Abstract
We developed a novel strategy for fabrication of microfluidic paper-based analytical devices (μPADs) by selective wet etching of hydrophobic filter paper using a paper mask having a specific design. The fabrication process consists of two steps. First, the hydrophilic filter paper was patterned hydrophobic by using trimethoxyoctadecylsilane (TMOS) solution as the patterning agent. Next, a paper mask penetrated with NaOH solution (containing 30% glycerol) was aligned onto the hydrophobic filter paper, allowing the etching of the silanized filter paper by the etching reagent. The masked region turned highly hydrophilic whereas the unmasked region remains highly hydrophobic. Thus, hydrophilic channels, reservoirs, and detection zones were generated and delimited by the hydrophobic barriers. The effects of some factors including TMOS concentration, etching temperature, etching time, and NaOH concentration on fabrication of μPAD were studied. Being free of any expensive equipment, metal mask and expensive reagents, this rapid, simple, and cost-effective method could be used to fabricate μPAD by untrained personnel with minimum cost. A flower-shaped μPAD fabricated by this presented method was applied to the glucose assay in artificial urine samples with good performance, indicating its feasibility as a quantitative analysis device. We believe that this method would be very attractive to the development of simple microfluidic devices for point-of-care applications in clinical diagnostics, food safety, and environmental protection.
I. INTRODUCTION
An increasingly important emerging area in chemical sensing is the development of rapid, simple, and cost-effective sensors for medical diagnostics, environmental testing, and food safety monitoring. Lab-on-a-chip, or micrototal analysis system (μTAS), has received much attention since this concept was first introduced by Manz et al.1 owing to the advantages of low sample/reagent consumption and fast analysis speed over the conventional analytical techniques.2–6 Although the proof-of-concept of lab-on-a-chip were widely demonstrated in the laboratories, far fewer real applications have been reported. One of the reasons is that the costly and time-consuming processes such as photolithography,7 chemical etching,8 and laser microfabrication9 are usually necessary in fabrication of microsystems on polymers, glass, and silicon. Moreover, expensive instruments and trained personnel are required. Microfluidic paper-based analytical device (μPAD) is an ideal alternative to the conventional lab-on-a-chip device fabricated on materials of polymers, glass, and silicon. Compared to the microfluidic devices fabricated on glass, polymers and silicon, μPADs have numerous advantages including low cost, easy and fast fabrication, portability and disposability. As a result, the recent years have witnessed fast development of μPADs in point-of-care diagnostics,10,11 environmental testing,12,13 and food monitoring14 since Martinez et al.15 first introduced the concept of μPAD in 2007.
Thus far, three strategies have been reported for the fabrication of μPADs. The first strategy is to generate hydrophilic-hydrophobic contrast by printing hydrophobic materials or reagents onto the hydrophilic substrate.16–22 Lu et al.16 transferred a pattern designed digitally on the filter paper using a wax printer. Although this method allows mass production of μPADs, it is limited by the expensive equipment and thus not suitable for applications in the developing countries and resource-limited regions. Recently, an inkjet printing method has become a relatively cheap alternative to the wax printing method. By inkjet printing method, various hydrophobic materials or reagents such as alkenyl ketene dimer (AKD)18,19 and poly(styrene) layer20 could be printed onto the filter paper substrate. The inkjet printing methods allows mass production of μPADs with a simple, rapid, and low-cost fabrication process. However, the commercial inkjet printers have to be modified by replacing the ink in the cartridge with the patterning agents dissolved in organic solvents. Thus, the printers may be easily damaged by the organic solvents. The second strategy for fabrication of μPADs is to deposit the hydrophobic materials or reagents onto the hydrophilic paper substrate using a metal mask having a specific design.23–27 By using a metal mask, various hydrophobic materials including wax,23–25 acrylic lacquer,26 and permanent ink27 could be deposited onto the filter paper to generate hydrophobic-hydrophilic contrast on the hydrophilic substrate. This strategy possesses the advantages such as low cost and simplicity; furthermore, no expensive equipment is required. Unfortunately, the metal masks have to be fabricated with expensive equipment such as linear cutting machine or laser cutting machine. To address this limitation, we recently described a novel method for fabrication of μPAD by silanization of filter cellulose using a paper mask.28 This method is simple and cost effective. Additionally, the paper mask is fabricated by cutting from a filter paper sheet with a common knife; thus, expensive cutting machine for fabricating masks are not required. The third strategy for fabrication of μPAD is to selectively pattern the hydrophobic substrate hydrophilic or vice versa by photolithography,15,29 laser etching,30 and plasma treatment.31 Nevertheless, a common limitation of this strategy is that the expensive equipments, such as lithographic equipment, CO2 laser, and plasma oxidizer, are required for the fabrication of μPADs; thus, this strategy is not suitable for the fabrication and application of μPADs in developing countries and resource-limited regions.
In this work, we described a novel, simple, and cost-effective method for fabrication of μPAD based on the selective wet etching of the hydrophobic filter paper using a paper mask. The filter paper sheet was silanized and turned highly hydrophobic by soaking in 2.0% trimethoxyoctadecylsilane (TMOS) solution. A filter paper mask having a specific design was then soaked in NaOH solution followed by aligning onto the hydrophobic paper, allowing to selectively etch the masked region of hydrophobic filter paper by the etching agent adsorbed on the paper mask. Thus, a hydrophilic-hydrophobic contrast was generated on the hydrophobic filter paper. Being free of any expensive instruments, metal masks, expensive reagents, and trained personnel, this method is novel, simple, and cost-effective, allowing rapid fabrication of μPAD within 5 min. A flower-shaped μPAD having six channels and detection zones was fabricated for determination of glucose in artificial urine samples, demonstrating its potential in biological assays.
II. EXPERIMENTAL
A. Chemicals and apparatus
All the chemicals used were of analytical grade unless mentioned otherwise, and demineralized water was used throughout. TMOS was purchased from Aladdin Industrial Co. (Shanghai, China). 2.0% (v/v) TMOS-heptane solution (containing 5% ethyl acetate) was used as patterning agent for silanization of hydrophilic filter paper. 0.1 mol l−1 NaOH solution (containing 30% glycerol) was used as the etching agents. A 200 mM phosphate buffer solution was prepared by combining 21.85 g Na2HPO4•12H2O and 6.08 g NaH2PO4•2H2O in 300 ml of H2O, and pH was adjusted to 7.0 and then was diluted to 500 ml. A 6.0 mol l−1 potassium iodide solution was prepared by dissolving 4.980 g potassium iodide in 5 ml of water. Glucose oxidase solution was prepared by dissolving 20 mg of glucose oxidase (Biological grade, Shanghai Jinsui Bio-Technology Co., Ltd., Shanghai, China) in 50 ml of buffer solution. Horseradish peroxidase solution was prepared by dissolving 13.4 mg of Horseradish peroxidase (Biological grade, Shanghai Jinsui Bio-Technology Co., Ltd., Shanghai, China) in 50 ml of buffer solution. The enzyme solutions were mixed by a ratio of 1:1 before use. The artificial urine solution was prepared according to the methods provided by references.15,32 Briefly, the artificial urine solution contains 1.1 mM lactic acid, 2.0 mM citric acid, 25 mM sodium bicarbonate, 170 mM urea, 10 mM CaCl2, 90 mM NaCl, 2.0 mM MgSO4, 10 mM NaSO4, 7.0 mM KH2PO4, 7.0 mM K2HPO4, and 25 mM NH4Cl. The pH of the urine solution was adjusted to 6.1 with 1.0 mol l−1 hydrochloric acid. A glucose stock standard solution (100 mmol l−1) was prepared by dissolving 1.9820 g glucose in 50 ml of artificial urine solution and diluted to 100 ml. The glucose working standard solutions were prepared by appropriate dilution of the stock standard solution with urine solution. A knife was used to fabricate the mask on filter paper (102, Hangzhou Xinhua Paper Limited, Hangzhou, China). A digital camera (Canon IXUS9515, Japan) was used to capture the images of glucose assays on μPADs. A contact angle meter (JC20001, Shanghai Zhongchen Digital Technic Apparatus Co., Ltd., Shanghai, China) was used to measure the contact angles based on the sessile drop method, using a water drop of 6 μl.
B. Fabrication of μPAD
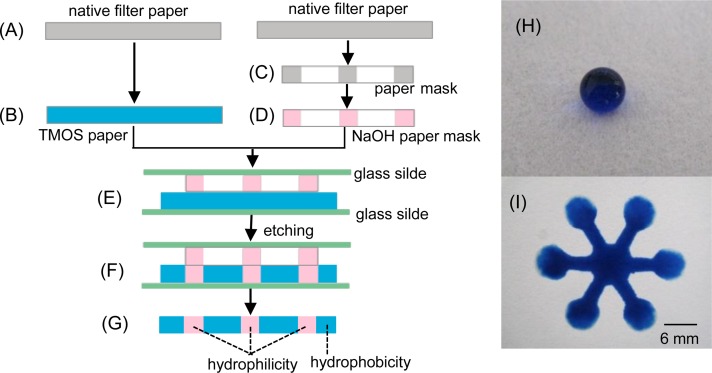
The principle of fabrication was schematically shown in Figure 1. The hydrophobic filter paper sheets were produced by immersing in 2.0% TMOS solution for 10 s (Figure 1(b)) and then air dried for 5 min, followed by heating for 1 h at 100 °C. The paper sheets could be used for the following etching procedure after 2 h of withdrawal from the heater. The paper mask was fabricated by printing a pattern designed digitally onto a filter paper with a laserJet printer (HP LaserJet 1020 plus, USA), followed by cutting along the printed pattern on the filter paper with a knife (Figure 1(c)). The paper mask was immersed into 0.1 mol l−1 NaOH solution (containing 30% glycerol) for 10 s (Figure 1(d)) and then put onto a glass slide, then a hydrophobic filter paper sheet and another glass slide were aligned onto the paper mask sequentially (Figure 1(e)). The paper mask and hydrophobic paper sheet sandwiched with glass slides were heated for 5 min at 60 °C, allowing NaOH adsorbed on paper mask to penetrate completely into the hydrophobic paper (Figure 1(f)). Thus, the hydrophilic-hydrophobic contrast was generated on the hydrophobic paper (Figure 1(g)). The fabricated μPAD was ready for use after washing with H2O.
FIG. 1.
Schematic diagram of fabricating μPAD by wet etching. Cross section: (a) native filter paper, (b) hydrophobic filter paper obtained by soaking in TMOS solution, (c) paper mask produced by cutting on the filter paper, (d) paper mask penetrated with NaOH solution, (e) Hydrophobic paper and paper mask sandwiched with glass slides, (f) Assembly after etching, and (g) μPAD with hydrophilic-hydrophobic contrast. (h) Photograph obtained by dropping 10 μl of aqueous blue solution on the hydrophobic filter paper patterned with TMOS. (i) Photograph of a flower-shaped μPAD after dropping aqueous blue solution onto the central unit of μPAD.
C. Glucose assay
A flower-shaped μPAD having 6 channels, 6 detection zones, and 1 central unit was fabricated and used for glucose assay. The principle for glucose assay was based on that described previously.15,33 Briefly, glucose is oxidized by glucose oxidase to produce gluconic acid and hydrogen peroxide. The produced hydrogen peroxide is then reduced to water by horseradish peroxidase, along with the oxidation of iodide to iodine.33 For the glucose assay, 50 μl of potassium iodide was initially spotted onto the central unit. After the solution flowed to the detection zones and air dried at room temperature for 8 min, 0.8 μl of 1:1 glucose oxidase/horseradish peroxidase solution and 0.8 μl of standard glucose solutions were spotted onto 6 detection zones, respectively. The image of the colorimetric assay was captured with a camera, and the gray values of the detection zones were measured with the ImageJ software for plotting the standard curve.
1. Safety consideration
The experiments of silanization of filter paper or preparation of TMOS solution should be performed with caution, while wearing protective goggles, gloves, and a long-sleeve lab coat.
III. RESULTS AND DISCUSSION
A. Generation of hydrophilic-hydrophobic contrast
In this work, generation of hydrophilic-hydrophobic contrast involves two steps. In the first step, the filter paper was soaked in TMOS solution and picked up; thus, the filter cellulose was penetrated with TMOS solutions. The filter paper penetrated with siloxane was then heated at 100 °C for 1 h, during which silanol groups (Si-OH) were produced by hydrolysis of Si-OR under the ambient water vapor. TMOS was immobilized onto the filter cellulose via the reaction between Si-OH of TMOS and C-OH of filter cellulose. Meantime, Si-OH on the paper may interconnect through the self-condensation of silanol groups.28,34,35 Thus, the filter cellulose was immobilized with the cross-linked TMOS and covered by the hydrophobic alkyl groups. In the second step, the produced hydrophobic paper was aligned onto a paper mask penetrated with NaOH solution, allowing selectively turning the masked region from hydrophobic to hydrophilic by wet etching whereas the unmasked region remains hydrophobic. As a result, the hydrophilic-hydrophobic contrast was generated on the filter paper.
B. Effect of TMOS concentration
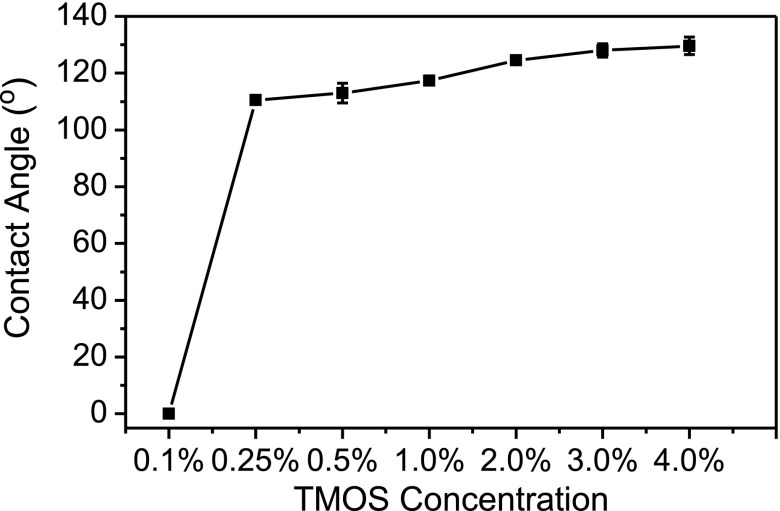
In this work, the hydrophilic paper substrate was patterned hydrophobic by soaking in TMOS solution. TMOS solutions in a range of 0.1%–4.0% were prepared to study the effect of TMOS concentration on hydrophobicity. Figure 2 shows that filter paper remains highly hydrophilic (contact angle = 0°) with 0.1% TMOS as patterning agent, while the filter paper could be patterned hydrophobic (contact angle = 110.5°) with 0.25% TMOS. The water contact angles increased slowly with the TMOS concentration in a range of 0.25%–4.0%. Bigger water contact angles are expected by further increasing the TMOS concentrations. On the other hand, the time required for the etching reagent (NaOH solution) to penetrate completely into the hydrophobic cellulose varied dramatically with the hydrophobicity or TMOS concentration. For example, only 1 min was required for the etching agent to penetrate completely into the hydrophobic paper (contact angle = 110.5°) patterned with 0.25% TMOS, while more than 30 min are needed to penetrate into the hydrophobic paper (contact angle = 129.6°) patterned with 4.0% TMOS. In this work, 2.0% TMOS was selected to pattern the filter paper by comprising the etching time required and the hydrophobicity of hydrophobic barrier.
FIG. 2.
Effect of TMOS concentrations on water contact angle on filter paper.
C. Effect of etching temperature
The effect of etching temperature in a range of 30–100 °C on water contact angle was studied by keeping NaOH concentration and etching time constant at 0.1 mol l−1 and 5 min, respectively. As shown in Figure 3, the water contact angle decreased with the increase of etching temperature in a range of 30–60 °C, and a water contact angle of 0° was observed at an etching temperature higher than 60 °C. These results indicated that the masked region of filter paper was completely etched and turned highly hydrophilic at a temperature higher than 60 °C. As a result, 60 °C was selected as the etching temperature in this work.
FIG. 3.
Effect of etching temperature on water contact angle. Etching time, 5 min; CNaOH = 0.1 mol l−1 (containing 30% glycerol); CTMOS = 2.0%.
D. Effect of NaOH concentration
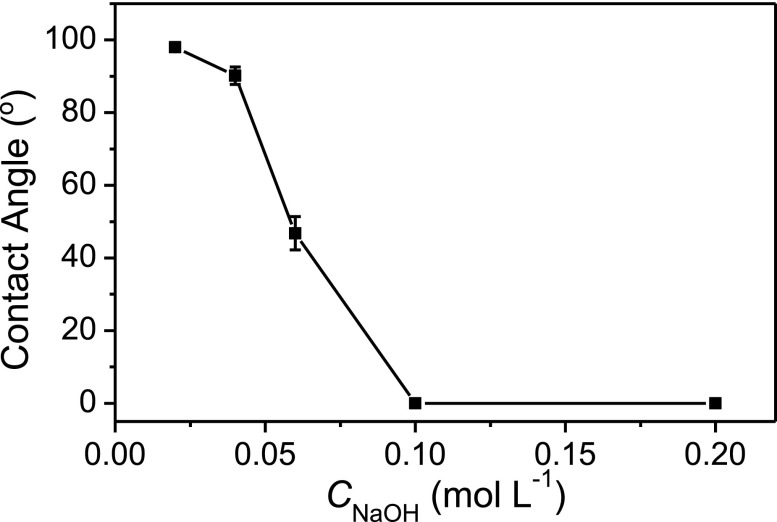
The effect of NaOH concentration in a range of 0.02–0.2 mol l−1 on the water contact angle was studied while keeping the etching time and temperature constant at 5 min and 60 °C, respectively. As shown in Figure 4, hydrophobicity decreased with the increase of NaOH concentration. The water drop rapidly penetrated into filter cellulose and a water contact angle of 0° was observed with 0.1 mol l−1 NaOH solution as the etching agent. Thus, 0.1 mol l−1 of NaOH solution was selected as the etching agent in this work.
FIG. 4.
Effect of NaOH concentration on water contact angle. Etching temperature: 60 °C; other conditions were the same as in Figure 3.
E. Effect of etching time
The effect of etching time in a range of 0.5–7.0 min on the water contact angle was studied by maintaining the etching temperature and NaOH concentration constant at 60 °C and 0.1 mol l−1, respectively. As shown in Figure 5, the water contact angles decreased with the increase of etching time in a range of 0.5–5.0 min and a water contact angle of 0° could be observed at an etching time higher than 5.0 min. In this work, 5.0 min was selected as the optimum etching time for selectively etching the hydrophobic paper.
FIG. 5.
Effect of etching time on the water contact angle. Etching temperature: 60 °C; other conditions were the same as Figure 3.
F. Glucose assay
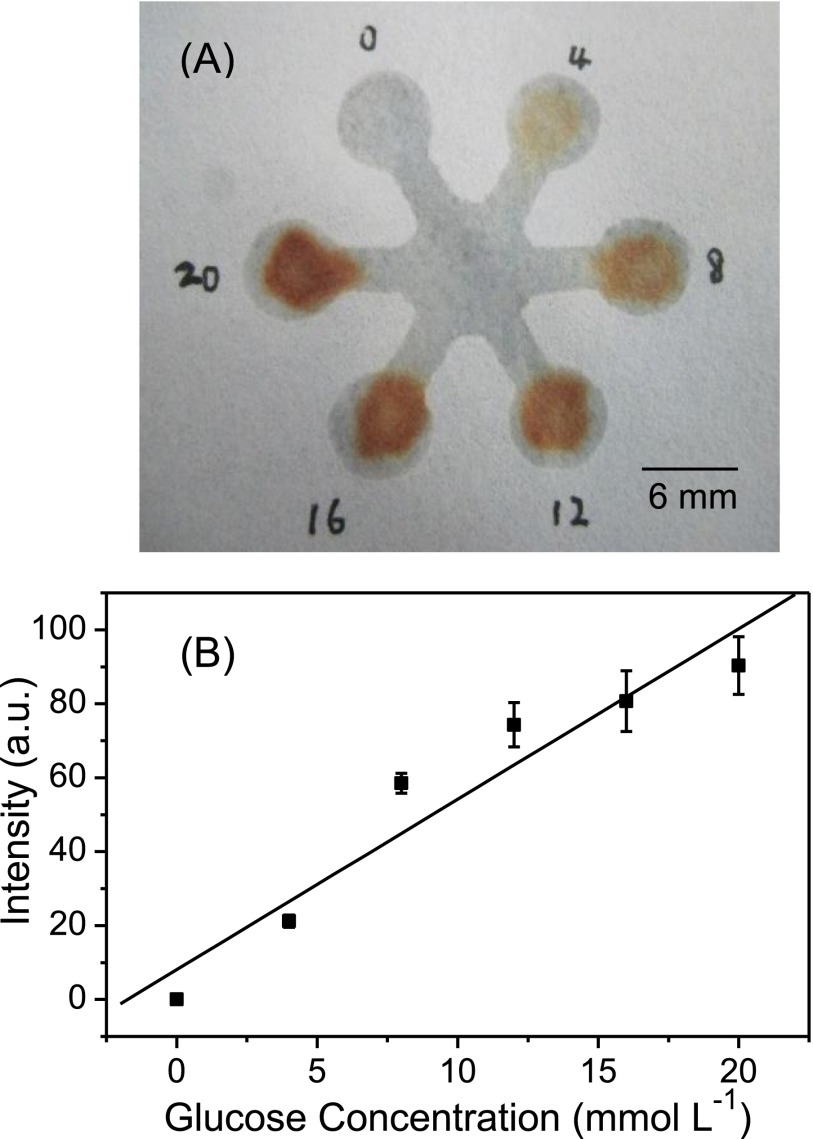
We measured the artificial urine samples of glucose in a clinically relevant range (0–20 mmol l−1) using a flower-shaped μPAD fabricated by this presented method. Figure 6(a) shows the image of glucose assay on μPAD. The gray intensity in detection zones were measured with the ImageJ software by subtraction of the blank value. A linear correlation between gray intensity (GI) and glucose concentration (C), GI = 4.6 C (mmol l−1) + 8.0 (n = 3), was obtained with a correlation coefficient of 0.980 (Figure 6(b)). The relative standard deviation (RSD) was 7.2% by determining 10 mmol l−1 of glucose in urine samples five times. The limit of detection for glucose in artificial urine was 2.47 mmol l−1 based on the 3Sb/K criterion (Sb is the standard deviation obtained from determining the blank solution 12 times and K is the slope of the standard curve). These results demonstrated that μPAD fabricated by this presented method could be used as a reliable and robust quantitative analysis platform in biological assays.
FIG. 6.
(a) Photograph of glucose assay performed on the μPAD with varied glucose concentration in a range of 0–20 mmol l−1. (b) Linear correlation between gray intensity and glucose concentration. The data were obtained from three repetitive experiments. The gray value was measured by the ImageJ software after the subtraction of blank gray value.
IV. CONCLUSIONS
We described a novel strategy for fabrication of μPAD by selective wet etching of hydrophobic filter paper using a paper mask. This method is free of any expensive equipment, metal mask, and expensive reagents, allowing rapid and simple fabrication of μPADs by the untrained personnel with minimum cost. These features are very attractive for applications of μPADs in the common labs, especially those in developing countries and resource-limited regions. Furthermore, other silane coupling agents with various functional groups could also be immobilized onto the filter cellulose and then selectively etched by etching agent. In addition, the etching agents could also be printed onto the hydrophobic paper for mass production of μPADs. Comparing to the method using paper mask reported previously,28 channels without disconnection and shrinkage could be easily fabricated by this presented method. Although the glucose assay in urine samples was chosen to demonstrate the feasibility of μPAD fabricated by this method as a quantitative analysis platform, this method would also be very attractive to the development of micro analytical devices for point-of care applications in clinical diagnostics, food safety testing, and environmental monitoring. One limitation of the μPADs fabricated by this method is the relative low resolution because the paper mask fabricated by using a common knife and scissor is in millimeter scale. The resolution of the fabricated μPADs could be improved by printing etching agents on the hydrophobic filter paper using an inkjet printer.
ACKNOWLEDGMENTS
The authors thank Professor Yunying Wu for the help in measurement of contact angles. Financial support from the Guangdong Provincial Natural Science Foundation of China (Grant Nos. S2012040007274 and S2013010012046) and the Research Start-up Fund of Hanshan Normal University (Grant No. QD20120521) is gratefully acknowledged.
References
- 1.Manz A., Graber N., and Widmer H. M., Sens. Actuators, B 1, 244 (1990). 10.1016/0925-4005(90)80209-I [DOI] [Google Scholar]
- 2.Hadd A. G., Raymond D. E., Halliwell J. W., Jacobson S. C., and Ramsey J. M., Anal. Chem. 69, 3407 (1997). 10.1021/ac970192p [DOI] [PubMed] [Google Scholar]
- 3.Dertinger S. K. W., Chiu D. T., Jeon N. L., and Whitesides G. M., Anal. Chem. 73, 1240 (2001). 10.1021/ac001132d [DOI] [Google Scholar]
- 4.Niu X. Z., Zhang B., Marszalek R. T., Ces O., Edel J. B., Klug D. R., and deMello A. J., Chem. Commun. 2009, 6159 (2009). 10.1039/b918100h [DOI] [PubMed] [Google Scholar]
- 5.Cai L. F., Zhu Y., Du G. S., and Fang Q., Anal. Chem. 84, 446 (2012). 10.1021/ac2029198 [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y. and Fang Q., Anal. Chem. 82, 8361 (2010). 10.1021/ac101902c [DOI] [PubMed] [Google Scholar]
- 7.Whitesides G. M., Ostuni E., Takayama S., Jiang X. Y., and Ingber D. E., Annu. Rev. Biomed. Eng. 3, 335 (2001). 10.1146/annurev.bioeng.3.1.335 [DOI] [PubMed] [Google Scholar]
- 8.Jia Z. J., Fang Q., and Fang Z. L., Anal. Chem. 76, 5597 (2004). 10.1021/ac0494477 [DOI] [PubMed] [Google Scholar]
- 9.Malek C. G. K., Anal. Bioanal. Chem. 385, 1362 (2006). 10.1007/s00216-006-0517-z [DOI] [PubMed] [Google Scholar]
- 10.Noh H. and Phillips S. T., Anal. Chem. 82, 8071 (2010). 10.1021/ac1005537 [DOI] [PubMed] [Google Scholar]
- 11.Li X., Ballerini D. R., and Shen W., Biomicrofluidics 6, 011301(2012). 10.1063/1.3687398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mentele M. M., Cunningham J., Koehler K., Volckens J., and Henry C. S., Anal. Chem. 84, 4474 (2012). 10.1021/ac300309c [DOI] [PubMed] [Google Scholar]
- 13.Sameenoi Y., Panymeesamer P., Supalakorn N., Koehler K., Chailapakul O., Henry C. S., and Volckens J., Environ. Sci. Technol. 47, 932 (2013). 10.1021/es304662w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jokerst J. C., Adkins J. A., Bisha B., Mentele M. M., Goodridge L. D., and Henry C. S., Anal. Chem. 84, 2900 (2012). 10.1021/ac203466y [DOI] [PubMed] [Google Scholar]
- 15.Martinez A. W., Phillips S. T., Butte M. J., and Whitesides G. M., Angew. Chem. Int. Ed. 46, 1318 (2007). 10.1002/anie.200603817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y., Shi W. W., Jiang L., Qin J. H., and Lin B. C., Electrophoresis 30, 1497 (2009). 10.1002/elps.200800563 [DOI] [PubMed] [Google Scholar]
- 17.Carrilho E., Martinez A. W., and Whitesides G. M., Anal. Chem. 81, 7091 (2009). 10.1021/ac901071p [DOI] [PubMed] [Google Scholar]
- 18.Delaney J. L., Hogan C. F., Tian J. F., and Shen W., Anal. Chem. 83, 1300 (2011). 10.1021/ac102392t [DOI] [PubMed] [Google Scholar]
- 19.Li X., Tian J. F., Garnier G., and Shen W., Colloids Surf. B. 76, 564 (2010). 10.1016/j.colsurfb.2009.12.023 [DOI] [PubMed] [Google Scholar]
- 20.Abe K., Suzuki K., and Citterio D., Anal. Chem. 80, 6928 (2008). 10.1021/ac800604v [DOI] [PubMed] [Google Scholar]
- 21.Bruzewicz D. A., Reches M., and Whitesides G. M., Anal. Chem. 80, 3387 (2008). 10.1021/ac702605a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe K., Kotera K., Suzuki K., and Citterio D., Anal. Bioanal. Chem. 398, 885 (2010). 10.1007/s00216-010-4011-2 [DOI] [PubMed] [Google Scholar]
- 23.Songjaroen T., Dungchai W., Chailapakul O., and Laiwattanapaisal W., Talanta 85, 2587 (2011). 10.1016/j.talanta.2011.08.024 [DOI] [PubMed] [Google Scholar]
- 24.Songjaroen T., Dungchai W., Chailapakul O., Henry C. S., and Laiwattanapaisal W., Lab Chip 12, 3392 (2012). 10.1039/c2lc21299d [DOI] [PubMed] [Google Scholar]
- 25.Dungchai W., Chailapakul O., and Henry C. S., Analyst 136, 77 (2011). 10.1039/c0an00406e [DOI] [PubMed] [Google Scholar]
- 26.Nurak T., Praphairaksit N., and Chailapakul O., Talanta 114, 291 (2013). 10.1016/j.talanta.2013.05.037 [DOI] [PubMed] [Google Scholar]
- 27.Nie J. F., Zhang Y., Lin L. W., Zhou C. B., Li S. H., Zhang L. M., and Li J. P., Anal. Chem. 84, 6331 (2012). 10.1021/ac203496c [DOI] [PubMed] [Google Scholar]
- 28.Cai L. F., Wang Y., Wu Y. Y., Xu C. X., Zhong M. H., Lai H. Y., and Huang J. S., Analyst 139, 4593 (2014). 10.1039/C4AN00988F [DOI] [PubMed] [Google Scholar]
- 29.He Q. H., Ma C. C., Hu X. Q., and Chen H. W., Anal. Chem. 85, 1327 (2013). 10.1021/ac303138x [DOI] [PubMed] [Google Scholar]
- 30.Chitnis G., Ding Z. W., Chang C. L., Savran C. A., and Ziaie B., Lab Chip 11, 1161 (2011). 10.1039/c0lc00512f [DOI] [PubMed] [Google Scholar]
- 31.Li X., Tian J. F., Nguyen T., and Shen W., Anal. Chem. 80, 9131 (2008). 10.1021/ac801729t [DOI] [PubMed] [Google Scholar]
- 32.Brooks T. and Keevil C. W., Lett. Appl. Microbiol. 24, 203 (1997). 10.1046/j.1472-765X.1997.00378.x [DOI] [PubMed] [Google Scholar]
- 33.Klasner S. A., Price A. K., Hoeman K. W., Wilson R. S., Bell K. J., and Culbertson C. T., Anal. Bioanal. Chem. 397, 1821 (2010). 10.1007/s00216-010-3718-4 [DOI] [PubMed] [Google Scholar]
- 34.Castellano M., Gandini A., Fabbri P., and Belgacem M. N., J. Colloid Interface Sci. 273, 505 (2004). 10.1016/j.jcis.2003.09.044 [DOI] [PubMed] [Google Scholar]
- 35.Abdelmouleh M., Boufi S., Belgacem M. N., Duarte A. P., Salah A. B., and Gandini A., Int. J. Adhes. Adhes. 24, 43 (2004). 10.1016/S0143-7496(03)00099-X [DOI] [Google Scholar]