0–100%) |
| Tenuibranchiurus | Australia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 100% (100–100%) |
| Virilastacus | South America | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0% (0–100%) |
| all species | 125 | 274 | 40 | 33 | 67 | 47 | 4 | 590 | 32% (24–47%) |
The majority (117 of 147 spp.) of threatened species (those classified as CR, EN or VU) were assessed using criterion B1 (geographical range size combined with fluctuations or declines). Only 13 species had adequate surveys from which to calculate AOO and thereby carry out assessments under criterion B2. Five species were assessed under criterion A (Astacus astacus, Austropotamobius pallipes, Astacopsis gouldi, Cambarus cracens and Engaeus granulatus); the other species had insufficient data on rates of population decline to meet this criterion. The assessment for Astacus astacus was based on population data from both systematic surveys and direct exploitation, whereas the other assessments were based on observed declines in EOO and AOO collected from systematic surveys over significant parts of the species' ranges. The remaining 12 threatened species were assessed under criterion D2 (i.e. species with a very small range—AOO <20 km2 or <5 locations—and subjected to rapidly becoming CR or EX as a result of future threat(s)). A minimum of three species in a genus were required to establish if the genera was at greater risk of extinction than expected by chance, and 10 species per genera to establish if the genera was less threatened than would be expected. This resulted in the exclusion of 18 of 30 genera from the analysis. Extinction risk was non-randomly distributed among genera (χ2 = 61.15, p < 0.001, d.f. = 28) with 11 of the remaining genera being more threatened than expected (table 2). Only the genus Cambarus showed a non-significant difference between the proportions of expected and observed threatened species.
Table 2.
Threat distribution across genera for which there were sufficient samples to determine whether species were more threatened than would be expected by chance, or under threatened: n.s., not significant; +, over threatened; −, under threatened.
| family | proportion observed | proportion expected | total species (non-DD) | >expected threat level p-value | <expected threat level p-value | over or under threatened |
|---|---|---|---|---|---|---|
| Pacifastacus | 0.333 | 0.009 | 3 | <0.001 | 1 | + |
| Cambarellus | 0.250 | 0.028 | 12 | <0.001 | 1 | + |
| Cambarus | 0.186 | 0.171 | 86 | 0.282 | 0.718 | n.s. |
| Fallicambarus | 0.188 | 0.031 | 16 | <0.001 | 1 | + |
| Hobbseus | 0.750 | 0.012 | 4 | <0.001 | 1 | + |
| Astacoides | 0.667 | 0.012 | 3 | <0.001 | 1 | + |
| Astacopsis | 0.333 | 0.005 | 3 | <0.001 | 1 | + |
| Cherax | 0.400 | 0.066 | 30 | <0.001 | 1 | + |
| Engaeus | 0.333 | 0.059 | 30 | <0.001 | 1 | + |
| Engaewa | 0.600 | 0.009 | 5 | <0.001 | 1 | + |
| Euastacus | 0.813 | 0.083 | 48 | <0.001 | 1 | + |
| Ombrastacoides | 0.333 | 0.019 | 9 | <0.001 | 1 | + |
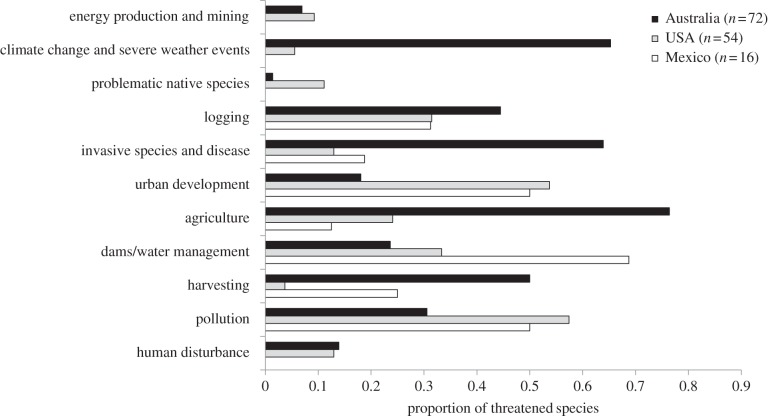
Sixty-five per cent of Australian threatened species were predicted to be at risk from climate-related threats, compared with only 5% of North American species. Similarly, invasive species, disease, agriculture and harvesting were found to impact a greater proportion of Australian threatened species than for Mexican and USA species. Threatened USA species were at greater threat from factors resulting in degradation and loss of habitat, notably urban development and pollution (figure 1). A similar pattern was observed in threatened Mexican species, but with dams and water management impacting a greater proportion of species. For Malagasy species, dominant threats were similar to those described for Australian species: invasive species, agriculture (i.e. land conversion for rice paddies) and harvesting but with no threat from climate change. On average, USA species were found to face fewer threats per threatened individual crayfish (2.1) than Mexican (2.2), Australian (3.9), Malagasy (4) and European (8) threatened species.
Figure 1.
Global threats affecting threatened species within the species-rich (>10 species) geographical regions.
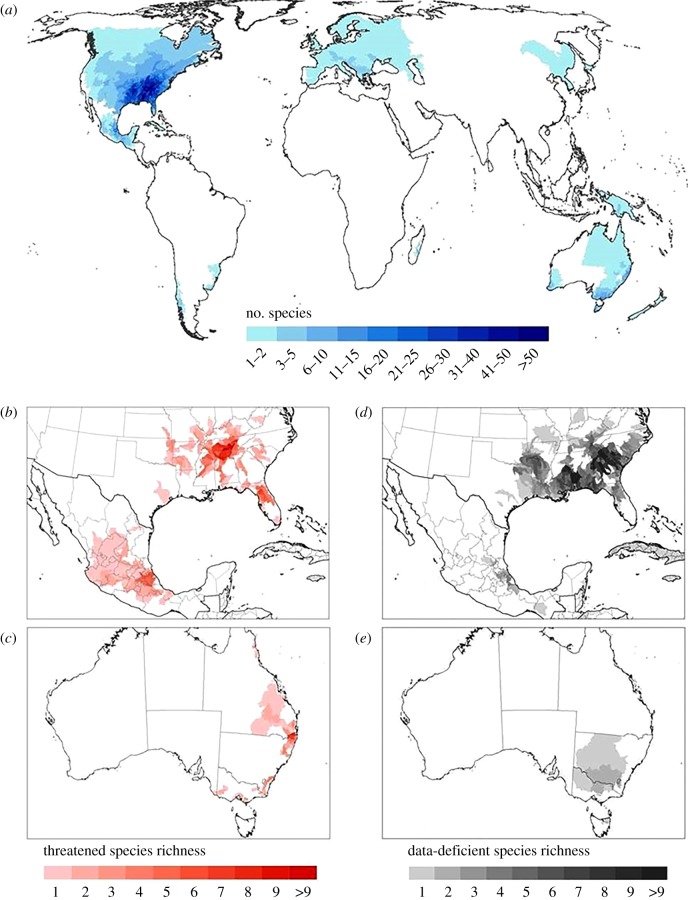
Crayfish were recorded in 60 countries, with 98% of species found to be endemic to a single country (562 of 590 spp.). In the USA, the major hotspot of diversity is in the southeast USA (notably Tennessee, Alabama and Mississippi; figure 2a) where 53% of species (189 of 357 spp.) are known from a single state. In Mexico, 95% (3 of 54 spp.) of species are endemic to the country with a major hotspot of diversity in the Gulf of Mexico region (figure 2a). In Australia, 84% (109 of 130 spp.) of species were found in only a single state with hotspots of diversity in the southeast and eastern Australia (southeast Victoria, Tasmania, northeastern New South Wales and southeastern Queensland; figure 2a). Distribution of threatened species richness (figure 2b,c) largely mirrors total species richness with higher numbers of threatened species in Australia (n = 60) than the USA (n = 56) or Mexico (n = 16). Numbers of DD species were highest in the USA (particularly Tennessee, South and North Carolina, the Florida Panhandle and Mobile River basin) and the Gulf of Mexico region (figure 2d) with 85% of DD species having an EOO of less than 20 000 km2. We observed relatively few DD species in Australia (figure 2e).
Figure 2.
Distribution of: (a) all species; (b) North American threatened species; (c) Australian threatened species; (d) North American data-deficient species; and (e) Australian data-deficient species. (Online version in colour.)
There was no correlation between data deficiency and centres of threatened species richness in Australia (r = 0.11, p = 0.60, d.f. = 24) or Mexico (r = 0.60, p = 0.086, d.f. = 710). However, there was a marginally non-significant correlation between data-deficiency and threatened species richness in the USA (r = 0.21, p = 0.06, d.f. = 141). There was low spatial overlap for both the USA (2%) and Australian (6.6%) threatened species and protected areas.
4. Discussion
(a). Patterns of threat and extinction risk
We found nearly one-third of the world's crayfish species are threatened with extinction. This level of threat exceeds that of most terrestrial and marine taxa, but is similar to that of the freshwater crabs and amphibians [5–7,13,43–45], highlighting the imperilled status of freshwater species. The taxonomically non-random distribution of extinction risk in crayfish suggests that certain intrinsic biological traits and external geographical factors might combine to influence risk. However, understanding the factors that drive high extinction risk and the synergistic effect of threats is complicated by a lack of spatial overlap between families [46], and by geographical variation in dominant threats; the biological traits that predict high risk under one threat type may not do so under another threat [47]. Notable differences in extinction risk between the genera of the Australian Parastacidae and the North American Cambaridae might be explained by levels of trait diversity, with both exhibiting considerable trait diversity across genera. For example, Parastacidae genera known only from Australia tend to exhibit small highly fragmented ranges, whereas South American and New Zealand genera exhibit large contiguous ranges (more than 20 000 km2). Differences in range size might be explained by the cooler climatic conditions of the Late Cretaceous and widescale flooding in both South America and New Zealand [48–50] both of which have facilitated crayfish dispersal. However, the Australian species-rich genera exhibit low trait diversity within genera, relative to genera of the Cambaridae [51]. For example, slow growth, apparent limited tolerances to increased temperatures [52], late sexual maturity and/or restricted ranges are all characteristic traits of the Australian genus Euastacus [53] (traits that tend to predict high risk of extinction in other taxa [33,34,54]), whereas the Australian Gramastacus and Geocharax are relatively small, have short lifespans and early sexual maturity, and can tolerate a wide range of environmental conditions as they occur in permanent and ephemeral freshwater systems [55]. Conversely, species of the North American genus Orconectes range from the cave-dwelling and long-lived (approx. 22 years) southern cave crayfish (Orconectes australis [56]), to the river- and lake-dwelling invasive spiny-cheek crayfish (Orconectes limosus) which lives for only 4 years [57].
Differences in the level of extinction risk between crayfish families might be partly explained by taxon age. A recent study of the world's marine lobsters dated the origin of Parastacidae to approximately 260 Ma and Cambaridae to approximately 160 Ma [58]. Older taxa might be expected to exhibit higher levels of extinction risk as all taxa must eventually go extinct [59]. A positive relationship between taxon age and extinction risk has been observed in birds [60]. However, in South Africa, the opposite relationship has been observed in plants where extinction risk is greater in the younger taxa [61]. The authors attribute this to the inherently small range size of rapidly diversifying lineages, a key trait for assessing extinction risk using the IUCN Red List Categories and Criteria [33]. There has been rapid diversification in the Cambaridae, resulting in 12 genera and 413 species (at the time of assessment; species lists are still growing), relative to the older Parastacidae (14 genera and 167 species). Congruence between areas of high human density and crayfish diversity might explain why the only known recent crayfish extinctions are from the USA and Mexico. With human density projected to increase within North America [62], continued loss and degradation of habitat (namely urban development, pollution, damming and water management) is likely not only to increase extinction rates but to impede future diversification.
While human density is lower in Australia than North America [62], Australian species face on average a greater number of threats. This complicates identifying the contribution of each threat to rates of decline as many threats act synergistically. For example, increasing temperatures and land conversion from natural state to agricultural use have increased the rate of irrigation, prompting water shortages and salinization of freshwater wetlands [63]. Similarly, increased logging of mature forests has increased the frequency of forest fires in southeast Australia [64]. While threats acting independently of one another may pose little danger to a species, threats acting synergistically can significantly increase rates of decline. In a recent study [65], declines in the population size of rotifers were 50 times faster when threats acted together. Uncertainty in the nature of dependency between threats poses a significant challenge to the effective allocation of conservation resources, and therefore may require action on multiple threats simultaneously.
Of all the geographical localities, European crayfish face the greatest number of threats, of which the most widespread is invasive species. Despite their large geographical ranges, declines of between 50% and 80% have been observed in the white-clawed crayfish (Austropotamobius pallipes) [66], and 50% and 70% in the noble crayfish (Astacus Astacus) [67]. The effect of interacting threats is particularly evident in the northern part of both species' ranges where populations have disappeared as rising temperatures have facilitated the range expansion of signal crayfish (Pacifastacus leniusculus) [68] and crayfish plague (Aphanomyces astaci) [69]. At present, invasive crayfish are not a widespread threat across the USA, although the invasive rusty crayfish (Orconectes rusticus) is currently expanding its range by up to 30 km per year [70]. The threat of invasive species was most evident in Australia, though invasive crayfish are a relatively minor threat relative to other species. Most of the Euastacus species are threatened by invasive predators such as cane toads (Rhinella marina) and feral pigs (Sus scrofa) which prey on young crayfish and destroy riparian habitat [53]. While invasive species are a prevalent threat to Australian crayfish, the impact of invasive species was often only attributed to localized declines [53].
(b). Deficits in knowledge
A high proportion of DD species can create taxonomic and geographical biases in the knowledge of extinction risk and the distribution of threat [46]. The proportion of DD crayfish was relatively similar to many previously assessed vertebrate groups (mammals, reptiles, amphibians and fish) [36], but low compared with other invertebrates, such as the freshwater crabs, dragonflies and freshwater molluscs [5–7]. Improved knowledge on the status of DD species is unlikely to significantly alter spatial patterns of extinction risk in the crayfish as there is already high spatial overlap between threatened and DD species in North America, and there are only small numbers of DD species elsewhere. However, the spatial overlap between threatened and DD North American species means there could be many more threatened species. An advantage of this close proximity means opportunities may exist to collect data on DD species while carrying out surveys on better known species, or species receiving survey attention because of conservation concern. Similarly, actions taken to protect better known species may positively benefit a number of these DD species. The majority of North American DD species have ranges smaller than 20 000 km2 and so may qualify for a threatened assessment under criterion B, if they are also found to be undergoing declines or fluctuations. However, a lack of information on whether threats are driving declines or fluctuations in range size, number of mature individuals or habitat quality prevented a threat assessment. There are entire genera for which there is little information on population trends, namely the Samastacus, Virilastacus and Cambaroides. Many of these species exhibit large continuous ranges and are therefore unlikely to qualify for a threat assessment under criteria B or D: threat assessments would only be possible under criterion A which would require detailed information on rates of population decline, or data sources from which to derive adequate proxies.
(c). Conservation
Despite the growing evidence for a freshwater biodiversity crisis, freshwater species remain a low priority on the conservation agenda. Freshwater species, particularly invertebrates, continue to be under-represented within protected area networks. In Africa, approximately one-third of threatened freshwater molluscs and freshwater crabs have 70% or more of their catchments within a protected area, compared with 75% of birds and 98% of mammals [3]. In this study, we observed even fewer crayfish within the boundaries of protected areas. Furthermore, our analysis was based on species ranges intersecting with protected areas which will overestimate the value of protected areas [71], so the proportion of species with greater than 70% of their catchments within protected area boundaries is almost certainly less. Even where species are within protected areas, these are unlikely to be managed for the preservation of freshwater biodiversity [72].
Similarly, freshwater invertebrates are under-represented on national endangered species lists. In the USA, 20% of mammals are listed on the Endangered Species Act list, compared with only 9% of molluscs and 1% of crayfish [73]. In Australia, 25% of terrestrial mammals are listed on the Environment Protection and Biodiversity Protection Act list, but only 5% of freshwater bivalves and 9% of crayfish [74]. Establishing effective conservation actions for many of the more threatened species is made complicated by the types of habitats occupied by some species. Many of the more threatened crayfish and freshwater molluscs are found in intermittent water bodies. Intermittent streams can support distinct and diverse biological communities, but despite their prevalence in the USA [75] they receive no protection under the US Clean Water Act [76].
Conservation of freshwater biodiversity is partly impeded by an inadequate understanding of the economic value of freshwater species and the services they provide [7]. To date, the majority of conservation effort is targeted towards charismatic species or those with a recognized economic value [77]. However, an economic valuation of biomes found freshwater systems were 34 times more valuable than terrestrial systems per unit area [78]. While placing an economic value on nature has its risks [79], realistic economic valuations of freshwater biodiversity and its services could be an important tool for moving freshwater conservation up the agenda.
Incorporating economics into conservation planning will aid the development of cost-effective measures. Conservation costs increase with extinction risk [80], and so actions focused on prevention rather than mitigation could present significant cost-saving opportunities. Invasive species are predicted to significantly increase extinction rates over the next century [81]. Every year, invasive species cost the USA economy $138 billion [82]. While the cost of eradication and control is often significantly higher than the cost of prevention [83], invasive species prevention is greatly under-funded [84]. A recent study estimated the cost of preventing zebra mussel (Dreissena polymorpha) invasion into one USA lake at $324 000 a year [84]. At present, the US Fish and Wildlife Service allocates $825 000 for the control and prevention of all invasive species in all lakes across the USA [84]. While it is not feasible to prevent invasion at all sites, not all sites are vulnerable to invasion. Prioritizing sites for protection from invasive species requires knowledge on the mechanisms of species colonization, suitability of habitat for invasive species, and the potential impact of the species [85]. A recent study employed machine learning methods for predicting sites most vulnerable to biological invasion by crayfish [85]. Methods such as these could be used to prioritize sites for protection by identifying hotspots of freshwater diversity that are most vulnerable to invasion by a range of aquatic invaders.
It is unlikely that actions against climate change can be implemented in a timescale that would avert significant biodiversity loss. A key strategy for tackling the effect of climate change will require the maintenance of ecological resilience—that is, the capacity of an ecosystem to withstand or recover from disturbance [86]. For many freshwater species, this will require maintenance of natural connectivity between freshwater habitats allowing for distributional shifts in response to changing environmental conditions. Two-thirds of Australian crayfish species are at risk from climate-mediated threats, a threat that is exacerbated by poor connectivity between areas of suitable habitat. However, identifying species most at risk is impeded by a lack of data on species' thermal limits and environmental parameters (e.g. moisture availability and temperature) [87]. Studies are needed to establish thermal tolerances in crayfish, whether thermal stress is already evident in Australian species, and establish current environmental parameters (primarily temperatures) for a representative selection of Australian ‘indicator’ species. These indicator species should include ‘at risk’ species from the various genera, and include the CR species of Euastacus that have been previously identified as ancient ‘climate refugees’ [52]. It would be prudent to develop management plans for the most CR species, and the need to consider maintaining captive populations and/or the relocation of species to more suitable habitats might be unavoidable given the nature and scale of the threats. With climate change now identified as one of the most significant threats affecting Australian freshwaters, developing baseline levels for a range of freshwater environmental parameters has been identified as a research priority [6,87–90]. Without action, it is predicted that climate change will increase in extent and intensity over the next century [91], and so many of the research gaps discussed here need to be considered in other freshwater biodiversity hotspots. Without efforts to address these data gaps, identification of ‘at risk’ species will be difficult and will limit future efforts to protect the ecological integrity of freshwaters.
This study highlights the major research gaps that hamper effective conservation planning for crayfish, many of which would positively benefit a range of freshwater taxa. Conservation planning needs to shift from a reactive to proactive approach if we are to safeguard freshwater systems against anthropogenic environmental damage.
Acknowledgements
The authors acknowledge the Esmée Fairbairn Foundation (N.I.R. and M.B.), the Rufford Foundation (B.C. and M.B.) and the Biodiversity Synthesis Center in Chicago who funded and hosted the workshop in which all North American species were assessed. For data compilation, we thank Alison Batchelor, Georgia Cryer, Ranmali De Silva, Ellie Dyer, Blythe Jopling, Gita Kasthala, Fiona Livingston, Maiko Lutz, Shane McGuinness, Harriet Milligan, Mala Ram, Jeremy Smith, Anne-Marie Soulsby, Kathryn Sullivan and Felix Whitton. We also acknowledge the vast network of data contributors who have been kind enough to review reports; Christopher Austin, Miklós Puky, Eva Kozubikova, William R. Elliott, John Foster, Holga Schulz, Ivana Maguire, Japo Jussila, Stephanie Peay, Peter Manko, Emmanuil Manos, Marina Paolucci, Martin Pugh, Naomi Wilson, Paul Bradley, Ralf Schulz, Boris Sket, Søren Berg, Adrian Hutchings, Angel Zaikov, Margaret Palmer, Daniel Hefti, Yoann Allanic, Markku Pursiainen, Liviu Miron, Lucian Pârvulescu, Peter Manko, Roger Cammaerts, Valery Fedotov, Olga Mandryka, Pedro Gutiérrez and Przemysław Śmietana. We thank our two anonymous referees for helpful comments on an earlier version of this paper.
References
- 1.Strayer D, Dudgeon D. 2010. Freshwater biodiversity conservation: recent progress and future challenges. J. N. Am. Benthol. Soc. BioOne 29, 344–358. ( 10.1899/08-171.1) [DOI] [Google Scholar]
- 2.Vörösmarty C, et al. 2010. Global threats to human water security and river biodiversity. Nature 467, 555–561. ( 10.1038/nature09440) [DOI] [PubMed] [Google Scholar]
- 3.Darwall WRT, et al. 2011. Implications of bias in conservation research and investment for freshwater species. Conserv. Lett. 4, 474–482. ( 10.1111/j.1755-263X.2011.00202.x) [DOI] [Google Scholar]
- 4.Holland RA, Darwall WRT, Smith KG. 2012. Conservation priorities for freshwater biodiversity: the key biodiversity area approach refined and tested for continental Africa. Biol. Conserv. 148, 167–179. ( 10.1016/j.biocon.2012.01.016) [DOI] [Google Scholar]
- 5.Cumberlidge N, et al. 2009. Freshwater crabs and the biodiversity crisis: Importance, threats, status, and conservation challenges. Biol. Conserv. 142, 1665–1673. ( 10.1016/j.biocon.2009.02.038) [DOI] [Google Scholar]
- 6.Clausnitzer V, et al. 2009. Odonata enter the biodiversity crisis debate: The first global assessment of an insect group. Biol. Conserv. 142, 1864–1869. ( 10.1016/j.biocon.2009.03.028) [DOI] [Google Scholar]
- 7.Darwall W, Seddon M, Clausnitzer V, Cumberlidge N. 2012. Chapter 2. Freshwater invertebrate life. In Spineless: status and trends of the world's invertebrates (eds Collen B, Böhm M, Kemp R, Baillie J.), pp. 26–33. London, UK: Zoological Society of London. [Google Scholar]
- 8.Ricciardi A, Rasmussen JB. 1999. Extinction rates of North American freshwater fauna. Conserv. Biol. 13, 1220–1222. ( 10.1046/j.1523-1739.1999.98380.x) [DOI] [Google Scholar]
- 9.Freyhof J, Brooks E. 2011. European Red List of freshwater fishes. Luxembourg: Publications Office of the European Union. [Google Scholar]
- 10.Convention on Biological Diversity. 2010. COP 10 decision X/2. Strategic plan for biodiversity 2011–2020. Montreal, Canada. [Google Scholar]
- 11.Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. ( 10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 12.Grenyer R, et al. 2006. Global distribution and conservation of rare and threatened vertebrates. Nature 444, 93–96. ( 10.1038/nature05237) [DOI] [PubMed] [Google Scholar]
- 13.Schipper J, et al. 2008. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230. ( 10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- 14.Oliver I, Beattie AJ, York A. 1998. Spatial fidelity of plant, vertebrate, and invertebrate assemblages in multiple-use forest in eastern Australia. Conserv. Biol. 12, 822–835. ( 10.1046/j.1523-1739.1998.97075.x) [DOI] [Google Scholar]
- 15.Whiting A, Lawler S, Horwitz P, Crandall K. 2000. Biogeographic regionalisation of Australia: assigning conservation priorities based on endemic freshwater crayfish phylogenetics. Anim. Conserv. 3, 155–163. ( 10.1111/j.1469-1795.2000.tb00240.x) [DOI] [Google Scholar]
- 16.Scholtz G. 2002. Phylogeny and evolution. In Biology of freshwater crayfish (ed. Holdich D.), pp. 30–52. Oxford, UK: Blackwell Science. [Google Scholar]
- 17.Ortmann AE. 1987. Ueber ‘Bipolarität’ in der Verbreitung mariner Tiere. Zool. Jahrb ü cher, Abteilung Syst. 9, 571–595. [Google Scholar]
- 18.Banarescu P. 1990. Zoogeography of fresh waters, vol. 1 Wiesbaden, Germany: Aula. [Google Scholar]
- 19.Hobbs H., Jr 1988. Crayfish distribution, adaptive radiation and evolution. In Freshwater crayfish: biology, management and exploitation (eds Holdich D, Lowery R.), pp. 55–82. London, UK: Croom Helm Ltd. [Google Scholar]
- 20.Adegboye D. 1983. On the non-existence of an indigenous species of crayfish on the continent of Africa. Freshw. Crayfish 5, 564–569. [Google Scholar]
- 21.Hobbs HH. 1989. An illustrated checklist of the American crayfishes (Decapoda, Astacidae, Cambaridae, Parastacidae). Smithsonian Contrib. Zool. 480, 1–236. ( 10.5479/si.00810282.480) [DOI] [Google Scholar]
- 22.Usio N, Townsend CR. 2001. The significance of the crayfish Paranephrops zealandicus as shredders in a New Zealand headwater stream. J. Crustac. Biol. 21, 354–359. ( 10.1163/20021975-99990135) [DOI] [Google Scholar]
- 23.Reynolds J, Souty-Grosset C, Richardson A. 2013. Ecological roles of crayfish in freshwater and terrestrial habitats. Freshw. Crayfish 19, 197–218. ( 10.5869/fc.2013.v19-2.197) [DOI] [Google Scholar]
- 24.Jones JPG, Andriahajaina FB, Ranambinintsoa EH, Hockley NJ, Ravoahangimalala O. 2006. The economic importance of freshwater crayfish harvesting in Madagascar and the potential of community-based conservation to improve management. Oryx 40, 168–175. ( 10.1017/S0030605306000500) [DOI] [Google Scholar]
- 25.Reynolds J, Souty-Grosset C. 2012. Management of freshwater biodiversity: crayfish as bioindicators. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Thies CG, Porche S. 2007. Crawfish tails: a curious tale of foreign trade policy making. Foreign Policy Anal. 3, 171–187. ( 10.1111/j.1743-8594.2007.00046.x) [DOI] [Google Scholar]
- 27.Taylor CA, et al. 2007. A reassessment of the conservation status of crayfishes of the United States and Canada after 10+ years of increased awareness. Fisheries 32, 372–389. ( 10.1577/1548-8446(2007)32[372:AROTCS]2.0.CO;2) [DOI] [Google Scholar]
- 28.Furse J. 2014. The freshwater crayfish fauna of Australia: update on conservation status and threats. In Advances in freshwater decapod systematics and biology. Crustaceana monographs 19 (eds Yeo D, Cumberlidge N, Klaus S.), pp. 273–296. Leiden, The Netherlands: Brill Publishers. [Google Scholar]
- 29.Furse J, Coughran J. 2011. An assessment of the distribution, biology, threatening processes and conservation status of the freshwater crayfish, genus Euastacus (Decapoda: Parastacidae), in Continental Australia. II. Threats, conservation assessments and key findings. In New Frontiers in Crustacean Biology: Proc. TCS Summer Meeting, Tokyo, Japan, 20–24 September 2009 Crustaceana Monographs 15 (ed. Akasura A.), pp 253–263. Leiden, The Netherlands: Brill Publishers; ( 10.1163/ej.9789004174252.i-354.172) [DOI] [Google Scholar]
- 30.Crandall K, Buhay J. 2008. Global diversity of crayfish (Astacidae, Cambaridae, and Parastacidae: Decapoda). Hydrobiologia 595, 295–301. ( 10.1007/s10750-007-9120-3) [DOI] [Google Scholar]
- 31.Holdich DM, Reynolds JD, Souty-Grosset C, Sibley PJ. 2009. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowledge Manage. Aquat. Ecosyst. 394–395, 11 ( 10.1051/kmae/2009025) [DOI] [Google Scholar]
- 32.IUCN. 2001. IUCN Red List Categories and Criteria v. 3.1. Gland, Switzerland: IUCN. [Google Scholar]
- 33.IUCN Standards and Petitions Subcommittee. 2014. Guidelines for Using IUCN Red List Categories and Criteria, v. 11 Gland, Switzerland: IUCN. [Google Scholar]
- 34.Darwall W, Smith K, Allen D, Seddon M, McGregor RG, Clausnitzer V, Kalkman V. 2009. Freshwater biodiversity: a hidden resource under threat. In Wildlife in a changing world: an analysis of the 2008 IUCN Red List of threatened species (eds Vie J, Hilton-Taylor C, Stuart S.), pp. 43–54. Gland, Switzerland: IUCN. [Google Scholar]
- 35.USGS EROS. 2010. HYDRO1k elevation derivative database. LP DAAC, Sioux Falls, South Dakota. See http://eros.usgs.gov/#/Find_Data/Products_and_Data_Available/gtopo30/hydro.
- 36.Hoffmann M, et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330, 1503–1509. ( 10.1126/science.1194442) [DOI] [PubMed] [Google Scholar]
- 37.Cardillo M, Meijaard E. 2012. Are comparative studies of extinction risk useful for conservation? Trends Ecol. Evol. 27, 167–171. ( 10.1016/j.tree.2011.09.013) [DOI] [PubMed] [Google Scholar]
- 38.Bielby J, Cunningham AA, Purvis A. 2006. Taxonomic selectivity in amphibians: ignorance, geography or biology? Anim. Conserv. 9, 135–143. ( 10.1111/j.1469-1795.2005.00013.x) [DOI] [Google Scholar]
- 39.Salafsky N, et al. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conserv. Biol. 22, 897–911. ( 10.1111/j.1523-1739.2008.00937.x) [DOI] [PubMed] [Google Scholar]
- 40.Clifford P, Richardson S, Hémon D. 1989. Assessing the significance of the correlation between two spatial processes. Biometrics 45, 123–134. ( 10.2307/2532039) [DOI] [PubMed] [Google Scholar]
- 41.Dudley N. 2008. Guidelines for applying protected area management categories. IUCN WCPA best practice guidance on recognising protected areas and assigning management categories and governance types, best practice protected area guidelines series No. 21. Gland, Switzerland. [Google Scholar]
- 42.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See www.R-project.org. [Google Scholar]
- 43.Butchart S, et al. 2004. Measuring global trends in the status of biodiversity: Red list indices for birds. PLoS Biol. 2, e383 ( 10.1371/journal.pbio.0020383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kemp R, Peters H, Allcock A, Carpenter K, Obura D, Polidoro B, et al. 2012. Chapter 3. Marine invertebrate life. In Spineless: status and trends of the world's invertebrates (eds Collen B, Böhm M, Kemp R, Baillie J.), pp. 34–45. London, UK: Zoological Society of London. [Google Scholar]
- 45.Böhm M, et al. 2013. The conservation status of the world's reptiles. Biol. Conserv. 157, 372–385. ( 10.1016/j.biocon.2012.07.015) [DOI] [Google Scholar]
- 46.Bland LM, Collen B, Orme CDL, Bielby J. 2012. Data uncertainty and the selectivity of extinction risk in freshwater invertebrates. Divers. Distrib. 18, 1211–1220. ( 10.1111/j.1472-4642.2012.00914.x) [DOI] [Google Scholar]
- 47.Isaac NJB, Cowlishaw G. 2004. How species respond to multiple extinction threats. Proc. R. Soc. Lond. B 271, 1135–1141. ( 10.1098/rspb.2004.2724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodburne MO, Case JA. 1996. Dispersal, vicariance, and the Late Cretaceous to early tertiary land mammal biogeography from South America to Australia. J. Mamm. Evol. 3, 121–161. ( 10.1007/BF01454359) [DOI] [Google Scholar]
- 49.Hamilton SK, Sippel SJ, Melack JM. 2002. Comparison of inundation patterns among major South American floodplains. J. Geophys. Res. D Atmos. 107, 1–14. ( 10.1029/2000JD000306) [DOI] [Google Scholar]
- 50.Burridge CP, Craw D, Jack DC, King TM, Waters JM. 2008. Does fish ecology predict dispersal across a river drainage divide? Evolution (NY) 62, 1484–1499. ( 10.1111/j.1558-5646.2008.00377.x) [DOI] [PubMed] [Google Scholar]
- 51.Adamowicz S, Purvis A. 2006. Macroevolution and extinction risk patterns in freshwater crayfish. Freshw. Crayfish 15, 1–23. [Google Scholar]
- 52.Bone J, Wild C, Furse J. 2014. Thermal limit of Euastacus sulcatus (Decapoda: Parastacidae), a freshwater crayfish from the highlands of central eastern Australia. Mar. Freshw. Res. 65, 645–651. ( 10.1071/MF13189) [DOI] [Google Scholar]
- 53.Furse J, Coughran J. 2011. An assessment of the distribution, biology, threatening processes and conservation status of the freshwater crayfish, genus Euastacus (Decapoda: Parastacidae), in Continental Australia. I. Biological background and current status. In New Frontiers in Crustacean Biology: Proc. TCS Summer Meeting, Tokyo, Japan, 20–24 September 2009 Crustaceana Monographs 15 (ed. Akasura A.), 241–252. Leiden, The Netherlands: Brill Publishers. [Google Scholar]
- 54.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. 2000. Predicting extinction risk in declining species. Proc. R .Soc. Lond. B. 267, 1947–1952. ( 10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston K, Robson B. 2009. Habitat use by five sympatric Australian freshwater crayfish species (Parastacidae). Freshw. Biol. 54, 1629–1641. ( 10.1111/j.1365-2427.2009.02213.x) [DOI] [Google Scholar]
- 56.Venarsky MP, Huryn AD, Benstead JP. 2012. Re-examining extreme longevity of the cave crayfish Orconectes australis using new mark–recapture data: a lesson on the limitations of iterative size-at-age models. Freshw. Biol. 57, 1471–1481. ( 10.1111/j.1365-2427.2012.02812.x) [DOI] [Google Scholar]
- 57.Smith DG. 1981. Life history parameters of the crayfish Orconectes limosus (Raf.) in Southern New England. Ohio J. Sci. 81, 169–172. [Google Scholar]
- 58.Bracken-Grissom H, et al. 2014. The emergence of the lobsters: phylogenetic relationships, morphological evolution and divergence time comparisons of an ancient group (Decapoda: Achelata, Astacidea, Glypheidea, Polychelida). Syst. Biol. 63, 457–479. ( 10.1093/sysbio/syu008) [DOI] [PubMed] [Google Scholar]
- 59.May R, Lawton J, Stork N. 1995. Assessing extinction rates. In Extinction rates (eds Lawton J, May R.), pp. 1–24. Oxford, UK: Oxford University Press. [Google Scholar]
- 60.Gaston KJ, Blackburn TM. 1997. Evolutionary age and risk of extinction in the global avifauna. Evol. Ecol. 11, 557–565. ( 10.1007/s10682-997-1511-4) [DOI] [Google Scholar]
- 61.Davies TJ, et al. 2011. Extinction risk and diversification are linked in a plant biodiversity hotspot. PLoS Biol. 9, e1000620 ( 10.1371/journal.pbio.1000620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Bank. 2014. Population density 2009–2013. World Development Indicators. See http://data.worldbank.org/ (accessed 20 April 2014). [Google Scholar]
- 63.Nielsen DL, Brock MA. 2009. Modified water regime and salinity as a consequence of climate change: prospects for wetlands of Southern Australia. Clim. Change 95, 523–533. ( 10.1007/s10584-009-9564-8) [DOI] [Google Scholar]
- 64.Lindenmayer DB, Hunter ML, Burton PJ, Gibbons P. 2009. Effects of logging on fire regimes in moist forests. Conserv. Lett. 2, 271–277. ( 10.1111/j.1755-263X.2009.00080.x) [DOI] [Google Scholar]
- 65.Mora C, Metzger R, Rollo A, Myers RA. 2007. Experimental simulations about the effects of overexploitation and habitat fragmentation on populations facing environmental warming. Proc. R. Soc. B. 274, 1023–1028. ( 10.1098/rspb.2006.0338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Füreder L, Gherardi F, Holdich D, Reynolds J, Sibley P. 2010. Souty-Grosset C. Austropotamobius pallipes. In IUCN 2013. IUCN Red List of Threatened Species, v. 2013.2. Downloaded on 19 February 2014 www.iucnredlist.org.
- 67.Edsman L, Füreder L, Gherardi F, Souty-Grosset C. 2010. Astacus astacus. In IUCN 2013. IUCN Red List of Threatened Species, v. 2013.2 www.iucnredlist.org Downloaded on 14 March 2014. [Google Scholar]
- 68.Bubb D, Thom T, Lucas M. 2004. Movement and dispersal of the invasive signal crayfish Pacifastacus leniusculus in upland rivers. Freshw. Biol. 49, 357–368. ( 10.1111/j.1365-2426.2003.01178.x) [DOI] [Google Scholar]
- 69.Bohman P, Nordwall F, Edsman L. 2006. The effect of the large-scale introduction of signal crayfish on the spread of crayfish plague in Sweden. Bull. Fr Pêche Piscic. 380–381, 1291–1302. ( 10.1051/kmae:2006026) [DOI] [Google Scholar]
- 70.Sorenson K, Bollens S, Counihan T. 2012. Rapid range expansion of rusty crayfish Orconectes rusticus (Girard, 1852) in the John Day River, Oregon, USA. Aquat. Invasions 7, 291–294. ( 10.3391/ai.2012.7.2.017) [DOI] [Google Scholar]
- 71.Brooks TM, et al. 2004. Coverage provided by the global protected-area system: is it enough? BioScience 54, 1081–1091. ( 10.1641/0006-3568(2004)054[1081:CPBTGP]2.0.CO;2) [DOI] [Google Scholar]
- 72.Abell R, Allan JD, Lehner B. 2007. Unlocking the potential of protected areas for freshwaters. Biol. Conserv. 134, 48–63. ( 10.1016/j.biocon.2006.08.017) [DOI] [Google Scholar]
- 73.US Fish and Wildlife Service. 2013. Endangered species. See http://www.fws.gov/endangered/
- 74.Australian Government Department of Sustainability Environment Water Population and Communities. 2013. EPBC act list of threatened fauna. See http://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl.
- 75.Meyer JL, Strayer DL, Wallace JB, Eggert SL, Helfman GS, Leonard NE. 2007. The contribution of headwater streams to biodiversity in river networks. J. Am. Water Resour. Assoc. 43, 86–103. ( 10.1111/j.1752-1688.2007.00008.x) [DOI] [Google Scholar]
- 76.CWA. 1972. Clean water act. See http://www2.epa.gov/laws-regulations/summary-clean-water-act (accessed 20 April 2014).
- 77.Bilz M, Nieto A, Sánchez S, Alexander KN, Cuttelod A, Kalkman VJ, et al. 2012. Chapter 5. Invertebrates: our natural capital. In Spineless: status and trends of the world's invertebrates (eds Collen B, Böhm M, Kemp R, Baillie JE.), pp. 60–71. London, UK: Zoological Society of London. [Google Scholar]
- 78.Costanza R, et al. 1997. The value of the world's ecosystem services and natural capital. Nature 387, 253–260. ( 10.1038/387253a0) [DOI] [Google Scholar]
- 79.Abell R. 2002. Conservation biology for the biodiversity crisis: a freshwater follow-up. Conserv. Biol. 16, 1435–1437. ( 10.1046/j.1523-1739.2002.01532.x) [DOI] [PubMed] [Google Scholar]
- 80.Brooks T, et al. 2006. Global biodiversity conservation priorities. Science 313, 58–61. ( 10.1126/science.1127609) [DOI] [PubMed] [Google Scholar]
- 81.Lodge D, Taylor C, Holdich D, Skurdal J. 2000. Nonindigenous crayfishes threaten north American freshwater biodiversity: lessons from Europe. Fisheries 25, 7–20. () [DOI] [Google Scholar]
- 82.Pimentel D, Lach L, Zuniga R, Morrison D. 2000. Environmental and economic costs of nonindigenous species in the United States. BioScience 50, 53–65. ( 10.1641/0006-3568(2000)050[0053:EAECON]2.3.CO;2) [DOI] [Google Scholar]
- 83.Allendorf FW, Lundquist LL. 2003. Introduction: population biology, evolution, and control of invasive species. Conserv. Biol. 17, 24–30. ( 10.1046/j.1523-1739.2003.02365.x) [DOI] [Google Scholar]
- 84.Leung B, Lodge DM, Finnoff D, Shogren JF, Lewis MA, Lamberti G. 2002. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proc. R. Soc. Lond. B 269, 2407–2413. ( 10.1098/rspb.2002.2179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vander Zanden MJ, Olden JD. 2008. A management framework for preventing the secondary spread of aquatic invasive species. Can. J. Fish Aquat. Sci. 65, 1512–1522. ( 10.1139/F08-099) [DOI] [Google Scholar]
- 86.Walker B, et al. 2002. Resilience management in social-ecological systems: a working hypothesis for a participatory approach. Conserv Ecol. 6, 14. [Google Scholar]
- 87.Bond N, Thomson J, Reich P, Stein J. 2011. Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Mar. Freshw. Res. 62, 1043–1061. ( 10.1071/MF10286) [DOI] [Google Scholar]
- 88.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 89.Koehn JD, Hobday AJ, Pratchett MS, Gillanders BM. 2011. Climate change and Australian marine and freshwater environments, fishes and fisheries: synthesis and options for adaptation. Mar. Freshw. Res. 62, 1148–1164. ( 10.1071/MF11139) [DOI] [Google Scholar]
- 90.Furse J, Coughran J. 2011. An assessment of the distribution, biology, threatening processes and conservation status of the freshwater crayfish, genus Euastacus (Decapoda: Parastacidae), in Continental Australia. III. Case studies and recommendations. In New Frontiers in Crustacean Biology: Proc. TCS Summer Meeting, Tokyo, Japan, 20–24 September 2009 Crustaceana Monographs 15 (ed. Akasura A.), pp. 265–274. Leiden, The Netherlands: Brill Publishers; ( 10.1163/ej.9789004174252.i-354.179) [DOI] [Google Scholar]
- 91.IPCC. 2007. Summary for policymakers. In Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S, Qin M, Manning M, Chen Z, Marquis M, Averyt K, et al.). Cambridge, UK: Cambridge University Press. [Google Scholar]