Abstract
Background/Aim
Medullary thyroid carcinoma (MTC) is a tumor associated with poor prognosis since it exhibits high resistance against conventional cancer therapy. Recent studies have shown that quinazolines exhibit a pro-apoptotic effect on malignant cells. The aim of the present study was to elucidate whether MTC cells are affected by quinazolines, in particular prazosin.
Materials and Methods
Proliferation, apoptosis and cell morphology of the MTC cell line TT were analyzed by WST-1 assay, caspase 3/7 activation tests and microscopy. Fibroblasts were used as control for non-malignant cells.
Results
Prazosin potently inhibited the growth of TT cells, induced apoptosis and caused vacuolization, as well as needle-like filopodia. Fibroblasts were affected by prazosin in the same way as MTC cells.
Conclusion
MTC cells are responsive to prazosin treatment similar to other malignancies. The fact that fibroblasts also respond to prazosin further highlights the importance to identify the unknown pro-apoptotic target of quinazolines.
Keywords: Medullary thyroid carcinoma, cell culture, α1-adrenergic receptors, quinazolines, prazosin, apoptosis
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor of the thyroid that accounts for 5-10% of all thyroid malignancies (1). MTC was described for the first time more than 100 years ago by Jaquet (2). Development of MTC can result from a spontaneous mutation (sporadic form) or can be transmitted hereditarily (25%) either alone (multi-generational familial MTC) or as part of a multiple endocrine neoplasia (MEN) type 2A or 2B syndrome (1-4). Even though it is well-known nowadays that MTC derives from calcitonin-producing para-follicular cells and that mutations in the RET proto-oncogene are the main driver mutations in MTC, widespread clinical expertise is so far limited because of the low incidence of MTC (2). Thus, no effective treatment for MTC with distant metastasis is as yet available (1-4). Knowledge about the importance of the RET kinase for the pathogenesis of MTC led to the development and investigation of multi-targeted inhibitors, which cross-react with RET (5, 6). Indeed, some clinical studies testing RET inhibitors were promising, but showed only modest results (5). Therefore, new therapeutic options are still needed in the therapy of MTC.
In order to find new therapeutic options for the treatment of malignancies, several strategies are nowadays pursued, including screening for new cancer drugs in natural products (7, 8) or the re-evaluation of known drugs – already authorized for other diseases – for potential anti-cancer effects. A good example for such a drug is thalidomide, which was initially authorized as a barbiturate but is nowadays used as an anti-cancer agent against multiple myeloma (9). Other examples for unexpected anti-cancer action of proved drugs are quinazolin-based α1-adrenergic antagonists, which are in the focus of the current study.
α1-adrenergic antagonists, including the quinazolines prazosin and doxazosin, are used in the treatment of hypertension. Furthermore, based on the observation that smooth muscle cells of the prostate predominately express α1-adrenoceptors (10), α1-adrenergic antagonist therapy was introduced for the first time in 1978 in order to reduce the muscle tonus of the prostate in patients with benign prostate hyperplasia (11). Kyprianou et al. demonstrated for the first time that α1-adrenergic antagonists are able to induce apoptosis in glandular epithelial and smooth muscle cells of the prostate in benign prostatic hyperplasia (BHP) patients (12). However, the group around Kyprianou et al. discovered in a follow-up study that the pro-apoptotic mechanism of α1-adrenergic antagonists on prostate cells is independent of α1-adrenoceptors (13). This is in line with our observation in leukaemia cells in which α1-adrenergic blockers induce apoptosis in the absence of α1-adrenoceptors (14-16). Further studies have revealed that α1-adrenergic antagonists also induce apoptosis in malignant prostate carcinoma cells (13). Based on observations in prostate cancer cells, other research groups investigated the impact of α1-adrenergic drugs on further human malignancies, such as pituitary adenoma, breast cancer, bladder cancer, as well as mesothelioma (17-20). The results of these investigations were promising since a pronounced pro-apoptotic effect of α1-adrenoceptor blockers on malignancies was documented in the respective studies (17-20).
The aim of the present study was to test, whether the MTC cell line TT is also sensitive towards treatment with quinazoline-based α1-adrenergic antagonists in a similar way, as already shown for other malignancies. For our study we have chosen prazosin, which exhibits a significantly higher potency to induce apoptosis in the K562 cell line than doxazosin according to recent studies in our lab (Zeller C, unpublished observation, 2013). This is in line with results obtained from human prostate cancer cell lines where prazosin exhibited supremacy against other common clinically used α1-adrenergic antagonists regarding the induction of apoptosis (21). Since information about possible growth inhibitory actions of quinazolines on non-malignant cells are sparse, we compared the effects of prazosin on TT cells with that on normal human skin fibroblasts.
Materials and Methods
Detection of α1-adrenergic receptor expression in TT cells using TaqMan® gene expression assays
Expression of α1-adrenergic receptors ADRA1A, ADRA1B and ADRA1D was assessed at the level of mRNA by TaqMan® gene expression assays (Life/Applied Biosystems, Foster City, CA, USA) using inventoried assays (ADRA1A: Hs00169124_m1, ADRA1B: Hs00171263_m1, ADRA1D: Hs00169865_m1). β-actin (ACTB, Hs03023943_g1) was used as internal control. Isolation of RNA, reverse transcription and real time polymerase chain reaction (PCR) were done as described previously (15). PCR reactions were run in a CFX96 Real Time PCR machine (Biorad, Hercules, CA, USA).
Cultivation of TT cells and skin fibroblasts
The human MTC cell line TT (presented for the first time at the congress “Advances in thyroid neoplasia”, Rome, 1981 by Leong) was obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK). TT cells were cultivated in Ham’s F-12 medium (Lonza, Verviers, Belgium) supplemented with 10% FBS (PAA, Pasching, Austria). The normal human skin fibroblast cell line HF-SAR, which was established at our institute (22), was maintained in Eagle’s MEM (Lonza) supplemented with 10% FBS. Both cell lines were cultivated under antibiotic-free conditions at 37°C, 5% CO2 in a fully humidified atmosphere in a cell culture incubator.
Analysis of growth of cells by means of the WST-1 proliferation assay
Relative cell proliferation, respectively viability, was analysed using the WST-1 cell proliferation reagent 4-(3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio)-1,3-benzene disulfonate (Roche Diagnostics, Mannheim, Germany) following the instructions of the manufacturer. For the WST-1 assay, cells were cultivated in 96-well plates for different periods of time, starting with a cell number of 1×104 cells in 100 μl medium. Absolute cell counts were assessed prior to seeding by a CASY® Cell Counter and Analyser System (Roche).
Detection of caspases 3/7 activation
Activation of effector caspases 3 and 7 was assessed by a luminescence-based Caspase-Glo® 3/7 assay (Promega, Fitchburg, WI, USA) according to the instructions of the manufacturer. Emitted luminescence was analysed with a GloMax®-Multi Detection System (Promega). Caspase-3/7-activation was further analysed using the fluorescent CellEvent® Caspase-3/7 Green ReadyProbes® Reagent (Life/Molecular Probes, Eugene, OR, USA). For this assay, cells were grown on glass chamber slides (Discovery Labware, Billerica, MA, USA) and surveyed for apoptotic cells, characterized by green fluorescent nuclei, with an Eclipse TE300 (Nikon, Tokyo, Japan) inverted microscope equipped with a FITC filter set.
Scanning electron microscopy (SEM) and live cell imaging
Preparation of cells for SEM was done following standard procedures (23, 24). Cells were grown on glass inserts in 24-well cell culture plates. After drug treatment, cells were fixed in 3% glutaraldehyde dissolved in 0.1 M cacodylate buffer, pH 7.2, overnight at 4°C and were post-fixed with osmium tetroxide. Cells were processed by critical point drying, finally coated with Au-Pd (23) and viewed by an Ultra 55 SEM (Zeiss, Jena, Germany).
Live cell imaging was done using a Cell Observer based on an Axiovert 200M inverted fluorescence microscope (Zeiss). Cells were cultivated on glass chamber slides and observed for a total period of 24 h. Every 10 min, a series of photos was taken of different positions on the slide.
Statistics
All data are presented as mean values and standard deviation (SD). Significance of obtained data was calculated using Sigma Plot 12.5 (Systat Software Inc., San Jose, CA, USA). Gaussian distribution of values was tested using the Shapiro-Wilk test. Multiple testing was performed by means of one way analysis of variance (ANOVA) with the Holm-Sidak post hoc testing procedure with an overall significance level of p≤0.05.
Results
TT cells express α1-adrenergic receptors
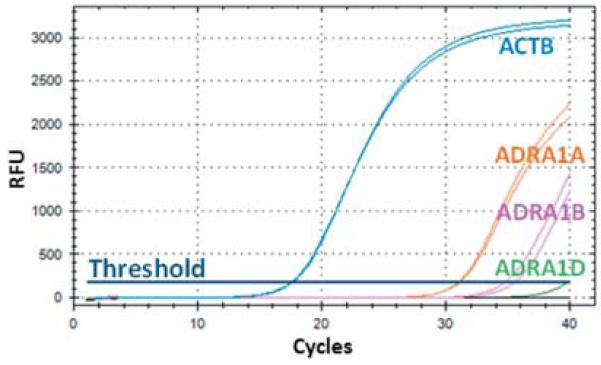
Even though it is well-documented that quinazoline-based α1-adrenergic antagonists induce apoptosis independent of adrenoceptors, we tested whether TT cells express α1-adrenoceptors. Using highly specific and sensitive TaqMan® gene expression assays, we were able to show that TT cells express the α1-adrenoceptor sub-types ADRA1A and ADRA1B but not ADRA1D (Figure 1).
Figure 1.
α1-adrenoceptor expression in the TT cell line. By means of real-time qRT-PCR we detected expression of α1-adrenergic receptors (ADRA) ADRA1A, ADRA1B but not ADRA1D. β-actin (ACTB) was analyzed as internal control (housekeeping gene). RFU: Relative fluorescence units.
Prazosin induces significant morphological changes and apoptosis in TT cells
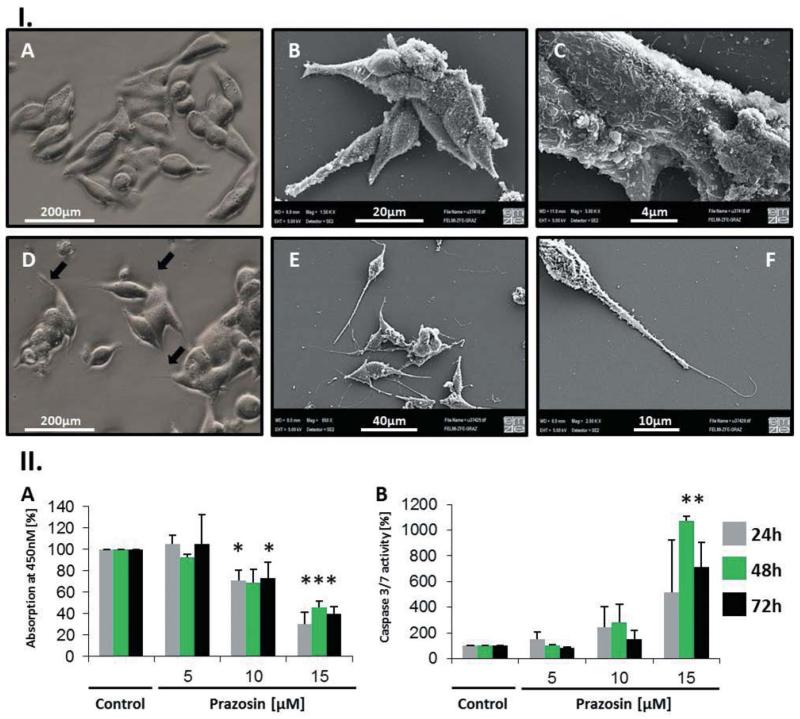
During and following a 24-h treatment with 15 μM prazosin, we observed vacuolization of cells, enhanced detachment and rounding, as well as the formation of typical spindle-like polar protrusions, most likely reminiscent to filopodia (Figure 2 and Supplementary Files, Video 2). In general, the phenotype of TT cells became more spindle-shaped following treatment with prazosin. The protrusions seemed not to originate from contraction of cells, but were mainly outgrowing from the polar endings of the cells. Ultrastructure analysis confirmed the fragile nature of these structures, which seemed to protrude from the spindle-like body of the cell (Figure 2). Further ultrastructure analysis revealed a highly complex surface structure of TT cells, which might reflect the secretory nature of these neuroendocrine cells (Figure 2). Live cell imaging of untreated and treated TT cells showed unexpected motility in vitro and the reversible formation of round multicellular aggregates (Supplementary Files, Video 1 and 2).
Figure 2.
Prazosin induced morphological alterations and apoptosis in the TT cell line. I. A: Light microscopic view of TT cells: Typical TT cells are small, spindle-shaped and growing in small clusters. B/C: Scanning electron microscopic (SEM) analysis of TT cells exhibited a highly complex cytoplasmic membrane showing many membrane-bound vesicular structures, which may reflect exocytotic processes. D: Light microscopic view of 24 h prazosin (15 μM)-treated TT cells: Prazosin-treated cells were generally more spindle-shaped and exhibited long filopodia like polar fibres (arrows). E/F: SEM analysis of 24 h prazosin-treated cells (15μM) confirmed the enhanced spindle-like character of TT cells upon prazosin treatment and showed that the formed fibres are very fragile structures. The fibres seem to protrude from the polar endings of the cells (F). II: A: Growth, respectively viability, of TT cells was tested using the WST-1 reagent over 72 h. A dose-dependent decrease of the optical density (OD) at 450 nm was evident when prazosin was added to the culture; n=4. B: Activation of effector caspases 3/7 indicates that prazosin induces apoptosis in TT cells; n=3. *p<0.05 according to one-way ANOVA with the Holm-Sidak post hoc test.
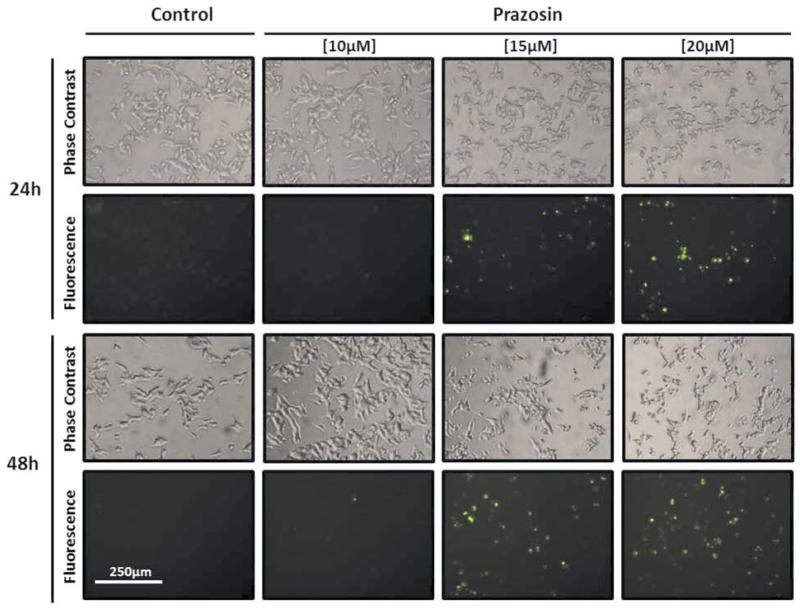
By means of the WST-1 assay, we observed a dose-dependent reduction of metabolic activity in prazosin-treated TT cells-containing wells indicating that prazosin induced growth inhibition and/or cell death. Methods to detect apoptosis, by means of caspase activation, showed that apoptosis occurs at prazosin concentrations ≥15 μM but not at 10μM concentrations (Figures 2 and 3). By using the fluorescence-based caspase activation assays, we confirmed the data obtained by luminescence assays clearly demonstrating that only cells with round phenotype – highly enriched in prazosin-treated wells – exhibited caspase-mediated fluorescence (Figure 3). Noticeably, cells with pronounced formation of protrusions were clearly devoid of fluorescence.
Figure 3.
Effector caspases 3/7 were activated in rounded cells but not in cells exhibiting prazosin-induced protrusions. TT cells were cultivated for 24 or 48 h with increasing concentrations of prazosin and stained with the CellEvent® Caspase-3/7 Green ReadyProbes reagent. In cells with activated caspases 3/7, a DNA-affine fluorescent dye is released from a substrate for caspases 3/7 and stains the nuclei of apoptotic cells green.
The pro-apoptotic action of prazosin is not limited to malignant cells
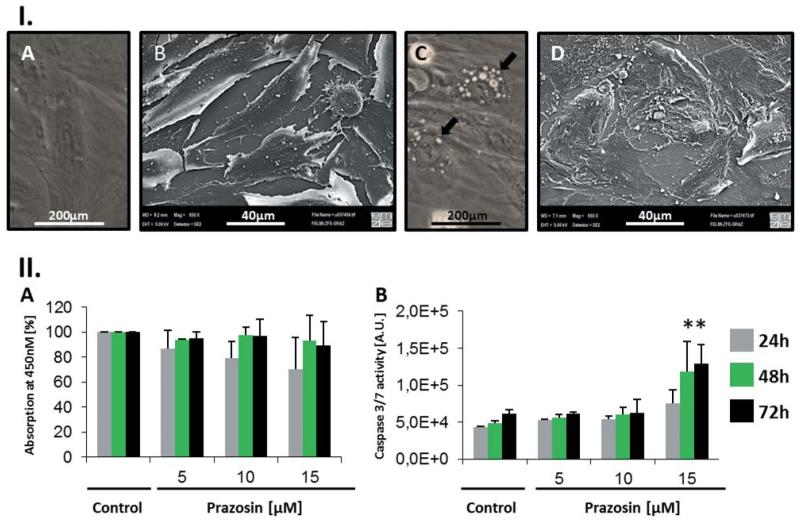
Since information about the pro-apoptotic action of prazosin on normal cells is sparse in comparison to malignant cells, we tested whether prazosin shows toxic effects on normal human skin fibroblasts. We observed no significant difference in the WST-1 assay, when testing untreated cells versus prazosin-treated cells over time (Figure 4). On the contrary, we found significantly (p<0.05) elevated effector caspase activity in prazosin-treated cells (15 μM), a dose that induced apoptosis in the TT cell line (Figure 4). Furthermore, normal human skin fibroblasts exhibited similar dramatic morphological alterations as the TT cell line (Figure 4). Cytoplasmic vacuolization was evident following prazosin treatment in the HF-SAR cells but, in contrast to the tested MTC cell line, HF-SAR cells showed no long protrusions as the TT cells did. Ultra-structural analysis revealed a more complex cell surface than untreated cells (Figure 4).
Figure 4.
The human skin fibroblast cell line HF-SAR is also sensitive towards prazosin treatment. I: A/B: Light (A), respectively scanning electron microscopy (SEM, B), view of HF-SAR cells. Fibroblasts exhibited a flattened phenotype with low contrast under the light microscope. C/D: Light (C), respectively SEM (D), view of 15μM prazosin-treated HF-SAR cells (24 h). Drug-treated cells exhibited huge vacuoles in the cytoplasm (C, arrows), fringy cell edges and an overall more complex cytoplasmic membrane. II: A: Growth, viability, of HF-SAR cells was tested using the WST-1 reagent over 72 h. No significant change of the OD at 450nm was seen when prazosin was added to the culture; n=3. B: Nevertheless, activation of effector caspases 3/7 indicates that prazosin induces apoptosis in HF-SAR cells too. The data presented in the graph show the mean values of triplicate luminescence measurements of one experiment. A second experiment ran under the same conditions confirmed the outcomes of the presented results. *p<0.05 according to one way ANOVA with the Holm-Sidak post hoc test. A.U.=arbitrary units.
Discussion
To the best of our knowledge, our study is the first to show that prazosin can induce apoptosis in a MTC cell line. This is an interesting observation in the light of the fact that MTC cells are described to be resistant towards conventional chemotherapy and radiotherapy (25).
Even though it is well-documented that prazosin induces apoptotic cell death independent of adrenergic receptors, we analyzed the expression of several known α1-adrenergic receptors in the TT cell line because no data exist in the literature assessing ADRA1-expression for this cell line. Indeed, we could demonstrate the expression of ADRA1A and ADRA1B but not ADRA1D in TT. This expression profile is similar, but not identical, to that shown for the rat MTC cell line 6-23, where the expression of ADRA1B and ADRA1D was postulated based on the results of ligand binding assays (26). Esbenshade et al. have shown that norepinephrine (NE) can induce a voltage-gated influx of calcium in 6-23 cells through α1-adrenergic receptors (26). Nevertheless, NE-induced calcitonin release was shown to be caused through β-adrenergic receptors but not through α-adrenergic receptors in MTC cells (27). Since calcium is an important cellular second messenger (28), we hypothesize that even though prazosin exerts its pro-apoptotic action mainly independent of adrenoceptors, blockade of α1-adrenoceptors may additionally negatively affect the cells.
When observing prazosin-treated cells under the microscope, large vacuoles and long polar needle-shaped polar protrusions could be seen. According to the literature, huge vacuoles can originate from different organelles of the endomembrane system including the endoplasmic reticulum, endosomes, lysosomes, Golgi stacks, as well as the trans-Golgi network (29, 30). Since prazosin is a weak base (31), it is most likely that the vacuoles originate from an organelle with acidic pH (mainly late endosomes or lysosomes) because weak bases are known to accumulate in an acidic environment (30). Accumulation of weak bases in acidic organelles causes swelling due to influx of water (30).
The formation of prazosin-induced fibres, which was also observed in an earlier study in the HEL erythroleukemia cell line (14), indicates that prazosin treatment may influence the cytoskeleton, which is responsible for the shape and movement of cells. Live cell imaging experiments revealed that TT cells are very mobile under in vitro conditions. The formation of fibres seemed to interfere with this mobility. Interestingly, cells exhibiting fibres were negative for caspase activation in the fluorescent caspase-3/7 assay. We assume that fibre formation is either an event prior to apoptosis induction or cells with fibres are more resistant towards prazosin treatment.
It is the desire of every oncologist to treat patients with drugs that are highly selective against malignant cells, and do not harm normal cells. Thus, in order to test the effect of prazosin on normal cells, we employed HF-SAR skin fibroblasts. Interestingly, when analyzing cells with the WST-1 assay, we could not observe any significant differences in the measured WST-1 absorption. These results suggested that prazosin exerts no significant toxic impact on normal skin fibroblasts, but this suggestion was dismissed when the analysis involved the caspase assay and microscopy. Prazosin induced apoptosis and dramatic morphological alterations in HF-SAR cells too. Our WST-1 results are in line with recommendations of the guidelines for the use and interpretation of assays for monitoring cell death of eukaryotic cells presented by a consortium around Galluzzi et al. (32). According to these guidelines, the conversion of WST-1 by mitochondrial enzymes may reflect metabolic alterations that do not necessarily correlate with the number of viable cells (32).
We conclude that prazosin is generally not selective for malignant cells concerning apoptosis induction, albeit normal prostate or sperm cells seem to be not or only slightly impacted by prazosin (13, 33). The sensitivity of cells towards prazosin-induced apoptosis may depend on differential expression of a still unknown protein with affinity towards prazosin and other quinazoline-based drugs. In the light of the high concentrations (μM-range) of prazosin to induce apoptosis, it would be careless to recommend the use of this drug for the treatment of cancer patients, since strong adverse effects may arise. Adverse effects of the quinazoline doxazosin were already observed in the ALLHAT study when doxazosin was tested at normal concentrations used for the treatment of hypertension (34, 35). The obtained results revealed that the combined cardiovascular disease risk had significantly increased in the doxazosin arm of the study compared to the chlorthalidone arm. Furthermore, the risk of heart failure was doubled (34).
Some research groups have already started to design new quinazoline-based anti-cancer drugs with higher efficiency (36). This approach, and also attempts for the identification of the still not identified main pro-apoptotic target of quinazoline-based α1-adrenergic antagonists, will hopefully pave the way of quinazoline-based drugs for use in cancer therapy in the future.
Supplementary Material
Video 1: Live cell imaging of the human MTC cell line TT. Cells were cultivated in chamber slides and observed using a Cell Observer for a total time of 24 h. Every 10 minutes a photo was taken of different positions on the slide.
Video 2: Live cell imaging of prazosin-treated TT cells. Cells were cultivated in chamber slides with 15 μM prazosin and observed using a Cell Observer for a total time of 24 h. Every 10 minutes a photo was taken of different positions on the slide.
Acknowledgements
This study was supported by a grant of the Austrian Science Fund (FWF): P24006, and the Franz Lanyar Foundation of the Medical University of Graz.
References
- 1.Leboulleux S, Baudin E, Travagli JP, Schlumberger M. Medullary thyroid carcinoma. Clin Endocrinol. 2004;61:299–310. doi: 10.1111/j.1365-2265.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- 2.American Thyroid Association Guidelines Task F. Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA., Jr. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 3.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88:1139–1148. doi: 10.1002/(sici)1097-0142(20000301)88:5<1139::aid-cncr26>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–375. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
- 5.Phay JE, Shah MH. Targeting RET receptor tyrosine kinase activation in cancer. Clin Cancer Res. 2010;16:5936–5941. doi: 10.1158/1078-0432.CCR-09-0786. [DOI] [PubMed] [Google Scholar]
- 6.Borrello MG, Ardini E, Locati LD, Greco A, Licitra L, Pierotti MA. RET inhibition: implications in cancer therapy. Expert Opin Ther Tar. 2013;17:403–419. doi: 10.1517/14728222.2013.758715. [DOI] [PubMed] [Google Scholar]
- 7.Kretschmer N, Rinner B, Deutsch AJ, Lohberger B, Knausz H, Kunert O, Blunder M, Boechzelt H, Schaider H, Bauer R. Naphthoquinones from Onosma paniculata induce cell-cycle arrest and apoptosis in melanoma Cells. J Nat Prod. 2012;75:865–869. doi: 10.1021/np2006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofer D, Schwach G, Ghaffari Tabrizi-Wizsy N, Sadjak A, Sturm S, Stuppner H, Pfragner R. Christia vespertilionis plant extracts as novel antiproliferative agent against human neuroendocrine tumor cells. Oncol Rep. 2013;29:2219–2226. doi: 10.3892/or.2013.2367. [DOI] [PubMed] [Google Scholar]
- 9.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. New Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 10.Caine M, Raz S, Zeigler M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Brit J Urol. 1975;47:193–202. doi: 10.1111/j.1464-410x.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- 11.Caine M, Perlberg S, Meretyk S. A placebo-controlled double-blind study of the effect of phenoxybenzamine in benign prostatic obstruction. Brit J Urol. 1978;50:551–554. doi: 10.1111/j.1464-410x.1978.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 12.Kyprianou N, Litvak JP, Borkowski A, Alexander R, Jacobs SC. Induction of prostate apoptosis by doxazosin in benign prostatic hyperplasia. J Urology. 1998;159:1810–1815. doi: 10.1016/S0022-5347(01)63162-8. [DOI] [PubMed] [Google Scholar]
- 13.Benning CM, Kyprianou N. Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an alpha1-adrenoceptor-independent action. Cancer Res. 2002;62:597–602. [PubMed] [Google Scholar]
- 14.Fuchs R, Stelzer I, Haas HS, Leitinger G, Schauenstein K, Sadjak A. The alpha1-adrenergic receptor antagonists, benoxathian and prazosin, induce apoptosis and a switch towards megakaryocytic differentiation in human erythroleukemia cells. Ann Hematol. 2009;88:989–997. doi: 10.1007/s00277-009-0704-z. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs R, Schraml E, Leitinger G, Stelzer I, Allard N, Haas HS, Schauenstein K, Sadjak A. alpha1-Adrenergic drugs modulate differentiation and cell death of human erythroleukemia cells through non adrenergic mechanism. Exp Cell Res. 2011;317:2239–2251. doi: 10.1016/j.yexcr.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs R, Schraml E, Leitinger G, Letofsky-Papst I, Stelzer I, Haas HS, Schauenstein K, Sadjak A. alpha1-adrenergic drugs exhibit affinity to a thapsigargin-sensitive binding site and interfere with the intracellular Ca2+ homeostasis in human erythroleukemia cells. Exp Cell Res. 2011;317:2969–2980. doi: 10.1016/j.yexcr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Fernando MA, Heaney AP. Alpha1-adrenergic receptor antagonists: novel therapy for pituitary adenomas. Mol Endocrinol. 2005;19:3085–3096. doi: 10.1210/me.2004-0471. [DOI] [PubMed] [Google Scholar]
- 18.Hui H, Fernando MA, Heaney AP. The alpha1-adrenergic receptor antagonist doxazosin inhibits EGFR and NF-kappaB signalling to induce breast cancer cell apoptosis. Eur J Cancer. 2008;44:160–166. doi: 10.1016/j.ejca.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui EJ, Shabbir M, Thompson CS, Mumtaz FH, Mikhailidis DP. Growth inhibitory effect of doxazosin on prostate and bladder cancer cells. Is the serotonin receptor pathway involved? Anticancer Res. 2005;25:4281–4286. [PubMed] [Google Scholar]
- 20.Masachika E, Kanno T, Nakano T, Gotoh A, Nishizaki T. Naftopidil induces apoptosis in malignant mesothelioma cell lines independently of alpha1-adrenoceptor blocking. Anticancer Res. 2013;33:887–894. [PubMed] [Google Scholar]
- 21.Lin SC, Chueh SC, Hsiao CJ, Li TK, Chen TH, Liao CH, Lyu PC, Guh JH. Prazosin displays anticancer activity against human prostate cancers: targeting DNA and cell cycle. Neoplasia. 2007;9:830–839. doi: 10.1593/neo.07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf C, Lederer K, Pfragner R, Schauenstein K, Ingolic E, Siegl V. Biocompatibility of ultra-high molecular weight polyethylene (UHMW-PE) stabilized with alpha-tocopherol used for joint endoprostheses assessed in vitro. J Mater Sci. 2007;18:1247–1252. doi: 10.1007/s10856-006-0098-6. [DOI] [PubMed] [Google Scholar]
- 23.Anderson TF. Techniques for the preservation of three-dimensional structure in preparing specimens for the electron microscope. Trans NY Acad Sci. 1951;13:130–134. [Google Scholar]
- 24.Pfragner R, Wirnsberger G, Niederle B, Behmel A, Rinner I, Mandl A, Wawrina F, Luo J, Adamiker D, Hoger H, Ingolic E, Schauenstein K. Establishment of a continuous cell line from a human carcinoid of the small intestine (KRJ-I) Int J Oncol. 1996;8:513–520. doi: 10.3892/ijo.8.3.513. [DOI] [PubMed] [Google Scholar]
- 25.Behr TM, Wulst E, Radetzky S, Blumenthal RD, Dunn RM, Gratz S, Rave-Frank M, Schmidberger H, Raue F, Becker W. Improved treatment of medullary thyroid cancer in a nude mouse model by combined radioimmunochemotherapy: doxorubicin potentiates the therapeutic efficacy of radiolabeled antibodies in a radioresistant tumor type. Cancer Res. 1997;57:5309–5319. [PubMed] [Google Scholar]
- 26.Esbenshade TA, Theroux TL, Minneman KP. Increased voltage-dependent calcium influx produced by alpha 1B-adrenergic receptor activation in rat medullary thyroid carcinoma 6-23 cells. Mol Pharmacol. 1994;45:591–598. [PubMed] [Google Scholar]
- 27.Zink-Lorenz A, Komitowska J, Raue F. Norepinephrine induced calcitonin secretion in rat medullary thyroid carcinoma 6-23 cells: interaction between intracellular calcium and cAMP. Exp Clin Endocrinol Diabetes. 1996;104:43–49. doi: 10.1055/s-0029-1211421. [DOI] [PubMed] [Google Scholar]
- 28.Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mimnaugh EG, Xu W, Vos M, Yuan X, Neckers L. Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol Cancer Res. 2006;4:667–681. doi: 10.1158/1541-7786.MCR-06-0019. [DOI] [PubMed] [Google Scholar]
- 30.Morissette G, Moreau E, R CG, Marceau F. Massive cell vacuolization induced by organic amines such as procainamide. J Pharmacol Exp Ther. 2004;310:395–406. doi: 10.1124/jpet.104.066084. [DOI] [PubMed] [Google Scholar]
- 31.Rao VM, Zannou EA, Stella VJ. Design of tablets for the delayed and complete release of poorly water-soluble weak base drugs using SBE7M-beta-CD as a solubilizing agent. J Pharm Sci. 2011;100:1576–1587. doi: 10.1002/jps.22375. [DOI] [PubMed] [Google Scholar]
- 32.Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren K, Borner C, Bredesen DE, Brenner C, Castedo M, Cidlowski JA, Ciechanover A, Cohen GM, De Laurenzi V, De Maria R, Deshmukh M, Dynlacht BD, El-Deiry WS, Flavell RA, Fulda S, Garrido C, Golstein P, Gougeon ML, Green DR, Gronemeyer H, Hajnoczky G, Hardwick JM, Hengartner MO, Ichijo H, Jaattela M, Kepp O, Kimchi A, Klionsky DJ, Knight RA, Kornbluth S, Kumar S, Levine B, Lipton SA, Lugli E, Madeo F, Malomi W, Marine JC, Martin SJ, Medema JP, Mehlen P, Melino G, Moll UM, Morselli E, Nagata S, Nicholson DW, Nicotera P, Nunez G, Oren M, Penninger J, Pervaiz S, Peter ME, Piacentini M, Prehn JH, Puthalakath H, Rabinovich GA, Rizzuto R, Rodrigues CM, Rubinsztein DC, Rudel T, Scorrano L, Simon HU, Steller H, Tschopp J, Tsujimoto Y, Vandenabeele P, Vitale I, Vousden KH, Youle RJ, Yuan J, Zhivotovsky B, Kroemer G. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009;16:1093–1107. doi: 10.1038/cdd.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellstrom WJ, Wang R, Peterson CA, Varady JC, Gesundheit N, Sikka SC. Effects of alprostadil and prazosin on motility, viability and membrane integrity of human sperm. J Urology. 1998;159:1559–1562. doi: 10.1097/00005392-199805000-00041. [DOI] [PubMed] [Google Scholar]
- 34.Piller LB, Davis BR, Cutler JA, Cushman WC, Wright JT, Jr., Williamson JD, Leenen FH, Einhorn PT, Randall OS, Golden JS, Haywood LJ, The ACRG Validation of Heart Failure Events in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Participants Assigned to Doxazosin and Chlorthalidone. Curr Control Trials Cardiovasc Med. 2002;3:10. doi: 10.1186/1468-6708-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Officers A, Coordinators for the ACRGTA. Lipid-Lowering Treatment to Prevent Heart Attack T Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Jama. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 36.Bilbro J, Mart M, Kyprianou N. Therapeutic value of quinazoline-based compounds in prostate cancer. Anticancer Res. 2013;33:4695–4700. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Live cell imaging of the human MTC cell line TT. Cells were cultivated in chamber slides and observed using a Cell Observer for a total time of 24 h. Every 10 minutes a photo was taken of different positions on the slide.
Video 2: Live cell imaging of prazosin-treated TT cells. Cells were cultivated in chamber slides with 15 μM prazosin and observed using a Cell Observer for a total time of 24 h. Every 10 minutes a photo was taken of different positions on the slide.