Abstract
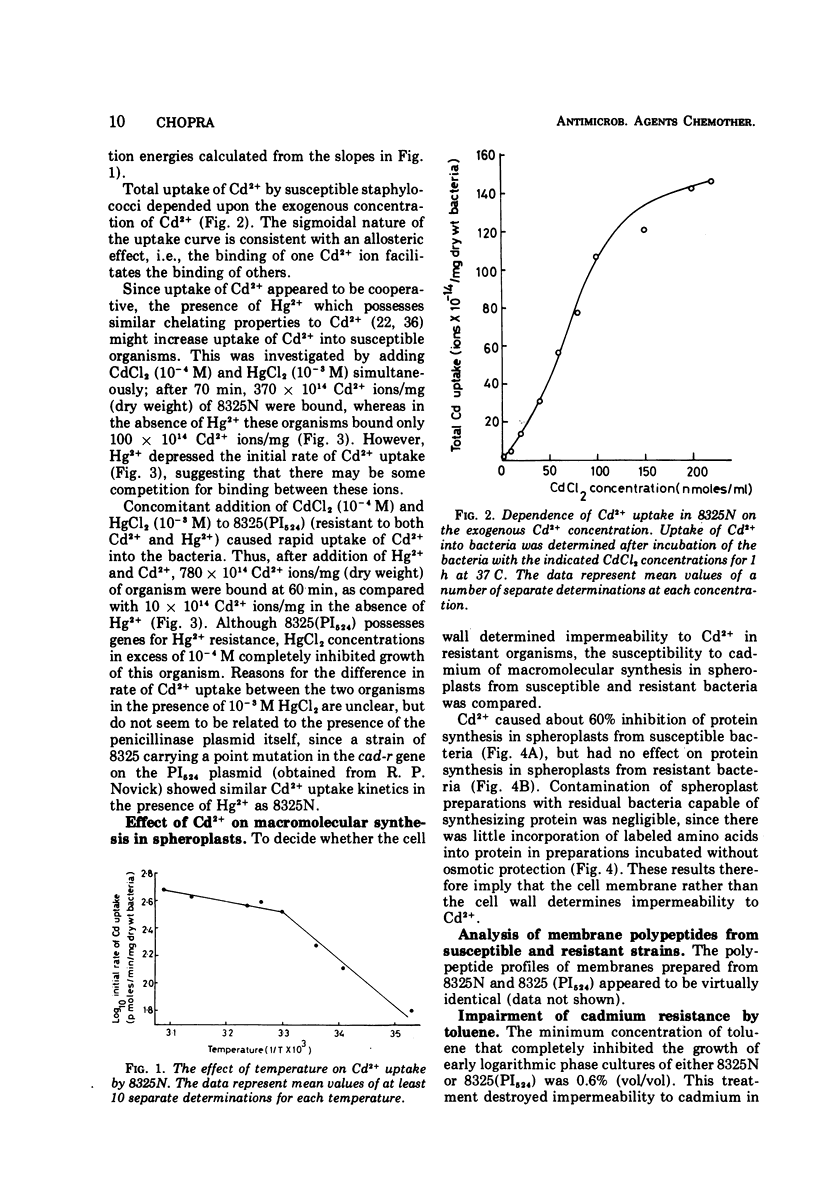
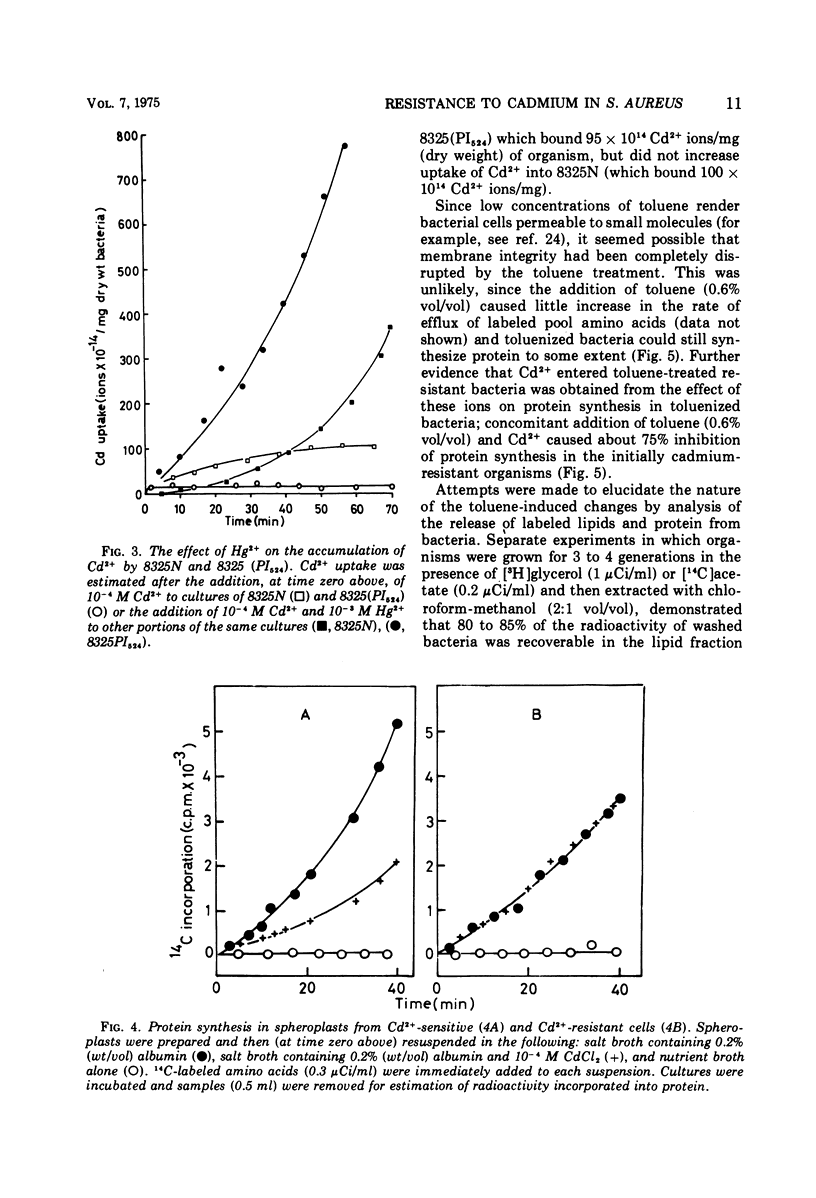
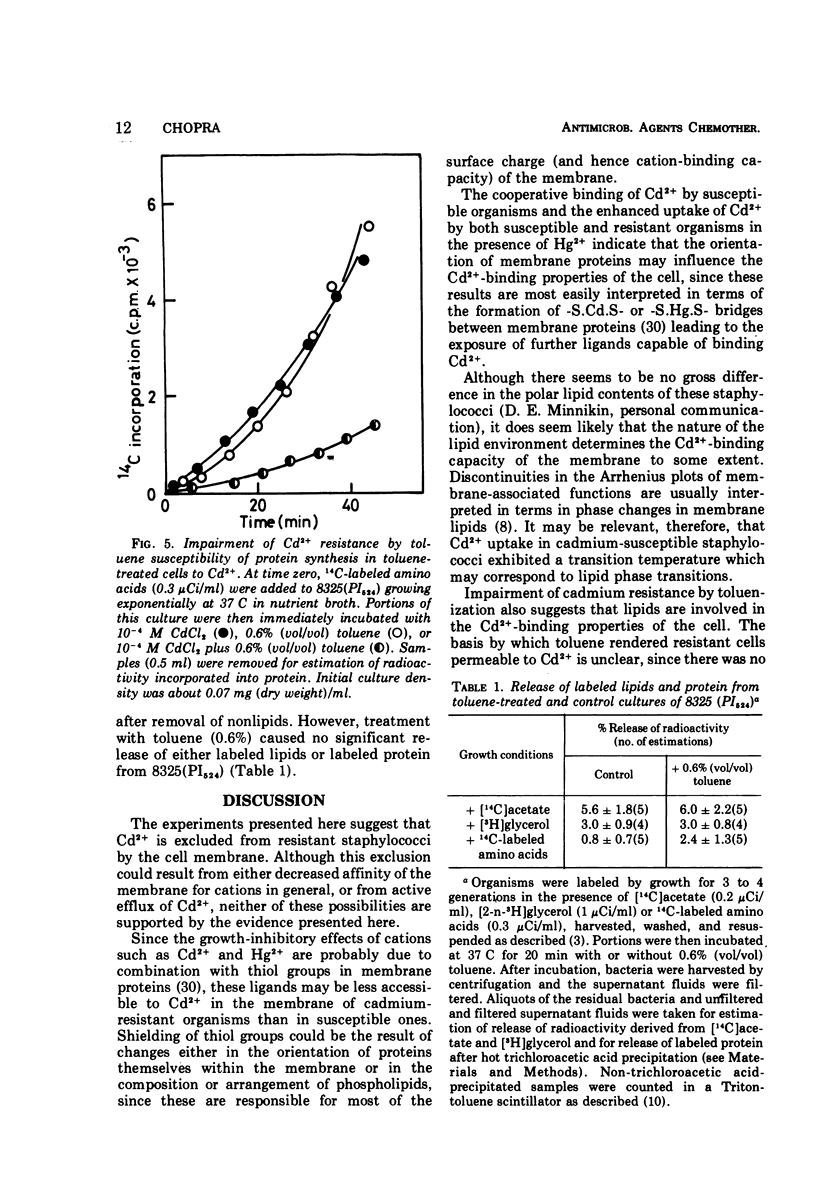
The mechanism of plasmid-mediated resistance to cadmium in Staphylococcus aureus was investigated. Protein synthesis in cell-free extracts from resistant or susceptible bacteria was equally susceptible to inhibition by Cd2+, but spheroplasts from resistant bacteria retained their resistance. Resistant bacteria did not have a decreased affinity for cations in general, nor was active metabolism required for exclusion of Cd2+. The kinetics of Cd2+ uptake into susceptible and resistant bacteria suggested that the conformation of membrane proteins in resistant bacteria may be important in the exclusion of Cd2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheshov E. H. The genetics of penicillinase production in Staphylococcus aureus strain PS80. J Gen Microbiol. 1969 Dec;59(3):289–301. doi: 10.1099/00221287-59-3-289. [DOI] [PubMed] [Google Scholar]
- Chopra I. Decreased uptake of cadmium by a resistant strain of Staphylococcus aureus. J Gen Microbiol. 1970 Oct;63(2):265–267. doi: 10.1099/00221287-63-2-265. [DOI] [PubMed] [Google Scholar]
- Chopra I., Lacey R. W., Connolly J. Biochemical and genetic basis of tetracycline resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):397–404. doi: 10.1128/aac.6.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Lacey R. W. Lack of correlation between methicillin resistance and susceptibility to lysostaphin in Staphylococcus aureus. J Gen Microbiol. 1974 Jun;82(2):419–420. doi: 10.1099/00221287-82-2-419. [DOI] [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- Connamacher R. H., Mandel H. G., Hahn F. E. Adaptation of populations of Bacillus cereus to tetracycline. Mol Pharmacol. 1967 Nov;3(6):586–594. [PubMed] [Google Scholar]
- Dyke K. G., Parker M. T., Richmond M. H. Penicillinase production and metal-ion resistance in Staphylococcus aureus cultures isolated from hospital patients. J Med Microbiol. 1970 Feb;3(1):125–136. doi: 10.1099/00222615-3-1-125. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried V. A., Novick A. Organic solvents as probes for the structure and function of the bacterial membrane: effects of ethanol on the wild type and an ethanol-resistant mutant of Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):239–248. doi: 10.1128/jb.114.1.239-248.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. M. Distribution of mercury resistance among Staphylococcus aureus isolated from a hospital community. J Hyg (Lond) 1970 Mar;68(1):111–119. doi: 10.1017/s0022172400028564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. M. Mercury resistance of Staphylococcus aureus. J Hyg (Lond) 1970 Mar;68(1):121–129. doi: 10.1017/s0022172400028576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOOS W. E., PATTEE P. A. A BIOCHEMICAL CHARACTERIZATION OF HISTIDINE-DEPENDENT MUTANTS OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1965 May;39:185–194. doi: 10.1099/00221287-39-2-185. [DOI] [PubMed] [Google Scholar]
- Kempner E. S. Trace metal analysis of nutrient broth. Appl Microbiol. 1967 Nov;15(6):1525–1526. doi: 10.1128/am.15.6.1525-1526.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I., Ishikawa T., Nakahara H. Mercury and cadmium resistances mediated by the penicillinase plasmid in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):1–7. doi: 10.1128/jb.117.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner D. J., Khan S. R. Proflavine Uptake and Release in Sensitive and Resistant Escherichia coli. J Bacteriol. 1968 Oct;96(4):1103–1114. doi: 10.1128/jb.96.4.1103-1114.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Chopra I. Evidence for mutation to streptomycin resistance in clinical strains of Staphylococcus aureus. J Gen Microbiol. 1972 Nov;73(1):175–180. doi: 10.1099/00221287-73-1-175. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Chopra I. Genetic studies of a multi-resistant strain of Staphylococcus aureus. J Med Microbiol. 1974 May;7(2):285–297. doi: 10.1099/00222615-7-2-285. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Grinsted J. Linkage of fusidic acid resistance to the penicillinase plasmid in Staphylococcus aureus. J Gen Microbiol. 1972 Dec;73(3):501–508. doi: 10.1099/00221287-73-3-501. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Transfer of tetracycline-resistance between strains of Staphylococcus aureus in mixed cultures. J Gen Microbiol. 1971 Dec;69(2):229–237. doi: 10.1099/00221287-69-2-229. [DOI] [PubMed] [Google Scholar]
- MARTELL A. E. Some factors governing chelating tendencies and selectivities in the interaction of ligands with metal ions. Fed Proc. 1961 Sep;20(3):35–38. [PubMed] [Google Scholar]
- MOORE B. A new screen test and selective medium for the rapid detection of epidemic strains of Staph. aureus. Lancet. 1960 Aug 27;2(7148):453–458. doi: 10.1016/s0140-6736(60)91591-9. [DOI] [PubMed] [Google Scholar]
- Machtiger N. A., Fox C. F. Biochemistry of bacterial membranes. Annu Rev Biochem. 1973;42:575–600. doi: 10.1146/annurev.bi.42.070173.003043. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Kennedy E. P. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem. 1971 May 10;246(9):3042–3049. [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyru G., Wexler L. F., Novick R. P. Naturally occurring penicillinase plasmids in Staphylococcus aureus. J Bacteriol. 1969 Apr;98(1):215–221. doi: 10.1128/jb.98.1.215-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H., JOHN M. CO-TRANSDUCTION BY A STAPHYLOCOCCAL PHAGE OF THE GENES RESPONSIBLE FOR PENICILLINASE SYNTHESIS AND RESISTANCE TO MERCURY SALTS. Nature. 1964 Jun 27;202:1360–1361. doi: 10.1038/2021360a0. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN A. Cell membrane as site of action of heavy metals. Fed Proc. 1959 Dec;18:1026–1038. [PubMed] [Google Scholar]
- VACZI L., FODOR M., MILCH H., RETHY A. Studies on the mercuric chloride resistance of Staphylococcus aureus. Acta Microbiol Acad Sci Hung. 1962;9:81–87. [PubMed] [Google Scholar]
- WILLIAMS R. J. Nature and properties of metal ions of biological interest and their coordination compounds. Fed Proc. 1961 Sep;2:5–14. [PubMed] [Google Scholar]
- Webb M. Interrelationships between the utilization of magnesium and the uptake of other bivalent cations by bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):428–439. doi: 10.1016/0304-4165(70)90133-9. [DOI] [PubMed] [Google Scholar]
- Webb M. The mechanism of acquired resistance to Co2+ and Ni2+ in Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):440–446. doi: 10.1016/0304-4165(70)90134-0. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E. Purification of lipids from nonlipid contaminants on Sephadex bead columns. J Lipid Res. 1966 Jul;7(4):558–561. [PubMed] [Google Scholar]



