Abstract
Cancer cells are strongly influenced by host cells within the tumour stroma and vice versa. This leads to the development of a tumour microenvironment with distinct physical and chemical properties that are permissive for tumour progression. The ability to migrate plays a central role in this mutual interaction. Migration of cancer cells is considered as a prerequisite for tumour metastasis and the migration of host stromal cells is required for reaching the tumour site. Increasing evidence suggests that transient receptor potential (TRP) channels and STIM/ORAI proteins affect key calcium-dependent mechanisms implicated in both cancer and stroma cell migration. These include, among others, cytoskeletal remodelling, growth factor/cytokine signalling and production, and adaptation to tumour microenvironmental properties such as hypoxia and oxidative stress. In this review, we will summarize the current knowledge regarding TRP channels and STIM/ORAI proteins in cancer and stroma cell migration. We focus on how TRP channel or STIM/ORAI-mediated Ca2+ signalling directly or indirectly influences cancer and stroma cell migration by affecting the above listed mechanisms.
Linked Articles
This article is part of a themed section on Cytoskeleton, Extracellular Matrix, Cell Migration, Wound Healing and Related Topics. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-24
Introduction
Cell migration is fundamental to cell and tissue homeostasis and plays a pivotal role in many physiological and pathophysiological processes. Thus, wound healing, immune surveillance and angiogenesis require the migration of fibroblasts, immune cells and endothelial cells respectively (Stupack and Cheresh, 2004; Martin and Leibovich, 2005; Friedl and Weigelin, 2008; Silva, 2010). However, there are also a number of pathologies that involve ‘too much’ migration of the ‘wrong’ cell types. This is particularly relevant for cancer progression. The migratory activity of tumour cells is a critical step within the metastatic cascade that leads to the settling of tumour cells in distant organs (Yamaguchi et al., 2005; Gupta and Massague, 2006; Hanahan and Weinberg, 2011). However, tumour cells do not act by themselves to acquire this aggressive migrating phenotype. They are strongly influenced by the tumour microenvironment (TME) and stromal cells of the host organ (e.g. fibroblasts, macrophages and other immune cells or endothelial cells). Tumour stroma cells can therefore be viewed as an active partner in promoting cancer metastasis (Gupta and Massague, 2006; Joyce and Pollard, 2009; Brabek et al., 2010). In fact, stroma cells are also found in metastases (Xu et al., 2010).
In recent years, it has become evident that proteins involved in ion transport are involved in the mechanisms underlying the metastatic cascade and the tumour–stroma interaction (Fraser and Pardo, 2008; Arcangeli, 2011; Pedersen and Stock, 2013). Calcium signalling plays a particularly prominent role in regulating cancer and stroma cell functions including cell migration (Prevarskaya et al., 2011; 2014,; Chen et al., 2013a).
Specifically, transient receptor potential (TRP) channels (see Alexander et al., 2013a) and the protein complex consisting of the stromal interaction molecule (STIM) and calcium release-activated calcium channel protein (ORAI) have evolved as new players in this context (Bodding, 2007; Prevarskaya et al., 2011; Bergmeier et al., 2013; Ouadid-Ahidouch et al., 2013). However, the molecular mechanisms by which they affect cancer and stroma cell migration as well as the mutual communication between these cell types are still far from being fully understood. The present review will focus on the role of TRP channels and STIM/ORAI proteins in regulating the mutual interplay between cancer and stroma cells with emphasis on cell migration. TRP channels are particularly interesting in this context since they are able to sense and respond to microenvironmental changes occurring during cancer development and progression. Further, we will discuss the role of STIM/ORAI proteins because they are also part of many of the growth factor signalling cascades underlying the tumour–stroma interplay. We refer to recent reviews for a comprehensive overview on the role of other ion channels and transporters in cell migration (Schwab et al., 2012; Stock et al., 2013; Schwab and Stock, 2014).
The tumour microenvironment (TME)
The progression of cancer requires genetic instability and a highly selective local TME. We therefore have to determine the environmental changes and corresponding adaptive cellular responses of cancer cells to explain their aggressive migrating phenotype (Gillies et al., 2012). This includes processes shown in Figure 1, which either depend on migration of tumour and stroma cells or that regulate their migratory activity. During tumour progression, the stroma evolves over time to actively support tumour growth. It can form up to 90% of total tumour volume as observed in pancreatic ductal adenocarcinoma (PDAC) (Li et al., 2010; Neesse et al., 2011). This excessive amount of PDAC stroma, also known as desmoplasia, is the result of a massive deposition of the extracellular matrix (ECM) components (predominantly collagen I) from constitutively active fibroblasts and so-called stellate cells (Bachem et al., 2005; Masamune et al., 2008). Desmoplasia leads to poor vascularization and thereby to the development of a progressively hypoxic and acidic environment, which further increases tumour aggressiveness, in part via hypoxia-inducible factor-1α (HIF-1α) and reactive oxygen species (ROS) signalling (Apte et al., 2004; Masamune et al., 2008; Webb et al., 2011; Erkan et al., 2012). A number of transport (and associated) proteins involved in stroma and tumour cell migration are HIF-1α-dependently up-regulated (Ivanov et al., 2001; Koukourakis et al., 2006; Tajima et al., 2006; Lauritzen et al., 2012). So far, there are only few reports on a similar HIF-1α dependence of TRP channel expression in tumours (Chigurupati et al., 2010).
Figure 1.
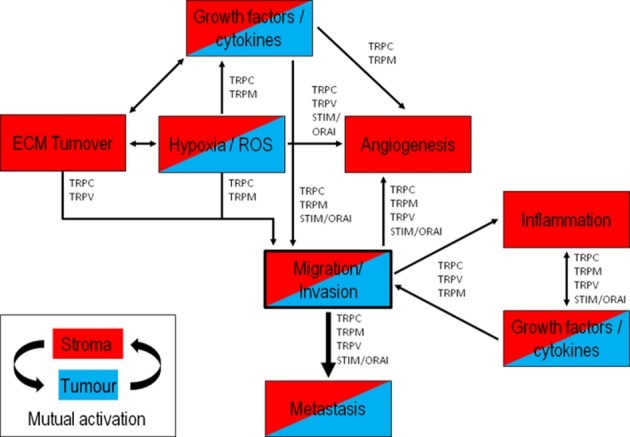
Major mechanisms of the tumour–stroma interplay in cancer progression. This illustration depicts major mechanisms in tumour progression involving either cancer cells (blue), stroma cells (red) or both (red/blue), all at some point being connected to cell migration. During tumour progression, cancer and stroma cells undergo a close mutual interaction with each other through continuous growth factor signalling. This shapes the tumour microenvironment and induces hypoxia and cellular oxidative stress, inflammatory responses, angiogenesis and ECM production/remodelling. The contributions of TRP channel families and STIM/ORAI proteins to different aspects of the cancer–stroma interplay underlying tumour invasion and metastasis are indicated.
The mutual interplay between cancer and stroma cells
Three classes of stromal cells can be distinguished: angiogenic vascular cells, cancer-associated fibroblastic cells and infiltrating immune cells (Shimoda et al., 2010; Hanahan and Coussens, 2012) such as tumour-associated macrophages and tumour-associated neutrophils (Joyce and Pollard, 2009; Roussos et al., 2011). One common feature of stroma cells is their ability to migrate, which enables them to reach the tumour site and then recruit more cells to the tumour area such as immune cells. At the same time, it supports migration of cancer cells during the metastatic cascade (Kalluri and Zeisberg, 2006; Fukuda et al., 2012).
The crosstalk between cancer and stroma cells largely depends on growth factors and cytokines secreted by both cell types (Joyce and Pollard, 2009; Sleeman et al., 2012). They act in an auto and paracrine way and lead to the mutual activation of tumour and stroma cells in the sense of a positive feedback loop. Prominent examples of TME-associated growth factors include the EGF, PDGF and VEGF families. They are complemented by different interleukins and chemokines predominantly secreted by immune cells (Mantovani et al., 2002; Bhowmick et al., 2004; Allavena et al., 2011). Growth factors are frequently released by matrix metalloproteinase (MMP) from ECM proteins to which they are bound (Li et al., 2007; Barkan et al., 2010; Kessenbrock et al., 2010). Binding of these factors to their receptors can lead to the activation of TRP channels and/or STIM/ORAI proteins and the initiation of (local) intracellular Ca2+ signalling cascades (Gkika and Prevarskaya, 2009; Tajeddine and Gailly, 2012; Lindemann et al., 2013). Sustained growth factor signalling of tumour and stromal cells culminates in the activation of the TME with induction of angiogenesis, ECM production/remodelling, sustained proliferation, tumour-promoting inflammation and migration/invasion. These processes can be linked to the functional expression of TRP channels and STIM/ORAI proteins as regulators of tumour and stroma cell migration (see Figure 1) (Wyckoff et al., 2004; Joyce and Pollard, 2009; Brabek et al., 2010; Chen et al., 2013a; Fioro and Gkika, 2013; Prevarskaya et al., 2014).
The discovery of the ability of stroma cells to co-metastasize to distant organs (Xu et al., 2010) and their possible role in guiding invasive cancer cells through the ECM has further highlighted the significance of the migration of stroma cells (Friedl and Wolf, 2009; Friedl and Alexander, 2011). Thus, in an in vitro setting, collective invasion of squamous cell carcinoma cells depended on the presence of fibroblasts. They created cell tracks within the matrix for the cancer cells to follow (Gaggioli et al., 2007). Breast cancer cells and macrophages employ a paracrine loop consisting of CSF-1 produced by carcinoma cells and EGF from macrophages to drive cell migration and invasion (Goswami et al., 2005). Finally, we would like to point out that activation of stroma cells within the tumour can also lead to altered ion channel expression. This is exemplified in a breast cancer-derived endothelial cell line (BTEC). In these cells, the expression of TRPV4 channels that are involved in tumour angiogenesis was significantly higher than in endothelial cells derived from normal breast tissue (Fiorio et al., 2008; 2012,).
Calcium-dependent signalling in cell migration
Polarization along the axis of movement together with cytoskeletal and membrane dynamics is fundamental for cell motility regardless of the respective (patho-) physiological function (Nabi, 1999; Pollard and Borisy, 2003; Anderson et al., 2008; Le Clainche and Carlier, 2008; Keren, 2011). This is in part mediated by a gradient of the intracellular calcium concentration ([Ca2+]i) within migrating cells with [Ca2+]i being higher at the rear end than at the front (Brundage et al., 1991; Schwab et al., 1997). It allows different components of the cellular migration machinery including focal adhesions, receptors and ion channels to be functional either at the cell front or rear end (Eddy et al., 2000; Broussard et al., 2008; Schwab et al., 2012; Stock et al., 2013). In addition, the front-rear Ca2+ gradient can be superimposed by locally elevated Ca2+ zones and short-lived Ca2+ flickers that play a role in regulating the directionality of migrating cells (Fabian et al., 2008; Wei et al., 2009; Tsai and Meyer, 2012). In that way, cells are able to fine-tune their molecular repertoire to the local microenvironment and extracellular guidance cues (Friedl and Wolf, 2009). Numerous components of the cellular migration machinery are Ca2+ sensitive. They affect cytoskeletal remodelling, focal adhesion turnover, matrix degradation, leading edge guidance or localized cell volume changes (Schwab et al., 2012; Falke and Ziemba, 2014). A rise of [Ca2+]i can trigger the dynamic formation of lamellipodia through Rac1 and thereby increase migration or induce stress fibres through RhoA activity and inhibit cell migration (Etienne-Manneville and Hall, 2002; Singh et al., 2007; Tian et al., 2010). Ca2+ signalling induces (i) contraction of the actomyosin network (Yang and Huang, 2005); (ii) activation of calpain (Jang et al., 2010), which is required for ECM matrix modelling by regulating MMP2 and 9 activity (Monet et al., 2010; Sukumaran et al., 2013), and regulation of focal adhesion turnover (Lawson and Maxfield, 1995; Giannone et al., 2002; 2004,; Wells et al., 2005; Svensson et al., 2010; Schafer et al., 2012; Zhao et al., 2012a); and (iii) induction of localized changes of the cell volume of migrating cells (Schwab et al., 1995; Schneider et al., 2000; Watkins and Sontheimer, 2011; Happel et al., 2013). These examples show that cell migration can be seen as a Ca2+-dependent signalling process, which can be linked to both Ca2+ influx through plasma membrane channels and/or Ca2+ release from internal Ca2+ stores.
TRP channels and STIM/ORAI proteins family
TRP channels are expressed ubiquitously throughout the body. They can be divided into subfamilies and subgroups based on amino acid sequence homology, mode of activation and function. The reader is referred to previous reviews on the subject (Pedersen et al., 2005; Nilius and Owsianik, 2011). Most TRP channels are non-selective cation channels that are permeable to Ca2+ and Na+ (PCa/PNa = 1–10). Nonetheless, most studies dealing with TRP channels in cell migration related the functional impact of TRP channels to their Ca2+ permeability. For the purpose of this review, it is noteworthy that TRPM6 and TRPM7 are also Mg2+ permeable (Owsianik et al., 2006).
TRP channels can be activated by diverse intra- and extracellular stimuli that are either of physical (e.g. temperature, osmotic pressure or mechanical stress) or chemical nature (e.g. pH, pO2, ROS, neurotransmitters, growth factors/cytokines, environmental irritants). Several of these stimulants are characteristic for the TME (as discussed earlier). This enables TRP channels to act as multifunctional cellular sensors, which are in an ideal position to respond to the evolving physical-chemical composition of the TME during tumour progression (Chen and Barritt, 2003; Dietrich et al., 2007; Bharate and Bharate, 2012). In this review, we will be focusing on members of the TRPC (Canonical), TRPV (Vanilloid) and TRPM (Melastatin) channel families as well as the STIM/ORAI complex (for nomenclature see Alexander et al., 2013a). These channel families are attractive candidates for probing the TME because at least some of their members are exquisitely sensitive to components of the TME. For example, TRPC channels are part of GPCR and receptor tyrosine kinase (RTK) signalling cascades mediating receptor-operated calcium entry (ROCE) (Ambudkar and Ong, 2007), and members of the TRPM family such as TRPM2 are activated by oxidative stress that is frequently encountered in tumours (Ray et al., 2012; Takahashi et al., 2012; Tochhawng et al., 2013).
Because GPCR signalling plays a central role in tumour pathophysiology, we will also address the role of the highly Ca2+-selective STIM/ORAI proteins. GPCR activation leads to the production of inositol-1,4,5 triphosphate (IP3) and Ca2+ release from intracellular stores into the cytosol. This, in turn, induces store-operated calcium entry (SOCE) (Minke and Cook, 2002; Clapham, 2003) mediated by STIM/ORAI proteins with STIM being the endoplasmatic reticulum (ER) Ca2+ sensor and ORAI the Ca2+-selective Ca2+ entry channel (Soboloff et al., 2006; 2012,; Cahalan, 2009; Sours-Brothers et al., 2009).
TRP channels and STIM/ORAI proteins functionally cooperate with other channels relevant for cell migration (Schwab et al., 2012). On the one hand, they supply Ca2+-sensitive channels such as KCa3.1, KCa2.3, CaCC (ANO/TMEM16) or ClC-3 with Ca2+ needed for their activation (Chantome et al., 2013; Cuddapah et al., 2013; Jacobsen et al., 2013; Turner and Sontheimer, 2013; Wanitchakool et al., 2014). On the other hand, TRP channels and STIM/ORAI proteins rely on the activity of K+ channels that hyperpolarize the cell membrane potential in order to maintain the electrochemical driving force for Ca2+ entry (Gao et al., 2010; Hammadi et al., 2012). Such functional cooperation has been shown to be needed for efficient migration, invasion and metastases of different cell types (Hammadi et al., 2012; Kuras et al., 2012; Siddiqui et al., 2012; Chantome et al., 2013; Chimote et al., 2013; Cuddapah et al., 2013; Turner and Sontheimer, 2013).
TRP channels and the cytoskeleton
TRP channels are engaged in a reciprocal interplay with the cytoskeleton. TRP channels can control the intracellular milieu for cytoskeletal dynamics (Clark et al., 2008). However, they can also be regulated by the cytoskeleton. For example, the interaction of TRPC1 channels with the calcium sensor STIM1 depends on an intact actomyosin cytoskeleton (Lopez et al., 2006). Actin depolymerization with calyculin A was reported to induce the internalization of TRPC channels, thereby blocking calcium entry in human neutrophils (Itagaki et al., 2004). TRPV1 and TRPV4 channels directly interact with actin and microtubule-enriched regions in larger signalling complexes synergistically regulating cell migration (Goswami et al., 2006; 2010,). The bidirectional regulation between TRP channels and the cytoskeleton mostly occurs through larger protein complexes in which TRP channels are linked to the actomyosin cytoskeleton, which thereby localizes signal transduction pathways and/or enhances the signal strength. These macromolecular protein complexes also include several signal transduction or scaffold proteins (Tang et al., 2000; Clark et al., 2006; Vandebrouck et al., 2007; Smani et al., 2013). Thus, Homer adaptor proteins are involved in the regulation of TRPC and ORAI channel gating in mammals (Yuan et al., 2003; 2012,; Jardin et al., 2012). However, the functional role of Homer proteins in SOCE regulation and coupling to TRPC, STIM and ORAI is still debatable and might depend on the idiosyncrasy of the cellular models investigated. Dissociation of TRPC1 from Homer1 has been proposed to be essential for SOCE activation by allowing TRPC1 to interact with STIM1 in HEK 293 cells (Yuan et al., 2003; 2012,). In human platelets, SOCE requires the association of Homer1 with TRPC1 and the IP3 receptor 2 (IP3R2, for nomenclature see Alexander et al., 2013b) together with its binding to STIM1 and ORAI1 (Jardin et al., 2012). This enables regulation of TRP channels together with the STIM/ORAI complex at multiple levels, as interference of these macromolecular complexes can affect all its members together with downstream effectors involved in cell migration.
TRP channels and STIM/ORAI proteins as sensors and effectors of the TME
Being membrane proteins, TRP channels and STIM/ORAI proteins have the ability to sense and react to various intra- and extracellular stimuli known to occur in the TME. Major stimulants characteristic for the TME include (i) hypoxia and, as a consequence of the resulting oxidative stress, (ii) ROS and (iii) ADP ribose (ADPr) (Waris and Ahsan, 2006). Hypoxia is a typical feature of solid tumours. It is due to an imbalance between oxygen demand and insufficient vascularization as well as tumour anaemia (Hanahan and Weinberg, 2011; Webb et al., 2011). So far, there is limited information about the impact of tumour hypoxia on TRP channel function although pO2 is one of the environmental factors that are of particular importance for tumour progression. TRP channels can act both as sensors and effectors of the above-mentioned hypoxia-related stimuli by increasing their expression and/or activity and thereby mediate the respective cellular response (Numata et al., 2013). These cell responses often involve elevated migratory activity and/or production/secretion of cytokines (illustrated in Figure 2) (Yamamoto et al., 2008; Chigurupati et al., 2010; Bauer et al., 2012; Tochhawng et al., 2013).
Figure 2.
Multiple sensor and effector functions of TRP channels and STIM/ORAI proteins in the tumour microenvironment. Illustration of major hypoxia and cellular oxidative stress-dependent mechanisms in cancer and stroma cells involving TRP channels and STIM/ORAI proteins. Hypoxic and oxidative stress can lead to up-regulation of TRP channels (e.g. TRPC1, TRPC3 and TRPC6) and STIM/ORAI proteins and mediate the production of ROS and ADPr. In cancer and stroma cells, TRP channels and STIM/ORAI proteins can have both a sensor function for extra-/intracellular stimuli mediating cellular responses and a effector function by increased expression and activation to induce chemokine/cytokine production in these cells. For the sake of clarity, this sketch does not include all signalling pathways mentioned in the text. Neither did we include all microenvironmental, growth factor- and chemokine-activated pathways involved in increased activation or expression of TRP channels and STIM/ORAI proteins. (Figure modified from Stock et al., 2013.)
Recently, TRP channels were found to function as sensors of oxygen availability (see Numata et al., 2013, for a review). TRP channels are either oxygen sensing themselves like TRPA1 channels in murine vagal and sensory neurons (Takahashi et al., 2011), or their expression is regulated by pO2 or their activity is indirectly regulated by pO2. Acute hypoxic pulmonary vasoconstriction involves the activation of TRPC6 channels (Weissmann et al., 2006) and the TRPC1-STIM1-ORAI1 complex is needed for regulating hypoxia-induced SOCE in pulmonary arterial smooth muscle cells (Lu et al., 2008; Ng et al., 2012). Hypoxic stress induces the expression of TRPM2 channels in cardiac fibroblasts leading to increased proliferation and ECM production (Takahashi et al., 2012). In glioblastoma, expression of TRPC6 channels is higher than in normal brain tissue. The elevated TRPC6 expression was replicated in in vitro experiments in which hypoxia increased TRPC6 channel expression in glioblastoma cells through a notch signalling pathway. Furthermore, suppression of TRPC6 greatly inhibited glioblastoma cell migration and invasion in response to hypoxia, possibly by inhibiting actin–myosin interactions (Chigurupati et al., 2010).
Notably, hypoxia facilitates the production of ROS (Cook et al., 2004; Waris and Ahsan, 2006; Yang et al., 2013b). ROS often lead to oxidative stress and can also be generated as a result of growth factor stimulation of RTKs and thereby transmit signals to induce cellular changes necessary for migration by affecting several of the previously mentioned Ca2+-sensitive effector molecules (Hurd et al., 2011; Ray et al., 2012; Tochhawng et al., 2013). This points towards a coupling between ROS and Ca2+ ions as stress-response messengers. This coupling is mediated at least in part by TRP channels and STIM/ORAI proteins (Figure 2) (Hawkins et al., 2010; Soboloff et al., 2012; Numata et al., 2013).
In PDAC cells, the expression of the NAD+-dependent stress responsive protein sirtuin 6 (SIRT6) enhances the production of ADPr. Furthermore, ADPr triggers Ca2+ signalling mediated by TRPM2 channels that promote the expression of the pro-inflammatory factors IL-8 and TNF-α and enhance cancer cell migration (Bauer et al., 2012). TRPM2, as well as TRPC3 channels, have also been demonstrated to serve as a sensor for oxidative stress in B-lymphoblasts which could enable the cells to reach or orient within the tumour (Roedding et al., 2012). ROS-dependent activation of TRPM2 channels leading to IL secretion has also been observed in other immune cells such as monocytes and neutrophils (Yamamoto et al., 2008; Wehrhahn et al., 2010; Knowles et al., 2011).
Hypoxic and pro-inflammatory conditions promote cellular stress and damage leading to an increase in intracellular NAD levels (Hong et al., 2009). The ectoenzyme CD38, which is up-regulated in immune and cancer cells, mediates increased cADPr and ADPr generation from NAD (for a review, see Malavasi et al., 2008; Vaisitti et al., 2011). ADPr binds to the TRPM2 channel leading to Ca2+ influx (Partida-Sanchez et al., 2007), which enhances the intracellular chemoattractant signal enabling chemotaxis of tumour and stroma cells (Vaisitti et al., 2011). Additionally, neutrophil and monocyte chemotaxis to ligands for several chemokine and chemoattractant receptors, including CCR1, CCR2, CCR5, CCR7, CXCR4, N-formyl peptide receptor (FPR) 1 and FPR2 (for receptor nomenclature see Alexander et al., 2013c), also requires CD38-dependent Ca2+ signalling (Partida-Sanchez et al., 2001). In granulocytes, the inflammatory process of NADPH oxidase-mediated superoxide production could be related to TRPC1, TRPC3, TRPC6 and ORAI1 channels (Brechard et al., 2008) (Figure 2). The activity of NADPH oxidase in ROS production is known to be relevant for cancer as well (Yang et al., 2013b), and its activity has been observed to be regulated by growth factors in pancreatic cancer (Edderkaoui et al., 2011). Taken together, these studies show that TRP channel expression and activity in both cancer and stroma cells is effectively regulated by ROS. The resulting cytokine/chemokine production can then support the recruitment of additional stroma cells. The chemosensitivity of TRP channels therefore probably constitutes an important element in securing the communication between stroma and cancer cells within the TME.
TRP channels in stroma cell migration
A substantial amount of data connects TRPC, TRPV and TRPM channels to stroma cell migration such as that of fibroblasts and immune cells like monocytes/macrophages, neutrophils and lymphocytes. Table 1 provides an overview of those TRP channels that are involved in stroma cell migration and cytokine/chemokine production. Some are illustrated in Figure 2.
Table 1.
TRP channels and STIM/ORAI proteins in stroma cell migration and function
| Channel | Stroma cell type(s) | Function | Mechanism | Reference |
|---|---|---|---|---|
| TRPC1 | HL-60 granulocytes | fMLP-mediated Ca2+ mobilization (TRPC1,3,6 and ORAI1) activates NAPD oxidase | ROS production | (Brechard et al., 2008) |
| Synovial fibroblasts | Stretch-mediated Ca2+ entry induces migration | Loss of TRPC1 decreases their mechanical stretch-induced change in the direction of migration | (Fabian et al., 2012) | |
| TRPC3 | Human monocytes | Increased expression leads to increased Ca2+ mobilization, Akt and ERK signalling, and chemotaxis | Chemotaxis | (Zhao et al., 2012b) |
| BLCL lymphocytes | ROS diminishes channel expression | ROS sensor | (Roedding et al., 2012) | |
| TRPC6 | Murine fibroblasts | Activation of p38 MAPK and SRF | Fibroblast-myofibroblast transformation | (Davis et al., 2012) |
| Murine neutrophils | Ca2+ mobilization regulates Akt and MAPK signalling | CXCR2-mediated chemotaxis | (Lindemann et al., 2013) | |
| Human neutrophils | Channel activation via selectin signalling | Chemotaxis | (McMeekin et al., 2006) | |
| TRPV2 | RAW264 macrophages | LPS induces TRPV2 Ca2+ mobilization leading to IL-6 and TNF-α production | Chemokine production | (Yamashiro et al., 2010) |
| Murine macrophages | Knock-down of TRPV2 impairs chemoattractant-elicited cell motility | Involved in phagocytosis and cell motility | (Link et al., 2010) | |
| TRPV4 | Breast cancer-derived endothelial cells | Arachidonic acid-induced actin remodelling and increase in TRPV4 expression and function | Increased migration | (Fiorio et al., 2012) |
| TRPM2 | Human monocytes | LPS induces TRPM2 Ca2+ mobilization leading to IL-6, IL-9, IL-10 and TNF-α production | Chemokine production | (Wehrhahn et al., 2010) |
| T-lymphocytes | T-cell receptor triggering activates TRPM2 | Cytokine secretion | (Melzer et al., 2012) | |
| U937 monocytes | ROS induces TRPM2 Ca2+ mobilization leading to IL-8 production via ERK signalling | Chemokine production | (Yamamoto et al., 2008) | |
| Murine T-cells | Regulation of IL-12 production | Cytokine production | (Knowles et al., 2011) | |
| Murine neutrophils | CD38 triggered by ADPr production TRPM2 Ca2+ mobilization | Chemotaxis | (Partida-Sanchez et al., 2007) | |
| BLCL lymphocytes | ROS diminishes TRPM2 channel activity | ROS sensor | (Roedding et al., 2012) | |
| TRPM4 | Jurkat T-cells | Down-regulation of Ca2+ signalling and IL-2 production | Regulation of chemokine production | (Launay et al., 2004) |
| Murine T-cells | NFAT regulation | Migration and chemokine production | (Weber et al., 2010) | |
| TRPM7 | 3T3 fibroblasts | Rac and Cdc42 activation | Regulation of polarization and migration | (Su et al., 2011) |
| WI-38 fibroblasts | Ca2+ flickers at the leading edge regulate turning of the cells | Regulation of PDGF chemotaxis | (Wei et al., 2009) | |
| Human T-cells | Ca2+ mobilization at the uropod | Cell migration | (Kuras et al., 2012) | |
| ORAI1 | Human and murine neutrophils | SOCE during neutrophil transition from rolling to arrest | Recruitment and actin polarization in intravascular crawling | (Schaff et al., 2010; Dixit et al., 2011) |
| STIM1/ORAI1 | Murine T-cells | Involved in SOCE | Loss of expression leads to reduced cytokine production | (Gwack et al., 2008; Oh-Hora et al., 2008) |
| STIM1/2 | Murine T-cells | STIM1 and STIM2 are critical for SOCE | Chemotaxis and cytokine production | (Ma et al., 2010) |
| Mast cells | STIM1 promotes the Ca2+ influx essential for mast cell activation and function | Lacking STIM1 leads to less degranulation and cytokine production | (Baba et al., 2008) | |
| Murine lymphocytes | STIM1 functions as a redox sensor to constitutively activate ORAI channels under oxidative stress | Mitochondrial Ca2+ overload and alterations in cellular bioenergetics | (Hawkins et al., 2010) |
Presently, it is assumed that the impact of most TRP channels on cell migration is due to their ability to mediate Ca2+ entry, for example, following the activation of growth factor or chemoattractant receptors (GPCR and RTK). Thereby, they are elements of the respective intracellular signalling cascades such as the phosphatidylinositol-3 kinase (PI3K) pathway, MAPK and the Ras-homologue-(Rho)-GTPases, which almost all depend on Ca2+ and affect cell migration (Falke and Ziemba, 2014). Rac and Cdc42 activation, for example, is regulated by guanine-nucleotide-exchange factors which can be activated by PI3K-mediated PIP3 production and via an increase in the [Ca2+]i (Benard et al., 1999; Schmidt and Hall, 2002; Fukata et al., 2003). PI3K activation could be linked to cytoskeletal reorganization, and ERK signalling regulates the actomyosin network by activation of Rho and myosin-II (Li et al., 2013). Several studies showed that different TRP channels elicit their effect on migration of stromal cells via these pathways. Examples include TRPM7-dependent polarization and migration of fibroblasts (Su et al., 2011), TRPC6-dependent chemotaxis of neutrophils towards ligands of the CXCR2 receptor (Damann et al., 2009; Lindemann et al., 2013), chemotaxis of monocytes towards fMLP relying on TRPC3 channels (Zhao et al., 2012b) or TRPV2-dependent migration of macrophages (Link et al., 2010).
In human neutrophils, platelet-activating factor-induced Ca2+ mobilization is prolonged by E-selectins in a TRPC6-dependent way (McMeekin et al., 2006) pointing to a role of TRPC6 channels in neutrophil extravasation. Such a mechanism would also be relevant for tumour cells when leaving blood or lymph vessels at the site of metastasis. Finally, we already mentioned the role of TRPM2 in cell migration of neutrophils and monocytes which is mediated via CD38-mediated production of ADPr (Partida-Sanchez et al., 2007; Vaisitti et al., 2011).
Growth factor and cytokine secretion
TRP channel-dependent secretion of cytokines constitutes an indirect mechanism by which TRP channels contribute to the regulation of (directed) cancer and stroma cell migration. Thus, LPS triggers RAW264 macrophages to produce IL-6 and TNF-α upon Ca2+ entry via TRPV2 channels (Yamashiro et al., 2010). Similarly, TRPM2 channels underlie enhanced cytokine/chemokine production in activated T-lymphocytes (Melzer et al., 2012) and monocytes (Yamamoto et al., 2008). The overexpression of a dominant-negative mutant of TRPM4 or elimination of TRPM4 using RNAi in Jurkat T-cells induces enhanced Ca2+ signalling and increased IL-2 production (Launay et al., 2004). In mouse T-cells, TRPM4 channels regulate the [Ca2+]i in a similar way and affect cell motility and IL-2 as well as IL-4 production by controlling the nuclear translocation of nuclear factor of activated T-cells (Weber et al., 2010). In addition, TRP channel activity itself can be regulated by cytokines. Myofibroblast transformation is supported by TRPC6 channels, which are activated by TGF-β (Davis et al., 2012).
TRP channels in cancer metastasis and invasion
TRP channel expression is altered during cancer progression. In fact, TRPM1 was originally identified as a tumour-suppressor gene in melanoma so that increased TRPM1 expression was associated with reduced metastatic and migratory potential (Duncan et al., 1998). Current knowledge indicates that increased or decreased TRP channel expression depends on the cancer type and cancer stage. TRP channels expression is particularly well studied in glioblastoma as well as in breast and prostate cancers. Even if their precise function has not yet been fully elucidated in all cancer types, their dysregulated expression represents a valuable diagnostic and/or prognostic marker. We refer to the following reviews for more details (Bodding, 2007; Prevarskaya et al., 2007; Van Haute et al., 2010; Santoni and Farfariello, 2011; Ouadid-Ahidouch et al., 2013). TRP channels whose expression in tumour cells is frequently dysregulated include TRPC1 and 6, TRPM7, TRPM8, and TRPV2 and 6. Their expression strongly correlates with tumour aggressiveness in different cancer types, as observed in human breast ductal adenocarcinoma. TRPV6 is mainly overexpressed in the invasive breast cancer cells and not in the corresponding non-invasive ones. Down-regulating TRPV6 in two breast cancer cell lines, MDA-MB-231 and MCF-7, reduced cell migration and invasion (Dhennin-Duthille et al., 2011). Table 2 provides an overview of those TRP channels that contribute to cancer cell migration.
Table 2.
TRP channels and STIM/ORAI proteins in cancer cell migration
| Channel | Cancer cell type(s) | Function | Mechanism | Reference |
|---|---|---|---|---|
| TRPC1 | Glioblastoma | EGF-stimulated localization to leading edge in migration | Chemotaxis towards EGF | (Bomben et al., 2011) |
| MDCK-F cells | Inhibition or down-regulation affects cell polarization, FGF-2 chemotaxis and stretch activation | Chemotaxis towards FGF-2 involved in mechanosignalling | (Fabian et al., 2008; 2011; 2012,,) | |
| BxPC3 PDAC cells | TGF-β-induced Ca2+ responses | Increased motility and invasion | (Dong et al., 2010) | |
| TRPC2 | FRTL-5 thyroid cells | Regulates Rac and calpain activity | Down-regulation decreases cell migration | (Sukumaran et al., 2013) |
| TRPC3 | MCF-7 breast cancer cell | SOCE/ROCE function. Polyunsaturated fatty acids inhibit TRPC3. | Increased migration and invasion | (Zhang et al., 2012) |
| TRPC6 | Glioblastoma | Increased expression through hypoxia-induced notch signalling | Knock-down inhibits migration and invasion | (Chigurupati et al., 2010) |
| Head and neck squamous cell carcinomas | Increased expression in cell lines and tumour tissue | Knock-down inhibits invasion | (Bernaldo de Quiros et al., 2013) | |
| TRPV1 | Hepatoblastoma(HepG2) | HGF increases TRPV1 channel activity | Increased migration | (Waning et al., 2007) |
| TRPV2 | PC3 and LNCaP prostate cancer cells | Lysophosphatidylcholine and lysophosphatidylinositol induced calcium influx by PI3,4K pathway | Increased expression and migration | (Monet et al., 2009) |
| PC3 xenograft tumours in mice | Induction of MMP2, MMP9 and cathepsin B | (Monet et al., 2010) | ||
| PC-3 prostate cancer cells and urothelial carcinoma cells T24/83 | Adrenomedullin induced membrane expression followed by increased activity | Increase in migration and invasion | (Oulidi et al., 2013) | |
| TRPV4 | Hepatoblastoma (HepG2) | Increased lamellipodial dynamics at frontal region of migrating cells | Increased migration | (Waning et al., 2007) |
| TRPV6 | MDA-MB-231 and MCF-7 breast cancer cells | Increased expression in non-invasive (MCF-7) and invasive (MDA-MB-231) cells | TRPV6 silencing reduced migration and invasion | (Dhennin-Duthille et al., 2011) |
| TRPM1 | B16-F1 melanoma cells | High expression in poor metastatic variants and increased expression in highly metastatic variants | Functional expression reduces metastasis and migratory potential and vice versa | (Duncan et al., 1998) |
| TRPM2 | BxPC-3 PDAC cells | Increased activation through SIRT6-elevated ADPr levels, an activator of TRPM2 | Increased migration | (Bauer et al., 2012) |
| TRPM7 | N1E-115 neuroblastoma cells | Activation affects actomyosin contractility and cell adhesion | Increased cell spreading through BK channel activation | (Clark et al., 2006) |
| MDA-MB-435 breast cancer cells | TRPM7 modulation involving the Src-MAPK signalling pathway | Silencing TRPM7 reduces cell migration and invasion | (Meng et al., 2012) | |
| MDA-MB-231 breast cancer cells | Polymerization of the cytoskeleton | Silencing TRPM7 impairs migratory and metastatic properties | (Middelbeek et al., 2012) | |
| BxPC-3 PDAC cells | Increased expression in PDAC and contribution to Mg2+ entry | Silencing TRPM7 reduced cell migration | (Rybarczyk et al., 2012) | |
| 5-8F and 6-10B nasopharyngeal carcinoma cells | Controlling Ca2+ influx | Increased migration | (Chen et al., 2010) | |
| A549 lung cancer cells | Basal and EGF-induced migration | Increased migration | (Gao et al., 2011) | |
| TRPM8 | Glioblastoma | Menthol and HGF/SF increases [Ca2+]i by activating TRPM8 | Increased migration through BK channel activation | (Wondergem et al., 2008; Wondergem and Bartley, 2009) |
| PC-3 prostate cancer cells | Overexpression of TRPM8 inactivates focal adhesion kinase | Decreased migration | (Yang et al., 2009b) | |
| PC-3 prostate cancer cells | PSA activated TRPM8 via the bradykinin 2 receptor signalling pathway | Decreased migration | (Gkika et al., 2010) | |
| STIM1/ORAI1/ | Glioblastoma | Increased expression of both ORAI1 and STIM1 | Increased migration | (Motiani et al., 2013a) |
| Hepatocellular carcinoma cells (HCC-LM3) | Regulate de-phosphorylation of focal adhesion kinase, and by that modulate focal adhesion turnover | STIM1 silencing and SOCE inhibitor inhibited migration and invasion | (Yang et al., 2013a) | |
| MDA-MB-231 breast cancer cells and mouse tumour | Implicated in serum-induced migration. Modulate focal adhesion turnover through Ras and Rac1 | Increased migration and invasion | (Yang et al., 2009a) | |
| ORAI1 | Human breast cancer cell line MDA-MB-435s | Colocalized in lipid rafts with KCa3.2 to regulate Ca2+ influx and calpain activity | Involved in migration and bone metastases | (Chantome et al., 2013) |
| STIM1/ORAI3/ | MCF-7 breast cancer cells (ER+ breast cancer cells) | EGF and thrombin mediated Ca2+ entry and ERK, focal adhesion kinase and NFAT regulation | Increase in tumourigenesis and invasion | (Motiani et al., 2010; 2013b,) |
In migrating glioblastoma cells, TRPC1 channels were detected in lipid rafts at the leading edge where they are needed for directed migration in an EGF gradient (Bomben et al., 2011). Their mode of action in controlling directional migration was investigated in more detail in transformed renal epithelial (MDCK-F) cells (Fabian et al., 2008; 2011; 2012,,). TRPC1 channels elicit a local Ca2+ microdomain at the leading edge. Its lack following TRPC1 ablation cannot be compensated for by external cues so that TRPC1-deficient MDCK-F cells are unable to chemotax towards FGF-2 (Fabian et al., 2011). TRPC1 channels also mediate TGF-β-induced Ca2+ responses associated with migration in human PDAC cells (Dong et al., 2010). Another member of the TRPC family, TRPC3, participates in two Ca2+ influx ways in MCF-7 breast cancer cells: SOCE and ROCE. Both ways are needed for cell migration so that their inhibition with polyunsaturated fatty acids impairs cell migration (Zhang et al., 2012). TRPC6 channels are also highly expressed in head and neck squamous cell carcinoma tumour samples and cancer cell lines. Their down-regulation inhibits cell invasion and cell migration (Bernaldo de Quiros et al., 2013). Moreover, in rat thyroid FRTL-5 cells, down-regulation of TRPC2 channels inhibits cell migration and invasion by decreasing Rac, calpain and MMP2 activity important for ECM remodelling (Sukumaran et al., 2013; Zhang et al., 2013).
TRPM7 channels are involved in migration of multiple cancers including lung cancer (Gao et al., 2011), nasopharyngeal carcinoma (Chen et al., 2010), breast cancer (Middelbeek et al., 2012) and PDAC (Rybarczyk et al., 2012). While the pro-migratory role of TRPM7 channels in nasopharyngeal carcinoma cells was linked to Ca2+ influx (Chen et al., 2010), that in PDAC cells was due to Mg2+ influx (Rybarczyk et al., 2012). TRPM7 has also been found to increase actomyosin reorganization and cell adhesion in spreading of N1E-115 neuroblastoma cells by cooperating with KCa1.1 channels (Clark et al., 2006). In MDA-MB-231 breast cancer cells, TRPM7 regulates myosin-II-based cellular tension and thereby modifies focal adhesion number, cell-cell adhesion and polarized cell movement (Middelbeek et al., 2012). TRPM7 knock-down increases focal adhesions and impairs migratory and metastatic properties of MDA-MB-231 and -435 breast cancer cells (Meng et al., 2012; Middelbeek et al., 2012). TRPM7 channels were therefore suggested to be part of a mechanosensory complex adopted by cancer cells to drive metastasis formation (Middelbeek et al., 2012). This is a similar role as seen in fibroblast migration (Wei et al., 2009) and for TRPC1 channels in MDCK-F cell migration (Fabian et al., 2012).
A connection to KCa1.1 channels was also observed for TRPM8 in glioblastoma cell migration. Menthol and hepatocyte growth factor induced TRPM8-mediated Ca2+ influx, which further activated KCa1.1 channels important for sustaining increased glioblastoma cell migration (Wondergem et al., 2008; Wondergem and Bartley, 2009). In contrast, increased expression of TRPM8 in PC-3 prostate cancer cells correlated with a decrease of migration efficiency via inactivation of focal adhesion kinase (Yang et al., 2009b). Similarly, TRPM8 activation with prostate-specific antigen also decreased cell migration of PC-3 prostate cancer cells (Gkika et al., 2010). Mice transplanted with TRPM8-overexpressing PC-3 cells developed tumours that were less vascularized than control (Zhu et al., 2011). The apparently discrepant findings with respect to the role of TRPM8 channels in tumour cell migration could be either due to the different cell types (glioma vs. prostate cancer cells) or due to the expression of different TRPM8 isoforms in the plasma membrane and the ER (Gkika and Prevarskaya, 2009; 2011,; Van Haute et al., 2010).
In prostate cancer cells, lysophosphatidylcholine and lysophosphatidylinositol activate TRPV2 channels and thereby increase migration via the PI3,4K pathway (Monet et al., 2009) and increased expression of MMP2, 9 and cathepsin B (Monet et al., 2010). Similarly, adrenomedullin, a peptide originally isolated from human phaeochromocytoma (Kitamura et al., 1993), stimulates prostate and urothelial cancer cell migration and invasion by increasing TRPV2 membrane expression and activity (Oulidi et al., 2013). Activation of TRPV1 channels in human hepatoblastoma (HepG2) cells enhances migration, possibly via dynamic regulation of microtubules (Goswami et al., 2006; Waning et al., 2007). Additionally, activation of the mechanosensitive TRPV4 channels led to increased lamellipodial dynamics pointing to the importance of the mechanosensitivity of the frontal region of migrating cells (Waning et al., 2007). This observation was later supported in F11 neuroblastoma x DRG neuron hybrid cells, where TRPV4 interacted with polymerized actin and tubulin filaments (Goswami et al., 2010).
STIM/ORAI in the tumour–stroma interplay
Several studies have addressed the role of STIM/ORAI proteins function in cells of the immune system (Feske, 2009; Chen et al., 2013a; Shaw et al., 2013). They showed, among others, that ORAI1 is required for the recruitment of neutrophils (Schaff et al., 2010; Dixit et al., 2011) or T-lymphocytes (Waite et al., 2013) from the blood stream. Murine T-cells lacking STIM1 or ORAI1 show severe defects in the production of IL-2, IL-4 and IFN-γ (Gwack et al., 2008; Oh-Hora et al., 2008). In addition, STIM1 and STIM2 are critical for chemotaxis of T-cells and pro-inflammatory cytokine production (Ma et al., 2010). STIM1 was also found to be a key factor in promoting Ca2+ influx essential for mast cell degranulation and cytokine production (Baba et al., 2008).
These observations are relevant for mechanisms underlying anti-tumour immunity. For example, high lactate levels in tumours suppress the proliferation and cytokine production of tumour-specific CD8+ cytotoxic T-lymphocytes (Fischer et al., 2007). This study did not yet address the role of STIM/ORAI in this process. However, it was later found that CD8+ T-cells lacking STIM1/2 have impaired SOCE leading to a defect in the anti-tumour immunity together with preventing tumour engraftment and growth (Weidinger et al., 2013). Moreover, the above mechanisms may also be relevant for the extravasation of tumour cells at their site of metastasis and for the recruitment of inflammatory cells to the tumour stroma. Accordingly, silencing of ORAI1 impaired the extravasation of nasopharyngeal cancer cells in a zebrafish model (Zhang et al., 2013). Indeed, altered expression and function of STIM/ORAI proteins in cancer cells is crucial for their behaviour and thereby for patient prognosis (McAndrew et al., 2011). In several tumour cell types including human primary glioblastoma, as well as cervical, hepatocellular or breast cancer cells, STIM/ORAI proteins were found to control invasion and migration and thereby metastases (Motiani et al., 2013a). At present, a likely explanation for these effects is the modulation of the turnover of focal adhesions by (local) regulation of the [Ca2+]i (Yang et al., 2009a; 2013a,; Motiani et al., 2010; 2013b,; Chen et al., 2011; 2013b,). Interestingly, in breast cancer cells, ORAI1 colocalizes with KCa2.3 independently from STIM1 within lipid rafts promoting cancer cell migration and bone metastases (Chantome et al., 2013).
Concluding remarks and clinical perspectives
Intracellular Ca2+ is one of the most versatile messengers regulating a plethora of cell functions including cell migration. It reflects the balance between Ca2+ influx and efflux across the plasma membrane as well as release from and uptake into intracellular stores. TRP and ORAI channels are important constituents of the Ca2+ influx pathways. Consequently, they are important regulators of Ca2+-dependent functions of both cancer cells and their surrounding stroma cells, such as migration, growth factor production and adaptation to microenvironmental changes. Their prominent role in cancer development and progression can be related to the fact that the expressions of TRP channels and STIM/ORAI proteins are frequently dysregulated in cancer in a stage and cancer type-dependent manner (Van Haute et al., 2010; Santoni and Farfariello, 2011; Ouadid-Ahidouch et al., 2013). They share this property with many other ion channels such as K+, Na+ or Cl– (Prevarskaya et al., 2010; Britschgi et al., 2013).
So far, there is only relatively limited information about TRP channels and STIM/ORAI proteins in tumour stroma cells. Despite a wealth of data on the function of ion channels in these cells, the elucidation of their role in cancer is still at its beginning. However, the observation that TRPV4 channel expression in tumour-derived endothelial cells differed from that in normal endothelial cells (Fiorio et al., 2012) highlights the importance of investigating the composition of the transportome of tumour-derived stroma cells in more detail. Until now, we largely rely on ‘proof of principle’ studies performed in ‘normal’ stroma cells showing that TRP channels and STIM/ORAI proteins are central for migration and/or growth factor secretion. In this review, we therefore attempted to synthesize the available knowledge from mostly ‘non-cancer’ studies in order to point to the potential importance of TRP channels and STIM/ORAI proteins in tumour stroma cells. However, depending on the degree of dysregulation in the cancer stroma, their role may be over- or underestimated. Profiling of TRP channels and STIM/ORAI proteins in tumour stroma cells needs to be complimented by the identification of downstream effector molecules of the cellular migration apparatus. The elucidation of the roles of these channel families in regulating tumour and stroma cell migration and other pro-metastatic behaviour therefore still constitutes a novel area of future research in oncology.
Nonetheless, the current knowledge allows us to propose that TRP channels and STIM/ORAI proteins represent potential therapeutic, diagnostic and/or prognostic targets with clinical potential in oncology. This is in part due to the fact that they are not only involved in cell migration but also in other functions critical for cancer progression such as tumour cell proliferation. This has, among others, been observed for TRPC1 and TRPC6 in glioblastoma (Bomben and Sontheimer, 2010; Chigurupati et al., 2010; Ding et al., 2010; Bomben et al., 2011), ORAI3 in breast cancer (Motiani et al., 2010; 2013b,), and TRPM8 and TRPV2 in prostate cancer (Yang et al., 2009b; Monet et al., 2010). Important functions of TRP channels within endothelial cells such as angiogenesis and vascularization of the tumour have also been observed (Fiorio et al., 2008; 2012,; Lodola et al., 2012) (reviewed in Fioro and Gkika, 2013). Moreover, targeting TRP channels or STIM/ORAI proteins expressed in both cancer and stroma cells offers the potential for a ‘double hit’ and the potential to break the vicious cycle of mutual cancer and stroma cell stimulation. Their dysregulated expression and function in tumours may also confer some degree of specificity over those channels expressed in healthy organs. Finally, being membrane proteins, TRP channels or ORAI proteins are easily accessible from the extracellular side, which reduces the risk of multidrug resistance due to drug export from the cytoplasm. Thus, there is an urgent demand to develop specific modulators of TRP channels or STIM/ORAI proteins that would ideally target splice variants or differently expressed channels only found in cancer as observed for TRPM8 (Shimoda et al., 2006).
Acknowledgments
Our work was supported by European Commission (ITN ‘IonTraC’), Deutsche Forshungsgemeinschaft, Cells-in-Motion Cluster of Excellence (EXC 1003 – CiM), University of Münster, Germany, IZKF Münster and IMF Münster.
Glossary
- ECM
extracellular matrix
- ER
endoplasmatic reticulum
- HIF-1α
hypoxia-inducible factor-1α
- IP3
inositol-1,4,5 triphosphate
- NAD
nicotinamide adenine dinucleotide
- ORAI1
calcium release-activated calcium channel protein 1
- ROCE
receptor-operated calcium entry
- RTK
receptor tyrosine kinase
- SOCE
store-operated calcium entry
- STIM1
stromal interaction molecule 1
- TME
tumour microenvironment
- TRP
transient receptor potential
Conflict of interest
The authors do not have a conflict of interest.
References
- Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Exp Cell Res. 2011;317:664–673. doi: 10.1016/j.yexcr.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013a;170:1607–1651. doi: 10.1111/bph.12447. and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14:Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1607. doi: 10.1111/bph.12446. and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013c;170:1459–1581. doi: 10.1111/bph.12445. and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar IS, Ong HL. Organization and function of TRPC channelosomes. Pflugers Arch. 2007;455:187–200. doi: 10.1007/s00424-007-0252-0. [DOI] [PubMed] [Google Scholar]
- Anderson TW, Vaughan AN, Cramer LP. Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts. Mol Biol Cell. 2008;19:5006–5018. doi: 10.1091/mbc.E08-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- Arcangeli A. Ion channels and transporters in cancer. 3. Ion channels in the tumor cell-microenvironment cross talk. Am J Physiol Cell Physiol. 2011;301:C762–C771. doi: 10.1152/ajpcell.00113.2011. [DOI] [PubMed] [Google Scholar]
- Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46:1181–1188. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer I, Grozio A, Lasiglie D, Basile G, Sturla L, Magnone M, et al. The NAD+-dependent histone deacetylase SIRT6 promotes cytokine production and migration in pancreatic cancer cells by regulating Ca2+ responses. J Biol Chem. 2012;287:40924–40937. doi: 10.1074/jbc.M112.405837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Bergmeier W, Weidinger C, Zee I, Feske S. Emerging roles of store-operated Ca2+ entry through STIM and ORAI proteins in immunity, hemostasis and cancer. Channels. 2013;7:379–391. doi: 10.4161/chan.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaldo de Quiros S, Merlo A, Secades P, Zambrano I, de Santa Maria IS, Ugidos N, et al. Identification of TRPC6 as a possible candidate target gene within an amplicon at 11q21-q22.2 for migratory capacity in head and neck squamous cell carcinomas. BMC Cancer. 2013;13:116. doi: 10.1186/1471-2407-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharate SS, Bharate SB. Modulation of thermoreceptor TRPM8 by cooling compounds. ACS Chem Neurosci. 2012;3:248–267. doi: 10.1021/cn300006u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodding M. TRP proteins and cancer. Cell Signal. 2007;19:617–624. doi: 10.1016/j.cellsig.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Bomben VC, Sontheimer H. Disruption of transient receptor potential canonical channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia. 2010;58:1145–1156. doi: 10.1002/glia.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomben VC, Turner KL, Barclay TT, Sontheimer H. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. J Cell Physiol. 2011;226:1879–1888. doi: 10.1002/jcp.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabek J, Mierke CT, Rosel D, Vesely P, Fabry B. The role of the tissue microenvironment in the regulation of cancer cell motility and invasion. Cell Commun Signal. 2010;8:22. doi: 10.1186/1478-811X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechard S, Melchior C, Plancon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008;44:492–506. doi: 10.1016/j.ceca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S, et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci U S A. 2013;110:E1026–E1034. doi: 10.1073/pnas.1217072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Brundage RA, Fogarty KE, Tuft RA, Fay FS. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991;254:703–706. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- Cahalan MD. STIMulating store-operated Ca2+ entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantome A, Potier-Cartereau M, Clarysse L, Fromont G, Marionneau-Lambot S, Gueguinou M, et al. Pivotal role of the lipid Raft SK3-Orai1 complex in human cancer cell migration and bone metastases. Cancer Res. 2013;73:4852–4861. doi: 10.1158/0008-5472.CAN-12-4572. [DOI] [PubMed] [Google Scholar]
- Chen J, Barritt GJ. Evidence that TRPC1 (transient receptor potential canonical 1) forms a Ca2+-permeable channel linked to the regulation of cell volume in liver cells obtained using small interfering RNA targeted against TRPC1. Biochem J. 2003;373:327–336. doi: 10.1042/BJ20021904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Luan Y, You CX, Chen XH, Luo RC, Li R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca2+ influx. Cell Calcium. 2010;47:425–432. doi: 10.1016/j.ceca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Chen YT, Chiu WT, Shen MR. Remodeling of calcium signaling in tumor progression. J Biomed Sci. 2013a;20:23. doi: 10.1186/1423-0127-20-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR. The ER Ca2+ sensor STIM1 regulates actomyosin contractility of migratory cells. J Cell Sci. 2013b;126:1260–1267. doi: 10.1242/jcs.121129. [DOI] [PubMed] [Google Scholar]
- Chigurupati S, Venkataraman R, Barrera D, Naganathan A, Madan M, Paul L, et al. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res. 2010;70:418–427. doi: 10.1158/0008-5472.CAN-09-2654. [DOI] [PubMed] [Google Scholar]
- Chimote AA, Hajdu P, Kucher V, Boiko N, Kuras Z, Szilagyi O, et al. Selective inhibition of KCa3.1 channels mediates adenosine regulation of the motility of human T cells. J Immunol. 2013;191:6273–6280. doi: 10.4049/jimmunol.1300702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Middelbeek J, van Leeuwen FN. Interplay between TRP channels and the cytoskeleton in health and disease. Eur J Cell Biol. 2008;87:631–640. doi: 10.1016/j.ejcb.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol. 2004;14:259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Cuddapah VA, Turner KL, Sontheimer H. Calcium entry via TRPC1 channels activates chloride currents in human glioma cells. Cell Calcium. 2013;53:187–194. doi: 10.1016/j.ceca.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damann N, Owsianik G, Li S, Poll C, Nilius B. The calcium-conducting ion channel transient receptor potential canonical 6 is involved in macrophage inflammatory protein-2-induced migration of mouse neutrophils. Acta Physiol (Oxf) 2009;195:3–11. doi: 10.1111/j.1748-1716.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–715. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, et al. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos Y, Schnitzler M, Salanova B, et al. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Ding X, He Z, Zhou K, Cheng J, Yao H, Lu D, et al. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst. 2010;102:1052–1068. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- Dixit N, Yamayoshi I, Nazarian A, Simon SI. Migrational guidance of neutrophils is mechanotransduced via high-affinity LFA-1 and calcium flux. J Immunol. 2011;187:472–481. doi: 10.4049/jimmunol.1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Shim KN, Li JM, Estrema C, Ornelas TA, Nguyen F, et al. Molecular mechanisms underlying Ca2+-mediated motility of human pancreatic duct cells. Am J Physiol Cell Physiol. 2010;299:C1493–C1503. doi: 10.1152/ajpcell.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- Edderkaoui M, Nitsche C, Zheng L, Pandol SJ, Gukovsky I, Gukovskaya AS. NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox. J Biol Chem. 2011;286:7779–7787. doi: 10.1074/jbc.M110.200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy RJ, Pierini LM, Matsumura F, Maxfield FR. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J Cell Sci. 2000;113:1287–1298. doi: 10.1242/jcs.113.7.1287. (Pt 7): [DOI] [PubMed] [Google Scholar]
- Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172–178. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Fabian A, Fortmann T, Dieterich P, Riethmuller C, Schon P, Mally S, et al. TRPC1 channels regulate directionality of migrating cells. Pflugers Arch. 2008;457:475–484. doi: 10.1007/s00424-008-0515-4. [DOI] [PubMed] [Google Scholar]
- Fabian A, Fortmann T, Bulk E, Bomben VC, Sontheimer H, Schwab A. Chemotaxis of MDCK-F cells toward fibroblast growth factor-2 depends on transient receptor potential canonical channel 1. Pflugers Arch. 2011;461:295–306. doi: 10.1007/s00424-010-0901-6. [DOI] [PubMed] [Google Scholar]
- Fabian A, Bertrand J, Lindemann O, Pap T, Schwab A. Transient receptor potential canonical channel 1 impacts on mechanosignaling during cell migration. Pflugers Arch. 2012;464:623–630. doi: 10.1007/s00424-012-1169-9. [DOI] [PubMed] [Google Scholar]
- Falke JJ, Ziemba BP. Interplay between phosphoinositide lipids and calcium signals at the leading edge of chemotaxing ameboid cells. Chem Phys Lipids. 2014 doi: 10.1016/j.chemphyslip.2014.01.002. doi: 10.1016/j.chemphyslip.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorio PA, Grange C, Antoniotti S, Tomatis C, Merlino A, Bussolati B, et al. Arachidonic acid-induced Ca2+ entry is involved in early steps of tumor angiogenesis. Mol Cancer Res. 2008;6:535–545. doi: 10.1158/1541-7786.MCR-07-0271. [DOI] [PubMed] [Google Scholar]
- Fiorio PA, Ong HL, Cheng KT, Brossa A, Bussolati B, Lockwich T, et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31:200–212. doi: 10.1038/onc.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioro PA, Gkika D. Emerging role of TRP channels in cell migration: from tumor vascularization to metastasis. Front Physiol. 2013;4:311. doi: 10.3389/fphys.2013.00311. . eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Pardo LA. Ion channels: functional expression and therapeutic potential in cancer. Colloquium on ion channels and cancer. EMBO Rep. 2008;9:512–515. doi: 10.1038/embor.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2009;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kobayashi A, Watabe K. The role of tumor-associated macrophage in tumor progression. Front Biosci. 2012;4:787–798. doi: 10.2741/s299. [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Gao H, Chen X, Du X, Guan B, Liu Y, Zhang H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium. 2011;50:559–568. doi: 10.1016/j.ceca.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Gao YD, Hanley PJ, Rinne S, Zuzarte M, Daut J. Calcium-activated K+ channel KCa3.1 activity during Ca2+ store depletion and store-operated Ca2+ entry in human macrophages. Cell Calcium. 2010;48:19–27. doi: 10.1016/j.ceca.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Giannone G, Ronde P, Gaire M, Haiech J, Takeda K. Calcium oscillations trigger focal adhesion disassembly in human U87 astrocytoma cells. J Biol Chem. 2002;277:26364–26371. doi: 10.1074/jbc.M203952200. [DOI] [PubMed] [Google Scholar]
- Giannone G, Ronde P, Gaire M, Beaudouin J, Haiech J, Ellenberg J, et al. Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J Biol Chem. 2004;279:28715–28723. doi: 10.1074/jbc.M404054200. [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkika D, Prevarskaya N. Molecular mechanisms of TRP regulation in tumor growth and metastasis. Biochim Biophys Acta. 2009;1793:953–958. doi: 10.1016/j.bbamcr.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Gkika D, Prevarskaya N. TRP channels in prostate cancer: the good, the bad and the ugly? Asian J Androl. 2011;13:673–676. doi: 10.1038/aja.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkika D, Flourakis M, Lemonnier L, Prevarskaya N. PSA reduces prostate cancer cell motility by stimulating TRPM8 activity and plasma membrane expression. Oncogene. 2010;29:4611–4616. doi: 10.1038/onc.2010.210. [DOI] [PubMed] [Google Scholar]
- Goswami C, Dreger M, Otto H, Schwappach B, Hucho F. Rapid disassembly of dynamic microtubules upon activation of the capsaicin receptor TRPV1. J Neurochem. 2006;96:254–266. doi: 10.1111/j.1471-4159.2005.03551.x. [DOI] [PubMed] [Google Scholar]
- Goswami C, Kuhn J, Heppenstall PA, Hucho T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS ONE. 2010;5:e11654. doi: 10.1371/journal.pone.0011654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadi M, Chopin V, Matifat F, Dhennin-Duthille I, Chasseraud M, Sevestre H, et al. Human ether à-gogo K+ channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J Cell Physiol. 2012;227:3837–3846. doi: 10.1002/jcp.24095. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Happel P, Moller K, Schwering NK, Dietzel ID. Migrating oligodendrocyte progenitor cells swell prior to soma dislocation. Sci Rep. 2013;3:1806. doi: 10.1038/srep01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, et al. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Brass A, Seman M, Haag F, Koch-Nolte F, Dubyak GR. Basal and inducible expression of the thiol-sensitive ART2.1 ecto-ADP-ribosyltransferase in myeloid and lymphoid leukocytes. Purinergic Signal. 2009;5:369–383. doi: 10.1007/s11302-009-9162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd TR, DeGennaro M, Lehmann R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2011;22:107–115. doi: 10.1016/j.tcb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki K, Kannan KB, Singh BB, Hauser CJ. Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J Immunol. 2004;172:601–607. doi: 10.4049/jimmunol.172.1.601. [DOI] [PubMed] [Google Scholar]
- Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen KS, Zeeberg K, Sauter DR, Poulsen KA, Hoffmann EK, Schwab A. The role of TMEM16A (ANO1) and TMEM16F (ANO6) in cell migration. Pflugers Arch. 2013;465:1753–1762. doi: 10.1007/s00424-013-1315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HS, Lal S, Greenwood JA. Calpain 2 is required for glioblastoma cell invasion: regulation of matrix metalloproteinase 2. Neurochem Res. 2010;35:1796–1804. doi: 10.1007/s11064-010-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin I, Albarran L, Bermejo N, Salido GM, Rosado JA. Homers regulate calcium entry and aggregation in human platelets: a role for Homers in the association between STIM1 and Orai1. Biochem J. 2012;445:29–38. doi: 10.1042/BJ20120471. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Keren K. Cell motility: the integrating role of the plasma membrane. Eur Biophys J. 2011;40:1013–1027. doi: 10.1007/s00249-011-0741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, et al. Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc Natl Acad Sci U S A. 2011;108:11578–11583. doi: 10.1073/pnas.1010678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- Kuras Z, Yun YH, Chimote AA, Neumeier L, Conforti L. KCa3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells. PLoS ONE. 2012;7:e43859. doi: 10.1371/journal.pone.0043859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- Lauritzen G, Stock CM, Lemaire J, Lund SF, Jensen MF, Damsgaard B, et al. The Na+/H+ exchanger NHE1, but not the Na+, HCO3− cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett. 2012;317:172–183. doi: 10.1016/j.canlet.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- Li J, Wientjes MG, Au JL. Pancreatic cancer: pathobiology, treatment options, and drug delivery. AAPS J. 2010;12:223–232. doi: 10.1208/s12248-010-9181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang S, Soto X, Woolner S, Amaya E. Erk and PI3K temporally coordinate different modes of actin-based motility during embryonic wound healing. J Cell Sci. 2013;126:5005–5017. doi: 10.1242/jcs.133421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann O, Umlauf D, Frank S, Schimmelpfennig S, Bertrand J, Pap T, et al. TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils. J Immunol. 2013;190:5496–5505. doi: 10.4049/jimmunol.1201502. [DOI] [PubMed] [Google Scholar]
- Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodola F, Laforenza U, Bonetti E, Lim D, Dragoni S, Bottino C, et al. Store-operated Ca2+ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS ONE. 2012;7:e42541. doi: 10.1371/journal.pone.0042541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L104–L113. doi: 10.1152/ajplung.00058.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, McCarl CA, Khalil S, Luthy K, Feske S. T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur J Immunol. 2010;40:3028–3042. doi: 10.1002/eji.201040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:G709–G717. doi: 10.1152/ajpgi.90356.2008. [DOI] [PubMed] [Google Scholar]
- McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, et al. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther. 2011;10:448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- McMeekin SR, Dransfield I, Rossi AG, Haslett C, Walker TR. E-selectin permits communication between PAF receptors and TRPC channels in human neutrophils. Blood. 2006;107:4938–4945. doi: 10.1182/blood-2005-09-3803. [DOI] [PubMed] [Google Scholar]
- Melzer N, Hicking G, Gobel K, Wiendl H. TRPM2 cation channels modulate T cell effector functions and contribute to autoimmune CNS inflammation. PLoS ONE. 2012;7:e47617. doi: 10.1371/journal.pone.0047617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Cai C, Wu J, Cai S, Ye C, Chen H, et al. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett. 2012;333:96–102. doi: 10.1016/j.canlet.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R, et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72:4250–4261. doi: 10.1158/0008-5472.CAN-11-3863. [DOI] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Monet M, Gkika D, Lehen'kyi V, Pourtier A, Vanden Abeele F, Bidaux G, et al. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim Biophys Acta. 2009;1793:528–539. doi: 10.1016/j.bbamcr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Monet M, Lehen'kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–1235. doi: 10.1158/0008-5472.CAN-09-2205. [DOI] [PubMed] [Google Scholar]
- Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285:19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiani RK, Hyzinski-Garcia MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, et al. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013a;465:1249–1260. doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, et al. Orai3 is an estrogen receptor alpha-regulated Ca2+ channel that promotes tumorigenesis. FASEB J. 2013b;27:63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR. The polarization of the motile cell. J Cell Sci. 1999;112:1803–1811. doi: 10.1242/jcs.112.12.1803. (Pt 12): [DOI] [PubMed] [Google Scholar]
- Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- Ng LC, O'Neill KG, French D, Airey JA, Singer CA, Tian H, et al. TRPC1 and Orai1 interact with STIM1 and mediate capacitative Ca2+ entry caused by acute hypoxia in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2012;303:C1156–C1172. doi: 10.1152/ajpcell.00065.2012. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata T, Ogawa N, Takahashi N, Mori Y. TRP channels as sensors of oxygen availability. Pflugers Arch. 2013;465:1075–1085. doi: 10.1007/s00424-013-1237-9. [DOI] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP channels: diagnostic markers and therapeutic targets for breast cancer? Trends Mol Med. 2013;19:117–124. doi: 10.1016/j.molmed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Oulidi A, Bokhobza A, Gkika D, Vanden Abeele F, Lehen'kyi V, Ouafik L, et al. TRPV2 mediates adrenomedullin stimulation of prostate and urothelial cancer cell adhesion, migration and invasion. PLoS ONE. 2013;8:e64885. doi: 10.1371/journal.pone.0064885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, et al. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol. 2007;179:7827–7839. doi: 10.4049/jimmunol.179.11.7827. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Stock C. Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res. 2013;73:1658–1661. doi: 10.1158/0008-5472.CAN-12-4188. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772:937–946. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16:107–121. doi: 10.1016/j.molmed.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Ouadid-Ahidouch H, Skryma R, Shuba Y. Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Philos Trans R Soc Lond B Biol Sci. 2014;369:20130097. doi: 10.1098/rstb.2013.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roedding AS, Gao AF, Au-Yeung W, Scarcelli T, Li PP, Warsh JJ. Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar Disord. 2012;14:151–161. doi: 10.1111/j.1399-5618.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybarczyk P, Gautier M, Hague F, Dhennin-Duthille I, Chatelain D, Kerr-Conte J, et al. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int J Cancer. 2012;131:E851–E861. doi: 10.1002/ijc.27487. [DOI] [PubMed] [Google Scholar]
- Santoni G, Farfariello V. TRP channels and cancer: new targets for diagnosis and chemotherapy. Endocr Metab Immune Disord Drug Targets. 2011;11:54–67. doi: 10.2174/187153011794982068. [DOI] [PubMed] [Google Scholar]
- Schafer C, Rymarczyk G, Ding L, Kirber MT, Bolotina VM. Role of molecular determinants of store-operated Ca2+ entry (Orai1, phospholipase A2 group 6, and STIM1) in focal adhesion formation and cell migration. J Biol Chem. 2012;287:40745–40757. doi: 10.1074/jbc.M112.407155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaff UY, Dixit N, Procyk E, Yamayoshi I, Tse T, Simon SI. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood. 2010;115:657–666. doi: 10.1182/blood-2009-05-224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Schneider SW, Pagel P, Rotsch C, Danker T, Oberleithner H, Radmacher M, et al. Volume dynamics in migrating epithelial cells measured with atomic force microscopy. Pflugers Arch. 2000;439:297–303. doi: 10.1007/s004249900176. [DOI] [PubMed] [Google Scholar]
- Schwab A, Stock C. Ion channels and transporters in tumour cell migration and invasion. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130102. doi: 10.1098/rstb.2013.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A, Gabriel K, Finsterwalder F, Folprecht G, Greger R, Kramer A, et al. Polarized ion transport during migration of transformed Madin-Darby canine kidney cells. Pflugers Arch. 1995;430:802–807. doi: 10.1007/BF00386179. [DOI] [PubMed] [Google Scholar]
- Schwab A, Finsterwalder F, Kersting U, Danker T, Oberleithner H. Intracellular Ca2+ distribution in migrating transformed epithelial cells. Pflugers Arch. 1997;434:70–76. doi: 10.1007/s004240050364. [DOI] [PubMed] [Google Scholar]
- Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Qu B, Hoth M, Feske S. Molecular regulation of CRAC channels and their role in lymphocyte function. Cell Mol Life Sci. 2013;70:2637–2656. doi: 10.1007/s00018-012-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L941–L949. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TA, Lively S, Vincent C, Schlichter LC. Regulation of podosome formation, microglial migration and invasion by Ca2+-signaling molecules expressed in podosomes. J Neuroinflammation. 2012;9:250. doi: 10.1186/1742-2094-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–7843. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- Sleeman JP, Christofori G, Fodde R, Collard JG, Berx G, Decraene C, et al. Concepts of metastasis in flux: the stromal progression model. Semin Cancer Biol. 2012;22:174–186. doi: 10.1016/j.semcancer.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Smani T, Dionisio N, Lopez JJ, Berna-Erro A, Rosado JA. Cytoskeletal and scaffolding proteins as structural and functional determinants of TRP channels. Biochim Biophys Acta. 2013;1838:658–664. doi: 10.1016/j.bbamem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours-Brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Exp Biol Med (Maywood) 2009;234:673–682. doi: 10.3181/0809-RM-279. [DOI] [PubMed] [Google Scholar]
- Stock C, Ludwig FT, Hanley PJ, Schwab A. Roles of ion transport in control of cell motility. Compr Physiol. 2013;3:59–119. doi: 10.1002/cphy.c110056. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207–238. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- Su LT, Liu W, Chen HC, Gonzalez-Pagan O, Habas R, Runnels LW. TRPM7 regulates polarized cell movements. Biochem J. 2011;434:513–521. doi: 10.1042/BJ20101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran P, Lof C, Pulli I, Kemppainen K, Viitanen T, Tornquist K. Significance of the transient receptor potential canonical 2 (TRPC2) channel in the regulation of rat thyroid FRTL-5 cell proliferation, migration, adhesion and invasion. Mol Cell Endocrinol. 2013;374:10–21. doi: 10.1016/j.mce.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Svensson L, McDowall A, Giles KM, Stanley P, Feske S, Hogg N. Calpain 2 controls turnover of LFA-1 adhesions on migrating T lymphocytes. PLoS ONE. 2010;5:e15090. doi: 10.1371/journal.pone.0015090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajeddine N, Gailly P. TRPC1 protein channel is major regulator of epidermal growth factor receptor signaling. J Biol Chem. 2012;287:16146–16157. doi: 10.1074/jbc.M112.340034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima N, Schonherr K, Niedling S, Kaatz M, Kanno H, Schonherr R, et al. Ca2+-activated K+ channels in human melanoma cells are up-regulated by hypoxia involving hypoxia-inducible factor-1alpha and the von Hippel-Lindau protein. J Physiol. 2006;571:349–359. doi: 10.1113/jphysiol.2005.096818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Sakamoto K, Kimura J. Hypoxic stress induces transient receptor potential melastatin 2 (TRPM2) channel expression in adult rat cardiac fibroblasts. J Pharmacol Sci. 2012;118:186–197. doi: 10.1254/jphs.11128fp. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, et al. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, et al. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2000;275:37559–37564. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochhawng L, Deng S, Pervaiz S, Yap CT. Redox regulation of cancer cell migration and invasion. Mitochondrion. 2013;13:246–253. doi: 10.1016/j.mito.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Tsai FC, Meyer T. Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr Biol. 2012;22:837–842. doi: 10.1016/j.cub.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KL, Sontheimer H. KCa3.1 modulates neuroblast migration along the rostral migratory stream (RMS) in vivo. Cereb Cortex. 2013 doi: 10.1093/cercor/bht090. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisitti T, Audrito V, Serra S, Bologna C, Brusa D, Malavasi F, et al. NAD+-metabolizing ecto-enzymes shape tumor-host interactions: the chronic lymphocytic leukemia model. FEBS Lett. 2011;585:1514–1520. doi: 10.1016/j.febslet.2011.04.036. [DOI] [PubMed] [Google Scholar]
- Van Haute C, De Ridder D, Nilius B. TRP channels in human prostate. ScientificWorldJournal. 2010;10:1597–1611. doi: 10.1100/tsw.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, et al. Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. FASEB J. 2007;21:608–617. doi: 10.1096/fj.06-6683com. [DOI] [PubMed] [Google Scholar]
- Waite JC, Vardhana S, Shaw PJ, Jang JE, McCarl CA, Cameron TO, et al. Interference with Ca release activated Ca (CRAC) channel function delays T-cell arrest in vivo. Eur J Immunol. 2013;43:3343–3354. doi: 10.1002/eji.201243255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waning J, Vriens J, Owsianik G, Stuwe L, Mally S, Fabian A, et al. A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium. 2007;42:17–25. doi: 10.1016/j.ceca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Wanitchakool P, Wolf L, Koehl GE, Sirianant L, Schreiber R, Kulkarni S, et al. Role of anoctamins in cancer and apoptosis. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130096. doi: 10.1098/rstb.2013.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Sontheimer H. Hydrodynamic cellular volume changes enable glioma cell invasion. J Neurosci. 2011;31:17250–17259. doi: 10.1523/JNEUROSCI.3938-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- Weber KS, Hildner K, Murphy KM, Allen PM. Trpm4 differentially regulates Th1 and Th2 function by altering calcium signaling and NFAT localization. J Immunol. 2010;185:2836–2846. doi: 10.4049/jimmunol.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrhahn J, Kraft R, Harteneck C, Hauschildt S. Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J Immunol. 2010;184:2386–2393. doi: 10.4049/jimmunol.0902474. [DOI] [PubMed] [Google Scholar]
- Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger C, Shaw PJ, Feske S. STIM1 and STIM2-mediated Ca2+ influx regulates antitumour immunity by CD8+ T cells. EMBO Mol Med. 2013;5:1311–1321. doi: 10.1002/emmm.201302989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A, Huttenlocher A, Lauffenburger DA. Calpain proteases in cell adhesion and motility. Int Rev Cytol. 2005;245:1–16. doi: 10.1016/S0074-7696(05)45001-9. [DOI] [PubMed] [Google Scholar]
- Wondergem R, Bartley JW. Menthol increases human glioblastoma intracellular Ca2+, BK channel activity and cell migration. J Biomed Sci. 2009;16:90. doi: 10.1186/1423-0127-16-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondergem R, Ecay TW, Mahieu F, Owsianik G, Nilius B. HGF/SF and menthol increase human glioblastoma cell calcium and migration. Biochem Biophys Res Commun. 2008;372:210–215. doi: 10.1016/j.bbrc.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–2596. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K, Sasano T, Tojo K, Namekata I, Kurokawa J, Sawada N, et al. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem Biophys Res Commun. 2010;398:284–289. doi: 10.1016/j.bbrc.2010.06.082. [DOI] [PubMed] [Google Scholar]
- Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y, et al. Blockade of store-operated Ca2+ entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett. 2013a;330:163–169. doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- Yang S, Huang XY. Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem. 2005;280:27130–27137. doi: 10.1074/jbc.M501625200. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009a;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Yang Y, Karakhanova S, Werner J, Bazhin AV. Reactive oxygen species in cancer biology and anticancer therapy. Curr Med Chem. 2013b;20:3677–3692. doi: 10.2174/0929867311320999165. [DOI] [PubMed] [Google Scholar]
- Yang ZH, Wang XH, Wang HP, Hu LQ. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J Androl. 2009b;11:157–165. doi: 10.1038/aja.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Lee KP, Hong JH, Muallem S. The closing and opening of TRPC channels by Homer1 and STIM1. Acta Physiol (Oxf) 2012;204:238–247. doi: 10.1111/j.1748-1716.2011.02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhou L, Shi W, Song N, Yu K, Gu Y. A mechanism underlying the effects of polyunsaturated fatty acids on breast cancer. Int J Mol Med. 2012;30:487–494. doi: 10.3892/ijmm.2012.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wei J, Kanada M, Yan L, Zhang Z, Watanabe H, et al. Inhibition of store-operated Ca2+ entry suppresses EGF-induced migration and eliminates extravasation from vasculature in nasopharyngeal carcinoma cell. Cancer Lett. 2013;336:390–397. doi: 10.1016/j.canlet.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Malinin NL, Meller J, Ma Y, West XZ, Bledzka K, et al. Regulation of cell adhesion and migration by Kindlin-3 cleavage by calpain. J Biol Chem. 2012a;287:40012–40020. doi: 10.1074/jbc.M112.380469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Ni Y, Chen J, Zhong J, Yu H, Xu X, et al. Increased migration of monocytes in essential hypertension is associated with increased transient receptor potential channel canonical type 3 channels. PLoS ONE. 2012b;7:e32628. doi: 10.1371/journal.pone.0032628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Wang X, Yang Z, Cao H, Meng Z, Wang Y, et al. Effects of TRPM8 on the proliferation and angiogenesis of prostate cancer PC-3 cells in vivo. Oncol Lett. 2011;2:1213–1217. doi: 10.3892/ol.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]



