Abstract
One of the hallmarks of cancer is the ability to activate invasion and metastasis. Cancer morbidity and mortality are largely related to the spread of the primary, localized tumour to adjacent and distant sites. Appropriate management and treatment decisions based on predicting metastatic disease at the time of diagnosis is thus crucial, which supports better understanding of the metastatic process. There are components of metastasis that are common to all primary tumours: dissociation from the primary tumour mass, reorganization/remodelling of extracellular matrix, cell migration, recognition and movement through endothelial cells and the vascular circulation and lodgement and proliferation within ectopic stroma. One of the key and initial events is the increased ability of cancer cells to move, escaping the regulation of normal physiological control. The cellular cytoskeleton plays an important role in cancer cell motility and active cytoskeletal rearrangement can result in metastatic disease. This active change in cytoskeletal dynamics results in manipulation of plasma membrane and cellular balance between cellular adhesion and motility which in turn determines cancer cell movement. Members of the tetraspanin family of proteins play important roles in regulation of cancer cell migration and cancer-endothelial cell interactions, which are critical for cancer invasion and metastasis. Their involvements in active cytoskeletal dynamics, cancer metastasis and potential clinical application will be discussed in this review. In particular, the tetraspanin member, CD151, is highlighted for its major role in cancer invasion and metastasis.
Linked Articles
This article is part of a themed section on Cytoskeleton, Extracellular Matrix, Cell Migration, Wound Healing and Related Topics. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-24
Introduction
Cell motility and invasion is an important biological process for the generation and development of the organism in normal and pathological conditions. Cancer cell motility and invasion is driven by similar cellular mechanisms to normal cell migration but is lacking in the inhibitory responsiveness that controls normal migration (Palmer et al., 2011). Therefore, the concept of targeting cancer cell migration as a therapeutic approach is unclear. However, the potential advantages of specifically targeting cancer cell migration are worth exploring. The cellular cytoskeleton provides cell structure and shape, and is essential for intrinsic cellular vesicle and organelle movements and also cellular external movement (Pardee, 2009).
The cytoskeleton and cancer metastasis
Single cell and collective cell movement are important features of cancer that enable cancer cells to invade and form metastases (Friedl and Gilmour, 2009). As stated by Friedl and Wolf (2010), the determining factors of cell migration include cell-cell adhesion (cadherins), cell-extracellular matrix (ECM) adhesion (integrins), cytoskeletal protrusion/contraction (Rac/Rho), traction force/propulsion and proteolysis. The subject of discussion in this review is the tetraspanin superfamily which has a role in various key determinants of cell migration. They interact directly with various integrins (Berditchevski and Odintsova, 1999; Berditchevski, 2001), matrix metalloproteinases (MMPs) (Takino et al., 2003; Yanez-Mo et al., 2008; Lafleur et al., 2009; Yáñez-Mó et al., 2011; Schroder et al., 2013) and E-cadherin (Greco et al., 2010). Specifically, the tetraspanin CD151 is important in maintaining the balance between RhoA and Rac-1 signalling in endothelial cells (Zhang et al., 2011a) and epidermal carcinoma cells (Johnson et al., 2009). In contrast, CD82 was found to modulate membrane composition leading to down regulation of cytoskeleton rearrangement via Src, p130CAS/Crk, and Rho/Rac pathway (Sridhar and Miranti, 2006; Liu et al., 2012). In addition, CD81 also interacts directly with Rac proteins which delays Rac inactivation, leading to enhanced cell migration (Tejera et al., 2013). Integrins are major partners of tetraspanins and they have been implicated in the control of cell adhesion and migration (Huttenlocher and Horwitz, 2011). They are best known to form focal adhesions, which are a complex of integrins, signalling proteins and actin cytoskeleton. This will be discussed in more detail in the following sections.
Tetraspanins
Tetraspanins are 4-transmembrane spanning proteins with short cytoplasmic N- and C- termini (Maecker et al., 1997; Berditchevski, 2001). They are expressed on the cell surface and/or intracellular vesicles (Wright et al., 2004b). This family contains 33 members in mammals (Berditchevski, 2001; Hemler, 2005; Levy and Shoham, 2005), each of which has a distinctive pattern of expression (Table 1). For example, CD9, CD81, TSPAN7, CD63, CD82, and CD151 are found in virtually all tissues whereas CD37 and CD53 are found in haemopoietic cells (Maecker et al., 1997; Bienstock and Barrett, 2001; Wright et al., 2004b). Tetraspanins are involved in fertilization, immune interaction and brain development (Yáñez-Mó et al., 2001) and have been linked to various processes including signal transduction pathways, cellular activation, proliferation, motility, adhesion, tissue differentiation, angiogenesis, tumour progression and metastasis (Hasegawa et al., 1998; Berditchevski, 2001; Yáñez-Mó et al., 2001; Ang et al., 2010).
Table 1.
Known human tetraspanins: distribution and function
| Tetraspanins | Other names | Tissue and organ distribution | Functions |
|---|---|---|---|
| TSPAN1 | TSP-1 NET-1 |
Endometrium, colon, kidney, heart, lung, pancreas, prostate, thyroid gland and trachea (Todd et al., 1998; Puls et al., 1999). |
|
| TSPAN2 | TSP-2 NET-3 |
Adrenal gland, brain, duodenum, intestine, liver, lung, ovary and testis. Weak expression in heart, pancreas, skin, stomach and uterus (Todd et al., 1998) Myeloid cells and T lymphocytes (Serru et al., 2000). |
|
| TSPAN3 | TSP-3 | Brain, endometrium, colon, kidney, heart, lung, melanocytes, pancreatic islets, pancreas, prostate, retina, thyroid gland, trachea and dendritic cells (Todd et al., 1998). Osteoclast precursor cells (Iwai et al., 2007) |
|
| TSPAN4 | TSP-4 NAG-2 TM4SF7 |
Brain, heart, melanocytes (Todd et al., 1998), osteoclast precursor cells (Iwai et al., 2007), spleen, colon, lymphocytes, pancreas, prostate and salivary gland (Tachibana et al., 1997), |
|
| TSPAN5 | TSP-5 NET-4 |
Brain, colon, liver/spleen, pancreas, retina, T-lymphoid cells (Todd et al., 1998; Serru et al., 2000) and osteoclast precursor cells (Iwai et al., 2007) | |
| TSPAN6 | TSP-6 | Brain, colon, liver/spleen, heart, lung, melanocytes, ovary, pancreas, prostate and retina (Maeda et al., 1998; Todd et al., 1998) | Not known |
| TSPAN7 | CD231 TALLA-1 A15 |
Brain, lung, kidney, skeletal muscle, spleen. Expression is also found in T-cell acute lymphoblastic leukaemia and neuroblastoma (Takagi et al., 1995) | |
| TSPAN8 | CO-029 | Gastric, oesophageal, hepatic, colorectal, and pancreatic carcinomas (Richardson et al., 2011) |
|
| TSPAN9 | NET-5 | Megakaryocytes, platelets and hematopoietic cells (Serru et al., 2000) |
|
| TSPAN10 | OCULOSPANIN | Retinal pigment epithelium and choroid (Wistow et al., 2002) | Not known |
| TSPAN11 | Not known | Not known | |
| TSPAN12 | NET-2 TM4SF12 |
Lymphoid cells (Serru et al., 2000) | |
| TSPAN13 | NET-6 | Osteoclast precursor cells and haematopoietic cells (Serru et al., 2000; Iwai et al., 2007) |
|
| TSPAN14 | DC-TM4F2 | Not known | |
| TSPAN15 | NET-7 | Myeloid cells, B-lymphoid cells and T-lymphoid cells (Serru et al., 2000) |
|
| TSPAN16 | TM4-B TM4SF16 |
Most tissues, strong expression in spinal cord, prostate, and salivary gland (Puls et al., 1999) | Not known |
| TSPAN17 | FBXO23 TM4SF17 |
Not known |
|
| TSPAN18 | N/A | Not known | Not known |
| TSPAN19 | N/A | Not known | Not known |
| UPK1B | UP1b TSPAN20 |
Bladder epithelium (Yu, 1994) |
|
| TSPAN21 | UP1a UPK1A, TSPAN21 |
Bladder epithelium (Yu, 1994) |
|
| PRPH2 | RDS ROCA-1 TSPAN22 |
The nervous system (Kaprielian and Patterson, 1993) and retina (Travis et al., 1991). | |
| TSPAN23 | ROM1 | Retina (Bascom et al., 1992) | |
| CD151 | PETA-3 SFA-1 MER2 TSPAN24 |
Most tissues including vascular endothelium, epidermis platelets and erythroid cells, except brain, red blood cells and lymphocytes (Sincock et al., 1997) |
|
| CD53 | TSPAN25 MOX44 |
Lymphocytes, monocytes, granulocytes and osteoclast precursor cells (Olweus et al., 1993; Iwai et al., 2007) | |
| CD37 | TSPAN26 | Mature B cells and osteoclast precursor cells (Iwai et al., 2007) | |
| CD82 | KAI1 ST6 SAR2 4F9 TSPAN27 C33 antigen |
Most tissues, except smooth muscle, adrenal cortex, urothelium, myelin of peripheral nerves, epithelium of amnion (Dong et al., 1995; Huang et al., 1997). Lymphocytes and monocytes (Imai et al., 1992). |
|
| CD81 | TAPA-1 TSPAN28 |
Most cells, including lymphoid cells (Oren et al., 1990; Berditchevski et al., 1996; Levy et al., 1998; Berditchevski, 2001; Hemler, 2005) |
|
| CD9 | MIC-3 MRP-1 TSPAN29 |
Most tissues including haematopoietic, osteoclast precursor cells and epithelial cells, except red blood cells and pancreas (Huang et al., 1997; Sincock et al., 1997; Nakamura et al., 2001) |
|
| CD63 | MLA1 TSPAN30 Ocular melanoma-associated antigen |
Haematopoietic cells, lymphoid tissues, osteoclast precursor cells and tissue macrophages (Metzelaar et al., 1991; Radford et al., 1996; Iwai et al., 2007). Bladder, gut, kidney, lung, ocular tissues, pancreas, prostate, salivary gland, spleen and uterus, except brain, red blood cells and lymphocytes (Donoso et al., 1985; Sincock et al., 1997) |
|
| TSPAN31 | SAS | Osteoclast precursor cells (Iwai et al., 2007) and sarcoma | Not known |
| TSPAN32 | valign="top"TSSC6 PHEMX |
High level of expression in haematopoietic tissues including peripheral blood leukocytes, thymus and spleen (Nicholson et al., 2000; Robb et al., 2001) | |
| TSPAN33 | PEN | Predominantly in erythroblasts (Heikens et al., 2007) |
|
Data sourced from cited references, the human protein atlas (http://www.proteinatlas.org) and the protein database (http://www.uniprot.org). N/A denotes data not available. ADAM10, A disintegrin and metalloprotease 10.
An overall rod-shaped structure of tetraspanins was revealed by a 6 Å resolution cryo-electron microscopy structure of uroplakin (Min et al., 2006). The rod-shaped structure consists of four close packed transmembrane helices that extend into the extracellular loops, capped by a disulfide-stabilized head domain (Figure 1). Of the 200–350 amino acids that are found in tetraspanins, 12–31 of them reside in the short extracellular loop for which structural information is not yet available.
Figure 1.
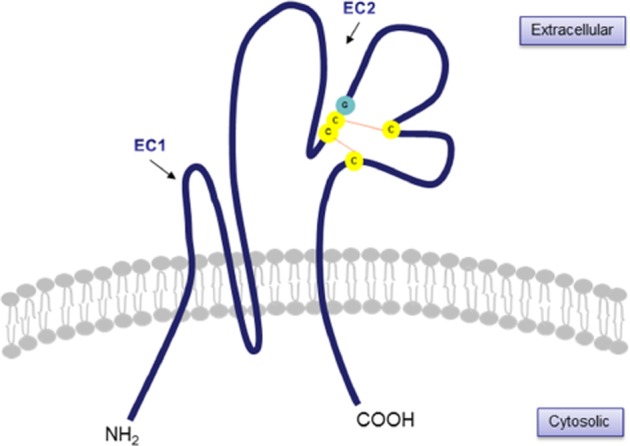
Representation of structural feature of tetraspanins. A variable domain (EC2) is stabilized by two disulfide bonds (orange lines) and consists of four invariant Cys residues (in yellow), two of which are in a Cys-Cys-Gly (CCG) motif (in yellow and green).
The large extracellular loop (EC2) of tetraspanins is of highly variable structure, despite conserved cysteine motifs, which may indicate tetraspanin-specific recognition processes (Kitadokoro et al., 2001). Conserved motifs include four invariant cysteine residues in the EC2 domain: CCG (Cys-Cys-Gly), PXSC (Phe-X-Ser-Cys) and EGC (Glu-Gly-Cys) (Hemler, 2001; Clark et al., 2004; Kovalenko et al., 2005) (Figure 1). The transmembrane domains stabilize heteromultimerization among different tetraspanins forming ‘tetraspanin webs’ (Fitter et al., 1998; Stipp et al., 2003b; Kovalenko et al., 2005). Besides conserved EC2 homology across species for any particular tetraspanin (Bienstock and Barrett, 2001), Seigneuret et al. suggested that the EC2 domain was organized into two subdomains: one conserved subdomain with a highly conserved fold despite significant residue differences and a second variable subdomain with extreme variability in size, amino acid sequence and protein fold governed by key disulfide bridges (Seigneuret et al., 2001). The EC2 regions of tetraspanins are required for interactions between tetraspanins and other transmembrane proteins such as integrins, and other signalling molecules (Maecker et al., 1997; Yáñez-Mó et al., 2001). In addition, mutations within transmembrane domains 1, 2 and 4 of peripherin/rds tetraspanin are linked to various types of retinal dystrophies (Stipp et al., 2003b). The short cytoplasmic tails show no obvious functional significance in signalling processes, suggesting association with other signalling molecules (Fitter et al., 1998). Tetraspanins are thought to act as molecular facilitators, recruiting groups of specific cell-surface proteins which stabilize functional signalling complexes (Maecker et al., 1997).
The tetraspanin ‘web’ or tetraspanin-enriched microdomain is an important biological feature of tetraspanin members and involves interactions with various leucocyte receptors, signalling molecules such as integrins, PKC, PI-4-kinase (PI4-K) and with each other (Wright et al., 2004b) (Figure 2). These interactions are important in determining fundamental biological activities such as cell adhesion, proliferation and cell motility. Interactions between tetraspanin members are important in maintaining the integrity of the tetraspanin web and providing binding sites for different ligands. However, these interactions are weaker than tetraspanin-partner interactions, such as CD151-α3β1 integrin (Wright et al., 2004b). Palmitoylation, post-translational acylation in most cases with cysteine residues, is found to be critical for organization of tetraspanin-enriched microdomains (Zhou et al., 2004) and the loss of palmitoylation affects tetraspanin-partner interactions, subcellular distribution, stability during biosynthesis and cell morphology (Yang et al., 2002; Stipp et al., 2003b).
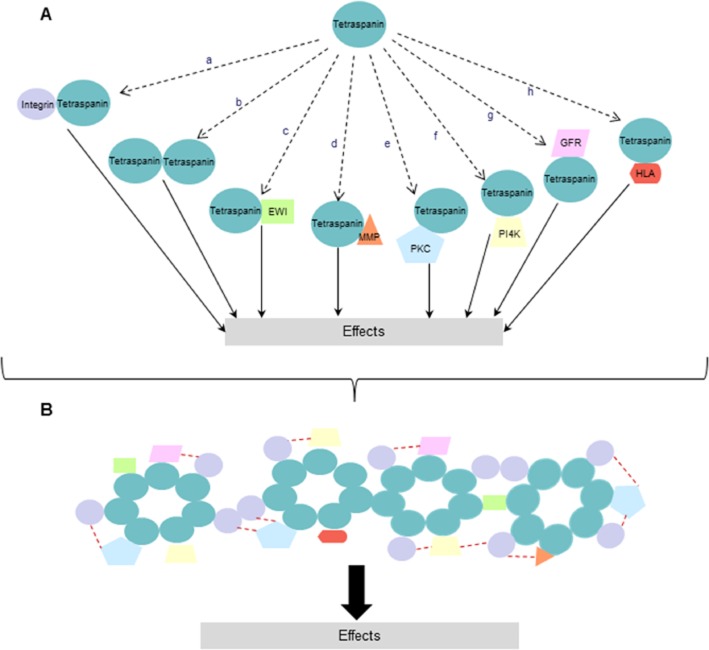
Figure 2.
Schematic representation of formation and complexity of the tetraspanin web. (A) Tetraspanins interact with various molecules including other members of tetraspanin superfamily (a–h) and these interactions can result in downstream signalling and biological function. (B) Representative formation of tetraspanin web forming specific signalling network of cell membrane and cytosolic proteins are shown. The network depending on tetraspanins and their partners are determinants of signal transduction and tetraspanins can bring together different signalling proteins into close proximity (red dotted lines). (EWI, a cell surface immunoglobulin SF protein; GFR, growth factor receptor; HLA, human leukocyte antigen).
Integrins are major partners of the tetraspanins and interact with a wide range of ECM proteins (Berditchevski, 2001; Hood and Cheresh, 2002) (see below for more discussion). The interaction of tetraspanins with laminin-binding integrins may be the pathway by which tetraspanins have an effect on migration and metastasis (Serru et al., 1999). Berditchevski emphasized that the tetraspanins CD81 and CD151 interact with integrins directly and as a consequence, may bring other family members into integrin proximity (Berditchevski, 2001). For example, the PKC family of phospholipid-dependent serine and threonine kinases is among the intracellular proteins which bind indirectly to tetraspanins and participate in various biological activities (Berditchevski, 2001). PKCs associate with several different tetraspanins such as CD9, CD81, CD82 and CD151, in which specificity resides within the intracellular domain of tetraspanins and tetraspanins act as linker molecules recruiting PKC into close proximity with β1, β2 and β3 integrins (Zhang et al., 2001b). PKCα and PKCε isoforms are found to interact with β1 integrins, promoting integrin-dependent cell motility and control of internalization of integrins (Ng et al., 1999; Ivaska et al., 2002).
Integrin involvement in cell invasion and metastasis is well established. Brakebusch et al., in particular, discussed the role of integrins in invasive growth in vivo and demonstrated the importance of integrin-mediated binding events in cell proliferation and invasion. For example, up-regulation of integrin α5β1 expression inhibits programmed cell death and β1 integrins promote metastasis (Brakebusch et al., 2002). Integrin αvβ4, when constitutively activated, promotes breast cancer metastatic activity in a human breast cancer cell line and mouse model (Felding-Habermann et al., 2001). β1 integrin is required for cancer cell adhesion and invasion by promoting formation of focal adhesion complexes to the extracellular matrix and mediating anti-apoptotic signal transduction pathways activating Akt/phosphoinositide 3-kinase (PI3-K), which may or may not involve focal adhesion kinase activation (Velling et al., 2004; Brockbank et al., 2005). Integrin expression profiles have been studied intensively and various integrin heterodimers, such as α3β1 and α6β1, are correlated with aggressiveness of prostate cancer, while expression of β4 integrin is often lost (Knox et al., 1994; Cress et al., 1995). A study of MDA-MB-231 cells, a breast cancer cell line, has shown that the α3β1-tetraspanin protein complex may be linked to an invasive phenotype of tumour cells via modulation of various signalling pathways, including degradation and activation of MMP-2 (a MMP family protein associated with supporting protrusive activity and invasive migration of the cells) and affecting PI3-K signalling pathways, which control actin cytoskeleton dynamics (Sugiura and Berditchevski, 1999). Mutagenesis of the α3 domain of integrin reveals a phosphorylation site within the conserved motif at the α3A cytoplasmic tail corresponding to integrin-related signalling, motility and morphology (Zhang et al., 2001a). In addition, the integrin α6β4 has novel functions in migration of epithelial and epithelial-derived carcinoma cells via the formation of adhesive structures, hemidesmosomes. These in turn link to the intermediate filament cytoskeleton and activate PI3-K which then stimulates other integrins, especially α3β1 (Mercurio et al., 2001). Co-localization of integrin β4 with CD151 also leads to activation of PKC which in turn promotes integrin internalization and increases cell motility (Gesierich et al., 2005). Binding of CD151 to α3β1 is highly stoichiometric and CD151 association with PI4-K brings PI4-K in closer proximity to α3β1 (Yauch et al., 1998), which may be one of the mechanisms by which CD151 promotes cell motility.
Expression and prognostic value of tetraspanins in various cancers
Many studies have found correlations between tetraspanins and progression of cancer. Most tetraspanins become down-regulated in metastatic tumours but the CD151 glycoprotein was the first tetraspanin member to be identified as a promoter of metastasis (Testa et al., 1999). Similarly, TSPAN8 is up-regulated in advanced stages in pancreatic, hepatic, oesophageal, gastric and colorectal carcinomas (Richardson et al., 2011). Although most studies have found CD9 to be down-regulated during cancer progression (see Tables 2 and 2002), the opposite has been found in osteosarcoma (Kubista et al., 2004), prostate cancer (Zvierev et al., 2005) and breast cancer (Kischel et al., 2012). These findings are supported by the finding that CD9 promotes expression of the matrix proteolytic enzyme, MMP-2 (Hong et al., 2005). Various tetraspanins have been investigated for their potential as prognostic factors in many cancers, as outlined in Table 2.
Table 2.
Tetraspanins are prognostic indicators in many cancers (↑ denotes increased levels = more progression and ↓ denotes decreased levels = more progression)
| Cancer type | Tetraspanins and correlation with prognosis | References |
|---|---|---|
| Astrocytoma | CD63↑ | (Rorive et al., 2010) |
| Breast cancer | CD9↓, CD82↓ | (Huang et al., 1998) |
| CD151↑ | (Sadej et al., 2009; Kwon et al., 2012) | |
| Clear cell renal cell carcinoma | CD151↑ | (Yoo et al., 2011) |
| TSPAN7 | (Wuttig et al., 2012) | |
| Colon cancer | CD9↓, CD82↓, CD151↑ | (Hashida et al., 2003) |
| TSPAN8↑ | (Greco et al., 2010) | |
| Colorectal cancer | TSPAN1↑ | (Chen et al., 2009) |
| Endometrial carcinoma | CD9↓ | (Miyamoto et al., 2001) |
| CD151↑ | (Voss et al., 2011) | |
| Gallbladder adenocarcinoma | CD9↓ | (Qiong et al., 2012) |
| Gastric cancer | CD9↓ | (Hori et al., 2004; Soyuer et al., 2010; Chen et al., 2011b) |
| TSPAN1↑ | (Chen et al., 2008b) | |
| CD151↑ | (Yang et al., 2013) | |
| Gastric gastrointestinal stromal tumour | CD9↓ | (Setoguchi et al., 2011) |
| Gingival squamous cell carcinoma | CD151↑ | (Hirano et al., 2009) |
| Glioblastoma | CD63↑ | (Rorive et al., 2010) |
| Head and neck squamous cell carcinoma | CD9↓ | (Mhawech et al., 2003) |
| Hepatocellular carcinoma | CD81↓ | (Inoue et al., 2001) |
| CD151↑ | (Ke et al., 2009; Shi et al., 2010; Devbhandari et al., 2011) | |
| TSPAN1↑ | (Chen et al., 2010a) | |
| TSPAN8↑ | (Kanetaka et al., 2001) | |
| Intrahepatic cholangiocarcinoma | CD151↑ | (Huang et al., 2010) |
| Lung adenocarcinoma | CD9↓ | (Higashiyama et al., 1997) |
| CD63↓ | (Kwon et al., 2007) | |
| Melanoma | CD9↓ | (Si and Hersey, 1993) |
| CD63↓ | (Radford et al., 1997) | |
| Merkel cell carcinoma | CD9↓, CD151↑ | (Woegerbauer et al., 2010) |
| Multiple myeloma | CD81↑ | (Paiva et al., 2012) |
| Non-small cell lung cancer | CD82↓ | (Adachi et al., 1996) |
| CD63↓ | (Kwon et al., 2007) | |
| CD151↑ | (Tokuhara et al., 2001) | |
| Oral squamous cell carcinoma | CD9↓ | (Kusukawa et al., 2001; Buim et al., 2010) |
| Oesophageal squamous cell carcinoma | CD9↓ | (Uchida et al., 1999) |
| CD82↓ | (Uchida et al., 1999) | |
| CD151↑ | (Suzuki et al., 2011) | |
| Ovarian carcinoma | CD9↓ | (Houle et al., 2002) |
| CD82↓ | (Schindl et al., 2001; Houle et al., 2002) | |
| TSPAN1↑ | (Scholz et al., 2009) | |
| CD63↓ | (Zhijun et al., 2007) | |
| Pancreatic cancer | CD9↓, CD82↓ | (Sho et al., 1998) |
| CD151↑ | (Zhu et al., 2011) | |
| Prostate cancer | CD82↓ | (Lijovic et al., 2002) |
| CD151↑ | (Ang et al., 2004) | |
| TSPAN13↓ | (Arencibia et al., 2009) | |
| Thyroid cancer | CD82↓ | (Chen et al., 2004) |
Association with integrins
Integrins are important in cell attachment and control cell migration, cell cycle progression and programmed cell death. They regulate these functions in synergy with other signalling pathways (Brakebusch et al., 2002), including tetraspanins such as CD81, CD9, CD53, CD63, CD82 and CD151, in various types of human cells (Maecker et al., 1997). They do not have any intrinsic activities but are present on the cell surface and respond to various ECM components and microenvironmental signals to form integrin-dependent signalling pathways to regulate proliferation, migration, invasion, apoptosis and angiogenesis (Brakebusch et al., 2002; Stupack and Cheresh, 2004). Major laminin-binding integrins (laminins are cell-adhesive proteins in basement membranes) are α3β1, α6β1, α6β4 and α7β1 (Nishiuchi et al., 2005). There is evidence that CD81 associates with the α4β1 integrin, and CD151 with α3β1, α6β1, α6β4 and α7β1 (Serru et al., 1999; Sterk et al., 2002; Wright et al., 2004b).
Evidence of a relationship between angiogenesis, lymphangiogenesis and cancer progression and involvement of integrins has become apparent in recent years. In endothelial cells, integrins have been found to be involved in the induction of angiogenesis (Dominguez-Jimenez et al., 2001; Wang et al., 2005; Mitchell et al., 2009; 2010,). Integrins α1β1, α2β1, α4β1, α5β1, α9β1, α6β4, αvβ3 and αvβ5 play a role in angiogenesis, while integrins α2β1, α4β1, α5β1 and α9β1 are involved in lymphangiogenesis (Hong et al., 2004; Jin and Varner, 2004; Dietrich et al., 2007; Avraamides et al., 2008; Okazaki et al., 2009; Garmy-Susini et al., 2010). Integrins α5β1, αvβ5 and αvβ3 bind provisional ECM components (the permissive basal ECM required for angiogenesis) such as fibronectin and vitronectin which are up-regulated during angiogenesis, while integrins that bind to basal ECM components collagen and laminin such as α1β1, α2β1, α3β1, α5β1 and α6β4 tend to be down-regulated (Stupack and Cheresh, 2004). Integrin α9β1 directly associates with VEGFs -A, -C and -D, which are major modulators of blood (VEGF-A) and lymph (VEGF-C and VEGF-D) vessel formation (Timoshenko et al., 2007; Vlahakis et al., 2007; Oommen, 2011; Majumder et al., 2012). Therefore, integrin protein expression is an important determinant to balance signal transduction pathways that occur within the cell.
Many studies have found various complexes of tetraspanins and integrins that are co-localized and together influence cell motility. In rat pancreatic adenocarcinoma, for example, the D6.1A tetraspanin (rat homologue of human CO-029 tetraspanin) and α6β4 integrin are co-expressed and together contribute to hematogenous spread of tumour cells (Gesierich et al., 2005). Interestingly, most of CD151's role is through association with integrins. Complexes of CD151 and integrin α3β1 are the focus of most studies as their interaction is strong, direct and stoichiometric (Yauch et al., 1998; Berditchevski et al., 2001). CD151 and the integrin α3β1 were directly associated and were important in recruiting various signalling molecules (including other tetraspanin members) into close proximity to each other to form a signalling complex (Serru et al., 1999). Loss of CD151 diminished the association of laminin-binding integrins (i.e. α3β1 and α6β1) with signalling proteins (Yauch et al., 1998; Takeda et al., 2007). CD151 was also associated with activation of various signalling molecules, PI3-K, PI4-K, PKB/Akt, endothelial nitric oxide synthase (eNOS), Rac and Cdc42 that are involved in cell migration, invasion and angiogenesis (Yauch et al., 1998; Takeda et al., 2007; Zheng and Liu, 2007a). The CD151-α3β1 complexes play a particular role in formation of focal contacts and intracellular signalling events, especially changes in actin-cytoskeleton dynamics leading to cell invasive migration (Berditchevski and Odintsova, 1999; Shigeta et al., 2003). CD151 is an essential molecular linker in integrin-dependent cell motility signalling (Kazarov et al., 2002) and important in determining integrin localization within cells (Sincock et al., 1997; Chometon et al., 2006; Hasegawa et al., 2007). Changes in integrin localization are believed to be a determinant of cancer invasion and metastasis.
Tetraspanin influence in forming complexes with integrins may regulate cellular integrin distribution and integrin trafficking, which in turn regulates cellular motility and invasion (Berditchevski and Odintsova, 1999; Winterwood et al., 2006; Berditchevski and Odintsova, 2007). Some of tetraspanin/integrin associations that are important in cancer cell motility/invasion and angiogenesis are shown in Tables 3 and 2010. Significance of integrin-tetraspanin association is well reviewed in Boucheix and Rubinstein (2001), Hemler (2008), Stipp (2010) and Bassani and Cingolani (2012).
Table 3.
Tetraspanin members have a role in cancer cell motility and invasion
| Tetraspanin | Cancer type | Promoter/Suppressor of motility/invasion | Proposed mechanism | Reference |
|---|---|---|---|---|
| CD9 | Small cell lung cancer | Suppressor | Not determined | (Zheng et al., 2005) |
| Fibrosarcoma | Suppressor | Inhibition of cell motility and colony formation via formation of complexes of CD9 and its partners TGFα, EGFR, EWI-2, EWIF and β1 and activation of Akt, p38, and EGFR pathways | (Chen et al., 2011a) | |
| Ovarian carcinoma | Suppressor | CD9 has a role in cell adhesion on ECM and down regulation of CD9 resulted in altered integrins β1, α2, α3β1, α 5, and α 6 expression and localizations | (Furuya et al., 2005) | |
| Prostate cancer | Promoter | Not determined | (Zvierev et al., 2005) | |
| Lung cancer | Suppressor | Association with β1 integrin | (Funakoshi et al., 2003) | |
| Breast cancer | Promoter | Not determined | (Kischel et al., 2012) | |
| Promoter | CD9/CD81 support MT1-MMP, a proteolytic enzyme, expression and CD9/CD81/MT1-MMP association enhances invasion in in vitro 3D collagen and fibrin gel | (Lafleur et al., 2009) | ||
| Promoter | CD9 and CD81 complex may independently promote α3β1 integrin association with PKCα | (Gustafson-Wagner and Stipp, 2013) | ||
| CD63 | Melanoma | Suppressor | Association with β1 integrin and may regulate β1 integrin expression | (Radford et al., 1997; Jang and Lee, 2003) |
| Colon cancer | Suppressor | CD63/α3 integrin complex regulates adhesion and migration on substrate laminin-5 | (Sordat et al., 2002) | |
| CD81 | Hepatocellular carcinoma | Suppressor | Interacting with PI4KIIβ and together affect actin cytoskeleton rearrangement | (Mazzocca et al., 2008) |
| Histiocytic lymphoma | Promoter | CD81 promotes cell membrane protrusive structures | (Bari et al., 2011) | |
| Breast cancer | Promoter | CD9/CD81 support MT1-MMP, a proteolytic enzyme, expression and CD9/CD81/MT1-MMP association enhances invasion in in vitro 3D collagen and fibrin gel | (Lafleur et al., 2009) | |
| Promoter | CD9 and CD81 complex may independently promote α3β1 integrin association with PKCα | (Gustafson-Wagner and Stipp, 2013) | ||
| CD82 | Ovarian cancer | Suppressor | Inhibiting αvβ3 integrin/vitronectin-mediated cell motility and proliferation | (Zlatna et al., 2009) |
| Non-small cell lung cancer | Suppressor | Regulating β1 integrin maturation and its cell surface expression | (Jee et al., 2007) | |
| Suppressor | Stabilizing E-cadherin–b-catenin complex (promoting cellular adhesion) | (Abe et al., 2008) | ||
| Oral squamous cell carcinoma line and non-small cell lung carcinoma |
Suppressor | Direct association with c-Met inhibiting HGF-promoted motility | (Takahashi et al., 2007 | |
| Prostate cancer | Suppressor | Not determined | (Dong et al., 1995; Lijovic et al., 2002; Bari et al., 2009) | |
| Suppressor | CD82 modulates integrin-mediated activations of c-Met and Src signallings | (Sridhar and Miranti, 2006) | ||
| Suppressor | Attenuates cell membrane protrusive structures | (Bari et al., 2011) | ||
| Suppressor | Direct association with EWI2/PGRL immunoglobulin member to mediate tumour cell migration | (Zhang et al., 2003) | ||
| Suppressor | Regulates β1 integrin activation at the cell surface affecting focal adhesion complex formation | (Lee et al., 2011) | ||
| CD151 | Melanoma | Promoter | Association with α3β1 and α6β1 integrins. Linking β1 integrins to Ras, Rac1 and Cdc42 |
(Hong et al., 2006; 2012,) |
| Skin squamous cell carcinoma | Promoter | CD151 supports PKCα-α6β4 integrin association and α6β4 integrin distribution | (Li et al., 2012) | |
| Gastric cancer | Promoter | Association with integrins α3 | (Yang et al., 2013) | |
| Prostate cancer | Promoter | Not determined | (Ang et al., 2004; 2010,) | |
| Pancreatic and colorectal carcinoma | Promoter | Association with α6β4 integrin and tetraspanin TSPAN8 | (Gesierich et al., 2005) | |
| Salivary gland cancer | Promoter | Association with c-Met and integrins α3/α6 and promotes HGF/c-Met signalling pathway | (Klosek et al., 2005) | |
| Breast cancer | Promoter | Association with c-Met and integrins α3/α6 and promotes HGF/c-Met signalling pathway | (Klosek et al., 2009) | |
| Promoter | Assisting ErbB2-integrin pathway through focal adhesion kinase (FAK) signalling | (Yang et al., 2010; Deng et al., 2012) | ||
| Intrahepatic cholangiocarcinoma | Promoter | Not determined | (Huang et al., 2010) | |
| Hepatocellular carcinoma | Promoter | Association with α6 integrin Increase Rac/Cdc42 activity |
(Ke et al., 2011; Fei et al., 2012) | |
| Colon cancer, glioblastoma and fibrosarcoma | Promoter | Possibly via FAK association | (Kohno et al., 2002) | |
| Human epidermoid carcinoma and fibrosarcoma | Promoter | Supports tumour cell detachment and tumour intravasation | (Zijlstra et al., 2008) | |
| Ovarian cancer | Promoter | Not determined | (Mosig et al., 2012) | |
| TSPAN1 | Colon cancer | Promoter | Not determined | (Chen et al., 2010b) |
| Hepatocellular carcinoma | Promoter | Not determined | (Wang et al., 2012) | |
| Skin squamous cell carcinoma | Promoter | Not determined | (Chen et al., 2010c) | |
| TSPAN8 | Oesophageal carcinoma | Promoter | ADAM12, a type of matrix metalloprotease enzyme is involved in TSPAN8's promotion of motility and invasion | (Zhou et al., 2008) |
| Colon cancer | Promoter | Modulating regulation of E-Cadherin/p120ctn complex on cell motility | (Greco et al., 2010) | |
| Colorectal cancer | Suppressor | Promotes cell motility through regulation of tumour cell-matrix and cell-cell adhesion | (Guo et al., 2012) | |
| TSPAN13 | Breast cancer | Suppressor | May promotes cell-matrix adhesion via down-regulation of MMPs | (Huang et al., 2007) |
MET, mesenchymal-epithelial transition.
Role of tetraspanins in cancer metastasis
Cellular changes of carcinogenesis involve various mechanisms, including regulatory gene mutations, gene over- and under-expression, endocrine activation and epigenetic alterations. This accumulation of changes results in a loss of balanced gene expression, allowing cells to undergo transformation (Golias et al., 2007). The cellular transformation together with changes in tumour microenvironment (TME) triggers invasive and/or metastatic phenotypes (Wall et al., 2003; Hugo et al., 2007). In order for tumour cells to create a progressive disease, they need to communicate with their surrounding cells. The TME plays an important part in the promotion of cancer cell growth, invasion, angiogenesis and survival (Fidler, 2002). Angiogenesis is one of the major characteristics of cancer metastasis and anti-angiogenic agents targeting various molecular targets are currently in clinical trials (Carmeliet and Jain, 2000; Detchokul and Frauman, 2011; Goel et al., 2011).
Members of the tetraspanin superfamily are implicated in regulation of cell proliferation, motility, adhesion, angiogenesis and tumour metastasis (Hemler et al., 1996; Hasegawa et al., 1998; Berditchevski, 2001; Boucheix et al., 2001; Longo et al., 2001; Tokuhara et al., 2001; Sadej et al., 2009). Tetraspanins are also associated with the regulation of the net proteolytic activity at the plasma membrane providing additional dimension of regulatory mechanisms for cell adhesion, migration and growth factor signalling (Yáñez-Mó et al., 2011; Dornier et al., 2012; Haining et al., 2012; Schroder et al., 2013). CD82/KAI1 was found to negatively correlate with the progression of prostate cancer, suggesting this tetraspanin has a tumour suppressor role (Lijovic et al., 2002). Since the first finding of the role of CD151 in the promotion of cancer migration and metastasis by Testa et al. (1999), numerous studies have been looking at this tetraspanin in various cancers: epidermoid, pancreatic, glioblastoma, breast, colorectal, amelanotic melanoma, osteosarcoma and hepatic fibrosarcoma (Testa et al., 1999; Kohno et al., 2002; Gesierich et al., 2005; Hong et al., 2006; Hasegawa et al., 2007; Yang et al., 2008; Shi et al., 2010; Zhang et al., 2010). CD151 affects cell motility and malignancy in non-small cell lung cancer (Sugiura and Berditchevski, 1999) and pancreatic and colorectal tumours (Gesierich et al., 2005) and also has a role in migration of neutrophils, fibroblasts and endothelial cells (Yáñez-Mó et al., 1998; Yauch et al., 1998; Kohno et al., 2002; Liu et al., 2007; Takeda et al., 2007; Zheng and Liu, 2007b; Zuo et al., 2010). A link between CD151 expression and prostate cancer prognosis has been demonstrated (Ang et al., 2004) and CD151 correlates with the prognosis and survival time of non-small cell lung cancer (Tokuhara et al., 2001), colon cancer (Hashida et al., 2003), renal cell carcinoma (Yoo et al., 2011), pancreatic adenocarcinoma (Zhu et al., 2011), oesophageal squamous cell carcinoma (Suzuki et al., 2011), intrahepatic cholangiocarcinoma (Huang et al., 2010), breast cancer (Sadej et al., 2010), hepatocellular carcinoma (Ke et al., 2009; Shi et al., 2010) and Merkel cell carcinoma (Woegerbauer et al., 2010) (see Table 5 below). CD151 has attracted much interest in cancer research and this will be discussed in the following sections. We have also summarized the significance of tetraspanins in various cancers (Tables 2002 and 4), according to their role in cancer invasion/metastasis and the angiogenesis process, respectively.
Table 5.
Prognostic value of CD151 in various cancers
| Cancer type | Associated proteins | Clinical correlation | Reference |
|---|---|---|---|
| Breast cancer | Integrins α3β1, α6β1 and α6β4 | Increased expression correlates with lower survival | (Sadej et al., 2010) |
| Not determined | Increased expression correlates with lower survival | (Kwon et al., 2012) | |
| Colon cancer | Not determined | Increased expression correlates with metastasis and lower survival | (Hashida et al., 2003) |
| Clear cell renal cell carcinoma | Not determined | Increased expression correlates with metastasis and lower survival | (Yoo et al., 2011) |
| Endometrial carcinoma | Expression correlates with E-cadherin expression | Increased expression correlates with aggressive forms and lower survival | (Voss et al., 2011) |
| Gastric cancer | Integrins α3 | Increased expression correlates with lower survival | (Yang et al., 2013) |
| Gingival squamous cell carcinoma | Not determined | Increased expression correlates with lower survival | (Hirano et al., 2009) |
| Hepatocellular carcinoma | Expression correlates with proto-oncogene c-Met expression | Increased expression correlates with metastasis, lower survival | (Ke et al., 2009) |
| Not determined | Increased expression concomitant with MMP9 and MVD correlates with lower survival | (Shi et al., 2010) | |
| Integrin β1 | Increased expression correlates with lower survival and high recurrence rate and expression of CD151/ integrin β1 complex greatly indicated poor prognosis | (Devbhandari et al., 2011) | |
| Intrahepatic cholangiocarcinoma | Expression correlates with proto-oncogene c-Met expression | Increased expression correlates with metastasis and lower survival | (Huang et al., 2010) |
| Non-small cell lung cancer | Not determined | Increased expression correlates with lower survival | (Tokuhara et al., 2001) |
| Oesophageal squamous cell carcinoma | Not determined | Increased expression correlates with metastasis and lower survival | (Suzuki et al., 2011) |
| Pancreatic ductal adenocarcinoma | Expression correlates with proto-oncogene c-Met and integrins α3/α6 expression | Increased expression correlates with metastasis and lower survival | (Zhu et al., 2011) |
| Prostate cancer | Not determined | Increased expression correlates with metastasis and lower survival | (Ang et al., 2004) |
Table 4.
Tetraspanins and their role in cancer angiogenesis
| Tetraspanin | Investigated cell types/animal models | Proposed mechanism | Reference |
|---|---|---|---|
| CD9 | Multiple myeloma | Involved in transendothelial invasion and CD9 expression was up-regulated upon contact with bone marrow endothelial cells | (De Bruyne et al., 2006) |
| Human cervical carcinoma | Not determined. Preferentially expressed near vascular and lymphatic tumour invasions which may suggest role in transendothelial migration | (Sauer et al., 2003b) | |
| Human umbilical vein endothelial cells (HUVECs) and melanoma cells | Promotes transendothelial migration of tumour cells and tumour-endothelial cells interactions | (Longo et al., 2001) | |
| Human dermal microvascular endothelial cells | Promotes endothelial cell VEGF- and HGF- induced motility and invasion but not proliferation | (Kamisasanuki et al., 2011) | |
| Human saphenous vein or mammary artery endothelial cells | Promotes endothelial cell motility via association with β1 and β3 integrins | (Klein-Soyer et al., 2000; Soyuer et al., 2010) | |
| Human gastric cancer cell xenografts in SCID mice | CD9 antibody treated mice had decreased angiogenesis. Mechanism was not determined | (Nakamoto et al., 2009) | |
| HUVECs and erythroleukemic cells | Associates with adhesion receptor ICAM-1 at the apical membrane as part of endothelial adhesion platforms (EAPs) that regulate cellular adhesion | (Barreiro et al., 2008) | |
| CD81 | HUVECs | Localised with CD151, CD9 and integrin α3β1 and promotes endothelial cellular migration | (Yáñez-Mó et al., 1998) |
| CD82 | HUVECs | Up-regulated CD82 gene expression under hypoxic condition (via HIF-2) and CD82 suppresses endothelial cell migration | (Nagao and Oka, 2011) |
| CD151 | HUVECs and erythroleukemic cells | Associates with adhesion receptor VCAM-1 at the apical membrane as part of endothelial adhesion platforms (EAPs) that regulate cellular adhesion | (Barreiro et al., 2008) |
| HUVECs | Complex of tetraspanins/α3β1 mediates Ang II promotion of tubulogenesis | (Dominguez-Jimenez et al., 2001) | |
| Hepatocellular carcinoma cells | Modulates MMP9 expression via the PI3-K/Akt/GSK-3b/Snail signal to promote angiogenesis | (Shi et al., 2010) | |
| Breast cancer cells | Responds to endothelial factors, maybe via association with α3β1and α6β4 integrins | (Sadej et al., 2010) | |
| HUVECs | Localized at cell-cell junction together with CD81, CD9 and α6β4 and promotes endothelial cell motility and ECM remodelling | (Yáñez-Mó et al., 1998) | |
| Mouse lung endothelial cells derived from CD151-null mice | Activates along with FAK, ERK, PI3K/Akt/eNOS, and Rac1/Cdc42 pathways to promote angiogenesis | (Takeda et al., 2007) | |
| Mouse lung endothelial cells derived from CD151-null mice and murine melanoma cells | Required for melanoma cell-endothelial cell adhesion and transendothelial migration | (Takeda et al., 2011) | |
| Human dermal microvascular endothelial cells (HMECs) and HUVECs | Promotes cell-matrix adhesion via stabilizing focal adhesion maturation and promotion of cadherin-independent cell-cell adhesion | (Zhang et al., 2011a) | |
| HUVECs | Localises at cell-cell junctions, promotes endothelial cell motility and promotes in vitro capillary tube formation | (Sincock et al., 1999) | |
| Rat/pig models of myocardial ischemia | CD151 gene delivery improved microvessel densities in animal models of myocardial ischemia | (Zheng and Liu, 2006; Zuo et al., 2009a,b2009b) | |
| HUVECs | CD151-integrin complex may be needed for the promotions of in vitro endothelial proliferation, migration and tube formation acting via ERK-dependent signalling pathway | (Zuo et al., 2010) | |
| Tspan8 | BDX-derived rat pancreatic adenocarcinoma | Induces angiogenic switch and promotes angiogenic factor expression | (Gesierich, 2006) |
| BDX-derived rat pancreatic adenocarcinoma and rat aortic ring endothelial cells | Exosomes expressing Tspan8 secreted by tumour cells activates endothelial cell activation, maturation and motility | (Nazarenko et al., 2010) |
Role as a promoter/suppressor of motility and invasion machinery
Changes in oncogene mutations, growth factor signalling, adhesion receptor profiles, actin cytoskeletal architecture, E-cadherin expression at cell-cell contacts and basement membrane composition are important determinants of tumour invasion and metastasis (Wells, 2006). Many tumours originate from epithelial cells, therefore it is important to look at changes in the development of intrinsic cellular transition (Hugo et al., 2007). Epithelial-mesenchymal transition (EMT) is a cellular transition of morphogenetic and organogenetic processes (Boyer et al., 2000), which results in induction of increased cell motility and dissociation from intercellular complexes in transformed cells. These changes include cell-cell dissociation, actin cytoskeleton reorganization and cell-substratum interactions (Savagner, 2001). The transformation was first recognised during gastrulation at an embryonic developmental stage where epithelial cells transform into embryonic mesoderm. However, this process also occurs during organogenesis and somitogenesis, somite formation in the embryo that differentiate into skeletal muscle, vertebrae and dermis of all vertebrates, and is involved in pathophysiological conditions such as wound healing, kidney fibrosis and cataract formation (Lee et al., 2006; Chaffer et al., 2007). Epithelial and mesenchymal cells are different in their appearance, composition adhesiveness and mechanism of migration (Lee et al., 2006). These differences allow detection of the occurrence of EMT and mesenchymal-epithelial transitions within the cells. In cancer, such transformation is recognized due to its similar changes in protein/gene expression as that observed during embryonic morphogenesis, although the process is typically not complete and often forms a metastable phenotype. However, in cancers this concept is still controversial due to limited knowledge of modulatory mechanisms (Lawrence et al., 2007) and difficulties in studying the process in the clinical setting. Delocalization of the adhesion molecule cadherin leads to a detachment of cells from cell-cell contacts and these cadherins are relocalized with the help of integrins (Borghi, 2010). Integrins play important parts in cellular interactions with the ECM and signalling pathways, supporting the promotion of tumour cell adhesion profiles and tumour cell motility (Jin and Varner, 2004). This in turn promotes tumour dissemination and metastasis.
Cell motility is one of the critical steps for cancer invasion and metastasis (Wells, 2006). Cell motility is a complex coordinated process in which cells acquire a motile phenotype involving changes in their protein expression profiles; these involve changes in oncogene mutations, growth factor signalling (including EGF and TGFα), adhesion proteins (e.g. integrins and E-cadherins), proteinases (e.g. MMPs and uPA), actin cytoskeletal proteins and structures (e.g. vimentin, microtubules and actin microfilaments), cell membrane proteins (e.g. tetraspanins) and basement membrane composition, which can lead to loss of cell-cell adhesion and promotion of cell-matrix interaction (Wells, 2006).
Tetraspanins are involved in cytoskeletal dynamics
Targeting actin rearrangement or dynamics is one of the initial approaches to target tumour cell motility and invasion (Fenteany and Zhu, 2003), as investigated in ovarian cancer using actin-targeting agent cytochalasin D (Bijman et al., 2008) and in prostate cancer looking at ZNF185, an actin-associated protein (Zhang et al., 2007), and SWAP70, a F-actin binding protein (Chiyomaru et al., 2011). However, severe cytotoxicity remains an issue and because actins exist in many isoforms, careful selection of an actin population or design of drugs that target actin will need to be elucidated (Fojo, 2006; Stehn et al., 2006; Terracciano et al., 2008; Blain et al., 2010). This has placed emphasis on actin rearrangement as a modulator of tumour cell motility. Tetraspanins are modulators of pathways that control actin remodelling and reorganization, which is an early step in cell migration machinery. These are demonstrated in Figure 3 showing the involvement of tetraspanins in regulation of the dynamics of cytoskeletal actin.
Figure 3.
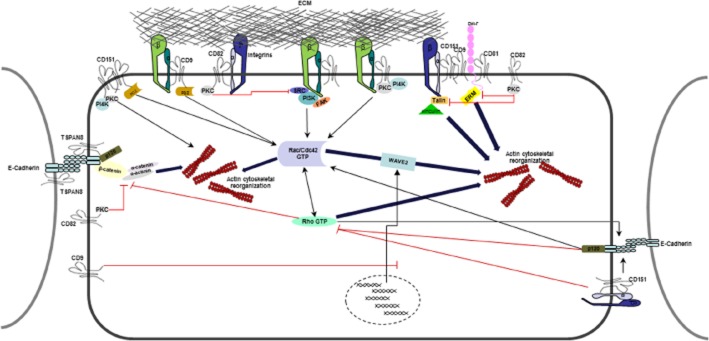
Tetraspanins play many roles in the regulation of the dynamics of cytoskeletal actin and thus in regulation of cell motility. Through binding to integrins, tetraspanins control the cytoskeletal rearrangements. Members of Rho family of GTPases (Rac/Rho/Cdc42 GTPases) mediate many aspects of actin dynamics. They also regulate cell-cell adhesion through cadherin-catenin complexes. E-cadherin-mediated cell-cell adherence involves reorganization of the actin cytoskeleton. p120ctn (represented as p120 in the diagram) associates directly with E-cadherins and plays a role in cell-cell adhesion and it also regulates Rho GTPase activity. The CD151-α3β1 integrin complex also affects the stability of E-cadherin-based junctions and Rho activation. CD151 also mediates activation of Rac/Cdc42 and CD151-PKC association mediates actin reorganization. CD151, CD9 and CD81 contain PDZ-domain-binding motifs and binding to PDZ-domain-containing proteins connects tetraspanins to the cytoskeleton. CD9 strengthens integrin adhesion to the ECM. CD9 also inhibits WAVE2 transcription affecting Rac-WAVE2-Arp2/3 complex associated activation of actin reorganization. There is evidence that TSPAN8 binds directly to E-cadherins. CD82 can regulate β-catenin/α-catenin via PKC. PKC also controls integrin-binding Talin and the EWI-F-binding protein ERM. PKC has been shown to inhibit, leading to inhibition of actin polymerisation. EWI-F, members of the Ig superfamily of proteins; FAK, focal adhesion kinase; PDZ, common structural domain of 80–90 amino acids; SRC, proto-oncogene encoding a tyrosine kinase.
Role in angiogenesis
Improved understanding of new blood vessel formation from existing vessels has changed the paradigm for cancer therapeutics with anti-angiogenesis drugs being used to complement traditional chemotherapy (Jones and Fujiyama, 1999). After carcinogenic transformation and growth, basement membrane degradation, invasion of the surrounding stroma and migration of endothelial cells in response to angiogenic stimuli, angiogenesis is a necessary step in order for a tumour mass to grow bigger than 1 mm (Fidler, 2002). Pathological angiogenesis is thought to be less tightly regulated than neovascularization occurring during development and wound healing (Stupack and Cheresh, 2004). In an adult, normal blood vessels remain dormant and the endothelial cells forming the lining of blood vessels enter a cell cycle at a rate of 1 in 103 cells. However, under pathological conditions, levels of pro-angiogenic factors are highly up-regulated allowing new vessel outgrowth (Stupack and Cheresh, 2004). There are many anti-angiogenic agents that are now available for cancer treatments including bevacizumab (for glioblastoma, renal cell carcinoma, non-squamous non-small cell lung cancer and colorectal cancer) (FDA, 2004), everolimus (for advanced renal cell carcinoma, pancreatic neuroendocrine tumours, subependymal giant cell astrocytoma) (FDA, 2009b), imatinib mesylate (for chronic myelogenous leukaemia, acute lymphoblastic leukaemia, dermatofibrosarcoma protuberans and metastatic gastrointestinal stromal cancer) (FDA, 2003), pazopanib (for advanced renal cell carcinoma) (FDA, 2009a), sunitinib mesylate (for advanced renal cell carcinoma, gastrointestinal stromal cancer and pancreatic neuroendocrine tumours) (FDA, 2006) and sorafenib (for hepatocellular carcinoma and advanced renal cell carcinoma) (FDA, 2005). These drugs target VEGF, VEGF receptors or PDGF receptors, which are known biomarkers of the angiogenic process.
Tumour-induced angiogenesis requires interaction and communication between endothelial cells, tumour cells and ECM (Jones and Fujiyama, 1999). Like tumour cells, endothelial cells express various tetraspanins including CD9, CD63, CD81, CD82, CD151, Tspan4 and Tspan8 and these tetraspanins have been shown to play a role in angiogenesis, leucocyte recognition, tumour-endothelial binding and vascular development (Bailey et al., 2011). These tetraspanins could potentially be a direct target in controlling the communication between endothelial cell, tumour cells and ECM. Longo et al. demonstrated involvement of CD9, CD151 and CD81 in angiogenesis (Longo et al., 2001) showing that CD9 facilitated tumour-endothelial transcellular migration. CD9 also regulated angiogenic activity, activated either by VEGF or hepatocyte growth factor (HGF) in endothelial cell migration and invasion assays in vitro and in vivo rat cornea micropocket angiogenesis assays (Kamisasanuki et al., 2011).
A study using CD151-null mice has indicated a role for CD151 in tumour angiogenesis (Takeda et al., 2007) and in vitro studies using endothelial cells have shown that CD151-integrin complexes have a role in endothelial cell proliferation, morphogenesis and migration, all of which are important in the angiogenesis process (Yáñez-Mó et al., 1998; Sincock et al., 1999; Zhang et al., 2002). Angiogenesis has been considered as a prognostic indicator in prostate cancer (Borre et al., 1998; Mehta et al., 2001; Bono et al., 2002) and correlates with the aggressiveness of the disease (Weidner et al., 1993; Ferrer et al., 1998). In animal models of myocardial ischaemia, introduction of CD151 via gene delivery improved capillary densities (Lan et al., 2005; Zuo et al., 2009a; Wei et al., 2011). Studies using CD151-null mice found that these mice were viable and no phenotypic change was reported. However, pathological angiogenesis in these mice was greatly affected, supporting a pro-angiogenic role for CD151 specifically in pathological conditions (Wright et al., 2004a; Takeda et al., 2007). The role of CD151 in angiogenesis is perhaps through assisting communication between tumour cells and endothelial cells (Sadej et al., 2009). Many studies found that CD151-integrin (especially α3β1 and α6β4) complexes are localized at the tumour cell and endothelial cell contact area (Yáñez-Mó et al., 1998; Longo et al., 2001; Sadej et al., 2009). CD151 is expressed in vascular endothelial cells and associates with integrin β1, β3, β4, α2, α3, α5 and α6 chains (Sincock et al., 1999). As discussed earlier, integrin expression is an important determinant to balance signal transduction pathways that occur within the cell. Expression of CD151 is required for integrin distribution within the endothelial intercellular contacts, which promotes angiogenesis (Takeda et al., 2007). While cross-talk between tumour cells and endothelial cells assisting in transendothelial migration of tumour cells is important in angiogenesis, CD151 has been found to be a membrane linker through which other signalling proteins stimulate the important regulator of endothelial cell function eNOS (Zheng and Liu, 2007b).
An important pathway that has been intensively studied and implicated in clinical trials for inhibition of angiogenesis and motility is extracellular-signal-regulated kinases 1/2 (ERK1/2), one of the MAPKs. Integrins can directly regulate ERK 1/2 stimulation or via integrin-mediated-growth-factor activation, especially αvβ3 integrin mediating VEGF and basic fibroblast growth factor signalling (Hood and Cheresh, 2002; Stupack and Cheresh, 2004). Peptide inhibitors, such as cilengitide, and humanized monoclonal antibodies against integrins αvβ3 and α5β1 have been tested in human clinical trials for various cancers (Hood and Cheresh, 2002; Stupack and Cheresh, 2004). The role of tetraspanins in angiogenesis has been investigated in various endothelial cells and tumour models, which are summarized in Table 4.
CD151 has a major role in cancer metastasis
The involvement of CD151 in the progression of cancer metastasis is well established. Besides its role in cancer invasion and metastasis, CD151 has also been associated with other important physiological and pathological conditions; primary glomerular disease (Baleato et al., 2008), hereditary nephrotic syndrome (Karamatic Crew et al., 2004) and wound healing (Penas et al., 2000). Loss of CD151 in mice results in defects in renal function but mice were viable and fertile, similar to humans with nonsense mutations in CD151 who develop end-stage hereditary nephropathy associated with pretibial epidermolysis bullosa and sensorineural deafness (Karamatic Crew et al., 2004; Sachs et al., 2006). Increasing interest in tetraspanins has marked their importance in the area of cancer biomarker research. It is increasingly appreciated that tetraspanins are potential candidates for therapeutic targeting to develop immunotherapies, biological agents, small molecule drugs and aptamers, which will be discussed in the following sections.
CD151 is a prognostic indicator in many cancers
So far, studies of solid tumours in humans suggest that CD151 expression increases as the disease progresses, with metastatic stages having the highest CD151 levels compared to primary tumours; this is true for non-small cell lung cancer (Tokuhara et al., 2001), colon cancer (Hashida et al., 2003), Merkel cell carcinoma (Woegerbauer et al., 2010), hepatocellular carcinoma (Ke et al., 2009) and prostate cancer (Ang et al., 2004) (see Table 5). The majority of studies have found a correlation between CD151 expression and cancer invasion and have thus contributed to the understanding that CD151 is a promotor of cell motility and tumour invasion processes (Sugiura and Berditchevski, 1999; Gesierich et al., 2005). Interestingly, there have been some contradictory findings that challenge this conclusion. A study in breast carcinoma found that decreased expression of CD151, CD9 and CD63 tetraspanins was associated with a more malignant phenotype (Sauer et al., 2003a). Another finding by Lin et al., also found an inverse relationship between CD151 expression and metastatic progression in colorectal cancer patients (Lin et al., 2011). However, in these studies, the levels of CD151 expression were not indicative of the disease outcomes.
Clinical application and potential use of tetraspanins in cancer drug development
Tetraspanins are potential targets for drug development in the area of infectious disease given that many tetraspanins are known to facilitate infection processes of various pathogens, for example, viral, bacterial and protozoan infections (Hassuna et al., 2009; Green et al., 2011). Hassuna et al. summarized how pathogens exploit tetraspanins and ways in which tetraspanins could be used to prevent infections via disruption of the tetraspanin web with antibodies to tetraspanin members, knock-down of tetraspanin expression via siRNA and use of tetraspanin to traffic target drugs (Hassuna et al., 2009). It may be possible to apply the same strategies for the treatments of cancer, which is regarded as a chronic disease. The variety of technologies that can be used to molecularly target cancer cells, including immunotherapy, aptamers and RNAi (Imai and Takaoka, 2006; Chen et al., 2010b; Kohmo et al., 2010; Seigneuric et al., 2011), in combination with novel delivery systems, provides exciting possibilities for targeting specific tetraspanins in a highly selective manner (Table 6).
Table 6.
Potential clinical application of tetraspanins for the development of cancer therapeutic agents
| Therapeutic approach | Tetraspanin application | Potential use in cancer therapeutics and mechanism of action |
|---|---|---|
| Prognostic markers | Markers of prognosis and clinical outcome |
|
| Immunotherapy |
Vaccine target Potential candidates as cancer vaccine target markers |
Vaccine target
|
|
Antibody-based immunotherapy Antibody against tetraspanins |
Antibody-based immunotherapy
|
|
| Exosome | Markers of exosomes |
|
| Aptamers | Potential use of tetraspanins in nucleic acid based aptamers for cancer cell recognition, which will offer better targeted treatment in a cell-type specific manner. Potential use as a cancer therapeutic target in inhibitory aptamer in the prevention of cancer metastasis. |
|
| RNAi therapeutics | Targets of RNAi therapy |
|
Current interest in cancer immunotherapy is growing, as evidence for cancer immunosurveillance is becoming stronger. Cancer immunosurveillance suggests that the immune system can detect and destroy precursors of cancer cells to stop progression to cancer (Zitvogel et al., 2006). Moreover, immunodeficient individuals are more susceptible to cancer incidence and development (Veenbergen and van Spriel, 2011). Immunotherapy involves biological treatments that stimulates or restores the patients' immune system in order to fight disease or infection (Shih et al., 2010; von Hofe, 2011). A number of novel drugs also offer more specific treatments targeting either cancer cell surface proteins or introduce irradiated tumour cells which may lead to activation of the host immune system. In the treatment of cancer, successful responses to immunotherapy require overcoming various factors including the tumour architecture, reduced antigen presentation, the immunosuppressive tumour microenvironment, resistance to cytotoxic T lymphocyte killing and active suppression of the immune system (Davis and Cebon, 2011). Hege et al. have summarized clinical trials that combined immunotherapy with other treatments to enhance the effectiveness of anti-tumour activity and this includes the use of CD40 and Toll-like receptors for DC activation, anti-CTLA4 and anti-CD25 antibodies to inhibit down-modulation of T-cell responses, VEGF blockade to prevent the inhibitory effects of the VEGF receptor and IFN-α for promotion of immunomodulatory responses (Hege et al., 2006). Furthermore, chemotherapy (e.g. docetaxel, a cytotoxic anti-microtubule agent) and anti-androgen therapy has been combined with cancer vaccines in clinical trials of prostate cancer to test whether combination of traditional therapy with immunotherapy can enhance anti-tumour responses (Arlen et al., 2005; 2006,; Madan et al., 2008). The results were encouraging and indicated beneficial outcome for patients receiving vaccine prior to conventional therapy. The administration routes for cancer immunotherapy can be via cancer vaccines using the patient's own cancer cells, allogeneic cancer cell lines or nucleic acid-based vaccines enabling expression of cancer-specific antigens or coupled with antibody-based immunotherapy. Recently, the US. Food and Drug Administration (FDA) approved Provenge® (sipuleucel-T), autologous CD54+ cells activated with PAP-GM-CSF [a stimulant made up of a unique prostate cancer antigen, prostate acid phosphatase (PAP) and an immune cell activator GM-CSF], in the treatment of asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer (FDA, 2010). This novel cancer vaccine comprises autologous cellular immunotherapy which involves manufacturing of the patient's own personalized cancer vaccine and it is the first therapeutic cancer vaccine to be approved by the FDA (Higano et al., 2010). Development of biological agents, including mAbs are still advancing in the area of cancer therapeutics. Twelve mAbs have been approved in the treatment of cancers including non-Hodgkin's lymphoma, metastatic breast cancer, chronic lymphocytic leukaemia, acute myeloid leukaemia and metastatic colorectal cancer (Shih et al., 2010).
Tetraspanins have various roles in immune responses and play different roles in infectious diseases facilitating microbial recognition, entry and stimulation of immune responses (van Spriel and Figdor, 2010; Veenbergen and van Spriel, 2011). CD81 is a co-receptor of the important human pathogen, hepatitis C virus (Pileri et al., 1998) and CD9 binds to diphtheria toxin (Cha, 2000). These roles of tetraspanins in activation of the immune system and antigen recognition are favourable indications for their potential use in cancer treatments of cancer biomarkers that is. for uses in therapeutic delivery vehicles such as RNA interference (RNAi), aptamers and exosomes. Potential clinical applications of tetraspanins and drug development strategies are summarized in Table 6.
Potential therapeutic benefits and limitations
Given the clinical heterogeneity of cancers, it is currently difficult to establish uniform and optimal diagnostic screening and treatment regimens for individual patients (Fidler, 2002). With the limitations of current approaches, researchers are searching for biomarkers that can more accurately indicate the prognosis of individual cases and thus lead to better personalized treatment options. This aim is very challenging and it is unlikely that any single biomarker will, by itself, be adequate for treatment decisions (Shariat et al., 2007).
We have recently reviewed new drugs targeting different aspects of prostate cancer development and potential biomarkers that have made it to clinical trials (Detchokul and Frauman, 2011). These drugs were administered in conjunction with traditional systemic therapies and targeted biomarkers were categorized into five therapeutic approaches; prostate cancer vaccines, epigenetic therapies, pro-apoptotic agents, prostate cancer antibodies and anti-angiogenesis approaches. These approaches have not exhibited therapeutic benefits over the mainstream cytotoxic treatments in prostate cancer due mainly to the participants recruited having developed castration-resistant prostate cancer. The beneficial outcome of these trials may become more obvious with patients with earlier stages of prostate cancer. The review emphasized the importance of biomarker targets and their potential use in the treatment of prostate cancer. A number of novel drugs also offer more specific treatment targeting either prostate cancer cell surface proteins or introduction of irradiated tumour cells which may lead to activation of the host immune system. The obvious benefits of targeted therapeutics would be the potential for minimization of adverse effects, hence better quality of life for patients. Although none of the drugs reviewed specifically targeted migration, it is undeniable that cell motility is at the heart of cancer invasion and dissemination, the most common cause of cancer morbidity and mortality. Notably, the successful story of cancer biomarkers is Her2/Neu in breast cancer. Trastuzumab, mAb to Her2/Neu, has been used in the treatment of Her2+ breast cancer patients in addition to first-line therapy and was found to prolong disease progression (Slamon et al., 2001; Baselga et al., 2012). This has encouraged more emphasis on individualization in cancer therapy.
Targeting cell migration in modifying or preventing metastasis is still in its infancy. Comprehensive reviews of tumour cell motility targets in metastasis treatment have been published recently (Palmer et al., 2011; Thiolloy and Rinker-Schaeffer, 2011). They have drawn attention to the increasing interest in cell migration research in cancer metastasis in the past two decades. Tetraspanins are known for their roles in tumour cell motility and invasion but their potential influence on tumour progression is still not well understood. Because cancer research has shifted from focusing mainly on the tumour itself to include the tumour's interaction with its microenvironment, cancer biomarkers not only are an indication of tumour origins but also of disease progression. Tetraspanins, having a role in two characteristics of cancer invasion/metastasis and angiogenesis, are possible biomarkers of tumour progression and therapeutic targets which may allow for more personalised therapy in the future.
Conclusions and future directions
The importance of tetraspanins in different aspects of cancer metastasis is integrated in this review. In particular, CD151 is a marker of disease progression clinically and encompasses diverse regulatory roles in the metastatic process. Inhibition of in vitro and in vivo motility and metastasis in various experimental settings highlight the major role of CD151 in the control of signalling complexes that drive cancer metastasis. CD151 also influences the localization and organization of its partner proteins, which are important determinants of metastatic behaviour in many cancers. The clinical applications of tetraspanin modulation are not limited to direct targeting via RNAi or antibodies, but will also be relevant to new classes of therapies, for example, biological agents, aptamers and exosomes. Tetraspanins are certainly a potential therapeutic target, which as cell surface molecules, can be targeted as a preventative and/or palliative strategy in cancer treatments.
Acknowledgments
S. D. is supported by the University of Melbourne Early Career Researcher grant and Austin Medical Research Foundation. This work was also supported by grant funding to EDW and a fellowship to M. W. P. from the National Health & Medical Research Council of Australia. We also acknowledge support through the Victorian Government's Operational Infrastructure Support Program.
Glossary
- EC2
large extracellular loop
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- MT1-MMP
membrane-type 1 MMP
- PI3-K
phosphoinositide 3-kinase
- PI4-K
PI-4-kinase
- RNAi
interference RNA
- TME
tumour microenvironment
Conflict of interest
We certify that there is no conflict of interest with any financial organizations regarding the materials discussed in the manuscript.
References
- Abe M, Sugiura T, Takahashi M, Ishii K, Shimoda M, Shirasuna K. A novel function of CD82/KAI-1 on E-cadherin-mediated homophilic cellular adhesion of cancer cells. Cancer Lett. 2008;266:163–170. doi: 10.1016/j.canlet.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Adachi M, Taki T, Ieki Y, Huang CL, Higashiyama M, Miyake M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996;56:1751–1755. [PubMed] [Google Scholar]
- Andre F, Schartz NEC, Movassagh M, Flament C, Pautier P, Morice P, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- Ang J, Lijovic M, Ashman LK, Kan K, Frauman AG. CD151 protein expression predicts the clinical outcome of low-grade primary prostate cancer better than histologic grading: a new prognostic indicator? Cancer Epidemiol Biomarkers Prev. 2004;13:1717–1721. [PubMed] [Google Scholar]
- Ang J, Fang BL, Ashman LK, Frauman AG. The migration and invasion of human prostate cancer cell lines involves CD151 expression. Oncol Rep. 2010;24:1593–1597. doi: 10.3892/or_00001022. [DOI] [PubMed] [Google Scholar]
- Arencibia JM, Martin S, Perez-Rodriguez FJ, Bonnin A. Gene expression profiling reveals overexpression of TSPAN13 in prostate cancer. Int J Oncol. 2009;34:457–463. [PubMed] [Google Scholar]
- Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RL, Herbert JM, Khan K, Heath VL, Bicknell R, Tomlinson MG. The emerging role of tetraspanin microdomains on endothelial cells. Biochem Soc Trans. 2011;39:1667–1673. doi: 10.1042/BST20110745. [DOI] [PubMed] [Google Scholar]
- Baleato RM, Guthrie PL, Gubler MC, Ashman LK, Roselli S. Deletion of Cd151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol. 2008;173:927–937. doi: 10.2353/ajpath.2008.071149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Zhang YH, Zhang F, Wang NX, Stipp CS, Zheng JJ, et al. Transmembrane interactions are needed for KAI1/CD82-mediated suppression of cancer invasion and metastasis. Am J Pathol. 2009;174:647–660. doi: 10.2353/ajpath.2009.080685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Guo Q, Xia B, Zhang YH, Giesert EE, Levy S, et al. Tetraspanins regulate the protrusive activities of cell membrane. Biochem Biophys Res Commun. 2011;415:619–626. doi: 10.1016/j.bbrc.2011.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro O, Zamai M, Yáñez-Mó M, Tejera E, López-Romero P, Monk PN, et al. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol. 2008;183:527–542. doi: 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom RA, García-Heras J, Hsieh CL, Gerhard DS, Jones C, Francke U, et al. Localization of the photoreceptor gene ROM1 to human chromosome 11 and mouse chromosome 19: sublocalization to human 11q13 between PGA and PYGM. Am J Hum Genet. 1992;51:1028–1035. [PMC free article] [PubMed] [Google Scholar]
- Bascom RA, Liu L, Heckenlively JR, Stone EM, Mcinnes RR. Mutation analysis of the ROM1 gene in retinitis pigmentosa. Hum Mol Genet. 1995;4:1895–1902. doi: 10.1093/hmg/4.10.1895. [DOI] [PubMed] [Google Scholar]
- Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass R, Werner F, Odintsova E, Sugiura T, Berditchevski F, Ellis V. Regulation of urokinase receptor proteolytic function by the tetraspanin CD82. J Biol Chem. 2005;280:14811–14818. doi: 10.1074/jbc.M414189200. [DOI] [PubMed] [Google Scholar]
- Bassani S, Cingolani LA. Tetraspanins: interactions and interplay with integrins. Int J Biochem Cell Biol. 2012;44:703–708. doi: 10.1016/j.biocel.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- Berditchevski F, Odintsova E. Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signalling. J Cell Biol. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Odintsova E. Tetraspanins as regulators of protein trafficking. Traffic. 2007;8:89–96. doi: 10.1111/j.1600-0854.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- Berditchevski F, Zutter MM, Hemler ME. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins) Mol Biol Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Gilbert E, Griffiths MR, Fitter S, Ashman L, Jenner SJ. Analysis of the CD151 center dot alpha(3)beta(1) integrin and CD151 center dot tetraspanin interactions by mutagenesis. J Biol Chem. 2001;276:41165–41174. doi: 10.1074/jbc.M104041200. [DOI] [PubMed] [Google Scholar]
- Bienstock RJ, Barrett JC. KAI1, A prostate metastasis suppressor: prediction of solvated structure and interactions with binding partners; integrins, cadherins, and cell-surface receptor proteins. Mol Carcinog. 2001;32:139–153. doi: 10.1002/mc.1073. [DOI] [PubMed] [Google Scholar]
- Bijman MNA, van Berkel MPA, van Nieuw Amerongen GP, Boven E. Interference with actin dynamics is superior to disturbance of microtubule function in the inhibition of human ovarian cancer cell motility. Biochem Pharmacol. 2008;76:707–716. doi: 10.1016/j.bcp.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Blain JC, Mok Y-F, Kubanek J, Allingham JS. Two molecules of lobophorolide cooperate to stabilize an actin dimer using both their ‘ring’ and ‘tail’ region. Chem Biol. 2010;17:802–807. doi: 10.1016/j.chembiol.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Boismenu R, Rhein M, Fischer WH, Havran WL. A role for CD81 in early T cell development. Science. 1996;271:198–200. doi: 10.1126/science.271.5246.198. [DOI] [PubMed] [Google Scholar]
- Bono AV, Celato N, Cova V, Salvadore M, Chinetti S, Novario R. Microvessel density in prostate carcinoma. Prostate Cancer Prostatic Dis. 2002;5:123–127. doi: 10.1038/sj.pcan.4500572. [DOI] [PubMed] [Google Scholar]
- Borghi N, Lowness M, Maruthamuthu V, Gardel ML, Nelson WJ. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc Natl Acad Sci U S A. 2010;107:13199–13200. doi: 10.1073/pnas.1002662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998;78:940–944. doi: 10.1038/bjc.1998.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–1205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucheix C, Soria C, Mirshahi M, Soria J, Perrot JY, Fournier N, et al. Characteristics of platelet aggregation induced by the monoclonal antibody ALB6 (acute lymphoblastic leukemia antigen p 24): inhibition of aggregation by ALB6Fab. FEBS Lett. 1983;161:289–295. doi: 10.1016/0014-5793(83)81027-8. [DOI] [PubMed] [Google Scholar]
- Boucheix C, Duc GHT, Jasmin C, Rubinstein E. Tetraspanins and malignancy. Expert Rev Mol Med. 2001;1:1–17. doi: 10.1017/S1462399401002381. [DOI] [PubMed] [Google Scholar]
- Boyer B, Valles AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000;60:1091–1099. doi: 10.1016/s0006-2952(00)00427-5. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockbank EC, Bridges J, Marshall CJ, Sahai E. Integrin beta 1 is required for the invasive behaviour but not proliferation of squamous cell carcinoma cells in vivo. Br J Cancer. 2005;92:102–112. doi: 10.1038/sj.bjc.6602255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buim MEC, Lourenço SV, Carvalho KC, Cardim R, Pereira C, Carvalho AL, et al. Downregulation of CD9 protein expression is associated with aggressive behavior of oral squamous cell carcinoma. Oral Oncol. 2010;46:166–171. doi: 10.1016/j.oraloncology.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, Chiaur DS, Guardavaccaro D, Parks W, Vidal M, Pagano M. Identification of a family of human F-box proteins. Curr Biol. 1999;9:1177–1179. doi: 10.1016/S0960-9822(00)80020-2. [DOI] [PubMed] [Google Scholar]
- Cha JH, Brooke JS, Ivey KN, Eidels L. Cell surface monkey CD9 antigen is a coreceptor that increases diphtheria toxin sensitivity and diphtheria toxin receptor affinity. J Biol Chem. 2000;275:6901–6907. doi: 10.1074/jbc.275.10.6901. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- Chambrion C, Le Naour F. The tetraspanins CD9 and CD81 regulate CD9P1-induced effects on cell migration. PLoS ONE. 2010;5:e11219. doi: 10.1371/journal.pone.0011219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SC, Vinayek N, Maher DM, Bell MC, Dunham KA, Koch MD, et al. Combined staining of TAG-72, MUC1, and CA125 improves labeling sensitivity in ovarian cancer: antigens for multi-targeted antibody-guided therapy. J Histochem Cytochem. 2007;55:867–875. doi: 10.1369/jhc.7A7213.2007. [DOI] [PubMed] [Google Scholar]
- Chen H, Dziuba N, Friedrich B, Lindern JV, Murray JL, Rojo DR, et al. A critical role for CD63 in HIV replication and infection of macrophages and cell lines. Virology. 2008a;379:191–196. doi: 10.1016/j.virol.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yuan D, Wang GL, Wang Y, Wu YY, Zhu J. Clinicopathological significance of expression of Tspan-1, Jab1 and p27 in human hepatocellular carcinoma. J Korean Med Sci. 2010a;25:1438–1442. doi: 10.3346/jkms.2010.25.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li X, Wang G-L, Wang Y, Zhu Y-Y, Zhu J. Clinicopathological significance of overexpression of TSPAN1, Ki67 and CD34 in gastric carcinoma. Tumori. 2008b;94:531–538. doi: 10.1177/030089160809400415. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhu Y-Y, Zhang X-J, Wang G-L, Li X-Y, He S, et al. TSPAN1 protein expression: a significant prognostic indicator for patients with colorectal adenocarcinoma. World J Gastroenterol. 2009;15:2270–2276. doi: 10.3748/wjg.15.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yuan D, Zhao R, Li H, Zhu J. Suppression of TSPAN1 by RNA interference inhibits proliferation and invasion of colon cancer cells in vitro. Tumori. 2010b;96:744–750. doi: 10.1177/030089161009600517. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhu YY, Li H, Wang GL, Wu YY, Lu YX, et al. Knockdown of TSPAN1 by RNA silencing and antisense technique inhibits proliferation and infiltration of human skin squamous carcinoma cells. Tumori. 2010c;96:289–295. doi: 10.1177/030089161009600217. [DOI] [PubMed] [Google Scholar]
- Chen SL, Sun YX, Jin ZG, Jing XH. Functional and biochemical studies of CD9 in fibrosarcoma cell line. Mol Cell Biochem. 2011a;350:89–99. doi: 10.1007/s11010-010-0685-1. [DOI] [PubMed] [Google Scholar]
- Chen Z, Mustafa T, Trojanowicz B, Brauckhoff M, Gimm O, Schmutzler C, et al. CD82, and CD63 in thyroid cancer. Int J Mol Med. 2004;14:517–527. [PubMed] [Google Scholar]
- Chen Z, Gu S, Trojanowicz B, Liu N, Zhu G, Dralle H, et al. Down-regulation of TM4SF is associated with the metastatic potential of gastric carcinoma TM4SF members in gastric carcinoma. World J Surg Oncol. 2011b;9 doi: 10.1186/1477-7819-9-43. doi: 10.1186/1477-7819-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyomaru T, Tatarano S, Kawakami K, Enokida H, Yoshino H, Nohata N, et al. SWAP70, actin-binding protein, function as an oncogene targeting tumor suppressive miR-145 in prostate cancer. Prostate. 2011;71:1559–1567. doi: 10.1002/pros.21372. [DOI] [PubMed] [Google Scholar]
- Chometon G, Zhang Z, Rubinstein E, Boucheix C, Mauch C, Aumailley M. Dissociation of the complex between CD151 and laminin-binding integrins permits migration of epithelial cells. Exp Cell Res. 2006;312:983–995. doi: 10.1016/j.yexcr.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Clark KL, Oelke A, Johnson ME, Eilert KD, Simpson PC, Todd SC. CD81 associates with 14-3-3 in a redox-regulated palmitoylation-dependent manner. J Biol Chem. 2004;279:19401–19406. doi: 10.1074/jbc.M312626200. [DOI] [PubMed] [Google Scholar]
- Cress AE, Rabinovitz I, Zhu WG, Nagle RB. The alpha-6-beta-1 and alpha-6-beta-4 integrins in human prostate-cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- Dang Z, Yagi K, Oku Y, Kouguchi H, Kajino K, Watanabe J, et al. Evaluation of Echinococcus multilocularis tetraspanins as vaccine candidates against primary alveolar echinococcosis. Vaccine. 2009;27:7339–7345. doi: 10.1016/j.vaccine.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Davis ID, Cebon JS. Developing cancer immunotherapies. Asia Pac J Clin Oncol. 2011;7:9–13. [Google Scholar]
- De Bruyne E, Andersen TL, De Raeve H, Van Valckenborgh E, Caers J, Van Camp B, et al. Endothelial cell-driven regulation of CD9 or motility-related protein-1 expression in multiple myeloma cells within the murine 5T33MM model and myeloma patients. Leukemia. 2006;20:1870–1879. doi: 10.1038/sj.leu.2404343. [DOI] [PubMed] [Google Scholar]
- Deng X, Li Q, Hoff J, Novak M, Yang H, Jin H, et al. Integrin-associated CD151 drives ErbB2-evoked mammary tumor onset and metastasis. Neoplasia. 2012;14:678–689. doi: 10.1593/neo.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HFG, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Detchokul S, Frauman AG. Recent developments in prostate cancer biomarker research: therapeutic implications. Br J Clin Pharmacol. 2011;71:157–174. doi: 10.1111/j.1365-2125.2010.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devbhandari RP, Shi G-M, Ke A-W, Wu F-Z, Huang X-Y, Wang X-Y, et al. Profiling of the tetraspanin CD151 web and conspiracy of CD151/integrin β1 complex in the progression of hepatocellular carcinoma. PLoS ONE. 2011;6:e24901. doi: 10.1371/journal.pone.0024901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer D, Stragies R, et al. Inhibition of inflammatory lymphangiogenesis by integrin alpha 5 blockade. Am J Pathol. 2007;171:361–372. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Jimenez C, Yanez-Mo M, Carreira A, Tejedor R, Gonzalez-Amaro R, Alvarez V, et al. Involvement of alpha 3 integrin/tetraspanins complexes in the angiogenic response induced by angiotensin II. FASEB J. 2001;15:1457–1459. doi: 10.1096/fj.00-0651fje. [DOI] [PubMed] [Google Scholar]
- Dong J, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884–886. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- Donoso LA, Folberg R, Edelberg K, Arbizo V, Atkinson B, Herlyn M. Tissue distribution and biochemical properties of an ocular melanoma-associated antigen. J Histochem Cytochem. 1985;33:1190–1196. doi: 10.1177/33.12.3905953. [DOI] [PubMed] [Google Scholar]
- Donoso LA, Felberg NT, Edelberg K, Borlinghaus P, Herlyn M. Metastatic uveal melanoma: an ocular melanoma associated antigen in the serum of patients with metastatic disease. J Immunoassay. 1986;7:273–283. doi: 10.1080/01971528608060472. [DOI] [PubMed] [Google Scholar]
- Dornier E, Coumailleau F, Ottavi JF, Moretti J, Boucheix C, Mauduit P, et al. TspanC8 tetraspanins regulate ADAM10/Kuzbanian trafficking and promote Notch activation in flies and mammals. J Cell Biol. 2012;199:481–496. doi: 10.1083/jcb.201201133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber P, Vonkova I, Stepanek O, Hrdinka M, Kucova M, Skopcova T, et al. SCIMP, a transmembrane adaptor protein involved in major histocompatibility complex class II signaling. Mol Cell Biol. 2011;31:4550–4562. doi: 10.1128/MCB.05817-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, Hahn LB, Kajiwara K, Berson EL. Dominant and digenic mutations in the peripherin/RDS and ROM1 genes in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1997;38:1972–1982. [PubMed] [Google Scholar]
- Duffield A, Kamsteeg EJ, Brown AN, Pagel P, Caplan MJ. The tetraspanin CD63 enhances the internalization of the H,K-ATPase beta-subunit. Proc Natl Acad Sci U S A. 2003;100:15560–15565. doi: 10.1073/pnas.2536699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Esposito CL, Passaro D, Longobardo I, Condorelli G, Marotta P, Affuso A, et al. A neutralizing RNA aptamer against EGFR causes selective apoptotic cell death. PLoS ONE. 2011;6:e24071. doi: 10.1371/journal.pone.0024071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhzad OC, Jon SY, Khademhosseini A, Tran TNT, LaVan DA, Langer R. Nanopartide-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res. 2004;64:7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- FDA. 2003;2012 FDA label information for Gleevec, Vol.: FDA. [Google Scholar]
- FDA. 2004;2012 FDA label information for Avastin, Vol.: FDA. [Google Scholar]
- FDA. 2005;2012 FDA label information for Nexavar, Vol.: FDA. [Google Scholar]
- FDA. 2006;2012 FDA label information for Sutent, Vol.: FDA. [Google Scholar]
- FDA. 2009a;2012 FDA label information for Votrient, Vol.: FDA. [Google Scholar]
- FDA. 2009b;2012 FDA label information of Afinitor, Vol.: FDA. [Google Scholar]
- FDA. 2010. FDA label information of Provenge: FDA.
- Fei Y, Wang J, Liu W, Zuo H, Qin J, Wang D, et al. CD151 promotes cancer cell metastasis via integrins α3β1 and α6β1 in vitro. Mol Med Rep. 2012;6:1226–1230. doi: 10.3892/mmr.2012.1095. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B, O'Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenteany G, Zhu S. Small-molecule inhibitors of actin dynamics and cell motility. Curr Top Med Chem. 2003;3:593–616. doi: 10.2174/1568026033452348. [DOI] [PubMed] [Google Scholar]
- Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, et al. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51:161–167. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- Fitter S, Seldin MF, Ashman LK. Characterisation of the mouse homologue of CD151 (PETA-3/SFA-1); genomic structure, chromosomal localisation and identification of 2 novel splice forms. Biochim Biophys Acta. 1998;1398:75–85. doi: 10.1016/s0167-4781(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Fojo T. Can mutations in γ-actin modulate the toxicity of microtubule targeting agents? J Natl Cancer Inst. 2006;98:1345–1347. doi: 10.1093/jnci/djj408. [DOI] [PubMed] [Google Scholar]
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T, Tachibana I, Hoshida Y, Kimura H, Takeda Y, Kijima T, et al. Expression of tetraspanins in human lung cancer cells: frequent downregulation of CD9 and its contribution to cell motility in small cell lung cancer. Oncogene. 2003;22:674–687. doi: 10.1038/sj.onc.1206106. [DOI] [PubMed] [Google Scholar]
- Furuya M, Kato F, Nishimura N, Ishiwata I, Ikeda H, Ito R, et al. Down-regulation of CD9 in human ovarian carcinoma cell might contribute to peritoneal dissemination: morphologic alteration and reduced expression of beta 1 integrin subsets. Cancer Res. 2005;65:2617–2625. doi: 10.1158/0008-5472.CAN-04-3123. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, et al. Integrin alpha 4 beta 1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010;70:3042–3051. doi: 10.1158/0008-5472.CAN-09-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartlan KH, Belz GT, Tarrant JM, Minigo G, Katsara M, Sheng KC, et al. A complementary role for the tetraspanins CD37 and Tssc6 in cellular immunity. J Immunol. 2010;185:3158–3166. doi: 10.4049/jimmunol.0902867. [DOI] [PubMed] [Google Scholar]
- Gesierich S, Berezovskiy I, Ryschich E, Zöller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. doi: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- Gesierich S, Paret C, Hildebrand D, Weitz J, Zgraggen K, Schmitz-Winnenthal FH, et al. Colocalization of the tetraspanins, CO-029 and CD151, with integrins in human pancreatic adenocarcinoma: impact on cell motility. Clin Cancer Res. 2005;11:2840–2852. doi: 10.1158/1078-0432.CCR-04-1935. [DOI] [PubMed] [Google Scholar]
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golias C, Charalabopoulos A, Stagikas D, Giannakopoulos X, Pescho D, Batistatou A, et al. Molecular profiling and genomic microarrays in prostate cancer. Exp Oncol. 2007;29:82–84. [PubMed] [Google Scholar]
- Greco C, Bralet MP, Ailane N, Dubart-Kupperschmitt A, Rubinstein E, Le Naour F, et al. E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Res. 2010;70:7674–7683. doi: 10.1158/0008-5472.CAN-09-4482. [DOI] [PubMed] [Google Scholar]
- Green LR, Monk PN, Partridge LJ, Morris P, Gorringe AR, Read RC. Cooperative role for tetraspanins in adhesin-mediated attachment of bacterial species to human epithelial cells. Infect Immun. 2011;79:2241–2249. doi: 10.1128/IAI.01354-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Xia B, Zhang F, Richardson MM, Li M, Zhang JS, et al. Tetraspanin CO-029 inhibits colorectal cancer cell movement by deregulating cell-matrix and cell-cell adhesions. PLoS ONE. 2012;7:e38464. doi: 10.1371/journal.pone.0038464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson-Wagner E, Stipp CS. The CD9/CD81 tetraspanin complex and tetraspanin CD151 regulate alpha3beta1 integrin-dependent tumor cell behaviors by overlapping but distinct mechanisms. PLoS ONE. 2013;8:e61834. doi: 10.1371/journal.pone.0061834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeuw JF, Goetsch L, Bailly C, Corvaia N. Tetraspanin CD151 as a target for antibody-based cancer immunotherapy. Biochem Soc Trans. 2011;39:553–558. doi: 10.1042/BST0390553. [DOI] [PubMed] [Google Scholar]
- Haining EJ, Yang J, Bailey RL, Khan K, Collier R, Tsai S, et al. The TspanC8 subgroup of tetraspanins interacts with A disintegrin and metalloprotease 10 (ADAM10) and regulates its maturation and cell surface expression. J Biol Chem. 2012;287:39753–39765. doi: 10.1074/jbc.M112.416503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Moyana T, Xiang J. Cancer immunotherapy by exosome-based vaccines. Cancer Biother Radiopharm. 2007;22:692–703. doi: 10.1089/cbr.2007.368-R. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Nomura T, Kishimoto K, Yanagisawa K, Fujita S. SFA-1/PETA-3 (CD151), a member of the transmembrane 4 superfamily, associates preferentially with α5β1 integrin and regulates adhesion of human T cell leukemia virus type 1- infected T cell to fibronectin. J Immunol. 1998;161:3087–3095. [PubMed] [Google Scholar]
- Hasegawa M, Furuya M, Kasuya Y, Nishiyama M, Sugiura T, Nikaido T, et al. CD151 dynamics in carcinoma-stroma interaction: integrin expression, adhesion strength and proteolytic activity. Lab Invest. 2007;87:882–892. doi: 10.1038/labinvest.3700657. [DOI] [PubMed] [Google Scholar]
- Hashida H, Takabayashi A, Tokuhara T, Hattori N, Taki T, Hasegawa H, et al. Clinical significance of transmembrane 4 superfamily in colon cancer. Br J Cancer. 2003;89:158–167. doi: 10.1038/sj.bjc.6601015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassuna N, Monk PN, Moseley GW, Partridge LI. Strategies for targeting tetraspanin proteins. BioDrugs. 2009;23:341–359. doi: 10.2165/11315650-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hato T, Ikeda K, Yasukawa M, Watanabe A, Kobayashi Y. Exposure of platelet fibrinogen receptors by a monoclonal antibody to CD9 antigen. Blood. 1988;72:224–229. [PubMed] [Google Scholar]
- Hege KM, Jooss K, Pardoll D. GM-CSF gene-modifed cancer cell immunotherapies: of mice and men. Int Rev Immunol. 2006;25:321–352. doi: 10.1080/08830180600992498. [DOI] [PubMed] [Google Scholar]
- Heikens MJ, Cao TM, Morita C, DeHart SL, Tsai S. Penumbra encodes a novel tetraspanin that is highly expressed in erythroid progenitors and promotes effective erythropoiesis. Blood. 2007;109:3244–3252. doi: 10.1182/blood-2006-09-046672. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Specific tetraspanin functions. J Cell Biol. 2001;155:1103–1107. doi: 10.1083/jcb.200108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Targeting of tetraspanin proteins – potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–758. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME, Mannion BA, Berditchevski F. Association of TM4SF proteins with integrins: relevance to cancer. Biochim Biophys Acta. 1996;1287:67–71. doi: 10.1016/0304-419x(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Hicke BJ, Stephens AW. Escort aptamers: a delivery service for diagnosis and therapy. J Clin Invest. 2000;106:923–928. doi: 10.1172/JCI11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, et al. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- Higashiyama M, Doi O, Kodama K, Yokouchi H, Adachi M, Huang C-L, et al. Immunohistochemically detected expression of motility-related protein-1 (MRP-1/CD9) in lung adenocarcinoma and its relation to prognosis. Int J Cancer. 1997;74:205–211. doi: 10.1002/(sici)1097-0215(19970422)74:2<205::aid-ijc12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Higginbottom A, Takahashi Y, Bolling L, Coonrod SA, White JM, Partridge LJ, et al. Structural requirements for the inhibitory action of the CD9 large extracellular domain in sperm/oocyte binding and fusion. Biochem Biophys Res Commun. 2003;311:208–214. doi: 10.1016/j.bbrc.2003.09.196. [DOI] [PubMed] [Google Scholar]
- Hirano C, Nagata M, Noman AA, Kitamura N, Ohnishi M, Ohyama T, et al. Tetraspanin gene expression levels as potential biomarkers for malignancy of gingival squamous cell carcinoma. Int J Cancer. 2009;124:2911–2916. doi: 10.1002/ijc.24297. [DOI] [PubMed] [Google Scholar]
- von Hofe E. A new ally against cancer. Sci Am. 2011;305:66–70. doi: 10.1038/scientificamerican1011-66. [DOI] [PubMed] [Google Scholar]
- Hong H, Goel S, Zhang Y, Cai W. Molecular imaging with nucleic acid aptamers. Curr Med Chem. 2011;18:4195–4205. doi: 10.2174/092986711797189691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong I, Jin Y, Byun H, Jeoung D, Kim Y, Lee H. Homophilic interactions of tetraspanin CD151 up-regulate motility and matrix metalloproteinase-9 expression of human melanoma cells through adhesion-dependent c-Jun activation signaling pathway. J Biol Chem. 2006;281:24279–24292. doi: 10.1074/jbc.M601209200. [DOI] [PubMed] [Google Scholar]
- Hong IK, Kim YM, Jeoung DI, Kim KC, Lee H. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp Mol Med. 2005;37:230–239. doi: 10.1038/emm.2005.31. [DOI] [PubMed] [Google Scholar]
- Hong IK, Jeoung DI, Ha KS, Kim YM, Lee H. Tetraspanin CD151 stimulates adhesion-dependent activation of Ras, Rac, and Cdc42 by facilitating molecular association between β1 integrins and small GTPases. J Biol Chem. 2012;287:32027–32039. doi: 10.1074/jbc.M111.314443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Hori H, Yano SJ, Koufuji K, Takeda J, Shirouzu K. CD9 expression in gastric cancer and its significance. J Surg Res. 2004;117:208–215. doi: 10.1016/j.jss.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Houle CD, Ding X-Y, Foley JF, Afshari CA, Barrett JC, Davis BJ. Loss of expression and altered localization of KAI1 and CD9 protein are associated with epithelial ovarian cancer progression. Gynecol Oncol. 2002;86:69–78. doi: 10.1006/gyno.2002.6729. [DOI] [PubMed] [Google Scholar]
- Hu CCA, Liang FX, Zhou G, Tu L, Tang CHA, Zhou J, et al. Assembly of urothelial plaques: tetraspanin function in membrane protein trafficking. Mol Biol Cell. 2005;16:3937–3950. doi: 10.1091/mbc.E05-02-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Taki T, Adachi M, Yagita M, Sawada S, Takabayashi A, et al. MRP-1/CD9 and KAI1/CD82 expression in normal and various cancer tissues. Int J Oncol. 1997;11:1045–1051. doi: 10.3892/ijo.11.5.1045. [DOI] [PubMed] [Google Scholar]
- Huang CL, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M. Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol. 1998;153:973–983. doi: 10.1016/s0002-9440(10)65639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Sossey-Alaoui K, Beachy SH, Geradts J. The tetraspanin superfamily member NET-6 is a new tumor suppressor gene. J Cancer Res Clin Oncol. 2007;133:761–769. doi: 10.1007/s00432-007-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XY, Ke AW, Shi GM, Ding ZB, Devbhandari RP, Gu FM, et al. Overexpression of CD151 as an adverse marker for intrahepatic cholangiocarcinoma patients. Cancer. 2010;116:5440–5451. doi: 10.1002/cncr.25485. [DOI] [PubMed] [Google Scholar]
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeyama S, Koyama M, Yamaoko M, Sasada R, Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med. 1993;177:1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- Imai T, Fukudome K, Takagi S, Nagira M, Furuse M, Fukuhara N, et al. C33 antigen recognized by monoclonal antibodies inhibitory to human T cell leukemia virus type 1-induced syncytium formation is a member of a new family of transmembrane proteins including CD9, CD37, CD53, and CD63. J Immunol. 1992;149:2879–2886. [PubMed] [Google Scholar]
- Inoue G, Horiike N, Onji M. The CD81 expression in liver in hepatocellular carcinoma. Int J Mol Med. 2001;7:67–71. doi: 10.3892/ijmm.7.1.67. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Ding L, Ikenaka K, Inoue Y, Miyado K, Mekada E, et al. Tetraspanin protein CD9 is a novel paranodal component regulating paranodal junctional formation. J Neurosci. 2004;24:96–102. doi: 10.1523/JNEUROSCI.1484-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Whelan RDH, Watson R, Parker PJ. PKCε controls the traffic of β1 integrins in motile cells. EMBO J. 2002;21:3608–3619. doi: 10.1093/emboj/cdf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai K, Ishii M, Ohshima S, Miyatake K, Saeki Y. Expression and function of transmembrane-4 superfamily (tetraspanin) proteins in osteoclasts: reciprocal roles of tspan-5 and NET-6 during osteoclastogenesis. Allergol Int. 2007;56:457–463. doi: 10.2332/allergolint.O-07-488. [DOI] [PubMed] [Google Scholar]
- Iwata S, Kobayashi H, Miyake-Nishijima R, Sasaki T, Souta-Kuribara A, Nori M, et al. Distinctive signaling pathways through CD82 and beta 1 integrins in human T cells. Eur J Immunol. 2002;32:1328–1337. doi: 10.1002/1521-4141(200205)32:5<1328::AID-IMMU1328>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Jang HI, Lee H. A decrease in the expression of CD63 tetraspanin protein elevates invasive potential of human melanoma cells. Exp Mol Med. 2003;35:317–323. doi: 10.1038/emm.2003.43. [DOI] [PubMed] [Google Scholar]
- Jee BK, Lee JY, Lim Y, Lee KH, Jo Y-H. Effect of KAI1/CD82 on the β1 integrin maturation in highly migratory carcinoma cells. Biochem Biophys Res Commun. 2007;359:703–708. doi: 10.1016/j.bbrc.2007.05.159. [DOI] [PubMed] [Google Scholar]
- Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Winterwood N, DeMali KA, Stipp CS. Tetraspanin CD151 regulates RhoA activation and the dynamic stability of carcinoma cell-cell contacts. J Cell Sci. 2009;122:2263–2273. doi: 10.1242/jcs.045997. [DOI] [PubMed] [Google Scholar]
- Jones A, Fujiyama C. Angiogenesis in urological malignancy: prognostic indicator and therapeutic target. BJU Int. 1999;83:535–556. doi: 10.1046/j.1464-410x.1999.00018.x. [DOI] [PubMed] [Google Scholar]
- Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, et al. TSPAN12 regulates retinal vascular development by promoting norrin- but not Wnt-induced FZD4/β-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Kamisasanuki T, Tokushige S, Terasaki H, Khai NC, Wang Y, Sakamoto T, et al. Targeting CD9 produces stimulus-independent antiangiogenic effects predominantly in activated endothelial cells during angiogenesis: a novel antiangiogenic therapy. Biochem Biophys Res Commun. 2011;413:128–135. doi: 10.1016/j.bbrc.2011.08.068. [DOI] [PubMed] [Google Scholar]
- Kanetaka K, Sakamoto M, Yamamoto Y, Yamasaki S, Lanza F, Kanematsu T, et al. Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J Hepatol. 2001;35:637–642. doi: 10.1016/s0168-8278(01)00183-0. [DOI] [PubMed] [Google Scholar]
- Kaprielian Z, Patterson PH. Surface and cytoskeletal markers of rostrocaudal position in the mammalian nervous system. J Neurosci. 1993;13:2495–2508. doi: 10.1523/JNEUROSCI.13-06-02495.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamatic Crew V, Burton N, Kagan A, Green CA, Levene C, Flinter F, et al. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assemly of human basement membranes in kidney and skin. Blood. 2004;104:2217–2223. doi: 10.1182/blood-2004-04-1512. [DOI] [PubMed] [Google Scholar]
- Kazarov AR, Yang XW, Stipp CS, Sehgal B, Hemler ME. An extracellular site on tetraspanin CD151 determines alpha 3 and alpha 6 integrin-dependent cellular morphology. J Cell Biol. 2002;158:1299–1309. doi: 10.1083/jcb.200204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu MY, et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology. 2009;49:491–503. doi: 10.1002/hep.22639. [DOI] [PubMed] [Google Scholar]
- Ke AW, Shi GM, Zhou J, Huang XY, Shi YH, Ding ZB, et al. CD151 amplifies signaling by integrin alpha 6 beta 1 to PI3K and induces the epithelial-mesenchymal transition in HCC cells. Gastroenterology. 2011;140:1629–1641. doi: 10.1053/j.gastro.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Kischel P, Bellahcene A, Deux B, Lamour V, Dobson R, De Pauw E, et al. Overexpression of CD9 in human breast cancer cells promotes the development of bone metastases. Anticancer Res. 2012;32:5211–5220. [PubMed] [Google Scholar]
- Kitadokoro K, Bordo D, Galli G, Petracca R, Falugi F, Abrignani S, et al. CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs. EMBO J. 2001;20:12–18. doi: 10.1093/emboj/20.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Soyer C, Azorsa DO, Cazenave J-P, Lanza F. CD9 participates in endothelial cell migration during in vitro wound repair. Arterioscler Thromb Vasc Biol. 2000;20:360–369. doi: 10.1161/01.atv.20.2.360. [DOI] [PubMed] [Google Scholar]
- Klosek SK, Nakashiro K, Hara S, Shintani S, Hasegawa H, Hamakawa H. CD151 forms a functional complex with c-Met in human salivary gland cancer cells. Biochem Biophys Res Commun. 2005;336:408–416. doi: 10.1016/j.bbrc.2005.08.106. [DOI] [PubMed] [Google Scholar]
- Klosek SK, Nakashiro K, Hara S, Goda H, Hasegawa H, Hamakawa H. CD151 regulates HGF-stimulated morphogenesis of human breast cancer cells. Biochem Biophys Res Commun. 2009;379:1097–1100. doi: 10.1016/j.bbrc.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, et al. Differential expression of extracellular-matrix molecules and the alpha(6)-integrins in the normal and neoplastic prostate. Am J Pathol. 1994;145:167–174. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Hosono O, Iwata S, Kawasaki H, Kuwana M, Tanaka H, et al. The tetraspanin CD9 is preferentially expressed on the human CD4(+)CD45RA(+) naive T cell population and is involved in T cell activation. Clin Exp Immunol. 2004;137:101–108. doi: 10.1111/j.1365-2249.2004.02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmo S, Kijima T, Otani Y, Mori M, Minami T, Takahashi R, et al. Cell surface tetraspanin CD9 mediates chemoresistance in small cell lung cancer. Cancer Res. 2010;70:8025–8035. doi: 10.1158/0008-5472.CAN-10-0996. [DOI] [PubMed] [Google Scholar]
- Kohno M, Hasegawa H, Miyake M, Yamamoto T, Fujita S. CD151 enhances cell motility and metastasis of cancer cells in the presence of focal adhesion kinase. Int J Cancer. 2002;97:336–343. doi: 10.1002/ijc.1605. [DOI] [PubMed] [Google Scholar]
- Kotha J, Longhurst C, Appling W, Jennings LK. Tetraspanin CD9 regulates β1 integrin activation and enhances cell motility to fibronectin via a PI-3 kinase-dependent pathway. Exp Cell Res. 2008;314:1811–1822. doi: 10.1016/j.yexcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Kovalenko OV, Metcalf DG, DeGrado WF, Hemler ME. Structural organization and interactions of transmembrane domains in tetraspanin proteins. BMC Struct Biol. 2005;5 doi: 10.1186/1472-6807-5-11. doi: 10.1186/1472-6807-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubista B, Erovic BM, Klinger H, Sulzbacher I, Trieb K. CD9 expression is not a prognostic factor in human osteosarcoma. Cancer Lett. 2004;209:105–110. doi: 10.1016/j.canlet.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Kusukawa J, Ryu F, Kameyama T, Mekada E. Reduced expression of CD9 in oral squamous cell carcinoma: CD9 expression inversely related to high prevalence of lymph node metastasis. J Oral Pathol Med. 2001;30:73–79. doi: 10.1034/j.1600-0714.2001.300202.x. [DOI] [PubMed] [Google Scholar]
- Kwon MJ, Park S, Choi JY, Oh E, Kim YJ, Park YH, et al. Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br J Cancer. 2012;106:923–930. doi: 10.1038/bjc.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MS, Shin S-H, Yim S-H, Lee KY, Kang H-M, Kim T-M, et al. CD63 as a biomarker for predicting the clinical outcomes in adenocarcinoma of lung. Lung Cancer. 2007;57:46–53. doi: 10.1016/j.lungcan.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Lafleur MA, Xu D, Hemler ME. Tetraspanin proteins regulate membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis. Mol Biol Cell. 2009;20:2030–2040. doi: 10.1091/mbc.E08-11-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- Lan RF, Liu ZX, Liu XC, Song YE, Wang DW. CD151 promotes neovascularization and improves blood perfusion in a rat hind-limb ischemia model. J Endovasc Ther. 2005;12:469–478. doi: 10.1583/04-1478R.1. [DOI] [PubMed] [Google Scholar]
- Lawrence MG, Veveris-Lowe TL, Whitbread AK, Nicol DL, Clements JA. Epithelial-mesenchymal transition in prostate cancer and the potential role of kallikrein serine proteases. Cells Tissues Organs. 2007;185:111–115. doi: 10.1159/000101311. [DOI] [PubMed] [Google Scholar]
- Lee HA, Park I, Byun HJ, Jeoung D, Kim YM, Lee H. Metastasis suppressor KAI1/CD82 attenuates the matrix adhesion of human prostate cancer cells by suppressing fibronectin expression and beta(1) Integrin activation. Cell Physiol Biochem. 2011;27:575–586. doi: 10.1159/000329979. [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, Shoham T. Protein-protein interactions in the tetraspanin web. Physiology. 2005;20:218–224. doi: 10.1152/physiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- Levy S, Todd SC, Maecker HT. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- Li JJ, Xu M, Huang HP, Zhou JJ, Abdel-Halim ES, Zhang JR, et al. Aptamer-quantum dots conjugates-based ultrasensitive competitive electrochemical cytosensor for the detection of tumor cell. Talanta. 2011;85:2113–2120. doi: 10.1016/j.talanta.2011.07.055. [DOI] [PubMed] [Google Scholar]
- Li Q, Yang XH, Xu F, Sharma C, Wang HX, Knoblich K, et al. Tetraspanin CD151 plays a key role in skin squamous cell carcinoma. Oncogene. 2012;32:1772–1778. doi: 10.1038/onc.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijovic M, Somers G, Frauman AG. KAI1/CD82 protein expression in primary prostate cancer and in BPH associated with cancer. Cancer Detect Prev. 2002;36:69–77. doi: 10.1016/s0361-090x(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Lin PC, Lin SC, Lee CT, Lin YJ, Lee JC. Dynamic change of tetraspanin CD151 membrane protein expression in colorectal cancer patients. Cancer Invest. 2011;29:542–547. doi: 10.3109/07357907.2011.606251. [DOI] [PubMed] [Google Scholar]
- Liu L, Liu WM, Zhou D, Cox J, Zhang XA. Tetraspanin CD151 promotes cell migration through regulating integrin trafficking. J Biol Chem. 2007;282:31631–31642. doi: 10.1074/jbc.M701165200. [DOI] [PubMed] [Google Scholar]
- Liu WM, Zhang F, Moshiach S, Zhou B, Huang C, Srinivasan K, et al. Tetraspanin CD82 inhibits protrusion and retraction in cell movement by attenuating the plasma membrane-dependent actin organization. PLoS ONE. 2012;7:e51797. doi: 10.1371/journal.pone.0051797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo N, Yanez-Mo M, Mittelbrunn M, De La Rosa G, Munoz M, Sanchez-Madrid F, et al. Regulatory role of tetraspanin CD9 in tumor-endothelial cell interaction during transendothelial invasion of melanoma cells. Blood. 2001;98:3717–3726. doi: 10.1182/blood.v98.13.3717. [DOI] [PubMed] [Google Scholar]
- Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- Maeda K, Matsuhashi S, Hori K, Xin Z, Mukai T, Tabuchi K, et al. Cloning and characterization of a novel human gene, TM4SF6, encoding a protein belonging to the transmembrane 4 superfamily, and mapped to Xq22. Genomics. 1998;52:240–242. doi: 10.1006/geno.1998.5415. [DOI] [PubMed] [Google Scholar]
- Majumder M, Tutunea-Fatan E, Xin X, Rodriguez-Torres M, Torres-Garcia J, Wiebe R, et al. Co-expression of α9β1 integrin and VEGF-D confers lymphatic metastatic ability to a human breast cancer cell line MDA-MB-468LN. PLoS ONE. 2012;7:e35094. doi: 10.1371/journal.pone.0035094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P, Gomes K, Ulrich H. Aptamers: from bench side research towards patented molecules with therapeutic applications. Expert Opin Ther Pat. 2009;19:1603–1613. doi: 10.1517/13543770903313746. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2011;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocca A, Liotta F, Carloni V. Tetraspanin CD81-regulated cell motility plays a critical role in intrahepatic metastasis of hepatocellular carcinoma. Gastroenterology. 2008;135:244–256. doi: 10.1053/j.gastro.2008.03.024. e241. [DOI] [PubMed] [Google Scholar]
- Mehta R, Kyshtoobayeva A, Kurosaki T, Small EJ, Kim H, Stroup R, et al. Independent association of angiogenesis index with outcome in prostate cancer. Clin Cancer Res. 2001;7:81–88. [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM. The α6b4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Metzelaar MJ, Schuurman HJ, Heijnen HF, Sixma JJ, Nieuwenhuis HK. Biochemical and immunohistochemical characteristics of CD62 and CD63 monoclonal antibodies. Expression of GMP-140 and LIMP-CD63 (CD63 antigen) in human lymphoid tissues. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61:269–277. [PubMed] [Google Scholar]
- Mhawech P, Dulguerov P, Tschanz E, Verdan C, Ares C, Allal AS. Motility-related protein-1 (MRP-1//CD9) expression can predict disease-free survival in patients with squamous cell carcinoma of the head and neck. Br J Cancer. 2003;90:471–475. doi: 10.1038/sj.bjc.6601542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min G, Wang H, Sun TT, Kong XP. Structural basis for tetraspanin functions as revealed by the cryo-EM structure of uroplakin complexes at 6-A resolution. J Cell Biol. 2006;173:975–983. doi: 10.1083/jcb.200602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Szekeres C, Milano V, Svenson KB, Nilsen-Hamilton M, Kreidberg JA, et al. Alpha 3 beta 1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci. 2009;122:1778–1787. doi: 10.1242/jcs.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Svenson KB, Longmate WM, Gkirtzimanaki K, Sadej R, Wang XH, et al. Suppression of integrin alpha 3 beta 1 in breast cancer cells reduces cyclooxygenase-2 gene expression and inhibits tumorigenesis, invasion, and cross-talk to endothelial cells. Cancer Res. 2010;70:6359–6367. doi: 10.1158/0008-5472.CAN-09-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Maruyama A, Okugawa K, Akazawa K, Baba H, Maehara Y, et al. Loss of motility-related protein 1 (MRP1/CD9) and integrin α3 expression in endometrial cancers. Cancer. 2001;92:542–548. doi: 10.1002/1097-0142(20010801)92:3<542::aid-cncr1353>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Molday RS, Warren R, Loewen C, Molday L. Cyclic GMP-gated channel and peripherin/rds-rom-1 complex of rod cells. Novartis Found Symp. 1999;224:249–261. doi: 10.1002/9780470515693.ch14. [DOI] [PubMed] [Google Scholar]
- Mosig RA, Li L, Senturk E, Shah H, Huang F, Schlosshauer P, et al. Application of RNA-Seq transcriptome analysis: CD151 is an Invasion/Migration target in all stages of epithelial ovarian cancer. J Ovarian Res. 2012;5:1–9. doi: 10.1186/1757-2215-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabokina SM, Senthilkumar SR, Said HM. Tspan-1 interacts with the thiamine transporter-1 in human intestinal epithelial cells and modulates its stability. Am J Physiol Gastrointest Liver Physiol. 2011;301:G808–G813. doi: 10.1152/ajpgi.00269.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K, Oka K. HIF-2 directly activates CD82 gene expression in endothelial cells. Biochem Biophys Res Commun. 2011;407:260–265. doi: 10.1016/j.bbrc.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Arai Y, Mori H, Matsushita Y, Kubo T, Nakanishi T. Small interfering RNA targeting CD81 ameliorated arthritis in rats. Biochem Biophys Res Commun. 2009;388:467–472. doi: 10.1016/j.bbrc.2009.06.150. [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Murayama Y, Oritani K, Boucheix C, Rubinstein E, Nishida M, et al. A novel therapeutic strategy with anti-CD9 antibody in gastric cancers. J Gastroenterol. 2009;44:889–896. doi: 10.1007/s00535-009-0081-3. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Handa K, Iwamoto R, Tsukamoto T, Takahasi M, Mekada E. Immunohistochemical distribution of CD9, heparin binding epidermal growth factor-like growth factor, and integrin α3β1 in normal human tissues. J Histochem Cytochem. 2001;49:439–444. doi: 10.1177/002215540104900403. [DOI] [PubMed] [Google Scholar]
- Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- Ng T, Shima D, Squire A, Bastiaens PIH, Gschmeissner S, Humphries MJ, et al. PKC alpha regulates beta 1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RH, Pantano S, Eliason JF, Galy A, Weiler S, Kaplan J, et al. Phemx, a novel mouse gene expressed in hematopoietic cells maps to the imprinted cluster on distal chromosome 7. Genomics. 2000;68:13–21. doi: 10.1006/geno.2000.6277. [DOI] [PubMed] [Google Scholar]
- Nikopoulos K, Gilissen C, Hoischen A, Erik van Nouhuys C, Boonstra FN, Blokland EAW, et al. Next-generation sequencing of a 40 Mb linkage interval reveals TSPAN12 mutations in patients with familial exudative vitreoretinopathy. Am J Hum Genet. 2010;86:240–247. doi: 10.1016/j.ajhg.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiuchi R, Sanzen N, Nada S, Sumida Y, Wada Y, Okada M, et al. Potentiation of the ligand-binding activity of integrin alpha 3 beta 1 via association with tetraspanin CD151. Proc Natl Acad Sci U S A. 2005;102:1939–1944. doi: 10.1073/pnas.0409493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima Y, Hirose T, Tachibana K, Tanaka T, Shi L, Doshen J, et al. The 4F9 antigen is a member of the tetra spans transmembrane protein family and functions as an accessory molecule in T cell activation and adhesion. Cell Immunol. 1993;152:249–260. doi: 10.1006/cimm.1993.1285. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Ni A, Ayeni OA, Baluk P, Yao LC, Vossmeyer D, et al. α5β1 integrin blockade inhibits lymphangiogenesis in airway inflammation. Am J Pathol. 2009;174:2378–2387. doi: 10.2353/ajpath.2009.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olweus J, Lund-Johansen F, Horejsi V. CD53, a protein with four membrane-spanning domains, mediates signal transduction in human monocytes and B cells. J Immunol. 1993;151:707–716. [PubMed] [Google Scholar]
- Oommen S, Gupta SK, Vlahakis NE. Vascular endothelial growth factor A (VEGF-A) induces endothelial and cancer cell migration through direct binding to integrin {alpha}9{beta}1: identification of a specific {alpha}9{beta}1 binding site. J Biol Chem. 2011;286:1083–1092. doi: 10.1074/jbc.M110.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren R, Takahashi S, Doss C, Levy R, Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva B, Gutierrez NC, Chen X, Vidriales MB, Montalban MA, Rosinol L, et al. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia. 2012;26:1862–1869. doi: 10.1038/leu.2012.42. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Ashby WJ, Lewis JD, Zijlstra A. Targeting tumor cell motility to prevent metastasis. Adv Drug Deliv Rev. 2011;63:568–581. doi: 10.1016/j.addr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee JD. The actin cytoskeleton in cell motility, cancer, and infection. Colloquium Series on the Cell Biology of Medicine. 2009;1:1–57. [Google Scholar]
- Penas PF, Garcia-Diez A, Sanchez-Madrid F, Yanez-Mo M. Tetraspanins are localized at motility-related structures and involved in normal human keratinocyte wound healing migration. J Invest Dermatol. 2000;114:1126–1135. doi: 10.1046/j.1523-1747.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- Petersen SH, Odintsova E, Haigh TA, Rickinson AB, Taylor GS, Berditchevski F. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur J Immunol. 2011;41:2556–2561. doi: 10.1002/eji.201141438. [DOI] [PubMed] [Google Scholar]
- Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- Powner D, Kopp PM, Monkley SJ, Critchley DR, Berditchevski F. Tetraspanin CD9 in cell migration. Biochem Soc Trans. 2011;39:563–567. doi: 10.1042/BST0390563. [DOI] [PubMed] [Google Scholar]
- Protty MB, Watkins NA, Colombo D, Thomas SG, Heath VL, Herbert JMJ, et al. Identification of Tspan9 as a novel platelet tetraspanin and the collagen receptor GPVI as a component of tetraspanin microdomains. Biochem J. 2009;417:391–400. doi: 10.1042/BJ20081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prox J, Willenbrock M, Weber S, Lehmann T, Schmidt-Arras D, Schwanbeck R, et al. Tetraspanin15 regulates cellular trafficking and activity of the ectodomain sheddase ADAM10. Cell Mol Life Sci. 2012;69:2919–2932. doi: 10.1007/s00018-012-0960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls KL, Ni J, Liu D, Morahan G, Wright MD. The molecular characterisation of a novel tetraspanin protein, TM4-B. Biochim Biophys Acta. 1999;1447:93–99. doi: 10.1016/s0167-4781(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Qiong Z, Li X, Zhulin Y, Fang L, Leping Y, Xiongying M. Expression levels of HMGA2 and CD9 and its clinicopathological significances in the benign and malignant lesions of the gallbladder. World J Surg Oncol. 2012;10:92–98. doi: 10.1186/1477-7819-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford KJ, Thorne RF, Hersey P. CD63 associates with transmembrane 4 superfamily members, CD9 and CD81, and with β1 integrins in human melanoma. Biochem Biophys Res Commun. 1996;222:13–18. doi: 10.1006/bbrc.1996.0690. [DOI] [PubMed] [Google Scholar]
- Radford KJ, Thorne RF, Hersey P. Regulation of tumor cell motility and migration by CD63 in a human melanoma cell line. J Immunol. 1997;158:3353–3358. [PubMed] [Google Scholar]
- Richardson M, Jennings LK, Zhang X. Tetraspanins and tumor progression. Clin Exp Metastasis. 2011;28:261–270. doi: 10.1007/s10585-010-9365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb L, Tarrant J, Groom J, Ibrahim M, Li R, Borobakas B, et al. Molecular characterisation of mouse and human TSSC6: evidence that TSSC6 is a genuine member of the tetraspanin superfamily and is expressed specifically in haematopoietic organs. Biochim Biophys Acta. 2001;1522:31–41. doi: 10.1016/s0167-4781(01)00306-2. [DOI] [PubMed] [Google Scholar]
- Rockey WM, Hernandez FJ, Huang S-Y, Cao S, Howell C. Rational truncation of an RNA aptamer to prostate-specific membrane antigen using computational structural modeling. Nucleic Acid Ther. 2011;21:299–314. doi: 10.1089/nat.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorive S, Lopez XM, Maris C, Trepant A-L, Sauvage S, Sadeghi N, et al. TIMP-4 and CD63: new prognostic biomarkers in human astrocytomas. Mod Pathol. 2010;23:1418–1428. doi: 10.1038/modpathol.2010.136. [DOI] [PubMed] [Google Scholar]
- Rubinstein E, Ziyyat A, Wolf J-P, Le Naour F, Boucheix C. Review: the molecular players of sperm–egg fusion in mammals. Semin Cell Dev Biol. 2006;17:254–263. doi: 10.1016/j.semcdb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Sachs N, Kreft M, Weerman MAV, Beynon AJ, Peters TA, Weening JJ, et al. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175:33–39. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadej R, Romanska H, Baldwin G, Gkirtzimanaki K, Novitskaya V, Filer AD, et al. CD151 regulates tumorigenesis by modulating the communication between tumor cells and endothelium. Mol Cancer Res. 2009;7:787–798. doi: 10.1158/1541-7786.MCR-08-0574. [DOI] [PubMed] [Google Scholar]
- Sadej R, Romanska H, Kavanagh D, Baldwin G, Takahashi T, Kalia N, et al. Tetraspanin CD151 regulates transforming growth factor beta signaling: implication in tumor metastasis. Cancer Res. 2010;70:6059–6070. doi: 10.1158/0008-5472.CAN-09-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Landrigan A, Levy R, Levy S. Complementary costimulation of human T-cell subpopulations by cluster of differentiation 28 (CD28) and CD81. Proc Natl Acad Sci U S A. 2012;109:1613–1618. doi: 10.1073/pnas.1121307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, Malhotra A. Fluorescent nanoparticle probes for imaging of cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:501–510. doi: 10.1002/wnan.134. [DOI] [PubMed] [Google Scholar]
- Sauer G, Kurzeder C, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. Expression of tetraspanin adaptor proteins below defined threshold values is associated with in vitro invasiveness of mammary carcinoma cells. Oncol Rep. 2003a;10:405–410. [PubMed] [Google Scholar]
- Sauer G, Windisch J, Kurzeder C, Heilmann V, Kreienberg R, Deissler H. Progression of cervical carcinomas is associated with down-regulation of CD9 but strong local re-expression at sites of transendothelial invasion. Clin Cancer Res. 2003b;9:6426–6431. [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Schindl M, Birner P, Breitenecker G, Oberhuber G. Downregulation of KAI1 metastasis suppressor protein is associated with a dismal prognosis in epithelial ovarian cancer. Gynecol Oncol. 2001;83:244–248. doi: 10.1006/gyno.2001.6366. [DOI] [PubMed] [Google Scholar]
- Scholz C-J, Kurzeder C, Koretz K, Windisch J, Kreienberg R, Sauer G, et al. Tspan-1 is a tetraspanin preferentially expressed by mucinous and endometrioid subtypes of human ovarian carcinomas. Cancer Lett. 2009;275:198–203. doi: 10.1016/j.canlet.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Schroder HM, Hoffmann SC, Hecker M, Korff T, Ludwig T. The tetraspanin network modulates MT1-MMP cell surface trafficking. Int J Biochem Cell Biol. 2013;45:1133–1144. doi: 10.1016/j.biocel.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Seigneuret M, Delaguillaumie A, Lagaudriere-Gesbert C, Conjeaud H. Structure of the tetraspanin main extracellular domain-a partially conserved fold with a structurally variable domain insertion. J Biol Chem. 2001;276:40055–40064. doi: 10.1074/jbc.M105557200. [DOI] [PubMed] [Google Scholar]
- Seigneuric R, Gobbo J, Colas P, Garrido C. Targeting cancer with peptide aptamers. Oncotarget. 2011;2:557–561. doi: 10.18632/oncotarget.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serru V, Le Naour F, Billard M, Azorsa DO, Lanza F, Boucheix C, et al. Selective tetraspan-integrin complexes (CD81/alpha4beta1, CD151/alpha3beta1, CD151/alpha6beta1) under conditions disrupting tetraspan interactions. Biochem J. 1999;340(Pt 1):103–111. [PMC free article] [PubMed] [Google Scholar]
- Serru V, Dessen P, Boucheix C, Rubinstein E. Sequence and expression of seven new tetraspans. Biochim Biophys Acta. 2000;1478:159–163. doi: 10.1016/s0167-4838(00)00022-4. [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Kikuchi H, Yamamoto M, Baba M, Ohta M, Kamiya K, et al. Microarray analysis identifies versican and CD9 as potent prognostic markers in gastric gastrointestinal stromal tumors. Cancer Sci. 2011;102:883–889. doi: 10.1111/j.1349-7006.2011.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariat AF, Karam JA, Margulis V, Karakiewicz PI. New blood-based biomarkers for the diagnosis, staging and prognosis of prostate cancer. BJU Int. 2007;101:675–683. doi: 10.1111/j.1464-410X.2007.07283.x. [DOI] [PubMed] [Google Scholar]
- Shi GM, Ke AW, Zhou JA, Wang XY, Xu Y, Ding ZB, et al. CD151 modulates expression of matrix metalloproteinase 9 and promotes neoangiogenesis and progression of hepatocellular carcinoma. Hepatology. 2010;52:183–196. doi: 10.1002/hep.23661. [DOI] [PubMed] [Google Scholar]
- Shigeta M, Sanzen N, Ozawa M, Gu J, Hasegawa H, Sekiguchi K. CD151 regulates epithelial cell-cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol. 2003;163:165–176. doi: 10.1083/jcb.200301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y-CT, Elting LS, Pavluck AL, Stewart A, Halpern MT. Immunotherapy in the initial treatment of newly diagnosed cancer patients: utilization trend and cost projections for non-Hodgkin's lymphoma, metastatic breast cancer, and metastatic colorectal cancer. Cancer Invest. 2010;28:46–53. doi: 10.3109/07357900902783187. [DOI] [PubMed] [Google Scholar]
- Sho M, Adachi M, Taki T, Hashida H, Konishi T, Huang C, et al. Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int J Cancer. 1998;79:509–516. doi: 10.1002/(sici)1097-0215(19981023)79:5<509::aid-ijc11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Si Z, Hersey P. Expression of the neuroglandular antigen and analogues in melanoma. CD9 expression appears inversely related to metastatic potential of melanoma. Int J Cancer. 1993;54:37–43. doi: 10.1002/ijc.2910540107. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Lim JWE, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- Sincock PM, Mayrhofer G, Ashman LK. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63 and α5β1 integrins. J Histochem Cytochem. 1997;45:515–525. doi: 10.1177/002215549704500404. [DOI] [PubMed] [Google Scholar]
- Sincock PM, Fitter S, Parton RG, Berndt MC, Gamble JR, Ashman LK. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J Cell Sci. 1999;112:833–844. doi: 10.1242/jcs.112.6.833. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Slupsky JR, Seehafer JG, Tang SC, Masellis-Smith A, Shaw AR. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb-IIIa complex. J Biol Chem. 1989;264:12289–12293. [PubMed] [Google Scholar]
- Soontornworajit B, Zhou J, Snipes MP, Battig MR, Wang Y. Affinity hydrogels for controlled protein release using nucleic acid aptamers and complementary oligonucleotides. Biomaterials. 2011;32:6839–6849. doi: 10.1016/j.biomaterials.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Sordat I, Decraene C, Silvestre T, Petermann O, Auffray C, Piétu G, et al. Complementary DNA arrays identify CD63 tetraspanin and α3 integrin chain as differentially expressed in low and high metastatic human colon carcinoma cells. Lab Invest. 2002;82:1715–1724. doi: 10.1097/01.lab.0000044350.18215.0d. [DOI] [PubMed] [Google Scholar]
- Soyuer S, Soyuer I, Unal D, Ucar K, Yildiz OG, Orhan O. Prognostic significance of CD9 expression in locally advanced gastric cancer treated with surgery and adjuvant chemoradiotherapy. Pathol Res Pract. 2010;206:607–610. doi: 10.1016/j.prp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- van Spriel AB, Figdor CG. The role of tetraspanins in the pathogenesis of infectious diseases. Microbes Infect. 2010;12:106–112. doi: 10.1016/j.micinf.2009.11.001. [DOI] [PubMed] [Google Scholar]
- van Spriel AB, Puls KL, Sofi M, Pouniotis D, Hochrein H, Orinska Z, et al. A regulatory role for CD37 in T cell proliferation. J Immunol. 2004;172:2953–2961. doi: 10.4049/jimmunol.172.5.2953. [DOI] [PubMed] [Google Scholar]
- Sridhar SC, Miranti CK. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and Src kinases. Oncogene. 2006;25:2367–2378. doi: 10.1038/sj.onc.1209269. [DOI] [PubMed] [Google Scholar]
- Stehn JR, Schevzov G, O'Neill GM, Gunning PW. Specialisation of the tropomyosin composition of actin filaments provides new potential targets for chemotherapy. Curr Cancer Drug Targets. 2006;6:245–256. doi: 10.2174/156800906776842948. [DOI] [PubMed] [Google Scholar]
- Sterk LM, Geuijen CAW, Van Den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the laminin-binding integrins {alpha}3{beta}1, {alpha}6{beta}1, {alpha}6{beta}4 and {alpha}7{beta}1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–1173. doi: 10.1242/jcs.115.6.1161. [DOI] [PubMed] [Google Scholar]
- Stipp CS. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med. 2010;12 doi: 10.1017/S1462399409001355. doi: 10.1017/S1462399409001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipp CS, Kolesnikova TV, Hemler ME. EWI-2 regulates α3β1 integrin–dependent cell functions on laminin-5. J Cell Biol. 2003a;163:1167–1177. doi: 10.1083/jcb.200309113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipp CS, Kolesnikova TV, Hemler ME. Functional domains in tetraspanin proteins. TRENDS Biochem Sci. 2003b;28:106–112. doi: 10.1016/S0968-0004(02)00014-2. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207–238. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Berditchevski F. Function of α3β1-tetraspanin protein conplexes in tumor cell invasion: evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2) J Cell Biol. 1999;146:1375–1389. doi: 10.1083/jcb.146.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Miyazaki T, Tanaka N, Sakai M, Sano A, Inose T, et al. Prognostic significance of CD151 expression in esophageal squamous cell carcinoma with aggressive cell proliferation and invasiveness. Ann Surg Oncol. 2011;18:888–893. doi: 10.1245/s10434-010-1387-3. [DOI] [PubMed] [Google Scholar]
- Tachibana I, Bodorova J, Berditchevski F, Zutter MM, Hemler ME. NAG-2, a novel transmembrane-4 superfamily (TM4SF) protein that complexes with integrins and other TM4SF proteins. J Biol Chem. 1997;272:29181–29189. doi: 10.1074/jbc.272.46.29181. [DOI] [PubMed] [Google Scholar]
- Tai XG, Yashiro Y, Abe R, Toyooka K, Wood CR, Morris J, et al. A role for CD9 molecules in T cell activation. J Exp Med. 1996;184:753–758. doi: 10.1084/jem.184.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Fujikawa K, Imai T, Fukuhara N, Fukudome K, Minegishi M, et al. Identification of a highly specific surface marker of T-cell acute lymphoblastic leukemia and neuroblastoma as a new member of the transmembrane 4 superfamily. Int J Cancer. 1995;61:706–715. doi: 10.1002/ijc.2910610519. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sugiura T, Abe M, Ishii K, Shirasuna K. Regulation of c-Met signaling by the tetraspanin KAI-1/CD82 affects cancer cell migration. Int J Cancer. 2007;121:1919–1929. doi: 10.1002/ijc.22887. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Kazarov AR, Butterfield CE, Hopkins BD, Benjamin LE, Kaipainen A, et al. Deletion of tetraspanin CD151 results in decreased pathological angiogenesis in vivo and in vitro. Blood. 2007;109:1524–1532. doi: 10.1182/blood-2006-08-041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Li QL, Kazarov AR, Epardaud M, Elpek K, Turley SJ, et al. Diminished metastasis in tetraspanin CD151-knockout mice. Blood. 2011;118:464–472. doi: 10.1182/blood-2010-08-302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takino T, Miyamori H, Kawaguchi N, Uekita T, Seiki M, Sato H. Tetraspanin CD63 promotes targeting and lysosomal proteolysis of membrane-type 1 matrix metalloproteinase. Biochem Biophys Res Commun. 2003;304:160–166. doi: 10.1016/s0006-291x(03)00544-8. [DOI] [PubMed] [Google Scholar]
- Tan W, Wang H, Chen Y, Zhang X, Zhu H. Molecular aptamers for drug delivery. Trends Biotechnol. 2011;29:634–640. doi: 10.1016/j.tibtech.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant JM, Groom J, Metcalf D, Li R, Borobokas B, Wright MD, et al. The absence of Tssc6, a member of the tetraspanin superfamily, does not affect lymphoid development but enhances in vitro T-cell proliferative responses. Mol Cell Biol. 2002;22:5006–5018. doi: 10.1128/MCB.22.14.5006-5018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- Tejera E, Rocha-Perugini V, López-Martín S, Pérez-Hernández D, Bachir AI, Horwitz AR, et al. CD81 regulates cell migration through its association with Rac GTPase. Mol Biol Cell. 2013;24:261–273. doi: 10.1091/mbc.E12-09-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Baracskay K, Kinter M, Melrose S, Brophy PJ, Boucheix C, et al. The tetraspanin protein, CD9, is expressed by progenitor cells committed to oligodendrogenesis and is linked to β1 integrin, CD81, and Tspan-2. Glia. 2002;40:350–359. doi: 10.1002/glia.10134. [DOI] [PubMed] [Google Scholar]
- Terracciano S, Bruno I, D'Amico E, Bifulco G, Zampella A, Sepe V, et al. Synthetic and pharmacological studies on new simplified analogues of the potent actin-targeting Jaspamide. Bioorg Med Chem. 2008;16:6580–6588. doi: 10.1016/j.bmc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Testa JE, Brooks PC, Lin J, Quigley JP. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 1999;59:3812–3820. [PubMed] [Google Scholar]
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Thiolloy S, Rinker-Schaeffer CW. Thinking outside the box: using metastasis suppressors as molecular tools. Semin Cancer Biol. 2011;21:89–98. doi: 10.1016/j.semcancer.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Timoshenko AV, Rastogi S, Lala PK. Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br J Cancer. 2007;97:1090–1098. doi: 10.1038/sj.bjc.6603993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff SK, Kaplan R, Kornblum HI, Bronstein JM. Developmental expression of OAP-1/Tspan-3, a member of the tetraspanin superfamily. J Neurosci Res. 2004;77:166–173. doi: 10.1002/jnr.20141. [DOI] [PubMed] [Google Scholar]
- Todd SC, Doctor VS, Levy S. Sequences and expression of six new members of the tetraspanin/TM4SF family. Biochim Biophys Acta. 1998;1399:101–104. doi: 10.1016/s0167-4781(98)00087-6. [DOI] [PubMed] [Google Scholar]
- Tokuhara T, Hasegawa H, Hattori N, Ishida H, Taki T, Tachibana S, et al. Clinical significance of CD151 gene expression in non-small cell lung cancer. Clin Cancer Res. 2001;7:4109–4114. [PubMed] [Google Scholar]
- Traggiai E, Lunardi C, Bason C, Dolcino M, Tinazzi E, Corrocher R, et al. Generation of anti-NAG-2 mAb from patients' memory B cells: implications for a novel therapeutic strategy in systemic sclerosis. Int Immunol. 2010;22:367–374. doi: 10.1093/intimm/dxq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Christerson L, Danielson PE, Klisak I, Sparkes RS, Hahn LB, et al. The human retinal degeneration slow (RDS) gene: chromosome assignment and structure of the mRNA. Genomics. 1991;10:733–739. doi: 10.1016/0888-7543(91)90457-p. [DOI] [PubMed] [Google Scholar]
- Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Uchida S, Shimada Y, Watanabe G, Li ZG, Hong T, Miyake M, et al. Motility-related protein (MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 1999;79:1168–1173. doi: 10.1038/sj.bjc.6690186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenbergen S, van Spriel AB. Tetraspanins in the immune response against cancer. Immunol Lett. 2011;138:129–136. doi: 10.1016/j.imlet.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Velling T, Nilsson S, Stefansson A, Johansson S. β1-integrins induce phosphorylation of Akt on serine473 independently of focal adhesion kinase and Src family kinases. EMBO Rep. 2004;5:901–905. doi: 10.1038/sj.embor.7400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, et al. Integrin alpha 9 beta 1 directly binds to vascular endothelial growth factor (VEGF)- a and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- Voss MA, Gordon N, Maloney S, Ganesan R, Ludeman L, McCarthy K, et al. Tetraspanin CD151 is a novel prognostic marker in poor outcome endometrial cancer. Br J Cancer. 2011;104:1611–1618. doi: 10.1038/bjc.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall SJ, Jiang Y, Muschel RJ, DeClerck YA. Meeting report: proteases, extracellular matrix, and cancer: an AACR special conference in cancer research. Cancer Res. 2003;63:4750–4755. [PubMed] [Google Scholar]
- Wang GL, Chen L, Wei YZ, Zhou JM, Wu YY, Zhang YX, et al. The effect of NET-1 on the proliferation, migration and endocytosis of the SMMC-7721 HCC cell line. Oncol Rep. 2012;27:1944–1952. doi: 10.3892/or.2012.1698. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu L, Che Y, Wang L, Jiang L, Dong C, et al. Egress of HSV-1 capsid requires the interaction of VP26 and a cellular tetraspanin membrane protein. Virol J. 2010;7:156–156. doi: 10.1186/1743-422X-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Ferreira AM, Shao Q, Laird DW, Sandig M. beta 3 integrins facilitate matrix interactions during transendothelial migration of PC3 prostate tumor cells. Prostate. 2005;63:65–80. doi: 10.1002/pros.20168. [DOI] [PubMed] [Google Scholar]
- Wei QA, Huang XL, Lin JY, Fei YJ, Liu ZX, Zhang XA. Adeno associated viral vector-delivered and hypoxia response element-regulated CD151 expression in ischemic rat heart. Acta Pharmacol Sin. 2011;32:201–208. doi: 10.1038/aps.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- Wells A. Cell motility in cancer invasion and metastasis. In: Wells A, editor. Cancer Metastasis-Biology and Treatment. 1st edn. Vol. 8. Dordrecht, The Netherland: Springer; 2006. pp. 1–351. Vol. [Google Scholar]
- Winterwood NE, Varzavand A, Meland MN, Ashman LK, Stipp CS. A critical role for tetraspanin CD151 in α3β1 and α6β4 integrin-dependent tumor cell functions on laminin-5. Mol Biol Cell. 2006;17:2707–2721. doi: 10.1091/mbc.E05-11-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G, Berstein SL, Wyatt MK, Farriss RN, Behal A, Touchman J, et al. Expressed sequence tag analysis of human RPE/choroid for the NEIBank Project: over 6000 non-redundant transcripts, novel genes and splice variants. Mol Vis. 2002;8:205–220. [PubMed] [Google Scholar]
- Woegerbauer M, Thurnher D, Houben R, Pammer J, Kloimstein P, Heiduschka G, et al. Expression of the tetraspanins CD9, CD37, CD63, and CD151 in Merkel cell carcinoma: strong evidence for a posttranscriptional fine-tuning of CD9 gene expression. Mod Pathol. 2010;23:751–762. doi: 10.1038/modpathol.2009.192. [DOI] [PubMed] [Google Scholar]
- Wright MD, Geary SM, Fitter S, Moseley GW, Lau L-M, Sheng K-C, et al. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol Cell Biol. 2004a;24:5978–5988. doi: 10.1128/MCB.24.13.5978-5988.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MD, Moseley GW, Van Spriel AB. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens. 2004b;64:533–542. doi: 10.1111/j.1399-0039.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Wuttig D, Zastrow S, Fussel S, Toma MI, Meinhardt M, Kalman K, et al. CD31, EDNRB and TSPAN7 are promising prognostic markers in clear-cell renal cell carcinoma revealed by genome-wide expression analyses of primary tumors and metastases. Int J Cancer. 2012;131:E693–E704. doi: 10.1002/ijc.27419. [DOI] [PubMed] [Google Scholar]
- Xu D, Sharma C, Hemler ME. Tetraspanin12 regulates ADAM10-dependent cleavage of amyloid precursor protein. FASEB J. 2009;23:3674–3681. doi: 10.1096/fj.09-133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo M, Barreiro O, Gonzalo P, Batista A, Megias D, Genis L, et al. MT1-MMP collagenolytic activity is regulated through association with tetraspanin CD151 in primary endothelial cells. Blood. 2008;112:3217–3226. doi: 10.1182/blood-2008-02-139394. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M, Alfranca A, Cabanas C, Marazuela M, Tejedor R, Ursa MA, et al. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/ PETA-3 with α3β1 integrin localized at endothelial lateral junctions. J Cell Biol. 1998;141:791–804. doi: 10.1083/jcb.141.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Mó M, Mittelbrunn M, Sánchez-Madrid F. Tetraspanins and intercellular interactions. Microcirculation. 2001;8:153–168. doi: 10.1038/sj/mn/7800076. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M, Gutiérrez-López MD, Cabañas C. Functional interplay between tetraspanins and proteases. Cell Mol Life Sci. 2011;68:3323–3335. doi: 10.1007/s00018-011-0746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Claas C, Kraeft S-K, Chen LB, Wang Z, Kreidberg JA, et al. Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol Biol Cell. 2002;13:767–781. doi: 10.1091/mbc.01-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XWH, Richardson AL, Torres-Arzayus MI, Zhou PC, Sharma C, Kazarov AR, et al. CD151 accelerates breast cancer by regulating alpha(6) integrin function, signaling, and molecular organization. Cancer Res. 2008;68:3204–3213. doi: 10.1158/0008-5472.CAN-07-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XWH, Flores LM, Li QL, Zhou PC, Xu FH, Krop IE, et al. Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists. Cancer Res. 2010;70:2256–2263. doi: 10.1158/0008-5472.CAN-09-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Zhang ZW, Liu QM, Sun YF, Yu JR, Xu WX. Overexpression of CD151 predicts prognosis in patients with resected gastric cancer. PLoS ONE. 2013;8:e58990. doi: 10.1371/journal.pone.0058990. doi: 10.1371/journal.pone.0058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin α3β1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell. 1998;9:2751–2765. doi: 10.1091/mbc.9.10.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Lee K, Chae JY, Moon KC. CD151 expression can predict cancer progression in clear cell renal cell carcinoma. Histopathology. 2011;58:191–197. doi: 10.1111/j.1365-2559.2011.03752.x. [DOI] [PubMed] [Google Scholar]
- Yu C, Hu Y, Duan J, Yuan W, Wang C, Xu H, et al. Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PLoS ONE. 2011;6:e24077. doi: 10.1371/journal.pone.0024077. . doi: 10.1371/journal.pone.0024077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lin JH, Wu XR, Sun TT. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J Cell Biol. 1994;125:171–182. doi: 10.1083/jcb.125.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MK, Kim D, Lee IH, So JS, Jeong YY, Jon S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small. 2011;7:2241–2249. doi: 10.1002/smll.201100472. [DOI] [PubMed] [Google Scholar]
- Zemni R, Bienvenu T, Vinet MC, Sefiani A, Carrié A, Billuart P, et al. A new gene involved in X-linked mental retardation identified by analysis of an X;2 balanced translocation. Nat Genet. 2000;24:167–170. doi: 10.1038/72829. [DOI] [PubMed] [Google Scholar]
- Zhang F, Michaelson JE, Moshiach S, Sachs N, Zhao W, Sun Y, et al. Tetraspanin CD151 maintains vascular stability by balancing the forces of cell adhesion and cytoskeletal tension. Blood. 2011a;118:4274–4284. doi: 10.1182/blood-2011-03-339531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Gong A, Young CYF. ZNF185, an actin–cytoskeleton-associated growth inhibitory LIM protein in prostate cancer. Oncogene. 2007;26:111–122. doi: 10.1038/sj.onc.1209769. [DOI] [PubMed] [Google Scholar]
- Zhang XA, Bontrager AL, Christopher SS, Stine-Kathrein K, Bazzoni G, Chen LB, et al. Phosphorylation of a conserved integrin α3 QPSXXE motif regulates signaling, motility, and cytoskeletal engagement. Mol Biol Cell. 2001a;12:351–365. doi: 10.1091/mbc.12.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta1 integrins. J Biol Chem. 2001b;276:25005–25013. doi: 10.1074/jbc.M102156200. [DOI] [PubMed] [Google Scholar]
- Zhang XA, Kazarov AR, Yang X, Bontrager AL, Stipp CS, Hemler ME. Function of the tetraspanin CD151-a6b1 integrin complex during cellular morphogenesis. Mol Biol Cell. 2002;13:1–11. doi: 10.1091/mbc.01-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, Lane WS, Charrin S, Rubinstein E, Liu L. EWI2/PGRL associates with the metastasis suppressor KAI1/CD82 and inhibits the migration of prostate cancer cells. Cancer Res. 2003;63:2665–2674. [PubMed] [Google Scholar]
- Zhang Y, Hong H, Cai W. Tumor-targeted drug delivery with aptamers. Curr Med Chem. 2011b;18:4185–4194. doi: 10.2174/092986711797189547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Zhang LJ, Hua YQ, Jia XF, Li JA, Hu S, et al. Comparative proteomic analysis of plasma membrane proteins between human osteosarcoma and normal osteoblastic cell lines. BMC Cancer. 2010;10:206. doi: 10.1186/1471-2407-10-206. doi: 10.1186/1471-2407-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Yano SJ, Zhang HL, Nakataki E, Tachibana I. CD9 overexpression suppressed the liver metastasis and malignant ascites via inhibition of proliferation and motility of small-cell lung cancer cells in NK cell-depleted SCID mice. Oncol Res. 2005;15:365–372. doi: 10.3727/096504005776449699. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Liu Z. Activation of the phosphatidylinositol 3-kinase/protein kinase akt pathway mediates CD151-induced endothelial cell proliferation and cell migration. Int J Biochem Cell Biol. 2007a;39:340–348. doi: 10.1016/j.biocel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Liu ZX. CD151 gene delivery increases eNOS activity and induces ECV304 migration, proliferation and tube formation. Acta Pharmacol Sin. 2007b;28:66–72. doi: 10.1111/j.1745-7254.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- Zheng ZZ, Liu ZX. CD151 gene delivery activates PI3K/Akt pathway and promotes neovascularization after myocardial infarction in rats. Mol Med. 2006;12:214–220. doi: 10.2119/2006-00037.Zheng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhijun X, Shulan Z, Zhuo Z. Expression and significance of the protein and mRNA of metastasis suppressor gene Me491/cd63 and integrin α5 in ovarian cancer tissues. Eur J Gynaecol Oncol. 2007;28:179–183. [PubMed] [Google Scholar]
- Zhou B, Liu L, Reddivari M, Zhang XA. The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility- and invasiveness-inhibitory activity. Cancer Res. 2004;64:7455–7463. doi: 10.1158/0008-5472.CAN-04-1574. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Ran YL, Hu H, Pan J, Li ZF, Chen LZ, et al. TM4SF3 promotes esophageal carcinoma metastasis via upregulating ADAM12m expression. Clin Exp Metastasis. 2008;25:537–548. doi: 10.1007/s10585-008-9168-0. [DOI] [PubMed] [Google Scholar]
- Zhu GH, Huang C, Qiu ZJ, Liu J, Zhang ZH, Zhao N, et al. Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Dig Dis Sci. 2011;56:1090–1098. doi: 10.1007/s10620-010-1416-x. [DOI] [PubMed] [Google Scholar]
- Zijlstra A, Lewis J, DeGryse B, Stuhlmann H, Quigley JP. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008;13:221–234. doi: 10.1016/j.ccr.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- Zlatna R, Pamina Xenia Charlotte G, Peter H, Matthias K, Birgit L, Manfred S, et al. Tumor suppressor KAI1 affects integrin αvβ3-mediated ovarian cancer cell adhesion, motility, and proliferation. Exp Cell Res. 2009;315:1759–1771. doi: 10.1016/j.yexcr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Zuo HJ, Liu ZX, Liu XC, Yang J, Liu T, Wen S, et al. Assessment of myocardial blood perfusion improved by CD151 in a pig myocardial infarction model. Acta Pharmacol Sin. 2009a;30:70–77. doi: 10.1038/aps.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo HJ, Liu ZX, Liu XC, Yang J, Liu T, Wen S, et al. CD151 gene delivery after myocardial Infarction promotes functional neovascularization and activates FAK signaling. Mol Med. 2009b;15:307–315. doi: 10.2119/molmed.2009.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo HJ, Lin JY, Liu ZY, Liu WF, Liu T, Yang J, et al. Activation of the ERK signaling pathway is involved in CD151-induced angiogenic effects on the formation of CD151-integrin complexes. Acta Pharmacol Sin. 2010;31:805–812. doi: 10.1038/aps.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvierev V, Wang JC, Chevrette M. Over-expression of CD9 does not affect in vivo tumorigenic or metastatic properties of human prostate cancer cells. Biochem Biophys Res Commun. 2005;337:498–504. doi: 10.1016/j.bbrc.2005.09.073. [DOI] [PubMed] [Google Scholar]