Abstract
India has compelling need and keen aspirations for indigenous clinical research. Notwithstanding this need and previously reported growth the expected expansion of Indian clinical research has not materialized. We reviewed the scientific literature, lay press reports, and ClinicalTrials.gov data for information and commentary on projections, progress, and impediments associated with clinical trials in India. We also propose targeted solutions to identified challenges. The Indian clinical trial sector grew by (+) 20.3% CAGR (compound annual growth rate) between 2005 and 2010 and contracted by (-) 14.6% CAGR between 2010 and 2013. Phase-1 trials grew by (+) 43.5% CAGR from 2005–2013, phase-2 trials grew by (+) 19.8% CAGR from 2005–2009 and contracted by (-) 12.6% CAGR from 2009–2013, and phase-3 trials grew by (+) 13.0% CAGR from 2005–2010 and contracted by (-) 28.8% CAGR from 2010–2013. This was associated with a slowing of the regulatory approval process, increased media coverage and activist engagement, and accelerated development of regulatory guidelines and recuperative initiatives. We propose the following as potential targets for restorative interventions:
Regulatory overhaul (leadership and enforcement of regulations, resolution of ambiguity in regulations, staffing, training, guidelines, and ethical principles [e.g., compensation]).
Education and training of research professionals, clinicians, and regulators.
Public awareness and empowerment.
After a peak in 2009-2010, the clinical research sector in India appears to be experiencing a contraction. There are indications of challenges in regulatory enforcement of guidelines; training of clinical research professionals; and awareness, participation, partnership, and the general image amongst the non-professional media and public. Preventative and corrective principles and interventions are outlined with the goal of realizing the clinical research potential in India.
Introduction
Challenges in India's clinical research environment
India's clinical research environment: The promise of an innovative, population-specific health care system supported by indigenous, evidence-based medical research is attractive for emerging economies such as Brazil, Russia, India, China, and South Africa, each presenting with a unique gene pool and health care environment characteristics and needs [1-3]. Unlike in the West, clinical research is a relatively recent venture for the Indian society. India represents 17.5% of the world's population but conducts only 1.4% of global clinical research (calculated for the period of August 7, 2011 to August 6, 2012) [4-6]. In India, numerous factors present advantages for home-grown medical research, specifically clinical research: English-speaking health care professionals; expert clinicians (including returning, Western-trained physicians); economic growth; access to world-class technologies; information technology and data management infrastructure; access to large, treatment-naïve and ethnically diverse patient populations with diseases of public health relevance; competitive operational costs; and internationally harmonized regulations [7]. However, these advantages have not translated into the expected growth in clinical trials in India.
Growth, stagnation, and decline: clinicaltrials.gov Analysis of Clinical Research in India
Methods
We accessed the ClinicalTrials.gov database on March 18, 2014 (Appendix A6) and used the “Advanced Search” feature, with “India” entered into the “Country 1” field, to conduct yearly searches (e.g., 01/01/2002 – 12/31/2002). The overall number of reported studies was recorded for each year from 2002–2013, and the yearly numbers by phase were broken down for 2005–2013 (due to the paucity of data in prior years). Compound annual growth rates (CAGRs) were determined using the following formula:
V(t0) : Start Value; V(tn) : Finish Value; tn − t0 : Number of Years
Results
a Indian clinical trial growth trends
There were 2378 trials registered with at least one site in India between 2002 and 2013. Only 44 trials were registered in the years 2002–2004, and these years were excluded from further analyses. The Indian clinical trial sector grew by (+) 20.3% CAGR of new trials between 2005 and 2010, and it contracted by (-) 14.6% CAGR between 2010 and 2013 (Figure 1). (The reduction brings 2013 numbers down to 2007 levels).
Figure 1.
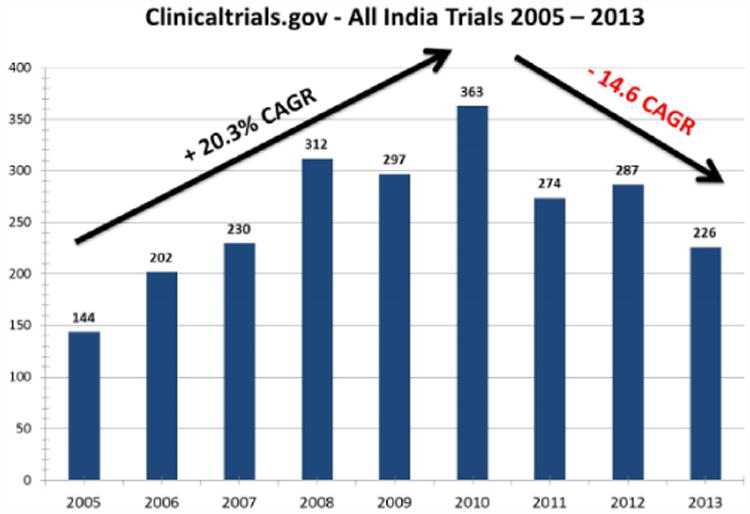
Indian clinical trials from ClinicalTrials.gov: all registered trials (2005–2013). CAGR, compound annual growth rate.
When broken down by phase of development (Figure 2), phase-1 trials grew by (+) 43.5% CAGR throughout the 2005-2013 period, but inspection of the individual trials revealed that these were almost exclusively Indian-based bioavailability/bioequivalence studies, whereas phase-2 and -3 studies were almost exclusively sponsored by international companies. Phase-2 trials grew by (+) 19.8% CAGR from 2005–2009 but contracted by (-) 12.6% CAGR from 2009–2013. Phase-3 trials grew by (+) 13.0% CAGR from 2005–2010 and contracted by (-) 28.8% CAGR from 2010–2013. Phase-4 trials remained at almost the same level, about 20-30 per year, throughout the 2005–2013 periods, except for a peak of 43 trials in 2009.
Figure 2.
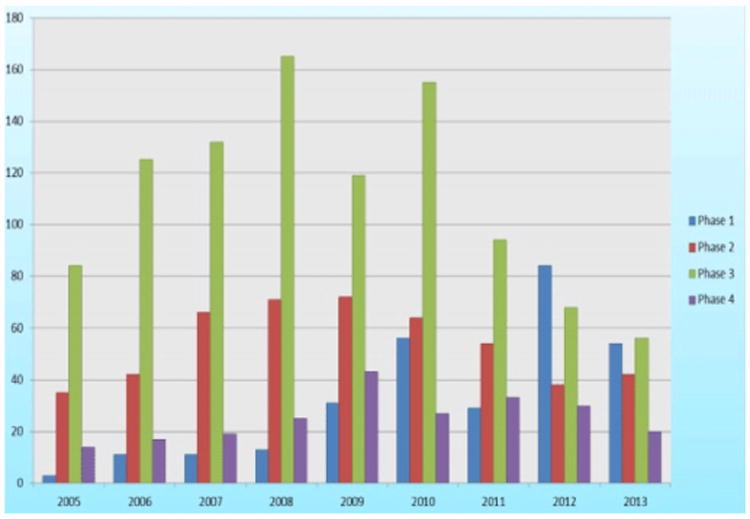
Indian clinical trials from ClinicalTrials.gov: trials by phase (2005–2013).
b US and Global Clinical Growth Trends
Between 2005 and 2013, global clinical trials grew by (+) 5.6% CAGR (from 12,921 to 20,066), and US clinical trials grew by (+) 2.7% CAGR (from 6330 to 7823) (Figure 3). In the United States, a flattening of growth was observed after 2008.
Figure 3.
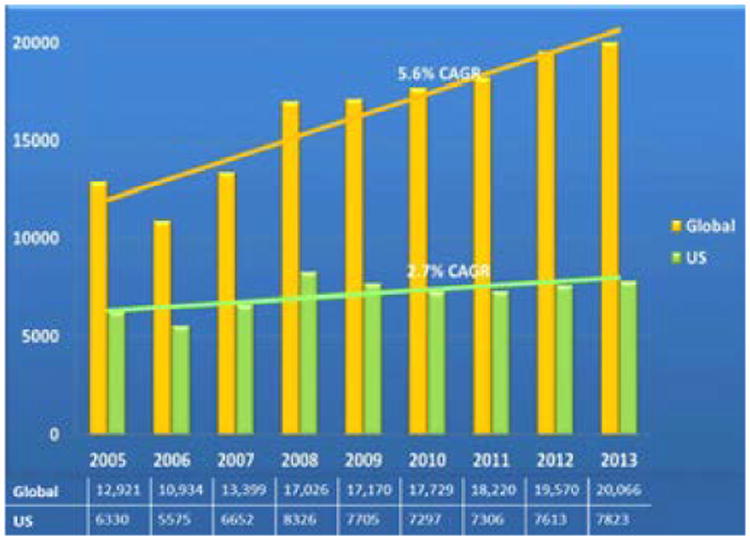
Global and US total clinical trials (2005–2013).
Recent challenges and negative developments in India's clinical research environment
The decline in the number of clinical trials was associated with an increase in reported clinical research mishaps [8-11], negative media coverage [8,9,12-16], activist protests [1,17-23], stagnation of the regulatory process [12,24,25] and departure of sponsors and collaborators [12,26,27].
In the same period there were increased attempts by regulatory [8,11,13,17,24,28-33], research professional [1,3,7,32,34-36] and public stakeholders [8,9,14,15,17-23,25,37-39,40-44] to understand and correct this reversal of fortunes. These events are summarized in Table 1 and described in the remainder of this article.
Table 1.
India clinical research environment: challenges and proposed solutions.
| Domains | Challenges | Characteristics of the Desired Research Environment | Proposed Solutions | |
|---|---|---|---|---|
| Regulatory (government, ethics committees, monitors, auditors) | Setting research agenda and standards |
|
||
| Evaluation of clinical research applications |
|
Review process:
|
|
|
| Enforcement of standards at research sites |
|
Enforcement of standards is:
|
Enforcement of research standards:
|
|
| Professional (industry, academia, health care: clinicians, investigators, research staff) | Compliance with research standards |
|
Strong research foundations and regular training in:
|
|
| Operations |
|
Sufficient time and resources for:
|
|
|
| Public/patients (patient advocacy groups, NGOs, the media) | Public awareness |
|
Public and patients are:
|
|
| Public partnership |
|
|
|
|
CRO: Clinical Research Organization; CREATE: Continuous Research Education and Training Exercises; NGO: Non-Governmental Organization; PARTAKE: Public Awareness of Research for Therapeutic Advancements through Knowledge and Empowerment.
Regulatory environment in India
There are three regulatory entities and respective guidelines that regulate clinical research in India. The main guideline is “Schedule Y” of the Drugs and Cosmetics Rules [43]. It was last revised in 2005. The second guideline, “Good Clinical Practices for Clinical Research in India” (of the Central Drugs Standard Control Organization) was established in 2002 and reflects many of the principles and recommendations of ICH E6 (International Conference on Harmonization's Good Clinical Practice guidelines) [44,45]. The third guideline is the “Ethical Guidelines for Biomedical Research on Human Participants” (of the Indian Council of Medical Research [ICMR]) from 2006 [46]. Overall, these guidelines reflect almost all internationally endorsed principles. Some Indian regulatory requirements are progressive in comparison to the rest of the world and are more protective of vulnerable populations and minorities, such as mandatory registration of all new clinical trials in the Clinical Trials Registry of India (as of 2009) [47]; registration of ethics committees; and use of language encouraging respect of participants' cultural, educational, and economic backgrounds [33]. Yet these regulations have still come under public and activist scrutiny [9,10,13,22,23,39].
The multiplicity and overlapping nature of the regulations (the three aforementioned guidelines) and sometimes ambiguous wording represent additional challenges [11,13,48]. This results in lengthy turnaround times for clinical trial approvals and under-enforcement of quality standards, which are features that have the potential to dissuade foreign sponsors from conducting clinical trials in India and may have contributed to the reduced number of clinical trials and departure of international collaborators [26,27,49].
The main challenge facing the Indian regulatory environment, hampered by understaffed and under-resourced agencies, is the ability to enforce regulations [11]. The Fifty-Ninth Report on the Functioning of the Central Drugs Standard Control Organization provides the following details. Regulatory workload is increasing at an annual rate of 20%, but there is no corresponding increase in manpower or infrastructure. Nine officers are handling approximately 20,000 applications per year. Furthermore, of 327 sanctioned posts, only 124 are occupied. Approval of new drugs and biologics, for which 1600 applications are submitted yearly, is handled by 25 staff and an additional 25 contractual technical staff. Media reports of unethical clinical research and activist petitions have led the Indian Supreme Court to put clinical research on hold and initiate regulatory overhaul [8-10,12,14,15,17,21,23-25,28-32,50].
Required Regulatory Guidelines
There are several areas that require regulatory guidelines to ensure parity with clinical research environments in other countries and response to special needs of the Indian environment. These include stem-cell, device, phase-0/microdosing, and integrative medicine research and compensation for adverse outcomes to participants in clinical trials (currently under development).
Science and regulatory challenge example: stem-cell therapy
Stem-cell research offers the potential to bring innovation to local context, make treatments more affordable and aiding in economic development. India demonstrates that stem-cell research and development is not confined to industrialized countries and has begun to harness stem cells to address its own health needs [51]. However, there are considerable scientific, operational, and regulatory gaps in stem-cell research in India compared with the developed world. India is responding to this challenge in a myriad of ways, including through the mushrooming of stem-cell clinics, establishing regulated and organized stem-cell research units, and creating task forces to establish guidelines and formalize regulation of the field.
Science environment: innovation, education, and centers of excellence
While many reviews of clinical research in India highlight the presence of highly skilled clinicians, it appears that the same cannot be said about the number of skilled investigators or that the building capacity for clinical research is as high of a priority in India as it is in other developing nations [34]. Clinical research is not an established health care career pathway in India. In the past, much of the clinical research activity was centered on development of generic medications rather than innovative therapeutics. There is an estimated pool of only 1500 qualified investigators in India, and there is a lack of government-accredited clinical-research training institutions, biostatisticians, and epidemiologists [7]. There is a need for clinical research centers to set standards of excellence, educate, train, and lead the emerging field of clinical research in India [34,51].
Negative reports in the media and professional press
Although the media have the means and responsibility to disseminate accurate information about clinical research and to help promote public awareness and engagement, unfavourable and inaccurate depictions abound and may undermine trust, support, participation in, and partnership in clinical research (Figure 4) [14,22,36,37,52-56]. For example, MedIndia.com, a website that describes itself as “Asia's premier health portal,” has the following quote in one of its articles: “…humans are becoming a source of experimental animals and being exploited. Due to intensive and strict Animal guidelines using animals in India too has become a very [sic] problem, so the drug companies have shifted their trials to humans rather [sic] to animals.”[57].
Figure 4.
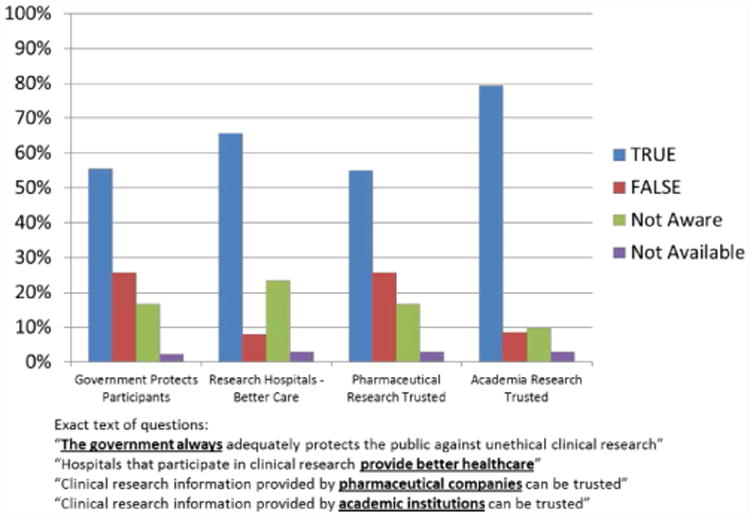
Indian public perception of clinical research: government, industry, academia, and hospitals. Adapted from PARTAKE Survey of Public Knowledge and Perceptions of Clinical Research in India [37].
Sometimes reports emphasize only the negative data when both positive and negative data are available. For example, in a review of public perceptions of clinical research, 44% of the cohort was reported to have an unfavourable impression of pharmaceutical companies, but the fact that 47% of the cohort had a favourable impression of pharmaceutical companies was not reported [16,58]. Likewise, the report that 39% of the cohort thought pharmaceutical companies failed to serve consumers (higher than in 1997 [19%]) did not include that 60% thought that pharmaceutical companies did a good job serving their consumers (higher than 2004 [44%]) [16,58].
Public/Patient Environment
The Indian public and patients have high stakes in a successful, indigenous clinical research environment that could bring about treatments suited to their needs, support an independent health care system, and contribute to the country's economic growth. Patient advocacy groups in particular have made significant contributions to clinical research in other countries [18-20]. In addition, a lack of knowledge about, awareness of, and participation in clinical research can have negative implications and lead to vulnerability to exploitation and/or perceptions of exploitation, reduced participation in clinical research, and impaired enforcement of standards of clinical trials [18,36-38].
Impact of Challenges and Deficiencies
It is likely that, faced with increasing regulatory turnaround timelines and increasing reports of incidents of unethical conduct of clinical research that are amplified and sensationalized by the media, sponsors are shying away from conducting research in India [9,22,27]. International companies are going elsewhere, and even Indian developers of new therapeutics are conducting their research outside of India [26]. And possibly, with inadequate resources to enforce regulations, the only recourse regulators have is to limit approvals and maintain a situation that discourages sponsors and operators from conducting research altogether [13,17,24].
Discussion
Our analyses show that after a peek in 2009-2010, clinical trials in India have experienced a decline, while global clinical trials continued to experience growth. We have identified a series of negative regulatory, professional, and public developments in the clinical trial sector in India that occurred during this same period (Table 1 and Figure 4). Although definitive causality cannot be established between these developments and trial growth trends, it is arguable that reversal of these negative developments could facilitate growth of the clinical trial sector in India. We propose several preventative and corrective measures that we believe are needed to realize the full potential of clinical research in India.
Proposed Solutions
We propose the following comprehensive approach to addresses each of the perceived challenges and each of the concerned stakeholders:
Develop a robust regulatory process with emphasis on expertise, training, enforcement, and availability.
Employ complementary self-regulation activities by industry and relevant professional research organizations.
Develop accreditation programs for research operators and ethics committees.
Develop quality education and training programs for research professionals and clinicians.
Involve journal editors and peer reviewers.
Develop awareness programs for patients, the public, and the media providing information about clinical research and empowering and encouraging participation (principles of autonomy, societal consent, community relevance, and shared responsibility).
Encourage proactive (rather than reactive) non-professional sector involvement in the dissemination and enforcement of clinical research standards.
Similarly, in 2004 Maggon [49,59] proposed the following recommendations for the conduct of clinical research in India:
Ensure that all patients are informed about their rights, obligations, and risks in their native languages.
Avoid commercial institutional review boards/ethics review committees.
Never perform a study in India that would not be approved in in the United States or Europe.
Ensure proper spacing of patients for safety, and avoid enrollment of large number of patients within a short period of time.
Arrange for provision of medication to responding patients for a certain period after termination of the trial.
Set up independent data monitoring and safety boards for large-scale studies.
Organize Good Clinical Practice training courses, investigator meetings, and protocol and case report form trainings.
Regulatory Reforms
Several progressive and unique regulatory initiatives—including clinical research organization legislation, registration of ethics committees [29], compensation legislation [17,28,30], pharmacovigilance, certification of research sites, and a clinical research ethics bill—are underway or in advanced stages of planning in India [7]. Increased interaction between regulators and sponsors is encouraged, especially in sensitive developmental milestones. Increased interaction between regulators and educators/trainers is also encouraged to ensure alignment with regulatory vision, policy, and guidelines and to facilitate enforcement of regulations.
Complementary Self-Regulation Activities by Industry
Indian regulatory authorities are in the process of building the infrastructure, resources, and expertise required for proper monitoring of clinical trials and enforcement of regulations. However, there remains a need for laws on compensation, censure of defaulters, and declaration of conflicts of interest by investigators and ethics committee members.
Developing regulations is a slow and evolving process; meanwhile, the clinical research industry could engage in activities utilizing its expertise, resources, and access to sites and investigators, such as:
Ensuring selection of sites with trained investigators and accredited ethics committees.
Educating and training clinical research operators, investigators, and ethics committee members.
Encouraging video and audio recording of the volunteer enrolment process and other means of ensuring study participants are adequately informed.
Ensuring adequate compensation for the trial participants.
Enhancing public awareness, knowledge, and engagement in clinical research.
Encouraging and supporting clinical site and ethics committee accreditation.
Conducting quality audits for all types of clinical trials, not only the regulatory critical ones.
Ensuring proper declaration of conflicts of interest by clinical research operators and investigators.
Establishing and/or supporting a unified database of study volunteers to avoid cross-participation.
Accreditation Programs for Research Operators and Ethics Committees
Programs such as the Association for the Accreditation of Human Research Protection Programs (AAHRPP), Forum for Ethical Review Committees in Asian and the Western Pacific (FERCAP), and Strategic Initiative for Developing Capacity in Ethical Review (SIDCER) of the ICMR are beginning to take on the role of accrediting and training ethics committees in India, but these changes are yet preliminary and purely voluntary [60-62]. A training and accreditation process that is transparent and mandatory will help raise the ethical review process to a much higher benchmark and create public faith in the processes of clinical research. The authors opine that independent ethics committees with no institutional affiliations must mandatorily undergo a continuous accreditation to minimize fly-by-night operators.
Education Training and Dissemination of Clinical Research Information
Clinical research education is already a part of clinical training in medical colleges, but experienced mentors need to be involved in the process. The rigor of research work and the importance of adhering to standards and guidelines must be emphasized early during training. A closely tied working and learning environment, collaboration projects, and programs involving both academia (e.g., medical, science, and biotechnology schools) and industry will enhance indigenous research. Also, minimizing red tape in the research processes is critical in the academic environment so that collaboration with scientists outside academia is seamless and enhances the development of indigenous intellectual property. In addition, exposure of Indian academia (and not just that of premier institutions) to the international research environment is critical so that the growth of research in India does not take place in silos. Finally, learning about and teaching of clinical research needs to be a continuous process, with CRE (continuing research education) being as important as CME (continuing medical education) programs. CRE should be made mandatory in medical schools and in tertiary care and research centres.
Journal Editors and Peer Reviewers
Journal editors also have a role in protecting the rights of research participants and disseminating quality research by ensuring publications conform to methodological and ethical principles and are transparent to professionals and the general public [35]. By gatekeeping the type of research that is published, journal editors and peer reviewers have the capability and obligation to improve the quality of conduct and reporting of clinical research.
Patient Advocacy, Non-Governmental Organizations, and the Public at Large: Empowering and Informing
The non-professional public—including patient advocacy groups, research activists, ethicists, non-governmental organizations, and the media—have an important role in clinical research as well. An empowered and informed public will actualize rights and obligations relevant to clinical research [37,38]. It will actively partner in the guidance of the sector in the following ways: identifying the vulnerability of sensitive groups; accessing resources; engaging in official policy-making; providing feedback to research sponsors, operators, and regulators about the values and preferences that are important to the non-professional public; reporting on the quality of research and enforcement of regulatory and ethical principles; and bringing to the attention of policy-makers any meaningful deviations, and thus helping monitor and oversee the clinical research sector and enforce regulatory guidelines and methodological standards. Important topics of education include:
The process of clinical research and its role in medical progress.
The rights of participants.
Compensation, including the differences between treatment-and illness-derived adverse events.
Confidentiality
Having adequate information about and knowledge of clinical research is essential for the proper function and partnership of all stakeholders involved in clinical research, professional and non-professional alike. Empowered public and patient sectors [18] (through advocacy groups) can contribute to recruitment efforts, sponsorship of research, and even establishment of research networks and competitive grant programs [19,38]. A recent survey of 201 genetic disease advocacy organizations reported 91% assisting in study recruitment, 75% collecting data, 60% providing financial support to researchers, and 56% assisting with study design [19]. Some have suggested that public and patient participation in clinical research implies a right to ownership of research data [38].
Considering India's expanding clinical research environment and the specific cultural challenges that face the conduct of clinical research in India, Mahaluxmivala has expressed an urgent need for an all-inclusive program and argues in favour of widespread and comprehensive Good Clinical Practice compliance [22]. Another element of the solution is educating and engaging the public in clinical research. This is being gradually realized by regulators, industry, and academia. According to the National Institutes of Health Director's Council of Public Representatives [63], it is believed that public understanding of research could contribute to earning public trust in the research enterprise and in the observance of human-protection measures in clinical research (Figure 4). An informed public could help monitor quality and ethics of clinical trials [21,23]. Feedback could be provided on parameters that are of value to clinical trial participants and patients—the ultimate recipients of clinical research products.
The public needs to understand that new, safe, and effective drugs to treat illnesses and address unmet health care needs can be produced only after clinical trials are conducted in humans [64]. Individuals who have participated in clinical research studies and are familiar with the conduct of clinical trials appear to have more positive perceptions of clinical research than do those of the general public [65], which again advocates for a more-informed public (one that may be more likely to endorse and partner in clinical research).
It is believed that participant protection in clinical research can be enhanced not only through adequate investigator technical and ethical knowledge, but also by increasing public awareness of relevant clinical research information [22]. Shah and Garg [20] have identified increasing awareness of clinical research as one of the key roles of patient advocacy groups. Also, Dr. Surinder Singh, the former Drug Controller General of India (DCGI), stated that the regulatory bodies in India are trying to generate awareness among patients regarding their rights as they pertain to clinical research, thus taking part in empowering prospective study participants to seek and enhance their knowledge and awareness of clinical research. Since India stands to benefit from these trials by much-needed investment into health care and access to beneficial drugs, there is an urgent need to create an agreeable environment by raising awareness and ensuring ethical clinical practice [17,37]. Educating the public on research and development, the inevitability and risks of human experimentation, and the promise of safe and effective new treatments for unmet health care needs would better position stakeholders to evaluate clinical research and become active partners in the process [64].
Conclusions
A sharp decline in clinical trial activity in India since 2009–2010 has been associated with reports of ethical improprieties, activist protests, and departure of international collaborators. Strong responses from regulators, research professionals, and the public have led to exploration of the causes and proposal of solutions to this downward trend. Although causality is difficult to establish, the main concerns appear to be related to enforcement of clinical trial standards, community awareness, and engagement of patients and the public in the clinical-trial process. Regardless of the causes, all stakeholders seem to agree that the key goals are protection of human research participants and generation of high-quality research results so India can respond to the need and realize the potential for indigenous, original, and high-quality clinical research.
Acknowledgments
Funding: National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Tal Burt, MD, confirms that he had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Author Contributions: Tal Burt, Pooja Sharma, and Savita Dhillon wrote the first draft with contributions from the other authors. All authors edited and approved the final draft.
References
- 1.Dandona L, Katoch VM, Dandona R. Research to achieve health care for all in India. Lancet. 2011;377:1055–1057. doi: 10.1016/S0140-6736(10)62034-X. [DOI] [PubMed] [Google Scholar]
- 2.Gupta V, Goel A, Bhoi S. Medical research in India. Lancet. 2006;368:644. doi: 10.1016/S0140-6736(06)69240-4. [DOI] [PubMed] [Google Scholar]
- 3.Dias A, Patel V. Closing the treatment gap for dementia in India. Indian J Psychiatry. 2009;51(Suppl 1):S93–97. [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Census Bureau. International Population Reports WP/02. U.S. Government Printing Office; Washington, DC: 2002. Global Population Profile. [Google Scholar]
- 5.Census Info India 2011.
- 6.ClinicalTrials.gov website.
- 7.Gupta YK, Padhy BM. India's growing participation in global clinical trials. Trends Pharmacol Sci. 2011;32:327–329. doi: 10.1016/j.tips.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Sharda S. HC takes strong note of clinical trials on Indians without consent. The Times of India; 2012. [Google Scholar]
- 9.Lakshmi R. India's drug trials fuel consent controversy. The Washington Post; 2012. [Google Scholar]
- 10.Shetty P. Vaccine trial's ethics criticized. Nature. 2011;474:427–428. doi: 10.1038/474427a. [DOI] [PubMed] [Google Scholar]
- 11.Parliament of India, Rajya Sabha. Fifty-Ninth Report on the Functioning of the Central Drugs Standard Control Organization (CDSCO) 2012 [Google Scholar]
- 12.Post stringent norms, clinical trials in India plummet. The Hindu; 2013. [Google Scholar]
- 13.Chowdhury N. Poor definitions threaten drug trial safety in India. Nat Med. 2013;19:15. doi: 10.1038/nm0113-15. [DOI] [PubMed] [Google Scholar]
- 14.Ramamurthy NV. Inept media trials of clinical trials. Perspect Clin Res. 2012;3:47–49. doi: 10.4103/2229-3485.96442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan V. India as world's laboratory. The Indian Express; 2010. [Google Scholar]
- 16.Getz KA. Public Confidence and Trust Today. Measuring Trust in Clinical Research. Monitor 2008 [Google Scholar]
- 17.Jesani A. New regulations on compensation for injury and death in drug trials. Indian J Med Ethics. 2013;10:76–79. doi: 10.20529/IJME.2013.026. [DOI] [PubMed] [Google Scholar]
- 18.Michaels M, Weiss ES, Guidry JA, Blakeney N, Swords L, et al. The promise of community-based advocacy and education efforts for increasing cancer clinical trials accrual. J Cancer Educ. 2012;27:67–74. doi: 10.1007/s13187-011-0271-6. [DOI] [PubMed] [Google Scholar]
- 19.Landy DC, Brinich MA, Colten ME, Horn EJ, Terry SF, et al. How disease advocacy organizations participate in clinical research: a survey of genetic organizations. Genet Med. 2012;14:223–228. doi: 10.1038/gim.0b013e3182310ba0. [DOI] [PubMed] [Google Scholar]
- 20.Shah K, Garg S. Patient advocacy groups: Need and opportunity in India. Perspect Clin Res. 2011;2:4–7. doi: 10.4103/2229-3485.76283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan S. Patient protection in clinical trials in India: some concerns. Perspect Clin Res. 2010;1:101–103. [PMC free article] [PubMed] [Google Scholar]
- 22.Mahaluxmivala N. Human subject protection in India - is it adequate? Perspect Clin Res. 2010;1:15–20. [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan S. Center for Studies in Ethics and Rights. Ethical concerns in clinical trials in India: an investigation. Centre for Research on Multinational Corporations website; 2009. 2009. [Google Scholar]
- 24.Ministry of Health and Family Welfare. Drugs and Cosmetics (First Amendment) Rules. 2013 [Google Scholar]
- 25.Bhattacharjee Y. Public health. Clinical trials paused as India adopts new rules. Science. 2013;341:327. doi: 10.1126/science.341.6144.327. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan V, Koshy PK. US agency NIH scraps nearly 40 clinical trials in India. LiveMint 2013 [Google Scholar]
- 27.Brennan Z. Quintiles shutters phase I unit in India. 2013 Outsourcing-Pharma.com.
- 28.Sugarman J, Bhan A, Bollinger R, Gupta A. India's new policy to protect research participants. BMJ. 2013;347:f4841. doi: 10.1136/bmj.f4841. [DOI] [PubMed] [Google Scholar]
- 29.Actions on the Recommendations of Prof Ranjit Roy Chaudhury Expert Committee to Formulate Policy and Guidelines for Approval of New Drugs, Clinical Trials and Banning of Drugs. New Delhi: Ministry of Health and Family Welfare; 2013. [Google Scholar]
- 30.Munshi R, Thatte U. Compensation for research related injury. Perspect Clin Res. 2013;4:61–69. doi: 10.4103/2229-3485.106392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Central Drugs Standard Control Organization. Draft Guidelines on Audio-Visual Recording of Informed Consent Process in Clinical Trial. 2014 [Google Scholar]
- 32.Bhatt A. Government's role in shaping public perceptions about clinical research. Perspect Clin Res. 2012;3:87–89. doi: 10.4103/2229-3485.100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandey A, Aggarwal AR, Maulik M, Gupta J, Juneja A, et al. The upgraded Clinical Trials Registry India: a summary of changes. Indian J Med Ethics. 2011;8:186. doi: 10.20529/IJME.2011.072. [DOI] [PubMed] [Google Scholar]
- 34.Ali R, Finlayson A Indox Cancer Research Network. Building capacity for clinical research in developing countries: the INDOX Cancer Research Network experience. Glob Health Action. 2012;5 doi: 10.3402/gha.v5i0.17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sathyanarayana Rao TS, Tharyan P. Editorial policies aimed at improving the transparency and validity of published research. Indian J Psychiatry. 2011;53:183–186. doi: 10.4103/0019-5545.86793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah JY, Phadtare A, Rajgor D, Vaghasia M, Pradhan S, et al. What leads Indians to participate in clinical trials? A meta-analysis of qualitative studies. PLoS One. 2010;5:e10730. doi: 10.1371/journal.pone.0010730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burt T, Dhillon S, Sharma P, Khan D, Mv D, et al. PARTAKE survey of public knowledge and perceptions of clinical research in India. PLoS One. 2013;8:e68666. doi: 10.1371/journal.pone.0068666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terry SF, Terry PF. Power to the people: participant ownership of clinical trial data. Sci Transl Med. 2011;3:69. doi: 10.1126/scitranslmed.3001857. [DOI] [PubMed] [Google Scholar]
- 39.Nundy S, Gulhati CM. A new colonialism?--Conducting clinical trials in India. N Engl J Med. 2005;352:1633–1636. doi: 10.1056/NEJMp048361. [DOI] [PubMed] [Google Scholar]
- 40.Al-Shahi Salman R, Beller E, Kagan J, Hemminki E, Phillips RS, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet. 2014;383:176–185. doi: 10.1016/S0140-6736(13)62297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jamerson PA, Vermeersch P. The role of the nurse research facilitator in building research capacity in the clinical setting. J Nurs Adm. 2012;42:21–27. doi: 10.1097/NNA.0b013e31823c180e. [DOI] [PubMed] [Google Scholar]
- 42.Burt T, Sharma P, Mittal S. Research Question, Study Design and Continuous Research Education and Training Exercises (CREATE) Program. J Clin Prev Card. 2012;1:35–43. [PMC free article] [PubMed] [Google Scholar]
- 43.Government of India. Drugs and Cosmetics Rules 1945. India: 2003. Schedule Y. Requirements and Guidelines for Permission to Import and/or Manufacture of New Drugs for Sale or to Undertake Clinical Trials. [Google Scholar]
- 44.Central Drugs Standard Control Organization. Good Clinical Practices for Clinical Research in India 2002 [Google Scholar]
- 45.ICH International Conference on Harmonization's Good Clinical Practice guidelines. 1996 [Google Scholar]
- 46.Indian Council of Medical Research. Ethical Guidelines for Biomedical Research on Human Participants 2006 [Google Scholar]
- 47.National Institutes of Medical Statistics, Indian Council of Medical Research. Clinical Trials Registry of India 2009 [Google Scholar]
- 48.Maggon K. Regulatory reforms and GCP clinical trials with new drugs in India. Clin Trials. 2004;1:461–467. doi: 10.1191/1740774504cn047oa. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee S, Hirschler B. Big Pharma pushes for U.S. action against India over patent worries. Reuters 2014 [Google Scholar]
- 50.Srinivasan S, Loff B. Medical research in India. Lancet. 2006;367:1962–1964. doi: 10.1016/S0140-6736(06)68861-2. [DOI] [PubMed] [Google Scholar]
- 51.Lander B, Thorsteinsdóttir H, Singer PA, Daar AS. Harnessing stem cells for health needs in India. Cell Stem Cell. 2008;3:11–15. doi: 10.1016/j.stem.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 53.Food and Drug Administration Amendments Act of 2007. Section 801, HR 3580. [Google Scholar]
- 54.Markman M, Petersen J, Montgomery R. An examination of the influence of patient race and ethnicity on expressed interest in learning about cancer clinical trials. J Cancer Res Clin Oncol. 2008;134:115–118. doi: 10.1007/s00432-007-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones JM, Nyhof-Young J, Moric J, Friedman A, Wells W, et al. Identifying motivations and barriers to patient participation in clinical trials. J Cancer Educ. 2006;21:237–242. doi: 10.1080/08858190701347838. [DOI] [PubMed] [Google Scholar]
- 56.Corbie-Smith G1, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 57.MedIndia website; 2005.
- 58.Views On Prescription Drugs And The Pharmaceutical Industry. Kaiser Public Opinions Spotlight 2008 [Google Scholar]
- 59.Maggon K. Investigator and site selection and performing GCP clinical studies in India. Control Clin Trials. 2004;25:366–377. doi: 10.1016/j.cct.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Accredited Organizations. Association for the Accreditation of Human Research Protection Programs, Inc. website.
- 61.Speers MA. Making human research safe: why we cannot afford to fail. Sci Eng Ethics. 2005;11:53–59. doi: 10.1007/s11948-005-0055-7. [DOI] [PubMed] [Google Scholar]
- 62.National Human Rights Commission. NHRC issues notices to the Union Health Secretary, ICMR and DCGI calling for reports on allegations of fatal drug trials in the coutry. NHRC website 2011 [Google Scholar]
- 63.Director's Council of Public Representatives website (2013) National Institutes of Health
- 64.Raghavan VV, Muralidharan G. Perception of clinical Trials in India. 2003 Pharmabiz.com website.
- 65.Mudd J. Reach out to the people: Who better to educate a wary public about clinical research than the industry's own? 2007 [Google Scholar]


