Abstract
Human immunodeficiency virus type 1 (HIV-1) and several simian immunodeficiency viruses (SIV) encode for a transmembrane protein known as Vpu. While the smallest of the HIV-1 proteins, it has two important functions within virus-infected cells. The first of these functions is the down-regulation of the CD4 receptor to prevent its interaction with the HIV-1 envelope glycoprotein. Vpu interacts with the CD4 receptor in the rough endoplasmic reticulum (RER), resulting in its re-translocation across the RER and subsequent degradation via the proteasomal pathway. The second major function of the Vpu protein is to facilitate release of virus from infected cells. Previous studies have shown that virus release is cell type specific, suggesting that certain cells may express a restriction factor that inhibits virus release in the absence of Vpu. Recently, bone marrow stromal antigen 2 (BST-2/HM124/CD317/tetherin) has been identified as this factor. This review will focus on new findings within the last four years on the role of Vpu in CD4 down-regulation and the restriction of virus release from cells. We will relate these findings to virus pathogenesis and propose questions regarding BST-2 as a restriction factor.
INTRODUCTION
The Vpu protein is a small transmembrane protein encoded by human immunodeficiency virus type 1 (HIV-1) but is not expressed by HIV-2 [10, 76]. Structural homologues have been detected in simian immunodeficiency virus (SIV) from chimpanzees (SIVcpz), the mona monkey (Cercopithecus mona; SIVmon), the greater spot-nosed monkey (Cercopithecus nictitans; SIVgsn), mustached monkeys (Cercopithecus cephus; SIVmus) and more recently Dent’s mona monkey (Cercopithecus mona denti; SIVden) [1, 13, 15]. Sequence analysis has shown that the vpu from SIVcpz is most closely related to the vpu from HIV-1 both in amino acid sequence and protein function [25, 50]. The Vpu protein has two established functions in the virus replication cycle. These functions are to disrupt CD4 trafficking and shunt it to the proteasome for degradation and to enhance virion release [43, 83]. In this review, we will focus primarily on new findings regarding CD4 down-regulation and enhanced virion release, and relate these to the pathogenesis of the virus.
THE VPU PROTEIN
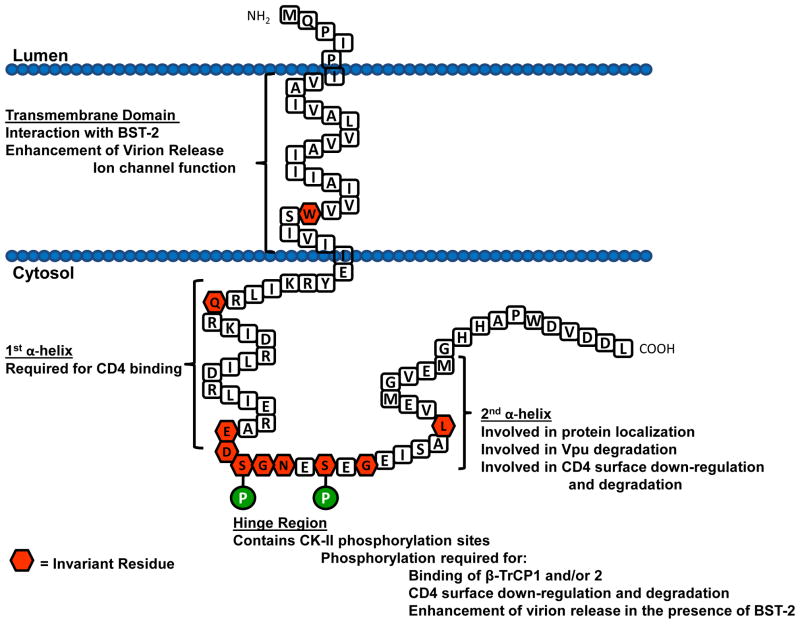
The Vpu protein is 77–86 amino acids in length and is comprised of a short N-terminal region, a transmembrane domain (TMD), and a longer cytoplasmic domain (CD) (Figure 1). Vpu is translated on the rough endoplasmic reticulum (RER) with the TMD also serving as an uncleaved leader sequence. The CD of Vpu has two predicted α-helical domains separated by a hinge region characterized by two canonical casein kinase II sites (S/T-X-X-E/D). The most variable regions of the protein are the N-terminal region (including parts of the transmembrane domain) and the far C-terminal region of the protein [50]. However, there are several highly conserved amino acids and domains among most vpu species. The most highly conserved region is the hinge region, which reflects its importance in Vpu function and will be discussed later. There are several invariant amino acids found in the transmembrane region and the cytoplasmic domain. The first is the invariant tryptophan at position 23 of the corrected HXB2 Vpu protein. This amino acid with its ring structure is probably involved in stabilizing the TMD within the lipid bilayer [63]. There is also an invariant glutamine at position 35 within the first α-helical domain and an invariant leucine at position 63 within the second α-helical domain. The function of Glu35 is unknown and the Leu63 will be discussed in a later section. The second highly conserved domain is the tyrosine based motif YXXL located at the TMD/CD interface. Unlike the other transmembrane glycoprotein of HIV-1, gp120/gp41, Vpu has no predicted N-linked glycosylation sites.
Fig. 1.
Schematic diagram of the membrane orientation of the consensus subtype B Vpu protein (strain HXB2 with a corrected methionine at the N-terminus). The amino acids within the hexagons represent amino acids that were found to be invariant among all vpu subtypes (50)
While the three-dimensional structure of the entire Vpu protein has yet to be solved, the structure of the TMD has been determined by nuclear magnetic resonance (NMR) spectroscopy in micelle and bilayer samples [62]. These investigators used the peptide Vpu2–30+, which was a 36-residue polypeptide that consists of residues 2–30 from the N terminus of Vpu and a six-residue “solubility tag” at its C terminus. They found that the Vpu2–30+ has a TM α-helix spanning residues 8–25 with an average tilt of 13°. They found that the helix was kinked slightly at the isoleucine at position 17, which results in tilts of 12° for residues 8–16 and 15° for residues 17–25. These investigators subsequently showed that the tilt angle of the helix was inversely proportional to hydrophobic thickness of the lipid bilayer [63].
IS VPU AN ION CHANNEL PROTEIN?
Several studies suggest that Vpu may have an ion channel activity. In the early descriptions of the Vpu protein, its structure was compared to the M2 protein of influenza as it had: a) a similar topology in the membrane having a short N-terminal region; b) an uncleaved leader/transmembrane domain and longer cytoplasmic domain; and c) a similar length and two casein kinase II phosphorylation sites. The H+ ion channel of M2 has been extensively characterized and is considered the prototypical “viroporin.” It is the best characterized of this class of viral proteins, which also includes the 6K protein of Sindbis virus (SV), and the 2B protein of poliovirus [26]. The initial evidence that Vpu could form an ion channel arose from studies showing that expression of Vpu in frog oocytes results in a conductance that is weakly selective for cations [20, 72]. Modeling studies have suggested that a pentameric structure for the Vpu TMD would be optimal for the formation of such a channel [11, 12, 27, 46, 70, 84]. These same investigators later showed that two derivatives of the Na+/K+ antiporter amiloride, dimethyl amiloride (DMA) and hexamethylene amiloride (HMA), inhibited Vpu-mediated virion release and replication in macrophages [21, 22]. In the most recent study, both DMA and HMA were shown to inhibit HIV-1 replication in monocyte-derived macrophage cultures [22]. However, we have found that these compounds affected the cell cycle regulation when expressed in T-cells at concentrations required to inhibit viral replication (unpublished data).
Previous studies of the M2 protein have shown that the His-X-X-X-Trp motif within the TMD was essential for ion channel activity with the histidine being the proton sensor and the tryptophan being the actual pore [58, 77, 78]. The drugs amantadine and rimantadine are known as M2 ion channel blockers [58, 77, 78]. Substitution of the M2 TMD histidine residue with an alanine resulted in a constitutively open channel, indicating its importance to channel activation [32, 33, 68, 78]. Using this as a basis, we first made a simian human immunodeficiency virus (SHIV) in which the TMD of Vpu was exchanged with the TMD of M2 [34]. This virus, SHIVM2, was capable of causing a severe loss of CD4+ T cells and AIDS when inoculated into macaques. In addition, we found that unlike the parental SHIVKU-1bMC33, replication of the SHIVM2 virus was sensitive to an M2 ion channel blocker, rimantadine. This study showed that drugs targeting the TMD of Vpu (in this case a chimeric Vpu with the TMD of the M2 protein) could reduce virus release from infected cells. If one compares the sequence of the Vpu and M2 TMDs, the subtype B Vpu contains the sequence Ala-X-X-X-Trp in approximately the same position as M2 with the tryptophan being invariant in Vpu [50]. We subsequently showed that substitution of the alanine in this motif with a histidine residue resulted in a SHIV (SHIVVpuA19H) that became more sensitive to rimantadine than the SHIVM2 virus [35]. These results indicate that a single amino acid substitution within the TMD of the Vpu protein converts a rimantadine-resistant SHIV to a rimantadine-sensitive SHIV. In a subsequent study, Park and Opella used NMR spectroscopy to analyze the structure of the TMD with the alanine to histidine substitution [64]. They showed that in C14 phospholipid bicelles, the unmodified TMD has a tilt angle of 30° but that the A18H had a tilt angle of 41°, indicating that the introduced histidine altered the structure of the helix in the bicelle. These investigators also showed that the isoleucine at position 15 and the tryptophan at position 22 of A18H but not the unmodified TMD were perturbed by the presence of rimantadine. Finally, they showed that the rotation angle of His18 and Trp22 in A18H were almost identical to His37 and Trp41 of the M2 protein (A18H, 41°, M2, 38°). Taken together, these studies indicate that the TMD of Vpu can be designed to bind the influenza antiviral rimantadine. Whether the ion channel activity of Vpu is functionally required for the virus release function is still controversial. However, these studies do provide “proof of concept” that Vpu could be a target for novel antiviral drugs.
THE ROLE OF VPU IN CD4 DOWN-REGULATION
During virus entry, the HIV-1 Env glycoprotein binds to the CD4 receptor and subsequently with a chemokine co-receptor, normally CCR5 on macrophages and CXCR4 on T helper cells. Thus, expression of CD4 molecules on the surface of cells is essential to HIV-1 infection, however, once infection has occurred, expression of CD4 poses several problems in the virus replication cycle. Newly synthesized CD4 molecules are capable of forming complexes with the Env precursor gp160 in the endoplasmic reticulum (ER) preventing transport and processing of the two cleavage products, gp120 and gp41, to the site of assembly [14, 37]. CD4 surface expression can lead to the retention of nascent virions at the plasma membrane and/or superinfection of cells by cell-free or cell-associated virus [43, 71, 82, 86]. HIV-1 has developed several mechanisms to down-modulate both intracellular and surface expression of CD4 molecules promoting productive virion assembly and release. These mechanisms are mediated by two accessory proteins encoded for by HIV-1: Nef and Vpu. These two proteins regulate CD4 expression at different cellular compartments and by distinct mechanisms. HIV-1 Nef functions by acting on mature CD4 molecules present at the plasma membrane or in late endosomes. Nef accelerates clathrin-mediated endocytosis of CD4 molecules from the cell plasma membrane and targets them to the lysosomes for destruction [23, 66, 67]. HIV-1 Vpu is expressed from the same mRNA as Env and is responsible for counteracting the effects of CD4 molecules early within the biosynthetic pathway. The general mechanism by which HIV-1 Vpu counteracts the problems presented by CD4 molecules has been well studied. Research conducted over the past several years has focused on the characterization of specific molecular components involved in this process and the consequences that this CD4 down-modulation has on pathogenesis and cellular homeostasis in vitro and in vivo (Figure 2).
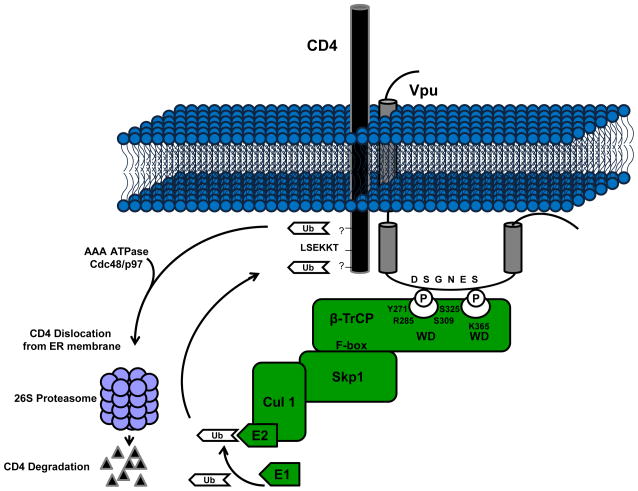
Fig. 2.
Down-modulation of cell surface CD4 expression by Vpu. In the rough endoplasmic reticulum, Vpu binds CD4 through an interaction between the first α-helix within the cytoplasmic domain of Vpu and a specific motif within the cytoplasmic domain of CD4 (LSEKKT). Vpu is phosphorylated by several different isoforms of casein kinase II. This phosphorylation recruits and binds the cellular F-box protein β-TrCP. CD4 is then ubiquitinated via the SCFβ-TrCP E3 Ub ligase complex after which it is shunted to the proteasome for degradation through an unknown mechanism.
Following synthesis of Vpu in the ER, it binds to a specific motif, LSEKKT, within the cytoplasmic domain of CD4 between residues 414 and 419 [5, 8, 45, 82]. Binding of the CD4 molecule by Vpu is required for its subsequent degradation; however, it is not sufficient [5, 6, 73, 79]. Previous studies have shown that both α-helical domains within the CD of HIV-1 Vpu are structurally important for CD4 down-modulation from the cell surface with only the first α-helix being required for binding [79]. These investigators showed that disruption of the predicted secondary structure of the two cytoplasmic α-helical domains eliminates Vpu-mediated CD4 surface down-modulation [79]. These investigators showed that expression of a Vpu protein with amino acid substitutions in the first α-helical domain but not in the second α-helical domain affected binding to CD4. Also, deletion of the C-terminal 23 residues within HIV-1 Vpu strain HXB2 or substitution of residues V65 thru M71 with the amino acid sequence ALGMAL, eliminates Vpu-mediated CD4 surface down-regulation, but not CD4 binding [61, 79]. Taken together, these studies indicate that primary and secondary structures are important for Vpu-mediated CD4 surface down-regulation and that the first α-helical region of the cytoplasmic domain of Vpu appears to be involved in the interaction with CD4. We recently completed a study that systematically analyzed the importance of each amino acid in the predicted second α-helical domain [31]. In this study, each amino acid was substituted with an alanine residue. We identified an additional amino acid, L63 within the second α-helix of HIV-1 Vpu strain HXB2 that is required for CD4 surface down-modulation [31]. In this study, we also identified a second amino acid, V68, which is necessary but is not sufficient for complete CD4 surface down-regulation. The L63 residue is conserved among all HIV-1 Vpu subtypes. While Tiganos and colleagues showed that substitution of this residue with a proline did disrupt Vpu-mediated CD4 degradation [80], a proline substitution eliminated the ability to distinguish between a primary role of the hydrophobic amino acid residue at this position and its structural role in the predicted second α-helical domain. Substitution of this residue with alanine or valine, which maintains the predicted secondary structure, did not disrupt binding to CD4 or β-TrCP, but did eliminate CD4 surface down-modulation. Interestingly, substitution of this residue with either an isoleucine or a glycine resulted in partial function in CD4 down-modulation, suggesting that the length of the side chain is important. Similar results were obtained for mutant Vpu with similar substitutions of the valine at position 68 although to a lesser extent. These mutants will be useful in determining the specific role of the second α-helix in the process of CD4 degradation.
The Vpu protein is phosphorylated at residues Ser52 and Ser56 (positions from HIVNL4-3 clone) present within two highly conserved casein kinase II phosphorylation sites (S/T-XX-D/E) [73]. This phosphorylation is required for CD4 degradation, but not CD4 binding [5, 65]. The phosphorylated Vpu protein recruits and binds the cellular F-box protein β-TrCP generating a CD4-Vpu-β-TrCP ternary complex [4, 49]. The signal for recognition of ligands, including HIV-1 Vpu, by β-TrCP is the phosphorylation of one or two serine residues present within a conserved motif, DSPGxxSP. Both phosphoserines have distinct orientations within the protein complex that allow them to make specific contacts with the WD-repeat β-propeller domain of β-TrCP [9, 19, 52, 85]. Biochemical analyses of the HIV-1 Vpu protein showed that the first phosphoserine interacts with the binding pocket of the β-TrCP WD-repeat domain (residues Y271, R285, S309 and S325). The second phosphoserine interacts with a basic patch in the β-TrCP WD-repeat domain centered around residue K365. The hydrophobic amino acids surrounding the DSPGxxSP motif also strengthen the interaction of Vpu with the β-TrCP by forming electrostatic, hydrogen and van der Waals bonds with specific residues within the binding pocket [9, 19, 52, 85].
The F-box protein β-TrCP recruits the other components of the E3 ubiquitin ligase complex (Skp1 and Cullin1) and mediates the ubiquitination of the CD4 molecule in trans. A homologue of β-TrCP known as β-TrCP2 or HOS was shown to be sufficient for Vpu-mediated CD4 down-modulation [3, 7]. Silencing of both homologues with siRNA was required for the inhibition of Vpu-mediated CD4 surface down-regulation [7]. The process by which Vpu mediates CD4 down-regulation resembles the ER-associated protein degradation process (ERAD); however, the exact mechanism by which CD4 is poly-ubiquitinated and transported to the cytosol for degradation is not completely understood. Meusser and Sommer reconstituted HIV-1 Vpu-mediated CD4 degradation in Saccharomyces cerevisiae to determine the role of ERAD proteins in this process [53]. In this system, CD4 was translocated into the ER, but, unlike human cells where CD4 is stable the CD4 was rapidly degraded in the absence of Vpu and depended on the presence of the yeast ERAD proteins and a functional proteasome. The rapid degradation of CD4 was eliminated in yeast defective in ERAD-dependent ubiquitination ligases, but could be restored by the expression of Vpu and human β-TrCP. Also, in the ERAD-defective yeast CD4 was shown to be ubiquitinated in the presence of phosphorylated Vpu and β-TrCP providing evidence supporting the trans-polyubiquitination of the CD4 by the SCFβ-TrCP E3 Ub ligase complex. The degradation of CD4 was shown to be dependent on the presence of four lysine residues within the cytosolic tail of CD4 [53]. Similar results were obtained for the polyubiquitination of the CD4 cytoplasmic domain mediated by the SCFβ-TrCP E3 Ub ligase complex in human cells [4]. However, CD4 down-regulation was not completely dependent on ubiquitination of the four cytoplasmic lysine residues suggesting a role for other serine or threonine residues present within the CD4 cytoplasmic tail. This study also provided evidence for the involvement of an ERAD protein, the AAA ATPase Cdc4/p97 by showing disruption of Vpu-mediated CD4 down-regulation through the expression of a transdominant negative mutant of this ATPase. This protein is involved in dislocation of ERAD substrates and this group also provides evidence for dislocation of the CD4 molecule that is dependent on its poly-ubiquitination. These data provide a better understanding of the specific mechanism by which Vpu mediates CD4 degradation and continued studies are needed to determine all proteins involved in the process and possible targets for therapeutics.
The HIV-1 Vpu protein has been found to escape degradation during the process of CD4 down-regulation and degradation [49, 73]. In a recent study Vpu degradation was shown to occur in cells arrested in early mitosis by a proteasome-mediated process [18]. The Vpu protein was shown to become phosphorylated at a downstream phosphoserine residue (Ser61 in HIVHXB2) inducing recruitment of an unknown E3 ubiquitin ligase complex for ultimate degradation [18]. Mutation of this residue resulted in the accumulation of HIV-1 Vpu within cells and increased release of HIV-1 particles from HeLa cells in comparison to HeLa cells infected with wild-type virus. Vpu-mediated CD4 degradation was not affected by mutation of this serine residue. These investigators indicated that phosphorylation of this residue was involved in regulating Vpu degradation. We previously analyzed the Vpu sequences from over 100 strains of HIV-1 group M with representation of all different subtypes. Our results indicate that a serine residue at this position is a relatively rare occurrence (BRU, HXB2 strains) and extrapolation to Vpu proteins from “clinical isolates” may not be possible. Whether the serine present at position 64 in the majority of HIV-1 Vpu proteins is phosphorylated and can substitute for the serine at position 61 remains to be elucidated. While our results are in agreement regarding serine 61 and degradation of Vpu, substitution of residues L63 and V68 with alanines also resulted in slower turnover of HXB2 Vpu in transfected cells [31]. Taken together, these results indicate that other amino acids within the second α-helical domain also contribute to the regulation of Vpu degradation.
THE CD4 DOWN-MODULATION FUNCTION IS CONSERVED IN VPU PROTEINS FROM SIVCPZ
Only HIV-1 and some SIVcpz strains express a Vpu protein, however, sequences and predicted secondary structures of the SIVcpz Vpu proteins are different from those of HIV-1 Vpu proteins. Also, some of the SIVcpz Vpu isolates only contain a single casein kinase II phosphorylation site. Therefore, our laboratory analyzed the ability of Vpu proteins from four SIVcpz isolates (CAM13, ANT, TAN1, and GAB1) to down-regulate CD4 from the cell surface and to induce degradation. SIVcpzCAM13, SIVcpzANT, and SIVcpzTAN1 only contain a single casein kinase II site. All four vpu isolates were fused in frame to the gene for enhanced green fluorescence protein (EGFP) and expressed under the control of a CMV promoter, similar to the HIV-1 subtype B vpu genes our laboratory has previously analyzed [34, 35, 74]. All four SIVcpz Vpu fusion proteins were membrane-associated, partially co-localized with DsRed-ER and ECFP-Golgi marker proteins, and completely co-localized with an ECFP-Membrane marker. Using immunostaining of live HeLa-CD4+ cells transfected with each SIVcpz Vpu fusion protein, we showed that all four Vpu proteins down-regulated CD4 surface expression. Analysis of CD4 molecules immunoprecipitated from 293 cells co-transfected with plasmids expressing either CD4 or each of the SIVcpz Vpu fusion proteins revealed that all four SIVcpz Vpu isolates induced CD4 degradation similar to HIV-1 Vpu (HXB2). Treatment of these cells with MG132 showed that this process was mediated by the proteasomal pathway similar to HIV-1 Vpu proteins. The SIVcpzCAM1 contains a serine residue immediately following the first casein kinase II site and SIVcpzANT and SIVcpzTAN both contain a string of five negatively charged amino acids immediately following the first casein kinase II site. We determined whether the CAM1 serine residue and the negatively charged amino acids in the ANT and TAN1 isolates substituted for the lack of a second casein kinase II site. Substitution of the serine residue at position 55 in the SIVcpzCAM13 Vpu with an alanine resulted in a decreased ability to down-regulate CD4 from the cell surface and an inability to degrade CD4. Similarly, substitution of the aspartic acid residues at positions 61 and 63 to positively charged lysines resulted in a Vpu protein that no longer down-regulated CD4 from the cell surface or mediated degradation of CD4 molecules [25]. Taken together, these results suggest that SIVcpz Vpu proteins do not require two canonical casein kinase II sites similar to HIV-1 Vpu proteins, but perhaps have adapted other mechanisms for this process.
THE ROLE OF VPU IN ENHANCED VIRUS RELEASE
The ability of Vpu to enhance the release of virions from infected cells exemplifies viral antagonism of an intrinsic host defense mechanism. The interferon-induced transmembrane protein BST-2 (CD317, HM1.24 or tetherin) restricts the release of nascent virions from the cell surface, while the Vpu protein relieves this restriction. Although the mechanisms involved in this process are not fully elucidated, current data suggest that BST-2 blocks release by directly retaining mature, nascent HIV-1 virions on the cell surface, while Vpu counteracts BST-2 by removing it from sites of virion assembly and budding at the plasma membrane. These relationships allow Vpu to be considered an antagonist of the innate, interferon-induced, immune response to enveloped viruses.
BST-2 is an interferon-induced, lipid raft-associated, type II integral membrane protein with an unusual topology. It contains a short cytoplasmic N-terminus followed by a transmembrane domain, a central extracellular domain predicted to form a coiled-coil that contains two N-linked glycosylation sites, and a C-terminal cleavage site predicted to form a glycosyl-phosphatidylinositol (GPI) anchor [36, 44]. This unusual membrane, topology, in which BST-2 interacts with the lipid bilayer twice, has suggested a membrane-spanning model of tethering in which one end of BST-2 is embedded in the membrane of the host cell and the other in the membrane of the budded virion. As a GPI-anchored protein, BST-2 is found within the cholesterol-enriched lipid domains from which HIV-1 (and other enveloped viruses) bud [44, 57, 59, 60]. The protein is found on the plasma membrane and within the endosomal system, including the trans-Golgi network [16, 44, 56, 81]. BST-2 co-localizes with HIV-1 Gag in a punctate distribution along the plasma membrane of cells expressing HIV-1, consistent with a direct tethering mechanism [40, 54, 56, 81]. This mechanism appears to involve the incorporation of BST-2 into nascent virions (Fitzpatrick et al., submitted). So far, the molecular requirements for virion-tethering within BST-2 include the cytoplasmic domain, the predicted coiled-coil ectodomain, and the GPI anchor [24, 56]. BST-2 forms disulfide linked dimers and is heterogeneously glycosylated [2, 44]. Kaletsky and colleagues treated 293T cells expressing the HIV Gag-Pol and human BST-2 with DTT and showed that it had no ability to elute restricted HIV from the cell surface, suggesting that cysteine-mediated dimerization alone does not mediate the restriction of virion release [41]. However, the exact role of glycosylation and dimerization in conjunction with other protein-protein interactions in the tethering process remain unknown. The ability of BST-2 to restrict the release of virus like particles (VLPs) derived from viral proteins from members of diverse viral families including all retroviruses tested, filoviruses, and an arenavirus (Lassa), suggests that it does not interact with any specific viral component, but rather the viral lipid envelope, an unknown receptor, or itself [40]. In the latter scenario, cell-associated BST-2 would interact with virion-associated BST-2, potentially via the protein’s coiled-coil extracellular domain. Although a direct tethering mechanism remains the most obvious and appealing model of restricted release, the precise topology of the BST-2 molecules that retain nascent virions at the cell surface and the molecular interfaces remain to be determined.
Vpu relieves the restriction of virion release imposed by BST-2. This relationship accounts for the long observed vpu phenotype of enhanced efficiency of viral release, and it explains why certain cell types such as HEK293 do not support this phenotype as they do not express BST-2 constitutively. To counteract BST-2, Vpu appears to remove the protein from sites of virion assembly along the plasma membrane [40, 54, 81]. In cells expressing Vpu, the overall level of BST-2 at the cell surface is reduced [2, 29, 54]. This reduction occurs rapidly, within sixteen hours of viral gene expression, consistent with the time frame in which progeny virions begin to bud from infected cells. The molecular mechanisms by which Vpu modulates BST-2 likely involves an interaction between the two proteins and the recruitment of a specific SCF E3 ubiquitin ligase complex containing the substrate adaptor β-TrCP to BST-2 by Vpu [29, 54]. In support of an interaction between Vpu and BST-2, the proteins co-localize microscopically in endosomal vesicles and can be co-immunoprecipitated from cellular lysates [17, 56, 81]. Though not yet formally shown, the transmembrane domains (TMDs) of Vpu and BST-2 likely mediate this interaction within the lipid bilayer. Conversely, BST-2 orthologues of non-human primates are neither virologically counteracted nor removed from the cell surface of Vpu [29, 51, 69]. This resistance to Vpu maps to the BST-2 TMD [29, 51, 69]. McNatt and colleagues constructed plasmids expressing human bst-2 genes with point mutations within the TMD that correlated with residues found in the TMD of the rhesus BST-2 protein [51]. This group concluded that no single change in the human BST-2 TMD abolished Vpu sensitivity. However, several combinatorial mutations elicited an increase in resistance including delGI, T45I (where residues G25 and I26 were deleted and residue T45 was substituted with an isoleucine) and delGI, I33V, I36L (where residues G25 and I26 were deleted, residue I33 was substituted with a valine and residue I36 was mutated to a leucine). McNatt also noted that residues V30 and P40 impart a distinct contribution to Vpu sensitivity. Exchange of the TMDs from human and rhesus BST-2 conferred complete resistance of human BST-2 and sensitivity of the rhesus BST-2 protein to HIV-1 Vpu [51]. Gupta also emphasized the importance of residue T45 in the human BST-2 protein, concluding that mutation of this residue to an isoleucine substantially reduces the sensitivity of human BST-2 to HIV-1 Vpu, without affecting antiviral activity [29]. These investigators also noted that substitution of this residue abrogates the Vpu mediated depletion of cellular steady state levels of BST-2, providing additional evidence in favor of the importance of BST-2 degradation for the virus to overcome its antiviral effects [29]. Both groups, as well as that of Rong and colleagues, agree that the TMD of human BST-2 is not only necessary but is also sufficient for the species specificity of Vpu-responsiveness [29, 51, 69]. These data support a model in which the TMDs of the two proteins interact, potentially directly, although the structural basis of this interaction remains to be elucidated.
The importance of Vpu-mediated surface down-regulation and the intracellular depletion of BST-2 with respect to the virologic effect of Vpu in spreading infections is not without controversy. Several groups have shown that HIV-1 Vpu causes intracellular depletion and surface down-regulation of human BST-2 in cell lines including HeLa, HEK 293T, and A3.01 CD4-positive T lymphoid cells, and these effects correlate with the enhancement of virion release [2, 17, 24, 29, 54, 81]. However, Miyagi and colleagues further investigated the roles of HIV-1 Vpu and the surface down-regulation and intracellular depletion of BST-2 on the kinetics of viral replication during spreading infections [55]. This group observed minimal changes in the surface expression of BST-2 during the course of infection of CEMx174 and H9 cells, while vpu clearly contributed to the net production of cell-free virions. The authors concluded that Vpu enhances virus release in the absence of either surface down-regulation or intracellular depletion of BST-2. This conclusion relies on the assumptions that the accumulation of cell-free virions during spreading infections is a simple correlate of the efficiency of virion-release from infected cells and that no other functions of Vpu are relevant to the rate of viral spread. These assumptions may not be correct: the absence of vpu enhances the rate of cell-to-cell viral spread [28] and the down-regulation of CD4 by Vpu may affect viral propagation, as reviewed above. In contrast to the conclusions of Miyagi et al., Rong and colleagues showed that BST-2 specifically decreased the replication rate of vpu-negative HIV-1 in a dose-dependent manner [69]. These investigators used an inducible system to express BST-2 in the CD4-positive T cell line SupT1, which lacks endogenous BST-2. The ability of Vpu to counteract BST-2 in this system directly correlated with the depletion of BST-2 levels.
Several questions regarding the mechanism of down-regulation of BST-2 by Vpu remain to be answered. In addition to interacting with BST-2, Vpu recruits to BST-2 a β-TrCP-containing E3 ubiquitin ligase complex to modulate the protein either directly or indirectly via ubiquitination [17, 47, 54]. As discussed above, the cytoplasmic domain of Vpu contains the sequence DSpGxxSP which recognizes the cellular proteins β-TrCP-1 and -2, which are F-box proteins and substrate adaptors for SCF E3 ubiquitin-ligase complexes. The interaction of Vpu with β-TrCP was first described as the basis for the down-regulation of CD4 by Vpu, however, evidence that a related process occurs in the case of BST-2 include the observations that: 1) the DSPGxxSP sequence in Vpu is required both for optimal virion release and the down-regulation of BST-2, and 2) expression of a dominant-negative β-TrCP or knockdown of endogenous β-TrCP inhibits both the Vpu-mediated enhancement of virion release and the down-regulation of BST-2. Although a consensus is now emerging regarding a key role of β-TrCP as a Vpu cofactor in counteracting BST-2, a number of questions remain. First, is BST-2 ubiquitinated in response to Vpu? Second, what is the consequence of such Vpu-mediated ubiquitination? Evidence has been presented in support of various outcomes: proteasomal degradation of BST-2, lysosomal degradation of BST-2, and/or post-endocytic endosomal trapping with partial lysosomal degradation [17, 24, 29, 54]. In support of the latter, the plasma membrane clathrin adaptor AP-2 and endosomal acidification are required for optimal down-regulation of BST-2 from the cell surface by Vpu [54]. The K5 protein of the Kaposi Sarcoma Virus has also been identified as an antagonist of human BST-2, and the data supporting this inhibition parallel to some extent the results reported for the Vpu protein [48]. The observation that lysines in the short amino-terminal domain of BST-2 are ubiquitinated by the K5 protein resulting in the rapid degradation of BST-2 provides increased incentive for determining if and how BST-2 is ubiquitinated in response to Vpu. Such information would provide a better understanding of the fate of BST-2 following its interaction with Vpu, and it might embrace multiple mechanisms for the down-regulation and degradation of BST-2, some of which could be cell-type specific. Notably, cell type differences and/or experimental formats (transient expression of exogenous BST-2 versus constitutive expression of endogenous protein) could contribute to the different mechanisms proposed thus far for the counteraction of BST-2 by Vpu. Nevertheless, a model is now apparent in which the transmembrane and cytoplasmic domains of Vpu each contribute to the counteraction of BST-2: the transmembrane domain via an interaction with BST-2 and the cytoplasmic domain via recruitment of an E3 ubiquitin ligase complex to cellular membranes containing BST-2. The effect of these interactions is the altered localization of BST-2 within the cell and the counteraction of restricted virion release (See Figure 3 for potential model).
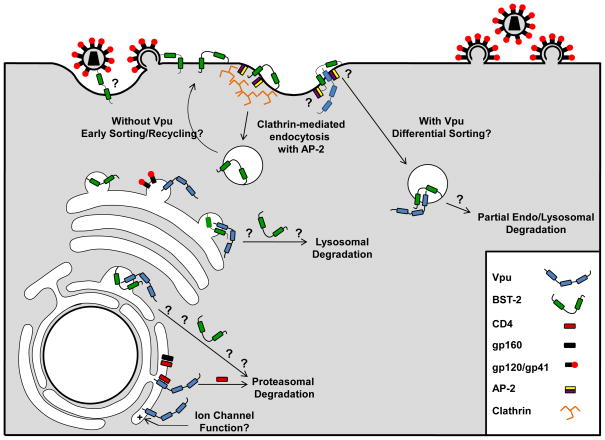
Fig. 3.
Possible mechanisms by which Vpu enhances virion release from cells. Vpu allows efficient transport of Env proteins to the site of virion assembly. Vpu is synthesized on the rough endoplasmic reticulum and binds CD4 releasing gp160 for processing and cleavage into gp120/gp41 which may be transported to the site of assembly. Vpu may function as a viroporin. Vpu resides within the rough endoplasmic reticulum, trans-Golgi network and at the cell surface. It is unknown whether the ability of Vpu to serve as an ion channel functions to enhance virion release at any of these intracellular locations. Vpu removes BST-2 from the site of virion assembly. In the absence of Vpu, BST-2 is expressed at the cell surface. The orientation used by BST-2 to “tether” virions to the surface remains unknown, however, the GPI anchor resides within lipid rafts where HIV-1 virions assemble and bud. This potentiates a model where the N-terminal transmembrane remains anchored within the cell membrane while the C-terminal GPI anchor is embedded in the virion membrane. BST-2 is internalized from lipid rafts on the cell surface by clathrin-mediated endocytosis. Two tyrosine residues within the N-terminal cytoplasmic tail are required for an interaction with a Δα-adaptin of the AP-2 complex. It is possible that in the presence of Vpu the targeting of endocytosed BST-2 containing vesicles is altered for endo-lysosomal degradation. Vpu may also potentially interact with BST-2 in trans-Golgi network and target it for lysosomal or proteasomal degradation.
As is a caveat of all in vitro studies, the importance and validity of these conclusions will not be fully substantiated until observed within a physiologically relevant in vivo host system. Pig-tailed (pt) and rhesus (rh) macaque BST-2 proteins have been shown to have antiviral effects on HIV-1 replication that are not Vpu sensitive [51]. However, recent studies have determined that SIV Nef is an inhibitor of these orthologues [38, 88]. More research is needed to increase our understanding of the mechanisms by which BST-2 restricts virion release and how Vpu and Nef antagonize this restriction. This will allow us to establish the most effective way to study the role of these interactions in vivo and to determine the potential of BST-2 as an antiviral therapeutic. Further research is also needed to determine if specific interactions (indirect or direct) exist between the Vpu and/or the Nef proteins and pt/rhBST-2 proteins that could be used to study the effects of BST-2 in vivo. The SHIV/macaque model of disease is an excellent tool for studying the in vitro and in vivo effects of human and non-human primate BST-2 proteins, because it expresses both the HIV-1 Vpu protein and the SIVmac239 Nef gene. SHIVs could potentially be engineered to express Vpu or Nef proteins with mutations that abrogate specific interactions without affecting other functions of both proteins. The intrinsic antiviral defenses encoded by human and non-human primate hosts now includes the TRIM family, APOBEC3 and BST-2 proteins, providing multiple avenues to the development of approaches to the design of novel therapies. The analysis of these endogenous antiviral agents also provides evidence of evolutionary pressure for multiple families of viruses to acquire new or positively selected genes that contribute to species tropism and the barriers to cross-species transmission.
THE ROLE OF BST-2 IN RELEASE OF PRIMATE LENTIVIRUSES LACKING A VPU PROTEIN
Recent studies have addressed the question of how retroviruses that lack a vpu gene, including multiple primate lentiviruses, are efficiently released from cells that express BST-2. Two reports have investigated the ability of SIVmac239 Env and Nef proteins to counteract multiple primate BST-2 proteins [38, 88]. While SIVmac239 Env was found to possess no antagonistic abilities towards either rhesus or pig-tailed BST-2 (rhBST-2; ptBST-2) proteins, the SIVmac239 Nef protein did. The ability of several different Nef proteins from different primate lentiviruses to counteract various primate BST-2 proteins revealed a species-specific bias towards antagonism [88]. Both groups showed that the release of SIVΔNef could be inhibited by several primate BST-2 proteins. Both groups investigated the potential for specific regions or amino acids within the BST-2 proteins that conferred susceptibility to the SIV Nef protein, and both identified an amino acid motif that included residues D/GDIW14-17, which is present within the cytoplasmic domain of pig-tailed and rhesus BST-2 but absent in human BST-2. Their results also revealed that this region could be inserted into the human BST-2 protein and impart susceptibility to the SIV Nef protein [38, 88]. Mutation of either the myristoylation site within the N-terminal region of SIV Nef or residues that affect the down-regulation of CD4 completely abolished the ability to counteract BST-2 whereas mutation of a site specifically involved in the down-regulation of MHC-I had no effect [88]. SIVmac239 Nef down-regulated surface BST-2 in 293T cells stably expressing HA-tagged rhesus BST-2, similar to the results obtained for cell surface down-regulation of human BST-2 by Vpu [38]. Thus, it appears that SIVmac239 Nef functions similarly to HIV-1 Vpu in the counteraction of BST-2 proteins in a species-specific manner.
UNANSWERED QUESTIONS OF THE VPU PROTEIN IN VIRUS PATHOGENESIS
The new exciting data from the last several years has brought some clarity to the role of Vpu in the virus replication cycle and its role in virus pathogenesis. One question that needs to be addressed is whether CD4 down-modulation is more important in vivo than the release of infectious virus from cells. Unfortunately, the macaque models do not express a BST-2 protein that is susceptible to HIV-1 Vpu. Thus, macaque models may be able to assess the role of CD4 down-modulation in virus pathogenesis but not virus release. Several studies have shown that a mutant Vpu with either asparagine or glycine substitutions at positions 52 and 56 (Vpu2/6) abolishes Vpu binding to β-TrCP and subsequently CD4 surface down-modulation. Previous studies suggested that the TMD and CD of Vpu were specifically responsible for virus release and CD4 down-modulation, respectively [71]. Our laboratory has used a simian-human immunodeficiency virus (SHIV) macaque model to study the specific contributions of the HIV-1 Vpu protein to pathogenesis. Using this model our laboratory showed that macaques inoculated with SHIV expressing a Vpu protein with the two serines of the CK-II sites changed to glycines did not lose circulating CD4+ T cells and maintained significantly lower viral loads than macaques inoculated with parental SHIV [74]. This study suggested that the ability of Vpu to down-modulate CD4 expression directly correlates to the progression of disease in macaques. More recent studies have presented data for the requirement of Vpu binding to β-TrCP for BST-2 surface down-regulation and subsequent release of virions from the cell surface, suggesting that CD4 down-modulation and virus release may involve a common cellular cofactor [17, 54]. Thus, elimination of the CK-II sites may have pleiotropic effects on different Vpu functions. These results extend the still unanswered question of “What is the specific contribution of Vpu mediated CD4 degradation to disease progression in vivo?” Therefore, the isolation of mutants that affect one function but not the other are needed to definitely address each function in its role in disease progression. It will be interesting to use the macaque model to study the pathogenesis of SHIVs expressing a Vpu protein that binds CD4 and β-TrCP, but is deficient in CD4 surface down-modulation (such as the VpuL63A and VpuV68A).
As discussed above, two recent studies have shown that SIV Nef proteins can counteract the BST-2 proteins of non-human primates; pointing to a new role for Nef in lentiviral pathogenesis [38, 88]. However, these studies raise an interesting question about how hBST-2 affects the replication of SIVmac239 in CEMx174 cells. This human B-T hybrid cell line expresses copious amounts of hBST-2, and hBST-2 inhibits the release of SIVmac239 derived virions [38, 55, 88]. This is somewhat surprising, as many researchers have grown SIVmac239 and SIVmac239ΔNef stock viruses in this cell line [30, 39, 42, 75, 87]. This apparent paradox again raises the question of the extent to which BST-2 restricts the rate of viral replication during multi-cycle, spreading infection.
Perhaps the key question is how the enhancement of virion release by Vpu relates to pathogenesis in the host. During HIV-1 infection of humans or SIV infection of macaques the depletion of CD4+ T cells in the secondary lymphoid organs ultimately results in AIDS, not the depletion of circulating CD4+ T cells. Since CD4+ T cells in these organs are generally in close proximity to one another (e.g., in the paracortical region of the lymph nodes, the periarterial lymphatic sheath of the spleen, and the lymphoid aggregates and Peyer’s patches of the gut associated lymphoid tissue), and tethered particles may be infectious, the efficient spread of virus by cell-to-cell transmission may occur even in the absence of Vpu. On the other hand, the observation that three lentiviral proteins, HIV-1 Vpu, SIV-Nef and HIV-2 Env, each counteract BST-2 to enhance virion release, weighs in favor of a key role for this phenotype in the replication of these viruses. Finally, few Vpu proteins from strains other than laboratory-adapted subtype B viruses have been studied. Subtype B HIV-1 isolates account for only 5–10% of the total HIV-1 infections worldwide. Future studies will determine if the counteraction of BST-2 and the enhancement of virion release are common to most or all HIV-1 subtypes and non-human primate lentiviruses that encode the Vpu protein, or if it is instead a phenotype of only a subset of vpu genes.
Acknowledgments
The work reported here is supported by grants NIH grants AI51981 to E.B.S. and AI081668 to JCG.
References
- 1.Barlow KL, Ajao AO, Clewley JP. Journal of Virology. 2003;77:6879–6888. doi: 10.1128/JVI.77.12.6879-6888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartee E, McCormack A, Früh K. PLoS Pathogens. 2006;10:e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besnard-Guerin C, Belaidouni N, Lassot I, Segeral E, Jobart A, Marchal C, Benarous R. Journal of Biological Chemistry. 2004;279:788–795. doi: 10.1074/jbc.M308068200. [DOI] [PubMed] [Google Scholar]
- 4.Binette J, Dube M, Mercier J, Halawani D, Latterich M, Cohen EA. Retrovirology. 2007;4:75. doi: 10.1186/1742-4690-4-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bour S, Schubert U, Strebel K. Journal of Virology. 1995;69:1510–1520. doi: 10.1128/jvi.69.3.1510-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buonocore L, Turi TG, Crise B, Rose JK. Virology. 1994;204(1):482–486. doi: 10.1006/viro.1994.1560. [DOI] [PubMed] [Google Scholar]
- 7.Butticaz C, Michielin O, Wyniger J, Telenti A, Rothenberger S. Journal of Virology. 2007;81:1502–1505. doi: 10.1128/JVI.01711-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen MY, Maldarelli F, Karczewski MK, Willey RL, Strebel K. Journal of Virology. 1993;67:3877–3884. doi: 10.1128/jvi.67.7.3877-3884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coadou G, Gharbi-Benarous J, Megy S, Bertho G, Evrard-Todeschi N, Segeral E, Benarous R, Girault JP. Biochemistry. 2003;42:14741–14751. doi: 10.1021/bi035207u. [DOI] [PubMed] [Google Scholar]
- 10.Cohen EA, Terwilliger EF, Sodroski JG, Haseltine WA. Nature. 1988;334:532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- 11.Cordes FS, Kukol A, Forrest LR, Arkin IT, Sansom MS, Fischer WB. Biochimica et Biophysica Acta. 2001;1512:291–298. doi: 10.1016/s0005-2736(01)00332-7. [DOI] [PubMed] [Google Scholar]
- 12.Cordes FS, Tustian AD, Sansom MS, Watts A, Fischer WB. Biochemistry. 2002;41:7359–7365. doi: 10.1021/bi025518p. [DOI] [PubMed] [Google Scholar]
- 13.Courgnaud V, Salemi M, Pourrut X, Mpoudi-Ngole E, Abela B, Auzel P, Bibollet-Ruche F, Hahn B, Vandamme AM, Delaporte E, Peeters M. Journal of Virology. 2002;76:8298–8309. doi: 10.1128/JVI.76.16.8298-8309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crise B, Buonocore L, Rose JK. Journal of Virology. 1990;64:5585–5593. doi: 10.1128/jvi.64.11.5585-5593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dazza MC, Ekwalanga M, Nende M, Shamamba KB, Bitshi P, Paraskevis D, Saragosti S. Journal of Virology. 2005;79:8560–8571. doi: 10.1128/JVI.79.13.8560-8571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dube M, Roy BB, Guiot-Guillain P, Mercier J, Binette J, Leung G, Cohen EA. Journal of Virology. 2009;83(9):4574–4590. doi: 10.1128/JVI.01800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas JL, Viswanathan K, McCaroll MN, Gustin JK, Fruh K, Moses AV. Journal of Virology. 2009;83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estrabaud E, Le Rouzic E, Lopez-Verges S, Morel M, Belaidouni N, Benarous R, Transy C, Berlioz-Torrent C, Margottin-Goguet F. PLoS Pathogens. 2007;3:995–1004. doi: 10.1371/journal.ppat.0030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evrard-Todeschi N, Gharbi-Benarous J, Bertho G, Coadou G, Megy S, Benarous R, Girault JP. Peptides. 2006;27:194–210. doi: 10.1016/j.peptides.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Ewart GD, Sutherland T, Gage PW, Cox GB. Journal of Virology. 1996;70:7108–7115. doi: 10.1128/jvi.70.10.7108-7115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewart GD, Mills K, Cox GB, Gage PW. European Biophysics Journal. 2002;31:26–35. doi: 10.1007/s002490100177. [DOI] [PubMed] [Google Scholar]
- 22.Ewart GD, Nasr N, Naif H, Cox GB, Cunningham AL, Gage PW. Antimicrobial agents and chemotherapy. 2004;48:2325–2330. doi: 10.1128/AAC.48.6.2325-2330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia JV, Miller AD. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 24.Goffinet C, Allespach I, Homann S, Tervo HM, Habermann A, Rupp D, Oberbremer L, Kern C, Tibroni N, Welsch S, Krijnse-Locker J, Banting G, Krausslich HG, Fackler OT, Keppler OT. Cell Host and Microbe. 2009;5:285–297. doi: 10.1016/j.chom.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Gomez LM, Pacyniak E, Flick M, Hout DR, Gomez ML, Nerrienet E, Ayouba A, Santiago ML, Hahn BH, Stephens EB. Virology. 2005;335:46–60. doi: 10.1016/j.virol.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez ME, Carrasco L. FEBS Letters. 2003;552:28–34. doi: 10.1016/s0014-5793(03)00780-4. [DOI] [PubMed] [Google Scholar]
- 27.Grice AL, Kerr ID, Sansom MS. FEBS Letters. 1997;405:299–304. doi: 10.1016/s0014-5793(97)00198-1. [DOI] [PubMed] [Google Scholar]
- 28.Gummuluru S, Kinsey CM, Emerman M. Journal of Virology. 2000;74(23):10882–10891. doi: 10.1128/jvi.74.23.10882-10891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta RK, Hue S, Schaller T, Verschoor E, Pillay D, Towers GJ. PLoS Pathogens. 2009;5(5):e1000443. doi: 10.1371/journal.ppat.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammes SR, Dixon EP, Malim MH, Cullen BR, Greene WC. Proceedings of the National Academy of Sciences, USA. 1989;86(23):9549–9553. doi: 10.1073/pnas.86.23.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill MS, Ruiz A, Schmitt K, Stephens EB. Virology. 2009 In press. [Google Scholar]
- 32.Holsinger LJ, Lamb RA. Virology. 1991;183:32–43. doi: 10.1016/0042-6822(91)90115-r. [DOI] [PubMed] [Google Scholar]
- 33.Holsinger LJ, Nichani D, Pinto LH, Lamb RA. Journal of Virology. 1994;68:1551–1563. doi: 10.1128/jvi.68.3.1551-1563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hout DR, Gomez ML, Pacyniak E, Gomez LM, Fegley B, Mulcahy ER, Hill MS, Culley N, Pinson DM, Nothnick W, Powers MF, Wong SW, Stephens EB. Virology. 2006;44:541–559. doi: 10.1016/j.virol.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Hout DR, Gomez LM, Pacyniak E, Miller JM, Hill MS, Stephens EB. Virology. 2006;348:449–461. doi: 10.1016/j.virol.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Ishikawa J, Kaisho T, Tomizawa H, Lee BO, Kobune Y, Inazawa J, Oritani K, Itoh M, Ochi T, Ishihara K, et al. Genomics. 1995;26:527–34. doi: 10.1016/0888-7543(95)80171-h. [DOI] [PubMed] [Google Scholar]
- 37.Jabbar MA, Nayak DP. Journal of Virology. 1990;64:6297–6304. doi: 10.1128/jvi.64.12.6297-6304.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. PLoS Pathogens. 2009;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joag SV, Stephens EB, Adams RJ, Foresman L, Narayan O. Virology. 1994;200(2):436–446. doi: 10.1006/viro.1994.1207. [DOI] [PubMed] [Google Scholar]
- 40.Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. Journal of Virology. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Proceedings of the National Academy of Sciences, USA. 2009;106(8):2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kestler HW, III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Cell. 1991;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 43.Klimkait T, Strebel K, Hoggan MD, Martin MA, Orenstein JM. Journal of Virology. 1990;64:621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. Traffic. 2009;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 45.Lenburg ME, Landau NR. Journal of Virology. 1993;67:7238–7245. doi: 10.1128/jvi.67.12.7238-7245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez F, Montal M, Blasie JK, Klein ML, Moore PB. Biophysical Journal. 2002;83:1259–1267. doi: 10.1016/S0006-3495(02)73898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. PLoS Pathogens. 2009;5(9):e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansouri M, Viswanathan K, Douglas JL, Hines J, Gustin J, Moses AV, Fruh K. Journal of Virology. 2009;83(19):9672–9681. doi: 10.1128/JVI.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. Molecular Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 50.McCormick-Davis C, Dalton SB, Singh DK, Stephens EB. AIDS Research and Human Retrovirology. 2000;16:1089–1095. doi: 10.1089/08892220050075363. [DOI] [PubMed] [Google Scholar]
- 51.McNatt MW, Zang T, Hatziioannou T, Bartlett M, Fofana IB, Johnson WE, Neil SJ, Bieniasz PD. PLoS Pathogens. 2009;5:e1000300. doi: 10.1371/journal.ppat.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Megy S, Bertho G, Gharbi-Benarous J, Evrard-Todeschi N, Coadou G, Segeral E, Iehle C, Quemeneur E, Benarous R, Girault JP. Journal of Biological Chemistry. 2005;280:29107–29116. doi: 10.1074/jbc.M501628200. [DOI] [PubMed] [Google Scholar]
- 53.Meusser B, Sommer T. Molecular Cell. 2004;14:247–258. doi: 10.1016/s1097-2765(04)00212-6. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, Stephens EB, Margottin-Goguet F, Benarous R, Guatelli JC. PLoS Pathogens. 2009;5(5):e10000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyagi E, Andrew AJ, Kao S, Strebel K. Proceedings of the National Academy of Sciences, USA. 2009;106:2868–2873. doi: 10.1073/pnas.0813223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neil SJ, Zang T, Bieniasz PD. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen DH, Hildreth JE. Journal of Virology. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okada A, Miura T, Takeuchi H. Biochemistry. 2001;40:6053–6060. doi: 10.1021/bi0028441. [DOI] [PubMed] [Google Scholar]
- 59.Ono A, Freed EO. Proceedings of the National Academy of Sciences, USA. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. Proceedings of the National Academy of Sciences, USA. 2004;101(41):14889–14894. doi: 10.1073/pnas.0405596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pacyniak E, Gomez ML, Gomez LM, Mulcahy ER, Jackson M, Hout DR, Wisdom BJ, Stephens EB. AIDS Research and Human Retroviruses. 2005;21:379–394. doi: 10.1089/aid.2005.21.379. [DOI] [PubMed] [Google Scholar]
- 62.Park SH, Mrse AA, Nevzorov AA, Mesleh MF, Oblatt-Montal M, Montal M, Opella SJ. Journal of Molecular Biology. 2003;333:409–424. doi: 10.1016/j.jmb.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 63.Park SH, Opella SJ. Journal of Molecular Biology. 2005;350:310–318. doi: 10.1016/j.jmb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Park SH, Opella SJ. Protein Science. 2007;16:2205–2215. doi: 10.1110/ps.073041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paul M, Jabbar MA. Virology. 1997;232:207–216. doi: 10.1006/viro.1997.8541. [DOI] [PubMed] [Google Scholar]
- 66.Piguet V, Chen YL, Mangasarian A, Foti M, Carpentier JL, Trono D. EMBO Journal. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier JL, Trono D. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 68.Pinto LH, Holsinger LJ, Lamb RA. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 69.Rong L, Zhang J, Lu J, Pan Q, Lorgeous RP, Aloysius C, Guo F, Liu SL, Wainberg MA, Liang C. Journal of Virology. 2009;83:7536–7546. doi: 10.1128/JVI.00620-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sansom MS, Forrest LR, Bull R. Bioessays. 1998;20(12):992–1000. doi: 10.1002/(SICI)1521-1878(199812)20:12<992::AID-BIES5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 71.Schubert U, Bour S, Ferrer-Montiel AV, Montal M, Maldarell F, Strebel K. Journal of Virology. 1996;70:809–819. doi: 10.1128/jvi.70.2.809-819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schubert U, Ferrer-Montiel AV, Oblatt-Montal M, Henklein P, Strebel K, Montal M. FEBS Letters. 1996;398:12–8. doi: 10.1016/s0014-5793(96)01146-5. [DOI] [PubMed] [Google Scholar]
- 73.Schubert U, Henklein P, Boldyreff B, Wingender E, Strebel K, Porstmann T. Journal of Molecular Biology. 1994;236:16–25. doi: 10.1006/jmbi.1994.1114. [DOI] [PubMed] [Google Scholar]
- 74.Singh DK, Griffin DM, Pacyniak E, Jackson M, Werle MJ, Wisdom B, Sun F, Hout DR, Pinson DM, Gunderson RS, Powers MF, Wong SW, Stephens EB. Virology. 2003;313:435–451. doi: 10.1016/s0042-6822(03)00339-8. [DOI] [PubMed] [Google Scholar]
- 75.Stephens EB, Sahni M, Leung K, Raghavan R, Joag SV, Narayan O. Journal of General Virology. 1998;79:1089–1100. doi: 10.1099/0022-1317-79-5-1089. [DOI] [PubMed] [Google Scholar]
- 76.Strebel K, Klimkait T, Martin MA. Science. 1988;241:1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- 77.Takeuchi H, Okada A, Miura T. FEBS Letters. 2003;552:35–38. doi: 10.1016/s0014-5793(03)00781-6. [DOI] [PubMed] [Google Scholar]
- 78.Tang Y, Zaitseva F, Lamb RA, Pinto LH. Journal of Biological Chemistry. 2002;277:39880–39886. doi: 10.1074/jbc.M206582200. [DOI] [PubMed] [Google Scholar]
- 79.Tiganos E, Yao X, Friborg J, Daniel N, Cohen EA. Journal of Virology. 1997;71:4452–4460. doi: 10.1128/jvi.71.6.4452-4460.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiganos E, Friborg J, Allain B, Daniel NG, Yao XJ, Cohen EA. Virology. 1998;251:96–107. doi: 10.1006/viro.1998.9368. [DOI] [PubMed] [Google Scholar]
- 81.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli JC. Cell Host and Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vincent MJ, Raja NU, Jabbar MA. Journal of Virology. 1993;67:5538–5549. doi: 10.1128/jvi.67.9.5538-5549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willey RL, Maldarelli F, Martin MA, Strebel K. Journal of Virology. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wray V, Kinder R, Federau T, Henklein P, Bechinger B, Schubert U. Biochemistry. 1999;38:5272–5282. doi: 10.1021/bi982755c. [DOI] [PubMed] [Google Scholar]
- 85.Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Molecular Cell. 2003;11(6):1445–56. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 86.Yao XJ, Garzon S, Boisvert F, Haseltine WA, Cohen EA. Journal of Acquired Immune Deficiency Syndrome. 1993;6(2):135–141. [PubMed] [Google Scholar]
- 87.Yoon K, Kestler HW, Kim S. Virus Research. 1998;57(1):27–34. doi: 10.1016/s0168-1702(98)00076-8. [DOI] [PubMed] [Google Scholar]
- 88.Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. Cell Host Microbe. 2009;6(1):54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]