Abstract
Cannabinoids and their synthetic analogues affect a broad range of physiological functions, including cardiovascular variables. Although direct evidence is still missing, the relaxation of a vast range of vascular beds induced by cannabinoids is believed to involve a still unidentified non-CB1, non-CB2 Gi/o protein-coupled receptor located on endothelial cells, the so called endothelial cannabinoid receptor (eCB receptor). Evidence for the presence of an eCB receptor comes mainly from vascular relaxation studies, which commonly employ pertussis toxin as an indicator for GPCR-mediated signalling. In addition, a pharmacological approach is widely used to attribute the relaxation to eCB receptors. Recent findings have indicated a number of GPCR-independent targets for both agonists and antagonists of the presumed eCB receptor, warranting further investigations and cautious interpretation of the vascular relaxation studies. This review will provide a brief historical overview on the proposed novel eCB receptor, drawing attention to the discrepancies between the studies on the pharmacological profile of the eCB receptor and highlighting the Gi/o protein-independent actions of the eCB receptor inhibitors widely used as selective compounds. As the eCB receptor represents an attractive pharmacological target for a number of cardiovascular abnormalities, defining its molecular identity and the extent of its regulation of vascular function will have important implications for drug discovery. This review highlights the need to re-evaluate this subject in a thoughtful and rigorous fashion. More studies are needed to differentiate Gi/o protein-dependent endothelial cannabinoid signalling from that involving the classical CB1 and CB2 receptors as well as its relevance for pathophysiological conditions.
Table of Links
| TARGETS | LIGANDS |
|---|---|
| 5-HT receptor | Abn-CBD |
| α1 adrenoceptor | Acetylcholine |
| Akt | Anandamide (AEA) |
| AT1 receptor | AM251 |
| BKCa channels | Apamin |
| CaV2.2 | Bradykinin |
| CaV3.1 | Cannabidiol |
| CaV3.2 | Carbachol |
| CaV3.3 | Charybdotoxin |
| CB1 receptor | Forskolin |
| CB2 receptor | HU-210 |
| ERK1/2 | Iberiotoxin |
| Glycine receptors | L-NAME |
| GPR18 | LPI |
| GPR55 | NaGly |
| GPR119 | NO |
| Ionotropic glutamate receptor | NS1619 |
| IP3 receptor | O-1602 |
| KCa channels | Oleamide |
| M1 muscarinic receptor | Oleoylethanolamide |
| M2 muscarinic receptor | Rimonabant (SR141716) |
| MAPK | Ryanodine |
| Na+/Ca2+ exchanger (NCX) | THC |
| NaV channel | WIN55212-2 |
| Nicotinic acetylcholine receptors | |
| NOS | |
| Opioid receptors | |
| PI3K | |
| PPARγ | |
| ROCK | |
| TRP channels | |
| TRPV channels | |
| VEGF receptor |
This Table lists key protein targets and ligands in this document, which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b,c,d,f,g,,,,,).
Introduction
Due to the diverse physiological effects of cannabinoids, the endocannabinoid system has attracted major attention as a potential therapeutic target for a broad range of diseases. While two Gi/o protein-coupled cannabinoid receptors, CB1 and CB2R receptors, commonly mediate the physiological actions of cannabinoids (Alexander et al., 2013a), their vasodilator effects in a wide range of vascular beds are not thought to involve these classical cannabinoid receptors (Jarai et al., 1999; Wagner et al., 1999; Ho and Hiley, 2003a; Offertaler et al., 2003). A site of action for cannabinoids distinct from CB1/CB2 receptors has also been demonstrated in the endothelium of different vascular beds, both in micro- and macrovessels, including the rat (Milman et al., 2006; Herradon et al., 2007; Lopez-Miranda et al., 2010) and rabbit aorta (Mukhopadhyay et al., 2002; McCollum et al., 2007), rat (Baranowska-Kuczko et al., 2012) and human pulmonary artery (Kozlowska et al., 2007). The molecular mechanisms, by which cannabinoids produce vasodilatation, have not been fully elucidated, but are believed to involve still unidentified CB receptors located on endothelial cells, so called endothelial cannabinoid receptors (eCB receptors).
Identification of the eCB receptor and its particualr signalling cascade is not merely a pure theoretical challenge. The selection of compounds with reduced psychoactivity has emerged as a promising therapeutic strategy for a vast number of diseases. Much interest in the identification of the eCB receptor and its pharmacological characterization stems from its promising therapeutic potential in a large number of disorders (Robson, 2013), especially considering its lack of psychiatric side effects, which result from stimulation of central CB1 receptors. Identification of the mechanisms of the CB1/CB2 receptor-independent actions of cannabinoids in the cardiovascular system is fuelled by discoveries of antioxidant, anti-inflammatory (Booz, 2011) and cardioprotective properties of cannabinoids that occur independently of CB1/CB2 receptors (Fouad et al., 2013). Accordingly, considerable effort is being assigned to identify the signalling mechanisms of eCB receptor-attributed effects, as selective eCB receptor targeting is thought to have great therapeutic potential.
Although 15 years have passed since the first publication of indications of the novel eCB receptor (Jarai et al., 1999; Wagner et al., 1999), its molecular identity is still unclear. Moreover, it is not yet conclusive that vascular effects commonly attributed to eCB receptor actually require G-protein coupled receptor (GPCR). The weak link is that our current knowledge on the eCB receptor, its pharmacology and signalling profile almost exclusively relies on the interpretation of data obtained in numerous arterial relaxation studies. These studies (i) commonly use a Gi/o protein inhibitor, pertussis toxin, to demonstrate the involvement of GPCRs (Herradon et al., 2007; Hoi et al., 2007; Kozlowska et al., 2007; O'Sullivan et al., 2009; Parmar and Ho, 2010; Alsuleimani and Hiley, 2013) and (ii) presume the eCB receptor agonists and antagonists used are selective. The sensitivity of the vasodilatation to pertussis toxin, the phytocannabinoid cannabidiol, its analogue O-1918 and the CB1 receptor antagonist rimonabant (SR141716A) became classic tools to propose and in further studies support the presence of non-CB1/non-CB2 endothelial G protein-coupled CB receptors referred to in the literature as ‘abnormal cannabidiol’, ‘endothelial anandamide’ or ‘atypical endothelial cannabinoid receptor’ (Jarai et al., 1999; Wagner et al., 1999; Mukhopadhyay et al., 2002; Herradon et al., 2007; Baranowska-Kuczko et al., 2012). Due to the abundant pharmacological effects of cannabinoids and cannabinoid-like compounds outlined by Alexander and Kendall (2007), possible interactions of these compounds with unspecified CB receptor-independent targets will not always be detected in relaxation studies. Vascular responsiveness to cannabinoids varies not only between various vascular beds/species, but also even within the same vascular bed. For example, in the rat isolated aorta, the maximal relaxation to anandamide varies from 22 (O'Sullivan et al., 2005b) to 60% (Milman et al., 2006) of the imposed contraction and may (Herradon et al., 2007) or may not depend (O'Sullivan et al., 2005b) on the presence of the endothelium. Similarly, in rat small mesenteric arteries, the relaxation to anandamide was shown to be either endothelium-dependent (O'Sullivan et al., 2004) or endothelium-independent (White and Hiley, 1997). A critical look at studies published reveals a number of further inconsistencies in the mechanisms of vasodilatation commonly attributed to eCB receptors, including the sensitivity to CB1 receptor antagonists rimonabant (Jarai et al., 1999; Harris et al., 2002; Ho and Hiley, 2003a; Milman et al., 2006) and AM251 (Ho and Hiley, 2003a; Hoi et al., 2007), NO synthase inhibitors (Jarai et al., 1999; Harris et al., 2002; Ho and Hiley, 2003a; Kozlowska et al., 2007; McCollum et al., 2007) and gap junction inhibitors (Brandes et al., 2002; Harris et al., 2002; Randall et al., 2002; Ho and Hiley, 2003a). Thus, anandamide was reported either to promote (Chaytor et al., 1999; Randall et al., 2002) or inhibit (Fleming et al., 1999; Brandes et al., 2002) gap junction communications, while rimonabant has an inhibitory effect (Chaytor et al., 1999). An ability of cannabinoids to affect gap junctions, a critical player in vascular electrical and mechanical responses, further emphasizes certain restrictions when using wire myography as the only approach to characterize the mechanisms of action of cannabinoids on endothelial cells and vascular pharmacology of CB receptor ligands. Table 1 summarizes the key findings on the pharmacology of relaxant responses to cannabinoids, highlighting substantial disparities in the pharmacological profiles of these responses.
Table 1.
Summary of pharmacology of CB1/CB2 receptor-independent relaxant responses to cannabinoids
| Ligands | SR141716A | AM-251 | O-1918 | L-NAME | De-endothelization | PTX | FAAH inhibitors | APA + ChTx | IbTx | TRPV1 antagonism | αGA | pEC50 (μM) | Rmax, % | Vascular bed | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AEA | Inhibition | No effect | No effect | Inhibition | No effect | No effect | 6.3 | 95 | RSMA | White and Hiley, 1997 | |||||
| AEA | Inhibition | No effect | Slight inhibition | Slight inhibition | Inhibition | 6.2 | 94 | RMA | Harris et al., 2002 | ||||||
| AEA | Inhibition | Inhibition | Inhibition | No effect | Inhibition | Inhibition | Inhibition | Inhibition | 5.7 | 89 | RSMA | O'Sullivan et al., 2004 | |||
| AEA | Inhibition | Inhibition | No effect | No effect | No effect | No effect | Inhibition | No effect | 5.0 | 33 | RMA | O'Sullivan et al., 2004 | |||
| AEA | No effect | 80 | RMA | Yang et al., 2007 | |||||||||||
| AEA | Inhibition | No effect | No effect | No effect | Inhibition | No effect | No effect | 6.8 | 45 | RCA | White et al., 2001 | ||||
| AEA | Inhibition | 6.7 | 97 | RSMA | White et al., 2001 | ||||||||||
| AEA | No effect | Inhibition | Inhibition | Inhibition | Inhibition | No effect | No effect | 5.9 | 51 | RA | Herradon et al., 2007 | ||||
| AEA | No effect | No effect | No effect | Inhibition | No effect | 5.9 | 22 | RA | O'Sullivan et al., 2005b | ||||||
| AEA | No effect | 5.2 | 90 | HPA | Kozlowska et al., 2007 | ||||||||||
| AEA | No effect | Inhibition | Inhibition | Inhibition | Inhibition | Inhibition | No effect | 5 | 90 | RPA | Baranowska-Kuczko et al., 2012 | ||||
| methAEA | Inhibition | Inhibition | Inhibition | Slight inhibition | No effect | 0.6 | 80 | RabA | Mukhopadhyay et al., 2002 | ||||||
| Abn-CBD | Inhibition | No effect | Inhibition | Inhibition | No effect | No effect | RPIMAB | Jarai et al., 1999 | |||||||
| Abn-CBD | Inhibition | 3.0 | 107 | RMA | Johns et al., 2007 | ||||||||||
| Abn-CBD | Inhibition | No effect | Inhibition | Inhibition | No effect | 6.7 | 93 | RMA | Offertaler et al., 2003 | ||||||
| Abn-CBD | Inhibition | Inhibition | No effect | No effect | Inhibition | No effect | No effect | 6.2 | 93 | RSMA | Ho and Hiley, 2003a | ||||
| Abn-CBD | No effect | Inhibition | Inhibition | Inhibition | Inhibition | Inhibition | No effect | 4.6 | 82 | RPA | Baranowska-Kuczko et al., 2012 | ||||
| Abn-CBD | Inhibition | no effect | 2 | RMA | Milman et al., 2006 | ||||||||||
| abn-cnd | Inhibition | No effect | Inhibition | Inhibition | Inhibition | 4.8 | 110 | HPA | Kozlowska et al., 2007 | ||||||
| NAGly | No effect | Inhibition | Inhibition | Inhibition | Inhibition | No effect | Inhibition | Inhibition | No effect | 5.8 | 88 | RSMA | Parmar and Ho, 2010 | ||
| ARA-S | Inhibition | Inhibition | 4.9 | 84 | RSMA | Parmar and Ho, 2010 | |||||||||
| ARA-S | Inhibition | No effect | 0.55 | 95 | RMA | Milman et al., 2006 | |||||||||
| ARA-S | No effect | No effect | Inhibition | 1.2 | 60 | RA | Milman et al., 2006 | ||||||||
| Oleamide | Inhibition | No effect | Inhibition | Inhibition | Inhibition | Inhibition | Inhibition | Inhibition | 1.2 | 99 | RSMA | Hoi and Hiley, 2006 |
Abn-CBD, abnormal cannabidiol; AEA, anandamide; APA + ChTx, apamin plus charybdotoxin; FAAH, fatty acid amide hydrolase; HPA, human pulmonary artery; IbTx, iberiotoxin; L-NAME, NG-Nitro-L-arginine methyl ester; RA, rat aorta; RCA, rat coronary artery; RPA, rat pulmonary artery; RSMA, rat small mesenteric artery; TRPV, transient receptor potential cation channel subfamily V.
This paper represents a critical overview of the relevant publications, drawing particular attention to certain discrepancies in the field. Numerous GPCR-independent actions of the compounds widely used as selective agonists and antagonists of the novel eCB receptor highlight the need for a re-evaluation of the subject in a thoughtful and rigorous fashion.
CB receptor-independent effect of rimonabant
The laboratory of G. Kunos was the first to propose the novel CB receptor in the vascular endothelium in 1999 (Jarai et al., 1999; Wagner et al., 1999). In the rat isolated perfused mesenteric arterial bed precontracted with phenylephrine, a bolus injection of anandamide was shown to produce a sustained and pronounced concentration-dependent vasodilatation (Wagner et al., 1999), which had an endothelium-dependent component sensitive to 1 μM of the CB1 receptor antagonist rimonabant. The receptor was suggested to be distinct from the CB1 receptor as the CB1 receptor agonists WIN55212-2 and HU-210 failed to reproduce the vasodilatation. These observations led the authors to conclude that the anandamide-induced mesenteric vasodilatation is mediated by a novel anandamide receptor located on endothelial cells. Consistent with this notion, abnormal cannabidiol (Abn-CBD), a synthetic analogue of the non-psychoactive phytocannabinoid cannabidiol, was shown to produce rimonabant-sensitive hypotension and endothelium-dependent mesenteric vasodilatation independently of NO (Jarai et al., 1999). The response was also preserved in mice lacking either CB1 receptors or both CB1 and CB2 receptors and, hence, appeared to depend on molecular target(s) distinct from CB1 and CB2 receptors. Based on these findings, it was concluded that Abn-CBD is a selective agonist, while cannabidiol is a selective antagonist of novel anandamide receptor present in endothelial cells (Jarai et al., 1999).
It is interesting to note that the authors also observed an antagonistic effect of 1 μM rimonabant on endothelium-dependent relaxation elicited by administration of a low concentrations of the Ca2+ ionophore ionomycin (Wagner et al., 1999). In isolated vessels, ionomycin evokes endothelial cell hyperpolarization, similar to that produced by acetylcholine (Bondarenko and Sagach, 2006). The antagonistic effect of 1 μM rimonabant on endothelium-dependent relaxation induced by the Ca2+ ionophore A23187, carbachol and bradykinin was also demonstrated by others (Randall et al., 1996; Randall and Kendall, 1997; White and Hiley, 1997), indicating that rimonabant may act through targets other than the CB1 receptor and putative eCB receptor. However, this possibility was discarded by the authors (Jarai et al., 1999) because of a reported failure of 1 μM rimonabant to inhibit the relaxation to NS1619, an opener of high-conductance Ca2+-dependent K+ channels (BKCa channels) (White and Hiley, 1998). At the same time, the contribution of BKCa channels to the Abn-CBD-evoked mesenteric vasodilatation was not confirmed because of its resistance to a combination of apamin plus iberiotoxin (Jarai et al., 1999). The inhibitory effect of 1 μM rimonabant on the relaxation induced by ionomycin was attributed to the stimulated release of anandamide from the endothelium in the presence of ionomycin (Wagner et al., 1999).
Of interest, earlier studies performed on the rat mesentery showed that the anandamide-induced vasodilatation is unaffected by endothelial cell denudation (Randall et al., 1996; White and Hiley, 1997) and NOS blockade (White and Hiley, 1997). Nevertheless, even in endothelium-denuded vessels, the response was antagonized by 0.1–1 μM rimonabant (Randall et al., 1996; Harris et al., 2002; Ho and Hiley, 2003b; O'Sullivan et al., 2004), strongly indicating a non-endothelial site of action of the blocker. Rimonabant was also shown to inhibit the relaxation of mesenteric arteries induced by the re-addition of Ca2+ (Bukoski et al., 2002). A similar blocking effect was observed for O-1918. The effect was detected in arteries from both control and CB1 receptor-deficient mice, excluding the effect of rimonabant on CB1 receptors. Additionally, in patch-clamp experiments, rimonabant inhibits an unidentified macroscopic K+ current other than that attributable to BKCa channels (Bukoski et al., 2002). In another early study (Chaytor et al., 1999), anandamide was shown to relax isolated rings of rabbit superior mesenteric artery through an endothelium-dependent and endothelium-independent mechanism. In this study, rimonabant at 10 μM attenuated endothelium-dependent, but not endothelium-independent, relaxation through inhibition of myoendothelial communications. Altogether, these data clearly indicate that rimonabant exhibits a number of effects independently of CB receptors and, hence, further work is needed to identify whether rimonabant-mediated suppression of eCB receptor-attributable vasodilatation is mediated via CB receptor-independent mechanisms. Pharmacological targets of putative antagonists of eCB receptors and their CB receptor-independent effect on vasodilaation are summarized in Table 2.
Table 2.
Cannabinoid receptor-independent molecular targets for the putative eCB receptor antagonists
| Putative antagonists of eCB receptor | Targets or CB receptor-independent effects | Reference |
|---|---|---|
| SR141716A | Antagonizes endothelium-dependent relaxation to carbachol, bradykinin, ionomycin and A23187 | Wagner et al., 1999 Randall et al., 1996 White and Hiley, 1997 |
| SR141716A | Antagonizes endothelium-independent relaxation to anandamide | Randall et al., 1996 Harris et al., 2002 Ho and Hiley, 2003a |
| SR141716A | Inhibition of myoendothelial communications | Chaytor et al., 1999 |
| SR141716A | Inhibits unidentified K+ channels | Bukoski et al., 2002 |
| SR141716A | Inhibits relaxation induced by Ca2+-re-addition | Bukoski et al., 2002 |
| O-1918 | Inhibits relaxation of denuded arteries to sodium nitroprusside Inhibits BKCa channels Inhibits Na+-Ca2+ exchanger |
Parmar and Ho, 2010 Godlewski et al., 2009 Bondarenko et al., 2013 |
| Cannabidiol | Acts as PPAR γ ligand Antagonist of α1 adrenoceptors Inhibits voltage-gated Na+ channels Inhibits 5-HT3 receptors Activates TRPV channels |
O'Sullivan et al., 2009 Pertwee et al., 2002 Hill et al., 2014 Yang et al., 2010 Hassan et al., 2014 |
BKCa, high-conductance Ca2+-dependent K+ channels; SR141716, rimonabant.
CB receptor-independent effect of O-1918
In 2003, the group of G. Kunos further substantiated the concept of the involvement of novel eCB receptor in the vasorelaxation induced by endocannabinoids by showing that in endothelium-intact, but not endothelium-denuded, rat mesenteric arterial segments, the cannabidiol analogue O-1918 concentration-dependently inhibits the relaxation to Abn-CBD, a ligand for the putative eCB receptor (Offertaler et al., 2003). These observations allowed the authors to propose O-1918 as a selective antagonist of the Abn-CBD receptor located on endothelial cells. Since then, numerous vascular relaxation studies replicated on different vascular beds employed O-1918 as a selective antagonist of eCB receptors (Hoi et al., 2007; Kozlowska et al., 2007; Zakrzeska et al., 2010; Baranowska-Kuczko et al., 2012; Caldwell et al., 2013), offering further support for existence of novel endothelially located CB receptors. However, in de-endothelized and permeabilized rabbit pulmonary artery strips, Abn-CBD still produced vasodilatation, the response was prevented by O-1918, and only partially reduced by AM251, SR141716A and pertussis toxin (Su and Vo, 2007), indicating a GPCR-independent action of Abn-CBD and O-1918 on molecular targets located on smooth muscle cells. Recently, GPCR-independent effects of O-1918 on distinct molecular targets located in the vasculature have been demonstrated (Godlewski et al., 2009; Bondarenko et al., 2013), indicating the possibility that the inhibitory action of O-1918 on vasodilatation is mediated by a non-specific effect. Consistent with this, at concentrations used to verify the role of eCB receptors (3–10 μM), O-1918 was also shown to significantly attenuate the relaxant responses of endothelium-denuded mesenteric arteries to sodium nitroprusside, a NO donor (Parmar and Ho, 2010). This observation suggests that O-1918 has CB receptor-independent target(s) located on smooth muscle cells. Indeed, in a concentration range used for inhibition of eCB receptor-attributable relaxation, O-1918 inhibits the activity of BKCa channels (Godlewski et al., 2009) and the Na+-Ca2+ exchanger (Bondarenko et al., 2013), key players regulating vascular contractility. These effects do not require GPCR, excluding O-1918 as a selective antagonist of eCB receptors and indicating that the interpretation of results where O-1918 has been assumed to be a selective eCB receptor antagonist should be treated with caution. The conclusions of authors on involvement of eCB receptors in relaxation are frequently based on the supposed selectivity of eCB receptor agonists and antagonists, while additional off-target effects of the compounds are not critically considered. For example, in a recent study performed on rat pulmonary artery, a vascular bed of endothelial cells expressing functional BKCa channels (Vang et al., 2010), the putative eCB receptor antagonist O-1918, which acts as a BKCa channel inhibitor (Godlewski et al., 2009), was shown to attenuate the anandamide-evoked relaxation (Baranowska-Kuczko et al., 2012). Conclusively, it is a general recommendation that the site of action of O-1918, a commonly used eCB receptor inhibitor claimed to be selective, needs to be unambiguously demonstrated.
CB receptor-independent effect of cannabidiol
Cannabidiol has a relatively low affinity for classical cannabinoid receptors, and is commonly used as an antagonist of the novel CB receptor. However, the relevant experimental data is also controversial and indicates the presence of multiple target sites for cannabidiol. Thus, unlike in rat mesentery (Jarai et al., 1999) and rat (Baranowska-Kuczko et al., 2012) and human (Kozlowska et al., 2007) pulmonary artery, where cannabidiol antagonizes the eCB receptor-attributed relaxation, in rat aorta, cannabidiol actually produces vasodilatation (O'Sullivan et al., 2009) by acting as a PPAR ligand. In contrast to findings of the earlier study (Jarai et al., 1999), cannabidiol was reported to produce relaxation in rat isolated mesenteric arteries (Offertaler et al., 2003), suggesting that this compound may act both as a silent antagonist and a partial agonist of eCB receptors. At 5–10 μM, concentrations commonly used to antagonize the eCB receptor-attributed vasodilatation, cannabidiol attenuates contractile responses to agonists of α1 adrenoceptors, as demonstrated in mouse isolated vas deferens (Pertwee et al., 2002). In addition, cannabidiol was shown to inhibit Nav channels (Hill et al., 2014), 5-HT3A receptor-mediated currents (Yang et al., 2010), and via activation of one or more transient receptor potential cation channel subfamily V (TRPV), enhance phagocytosis of mouse microglial BV-2 cells (Hassan et al., 2014).
KCachannels in the eCBR-attributable relaxation
The identity of the K+ channel subtypes involved in the relaxation to cannabinoids is still controversial. In rat isolated small mesenteric artery, the relaxation to N-arachidonylglycine (NAGly) is sensitive to iberiotoxin, a blocker of Ca2+-activated K+ (KCa) channels of large (BKCa) conductance, and has endothelium-dependent component (Parmar and Ho, 2010). In contrast, the relaxation to anandamide was reported to be endothelium-independent and insensitive to iberiotoxin either alone or in combination with apamin (White and Hiley, 1997). In rat isolated mesenteric arteries, charybdotoxin, the BKCa and IKCa channel blocker, inhibited the relaxation to Abn-CBD (Offertaler et al., 2003). The effect was slightly potentiated by the additional presence of apamin, while apamin alone had no effect. The combination of charybdotoxin plus apamin was effective at inhibiting the eCB receptor-attributed vasodilator responses in a number of other vascular beds, including human (Kozlowska et al., 2007) and rat (Baranowska-Kuczko et al., 2012) pulmonary artery, implicating KCa channels of small (SKCa), intermediate (IKCa) and large conductance. In rat coronary arteries, the relaxation to anandamide was shown to be endothelium-independent and sensitive to iberiotoxin (White et al., 2001). Interestingly, the endothelial cells of rat aorta express functional IKCa and SKCa channels (Marchenko and Sage, 1996; Bondarenko et al., 2012), and anandamide-induced aortic relaxation is insensitive to a combination of apamin and charybdotoxin (Herradon et al., 2007).
The role of NO in relaxation to cannabinoids
In rat isolated small mesenteric artery, the relaxation attributable to novel CB receptors was reported to be either sensitive (Hiley and Hoi, 2007; Parmar and Ho, 2010) or insensitive to NOS inhibition (Ho and Hiley, 2003a; 2004,; O'Sullivan et al., 2004). In macrovessels, such as rat (Herradon et al., 2007) and rabbit (Mukhopadhyay et al., 2002) aorta and rat pulmonary artery (Baranowska-Kuczko et al., 2012), the CB1/CB2 receptor-independent endothelium-dependent relaxation is diminished or fully inhibited by inhibition of NOS and was shown to be accompanied by an increased NO release (McCollum et al., 2007; Baranowska-Kuczko et al., 2012). Conversely, in human pulmonary artery, the relaxation was reported to be insensitive to NOS inhibition (Kozlowska et al., 2007). The reasons for these discrepancies are not clear but probably represent regional/species differences.
Is GPCR 55 (GPR55) a novel CB receptor?
In addition to CB1 and CB2 receptors, GPR55 has been described as a target for cannabinoid ligands (Ryberg et al., 2007; Pertwee, 2010; Liu et al., 2014). Initially, GPR55, which, in addition to classical cannabinoids and cannabinoid-like compounds [Abn-CBD, O-1602, N-arachidonyl serine (ARA-S)], is activated by lysophosphatidylinositol (LPI), an endogenous lysophospholipid not related to CB receptor ligands, has been proposed to represent the eCB receptor (Baker et al., 2006). Activation of GPR55 by several ligands was shown to increase intracellular Ca2+ in several cell types (Kohno et al., 2006; Lauckner et al., 2008), including endothelial cell line EA.hy926 (Waldeck-Weiermair et al., 2008), where GPR55 stimulation by LPI produces transient hyperpolarization (Bondarenko et al., 2010), suggesting the functional importance of GPR55 in vascular physiology. In human dermal microvascular endothelial cells, GPR55 knockdown partially inhibited ARA-S-induced migration and activation of Akt and ERK1/2 (Zhang et al., 2010). Additionally, in GPR55-knockdown cells, the ARA-S-induced VEGF production was significantly decreased as compared with the control GPR55-expressing cells (Zhang et al., 2010). However, the BP lowering effect and the vasodilator responses of isolated mesenteric arteries to O-1602 and Abn-CBD appeared to be unaltered in GPR55-deficient mice (Johns et al., 2007). These results indicate that although current knowledge implicates GPR55 in cell migration and growth, GPR55 is not critically involved in the vasodilatation to O-1602 and abn-cbd, and accordingly, GPR55, perhaps, is unlikely to represent the molecular identity of the eCB receptor. An additional set of observations at first glance arguing against GPR55 as the eCB receptor is a discrepancy between the reported Gi/o protein requirement for the vasodilator responses (Mukhopadhyay et al., 2002; Offertaler et al., 2003) and non-Gi/o protein-mediated GPR55 signalling cascade reported in a number of studies (Lauckner et al., 2008; Henstridge et al., 2009; Brown et al., 2011). However, given the uncertainties over the requirement of Gi/o proteins in the eCB receptor-attributed vasodilatation discussed further below and the ability of shared GPR55/GPR18 signalling through Gq protein to increase intracellular Ca2+ (Lauckner et al., 2008; Console-Bram et al., 2014), the association between GPR55 and the eCB receptor should still be considered.
Is GPCR 18 (GPR18) a novel CB receptor?
Another recently emerged candidate for the eCB receptor is GPR18 activated by Δ9-tetrahydrocannabinol (THC), anandamide, Abn-CBD and the anandamide metabolite NAGly (Kohno et al., 2006; McHugh et al., 2012; Takenouchi et al., 2012). Stimulation of GPR18 by several ligands increases intracellular Ca2+ in several cell types (Kohno et al., 2006; Console-Bram et al., 2014), suggesting that vascular GPR18-dependent signalling may have functional implications. In microglial cells, at picomolar and low nanomolar concentrations range, NAGly and Abn-CBD drive cell migration in a GPR18-dependent manner (McHugh et al., 2010). Pertussis toxin was found to attenuate the Abn-CBD- and NAGly-induced cell migration (Mo et al., 2004; McHugh et al., 2010). Additionally, the toxin antagonized the NAGly-induced inhibition of forskolin-stimulated cAMP production (Kohno et al., 2006) and activation of MAPK by Abn-CBD (Offertaler et al., 2003), anandamide and NAGly (McHugh et al., 2010; 2012,), suggesting that the effect is mediated by Gi/o protein.
Based on observations made on microglial cell migration, GPR18 was proposed to be a molecular candidate for the Abn-CBD receptor (McHugh et al., 2010). Notably, both GPR18 mRNA and protein have recently been identified in isolated arteries and endothelial cells (Ho and Yeung, 2009; MacIntyre et al., 2014; Wilhelmsen et al., 2014). However, a correlation between the GPR18 level and the dilator responses is missing and, hence, the role of GPR18 in vasoactivity and other vascular functions is still unclear. There is also inconsistency in the effects of GPR18 ligands on endothelium-dependency of the dilator responses even within the same vascular bed. Thus, in rat isolated small mesenteric arteries, the relaxation to GPR18 agonists anandamide (O'Sullivan et al., 2004), NAGly (Parmar and Ho, 2010) and Abn-CBD (Ho and Hiley, 2003a) has a strong endothelium-dependent component, while the dilator responses of the same arterial bed to THC (O'Sullivan et al., 2005a) and, curiously, anandamide (White and Hiley, 1997; Ho and Hiley, 2003b) were shown to be endothelium-independent.
The stimulating effects of NAGly and Abn-CBD on cell migration were insensitive to rimonabant, but antagonized by O-1918 and ARA-S, a structurally related to anandamide non-psychoactive endocannabinoid with weak affinity for GPR18 (McHugh et al., 2010). However, in rat isolated aorta and mesenteric artery, ARA-S produces a significant endothelium-dependent vasodilatation with potency similar to that evoked by Abn-CBD (Milman et al., 2006), an observation which is not consistent with the ARA-S being a GPR18/Abn-CBD receptor antagonist. Within the same concentration range, ARA-S directly stimulates the BKCa channel (Godlewski et al., 2009). Unlike in the mesentery, the aortic relaxation to ARA-S was blocked by pertussis toxin, suggesting the involvement of a GPCR other than GPR18 in aortic, but not mesenteric, vasodilatation. Oleamide, an endogenous lipid that is structurally related to anandamide, produces vasorelaxation with an endothelium-dependent component in rat isolated small mesenteric arteries (Hoi and Hiley, 2006). Consistent with the pharmacological profile of eCB receptors, the relaxation was sensitive to rimonabant, O-1918 and pertussis toxin pretreatment. There are, unfortunately, no reports that oleamide binds GPR18 and, hence, it is difficult to assign the mechanism of relaxation to GPR18 or another putative GPCR. The identity of eCB receptor as GPR18 was further questioned by the demonstration that oleoylethanolamide, the anandamide-like monounsaturated fatty acid, which was shown to be a GPR119, but not a GPR18 agonist (Overton et al., 2008), produces O-1918-sensitive endothelium- and NO-dependent relaxation in third branches of rat superior mesenteric artery (Alsuleimani and Hiley, 2013). The recent demonstration of an inability of the GPR55/GPR18 agonist O-1602 to mimic the effects of Abn-CBD and NAGly in ocular signalling system (Caldwell et al., 2013; MacIntyre et al., 2014) casts further doubts on the selectivity of the compounds involved, further challenging the concept that the effects of NAGly and Abn-CBD are solely mediated by GPR18. Given the discrepancy between the levels of GPR18 expression and NAGly in different tissues (Alexander, 2012), a link between NAGly and GPR18 and the identification of GPR18 as the Abn-CBD receptor, the stimulation of which causes vasodilation, seems not so straightforward. Activation of GPR18 by NAGly was not established in a study employing high-throughput β-arrestin-based screening (Yin et al., 2009). The application of NAGly to GPR18-expressing rat sympathetic neurons did not inhibit N-type (Cav2.2) Ca2+ currents, a primary downstream effector of Gi/o proteins, but instead potentiated the currents (Lu et al., 2012). Recent findings indicate that GPR18 is coupled to several effector pathways through both Gi/o and Gαq proteins (Console-Bram et al., 2014). Signalling via a specific pathway is likely to be context-specific. Signalling via a single receptor coupled to different G-protein subunits may partially explain the controversies in eCB receptor pharmacology. Nevertheless, the association between GPR18 and the eCB receptor-attributed signalling is not yet proven. A schematic figure explaining the current status of the possible existence and signalling through eCB receptors is shown in Figure 1.
Figure 1.
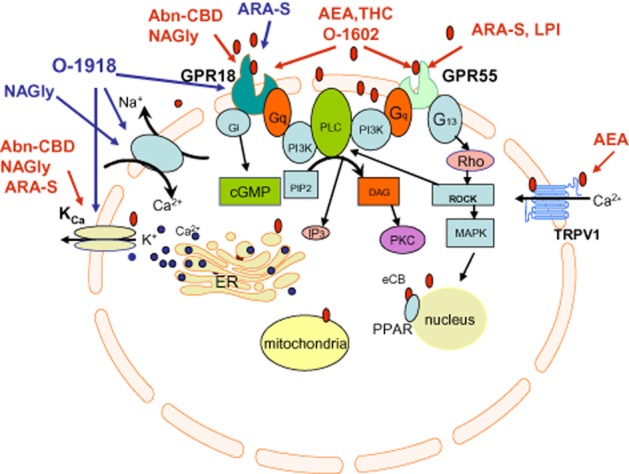
Schematic illustration of current status of research of CB1/CB2 receptor-independent endothelial cannabinoid signalling. Red arrows indicate stimulating effects, blue arrows indicate inhibitory effects.
Limits of cell culture models
An additional caveat in vascular cannabinoid research is the common use of cell culture models, a condition that may profoundly affect the signalling profile, not reflecting the situation in situ but rather a diseased state. Many aspects of endothelial cell signalling, such as functional expression of ion channels/receptors, change dramatically in culture conditions (Sandow and Grayson, 2009). It is well known that endothelial cells grown in culture cease to produce mRNA for M1 and M3 muscarinic receptors and, consequently, cultured endothelial cells are unresponsive to acetylcholine (Marchenko and Sage, 1993). Cell culture-associated abnormalities in endothelial cell signalling are also associated with TRPV3 channels and ryanodine-sensitive stores (Bondarenko and Sagach, 1997; Kohler et al., 2001). In addition, the alterations are undoubtedly relevant for endocannabinoid signalling mechanisms. Notably, while some reports utilizing the human endothelial cell line EA.hy926 indicate the expression of the CB1 receptor and its involvement in the mobilization of intracellular Ca2+ in response to anandamide (Waldeck-Weiermair et al., 2008), other studies failed to detect CB1 receptor expression in the same cell line (Liu et al., 2000). A further potential limitation is associated with the studying of GPCR signalling pathways in heterologous expression systems. It is worth noting that in transfected cells, recombinant opioid and CB receptors operate through the same pool of G proteins, while in cells endogenously co-expressing CB1 with opioid receptors, the signalling occurs through distinct pools of G proteins (Shapira et al., 2000).
An additional caveat associated with utilizing heterologous expression systems is an essential dependency of CB1 receptor- and GPR55-mediated cannabinoid signalling on the lipid composition of the plasma membrane (Gasperi et al., 2012). Keeping in mind that cannabinoids target distinct GPCR-independent ion transport systems, the functional activity of which is regulated by membrane cholesterol, cell culture models may not be able to represent the ‘native’ environment and, most likely, the evoked response is context-dependent. Conclusively, studying cannabinoid signalling in a non-native environment may not reflect a true picture and complicates data interpretation, indicating a need for alternative, more physiological, methodological approaches.
GPCR-independent targets of CB receptor ligands
The mechanism of action of endocannabinoids is not limited to GPCRs. Recently, a growing number of studies have convincingly shown that, at physiologically relevant concentrations, in addition to GPCR-dependent effects, bioactive lipids affect the properties of various ion transport mechanisms independent of GPCRs. The targets affected include voltage- and ligand-gated ion channels, namely the nicotinic acetylcholine, 5-HT and glycine receptors and ionotropic glutamate receptors (Oz, 2006; Alexander and Kendall, 2007; Barana et al., 2010). Anandamide in the concentration range of 1–10 μM causes a significant inhibition of voltage-gated Na+ channels in mouse cortical (Nicholson et al., 2003) and rat dorsal root ganglion neurons (Nicholson et al., 2003; Kim et al., 2005). Various lipoamino acids, including NAGly, were recently shown to inhibit Cav3.1, Cav3.2 and Cav3.3 channels (Ross et al., 2009) and currents mediated by recombinant GABAA receptors (Baur et al., 2013). NAGly enhances the inhibitory glycinergic synaptic transmission by blocking glycine uptake (Jeong et al., 2010).
While much is known about the GPCR-independent effects of endocannabinoids and bioactive lipids on ion transport mechanisms in the nervous system, the vascular molecular targets are much less explored. In recent years, evidence has accumulated suggesting that endocannabinoids and lipid compounds exert their effect on endothelial cells by targeting both GPCR-dependent and GPCR-independent targets, including non-selective transient receptor potential (TRP) channels (Bondarenko et al., 2010), Na+-K+-ATPase (Bondarenko et al., 2010) and different KCa channels (Bondarenko et al., 2011a,b,). In a GPCR-independent manner, anandamide and NAGly inhibit both forward and reverse modes of Na+-Ca2+ exchanger in endothelial cells (Bondarenko et al., 2013). In addition, NAGly was shown to directly potentiate the BKCa channels (Bondarenko et al., 2013) and inhibit store-operated Ca2+ entry in different cell types (Deak et al., 2013). Because vascular signalling and function are chiefly regulated by Na+-Ca2+ exchanger (Bondarenko, 2004; Andrikopoulos et al., 2011), IKCa, BKCa (Vang et al., 2010; Bondarenko et al., 2012; Wulff and Kohler, 2013) and different members of TRP channels, identification of their contribution to GPCR-dependent and -independent cannabinoid signalling still needs to be clarified.
There is also a certain disparity in EC50 values of some endocannabinoids as GPR18 ligands and the EC50 values of the evoked relaxation. The original study of Kohno et al. (2006), who first identified NAGly as a ligand of GPR18, showed that this lipoamino acid concentration-dependently inhibits cAMP formation in a pertussis toxin-sensitive manner, with a calculated IC50 value of 20 nM. However, in rat small mesenteric arteries, NAGly evoked a concentration-dependent relaxation with an EC50 value of 5.8 μM (Parmar and Ho, 2010), which is several orders higher than that required to activate GPR18. At these concentrations, NAGly exhibits a direct GPCR-independent effect on a number of ion transport systems expressed in both endothelial and smooth muscle cells (Bondarenko et al., 2013; Deak et al., 2013). In endometrial cell line, HEC-1B, THC was shown to be a full agonist for GPR18, with a calculated EC50 value of 0.96 μM (McHugh et al., 2012). However, the THC-induced relaxation occurred at higher concentrations, with an EC50 value of 5.8 μM (O'Sullivan et al., 2005b). Similar observations were made with another GPR18 agonist, Abn-CBD. Abn-cbd binds GPR18 with an EC50 value of 835.8 nM (McHugh et al., 2012), while it produces relaxation with EC50 value of 6.2 μM (Ho and Hiley, 2003a). As the concentrations needed for the vasodilator effects are far beyond the values sufficient to activate GPR18, the conclusion that the vascular effects of GPR18 agonists are indeed GPR18-dependent is rather speculative. Altogether, the data available do not yet allow the eCB receptor, the stimulation of which is required for endothelium-dependent relaxation to cannabinoids, to be classified as GPR18.
Sensitivity of the relaxant responses to pertussis toxin
In addition to sensitivity of the relaxant responses to the ‘selective’ antagonists of eCB receptors, the concept of the involvement of novel eCB receptors in the responses is largely supported by inhibition of cannabinoid-induced vasodilatation following pretreatment with pertussis toxin. However, a number of studies did not confirm the pertussis toxin sensitivity of mesenteric vasodilatation to Abn-CBD (Ho and Hiley, 2003a; Milman et al., 2006), ARA-S (Milman et al., 2006) and a synthetic cannabinoid-like compound VSN16 (3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide), which produces endothelium-dependent vasodilatation sensitive to rimonabant, O-1918 and AM-251 (Hoi et al., 2007), thus questioning the involvement of a GPCR. Interestingly, unlike in the mesentery, the aortic relaxant effects of ARA-S and Abn-CBD in the study of Milman et al. were antagonized by pertussis toxin pretreatment. However, the vasorelaxation was insensitive to O-1918 and rimonabant, commonly used selective antagonists of eCB receptors, suggesting distinct mechanisms for the vasodilatation in rat aorta and mesenteric artery (Milman et al., 2006). In rat small mesenteric artery, pertussis toxin was shown to antagonize the relaxation to the Ca2+ ionophore A23187 (White and Hiley, 1997). The recently demonstrated multiplicity of GPR18- and GPR55-activated cellular pathways, which include either Gi/o- and Gαq- (Console-Bram et al., 2014) or Gα12/13- and Gαq- (Lauckner et al., 2008) mediated signalling, respectively, may also partially explain the discrepancies in the reported sensitivity of eCB receptor-attributed relaxant responses to pertussis toxin.
Notably, the use of pertussis toxin in myographic studies as an indicator for GPCR involvement is associated with several flaws. Firstly, pertussis toxin is not a universal GPCR inhibitor but is only an inhibitor of the majority of the members of Gi/o protein family. The ADP-ribosylating toxin, pertussis toxin, catalyzes the ADP-ribosylation of the α subunits of the heterotrimeric Gi/o protein family (Gαi, Gαo and Gαt, but not Gαz), thereby preventing the G proteins from interacting with the related GPCRs (Mangmool and Kurose, 2011). It should be noted that cannabinoid signalling is not limited to Gi/o proteins and involves Gαs and Gαq proteins. Both CB1 and CB2 receptors activate multiple signal transduction pathways such as both inhibition and stimulation of adenylyl cyclase and protein kinase pathways via Gi/o and Gαs proteins respectively (Bosier et al., 2010). Coupling of the CB receptor to Gαq proteins results in stimulation of the activity of PLC (Bosier et al., 2010), which hydrolyzes phosphatidylinositol 4,5-bisphosphate to diacyl glycerol and inositol 1,4,5-trisphosphate (IP3) with subsequent activation of IP3-operated Ca2+ permeable channels. Hence, pertussis toxin as a tool for detection of the involvement of Gαs and Gαq in the response has limited benefits, unless suppression of the signalling via Gi/o proteins unmasks coupling of CB receptors to non-Gi/o proteins. Secondly, pertussis toxin is not specific for Gi/o. proteins and exhibits a number of GPCR-independent effects on key signalling mechanisms, including modulation of Ca2+ entry, cAMP and diacyl glycerol synthesis (Garcia et al., 2001; Mangmool and Kurose, 2011), activation MAPK (Garcia et al., 2001) and up-regulation of angiotensin type 1 (AT1) receptor signalling (Nishida et al., 2010). Consistent with these non-specific effects, pre-incubation of mesenteric arteries with pertussis toxin was found to inhibit endothelium-dependent relaxation to the Ca2+ ionophore A23187 (White and Hiley, 1997). Collectively, the sensitivity of the relaxant responses to pertussis toxin is likely to serve as an unreliable indicator for GPCR involvement.
In view of the conflicting pharmacology on the presence of eCB receptors, the data either supporting or non-supporting the concept of an eCB receptor was summarized in Tables 2013a and 2013b respectively.
Table 3.
Data supporting the presence of a novel endothelial cannabinoid receptor
| Read-out assay | Species and vascular bed | Agonist | pEC50 | Reference |
|---|---|---|---|---|
| PTX-sensitive CB1/CB2 receptor-independent endothelium-dependent relaxation | RSMA | AEA | 6.3 μM | White and Hiley, 1997 |
| RSMA | NAGly | 5.8 μM | Parmarand Ho, 2010 | |
| RSMA | ARA-S | 4.9 μM | Parmar and Ho, 2010 | |
| RA | AEA | 5.9 μM | Herradon et al., 2007 | |
| RA | THC | 5.81 μM | O'Sullivan et al., 2005b | |
| RA | Abn-CBD | not presented | Milman et al., 2006 | |
| RA | ARA-S | 1–50 μM | Milman et al., 2006 | |
| RMA | Abn-CBD | 5.6 μM | Offertaler et al., 2003 | |
| RabA | AEA | 0.6 μM | Mukhopadhyay et al., 2002 | |
| PTX-sensitive activation of p42/44 MAPK | Cell type | Agonist | Concentrations | Reference |
| HUVEC | Abn-CBD | 30 μM | Mo et al., 2004 | |
| HUVEC | Abn-CBD | 10 μM | Offertaler et al., 2003; | |
| HEK293-GPR18, BV-2 | NAGly | 10 nM–10 μM | McHugh et al., 2010 | |
| HEC-1B | NAGly, AEA | 10 nM | McHugh et al., 2012 | |
| PTX-sensitive cell migration | Cell type | Agonist | Concentrations | Reference |
| HUVEC | Abn-CBD | 10–50 μM | Mo et al., 2004 | |
| BV-2 | NAGly | 0.17 nM | McHugh et al., 2010 | |
| BV-2 | Abn-CBD | 13.1 nM | McHugh et al., 2010 | |
| HEK293-GPR18 | NAGly | 1 μM | McHugh et al., 2010 | |
| HEK293-GPR18 | Abn-CBD | 1 μM | McHugh et al., 2010 | |
| Cell migration sensitive to PI3K/Akt inhibitors | Cell type | Agonist | Concentrations | Reference |
| HUVEC | Abn-CBD | 30 μM | Mo et al., 2004 |
Abn-CBD, abnormal cannabidiol; AEA, anandamide; ARA-S, N-arachidonyl serine; BV-2, mouse microglial cells; CB1R-cannabinoid receptor type 1; CB2R-cannabinoid receptor type 2; PTX, pertussis toxin; RA, rat aorta; RabA, rabbit aorta; RMA, rat mesenteric artery; RSMA, rat small mesenteric artery; THC, Δ9-tetrahydrocannabinol.
Table 4.
Data non-supporting the presence of novel endothelial cannabinoid receptor
| Read-out assay | Species/vascular bed | Agonist | pEC50 | Reference |
|---|---|---|---|---|
| PTX-insensitive relaxation to CB | RSMA | Abn-CBD | 6.2 μM | Ho and Hiley, 2003a; |
| RMA | Abn-CBD | 2 μM | Milman et al., 2006 | |
| RMA | ARA-S | 0.55 μM | Milman et al., 2006 | |
| RSMA | VSN16 | 88 nM | Hoi et al., 2007 | |
| RSMA | THC | 5.37 μM | O'Sullivan et al., 2005a | |
| PTX-sensitive EDR to A23187 | RSMA | A23187 | 6.46 μM | White and Hiley, 1997 |
| pEC50 | ||||
| Lack or minor EDR to CB | RabA | Abn-CBD | 0.01–0.3 μM tested | Su and Vo, 2007 |
| RCA | AEA | 6.8 μM | White et al., 2001 | |
| RSMA | AEA | 6.3 μM | White and Hiley, 1997 | |
| RMA | AEA | 5.0 μM | O'Sullivan et al., 2004 | |
| RMA | AEA | 0.3 μM tested | Randall et al., 1996 | |
| RA | AEA | 5.9 μM | O'Sullivan et al., 2005b | |
| RMA | AEA | 6.2 μM | Harris et al., 2002 | |
| RSMA | AEA | 6.7 μM | Ho and Hiley, 2003b | |
| RSMA | THC | 5.37 μM | O'Sullivan et al., 2005a | |
| RMA | THC | 1–10 μM tested | O'Sullivan et al., 2005a | |
| Rimonabant, μM | ||||
| Rimonabant inhibits vasodilatation to non-CB agonists/Ca2+ ionophores | RMA | Ionomycine | 5 μM | Wagner et al., 1999 |
| RCA | Bradykinin | 1 μM | Randall et al., 1997 | |
| RMA | Bradykinin | 1 μM | Randall et al., 1996 | |
| RMA | A23187 | 1 μM | Randall et al., 1996 | |
| RMA | Carbachol | 1 μM | Randall et al., 1996 | |
| RSMA | Carbachol | 1 μM | White and Hiley, 1997 | |
| O-1918, μM | ||||
| No effect of O-1918 on EDR O-1918 inhibits endothelium-independent relaxation | RA | ARA-S | 10 μM | Milman et al., 2006 |
| MA | ARA-S | 10 μM | Milman et al., 2006 | |
| RabPA | Abn-CBD | 0.1–1 μM | Su and Vo, 2007 | |
| RSMA | NAGly | 3 μM | Parmar and Ho, 2010 | |
| RSMA | SNP | 3 μM | Parmar and Ho, 2010 | |
| GPCR-independent targets for O-1918 | Targets | Effect | Concentration tested | |
| NCX | Inhibition | 10 μM | Bondarenko et al., 2013 | |
| BKCa | Inhibition | pIC50 = 6.6 μM | Godlewski et al., 2009 |
Abn-CBD, abnormal cannabidiol; AEA, anandamide; ARA-S, N-arachidonyl serine; BKCa, high-conductance Ca2+-dependent K+ channels; CB, cannabinoid; EDR, endothelium-dependent relaxation; NCX, Na+-Ca2+ exchanger; PTX, pertussis toxin; RA, rat aorta; RabA, rabbit aorta; RabPA, rabbit pulmonary artery; RCA, rat coronary artery; RMA, rat mesenteric artery; RSMA, rat small mesenteric artery; SNP, sodium nitroprussid; THC, Δ9-tetrahydrocannabinol; VSN16, 3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide.
Pertinent unsolved questions
Despite great efforts, our understanding of the vascular pharmacology of cannabinoid signalling and the mechanisms through which endocannabinoids affect vascular function is limited by assessment of vascular contractility as the technique of choice and utilization of non-vascular cell culture models. The variable effects of putative eCB receptor inhibitors and pertussis toxin indicate the complexity of the mechanisms of vascular cannabinoid signalling and possible simultaneous activation of several molecular targets, which may be expressed both in endothelial and smooth muscle cells. The largely accepted view on the presence of a novel CB receptor in vascular endothelium depends on key assumptions on the selectivity of eCB receptor agonists/antagonists, which appear to be rather premature. Recently identified multiple GPCR-independent targets for compounds, which have been used as selective agonists and antagonists for the eCB receptor, challenge the concept of the existence of an eCB receptor and its role in vascular responses to cannabinoids needs further support. Direct evidence for the existence of an eCB receptor is still missing and the mechanisms responsible for eCB receptor-attributed relaxation still need to be elucidated. Meanwhile, several key questions remain unanswered.
Do the vasodilator effects of cannabinoids commonly attributed to eCB receptors require a novel GPCR or do the effects, at least partially, depend on the stimulation of GPCR-independent targets? Clear dissection of GPCR-dependent and -independent endothelial signalling to cannabinoids at the level of ion channels and the molecular identity of the respective targets are ultimately required.
If a novel GPCR is involved in the vasodilatation induced by cannabinoids, is it only one or several eCB receptors? What is the molecular identity of the GPCRs and the respective signal transduction pathways? What is their role in vascular reactivity, angiogenesis and inflammation?
What are the multiple intracellular targets for endocannabinoids, the identity of these targets and their functional role?
Considering that in blood vessels, endothelial cell signalling and function is largely influenced by the smooth muscle cells, what are the molecular targets located on smooth muscle cells and their role in endothelial cell responses to cannabinoids?
Concluding remarks and future directions
In conclusion, substantial data have been accumulated suggesting that a site distinct from CB1 and CB2 receptors mediates the vasodilator effect of cannabinoids. There is evidence that cannabinoids bind to non-CB1/CB2 GPCRs, including GPR18 and GPR55, and their expression has been shown in vascular cells. Ligands and blockers of the putative eCB receptor widely used as selective compounds, often have GPCR-independent effects, influence functional properties of a number of ion channels/transporters expressed in the vasculature with potencies similar to that required to influence vascular reactivity, but higher than that required to activate respective GPCR. The observations that demonstrate Abn-CBD and NAGly are capable of activating GPR18 and inducing vasodilatation do not unequivocally indicate that the vasodilator effect of these compounds is mediated by GPR18. In this regard, it is unhelpful that due to a lack of selective GPR18 antagonists, O-1918 and cannabidiol are increasingly used to support the presence of eCB receptors in in vitro and in vivo experiments. Clearly, there is a need to attribute cannabinoid-induced vascular responses to stimulation of a specific orphan GPCR. To tackle this problem, it would be very helpful to develop and test selective agonists and antagonists of GPR18 and GPR55. Future studies on arteries isolated from GPR18 knockout mice would help to understand the role of GPR18 in the vasodilator effects of cannabinoids commonly attributed to eCB receptors. In addition, as endothelial electrical signalling has a key role in cell function and as there are limitations in cell culture models, systematic characterization of electrical events at the level of in situ vascular endothelium initiated by CB1/CB2 receptor-inactive cannabinoids with the identification of GPCR-dependent signalling is ultimately required. This would pinpoint the responses either to specific GPCR stimulation or GPCR-independent targets. It would be important to determine whether manipulations in GPR18 expression in excised vessels affect endothelial cell electrical responses and endothelium-dependent relaxation and other vascular functions. It is also important to extrapolate any signalling crosstalk between heterologously expressed GPR55 and CB1/CB2 receptors (Kargl et al., 2012; Martinez-Pinilla et al., 2014) and determine the biased agonism of GPR18 signalling reported for GPR18-expressing HEK293 cells (Console-Bram et al., 2014) to native GPCRs expressed in the vascular endothelium.
In vascular preparations, molecular targets for cannabinoids may be located both on endothelial and smooth muscle cells. In the latter case, the signal initiated can be transmitted to the endothelial cell layer either through gap junctions or a diffusible messenger to produce an endothelium-dependent response. It is essential, therefore, to differentiate endothelium-derived signalling from that originating within the smooth muscle cells. Further work on understanding the mechanisms of the regulation of CB1/CB2 receptor-independent vascular effects of cannabinoids would provide further insights into the elucidation of the role of novel GPCRs sensitive to cannabinoids in health and disease.
Conflict of interest
None.
Glossary
- Abn-CBD
abnormal cannabidiol
- ARA-S
N-arachidonyl serine
- BKCa
high-conductance Ca2+-dependent K+ channels
- CB1 receptor
cannabinoid receptor type 1
- CB2 receptor
cannabinoid receptor type 2
- eCB receptor
endothelial cannabinoid receptor
- GPR18
GPCR 18
- NAGly
N-arachidonyl glycine
- O-1918
1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene
- SR141716
rimonabant
- THC
Δ9-tetrahydrocannabinol
- TRP
transient receptor potential
References
- Alexander SP. So what do we call GPR18 now? Br J Pharmacol. 2012;165:2411–2413. doi: 10.1111/j.1476-5381.2011.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol. 2007;152:602–623. doi: 10.1038/sj.bjp.0707456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear hormone receptors. Br J Pharmacol. 2013d;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013f;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013g;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsuleimani YM, Hiley CR. Mechanisms of vasorelaxation induced by oleoylethanolamide in the rat small mesenteric artery. Eur J Pharmacol. 2013;702:1–11. doi: 10.1016/j.ejphar.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos P, Baba A, Matsuda T, Djamgoz MB, Yaqoob MM, Eccles SA. Ca2+ influx through reverse mode Na+/Ca2+ exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J Biol Chem. 2011;286:37919–37931. doi: 10.1074/jbc.M111.251777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Barana A, Amorós I, Caballero R, Gómez R, Osuna L, Lillo MP, et al. Endocannabinoids and cannabinoid analogues block cardiac hKv1.5 channels in a cannabinoid receptor-independent manner. Cardiovasc Res. 2010;85:56–67. doi: 10.1093/cvr/cvp284. [DOI] [PubMed] [Google Scholar]
- Baranowska-Kuczko M, MacLean MR, Kozlowska H, Malinowska B. Endothelium-dependent mechanisms of the vasodilatory effect of the endocannabinoid, anandamide, in the rat pulmonary artery. Pharmacol Res. 2012;66:251–259. doi: 10.1016/j.phrs.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Baur R, Gertsch J, Sigel E. Do N-arachidonyl-glycine (NA-glycine) and 2-arachidonoyl glycerol (2-AG) share mode of action and the binding site on the β2 subunit of GABAA receptors? PeerJ. 2013;1:e149. doi: 10.7717/peerj.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A. Sodium-calcium exchanger contributes to membrane hyperpolarization of intact endothelial cells from rat aorta during acetylcholine stimulation. Br J Pharmacol. 2004;143:9–18. doi: 10.1038/sj.bjp.0705866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A, Sagach V. Na+-K+-ATPase is involved in the sustained ACh-induced hyperpolarization of endothelial cells from rat aorta. Br J Pharmacol. 2006;149:958–965. doi: 10.1038/sj.bjp.0706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A, Waldeck-Weiermair M, Naghdi S, Poteser M, Malli R, Graier WF. GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells. Br J Pharmacol. 2010;161:308–320. doi: 10.1111/j.1476-5381.2010.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A, Panasiuk O, Stepanenko L, Goswami N, Sagach V. Reduced hyperpolarization of endothelial cells following high dietary Na+: effects of enalapril and tempol. Clin Exp Pharmacol Physiol. 2012;39:608–613. doi: 10.1111/j.1440-1681.2012.05718.x. [DOI] [PubMed] [Google Scholar]
- Bondarenko AI, Sagach VF. Caffeine-induced electrical responses of intact endothelial cells. Neurophysiology. 1997;29:156–161. [Google Scholar]
- Bondarenko AI, Malli R, Graier WF. The GPR55 agonist lysophosphatidylinositol acts as an intracellular messenger and bidirectionally modulates Ca2+-activated large-conductance K+ channels in endothelial cells. Pflugers Arch. 2011a;461:177–189. doi: 10.1007/s00424-010-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko AI, Malli R, Graier WF. The GPR55 agonist lysophosphatidylinositol directly activates intermediate-conductance Ca2+-activated K+ channels. Pflugers Arch. 2011b;462:245–255. doi: 10.1007/s00424-011-0977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko AI, Drachuk K, Panasiuk O, Sagach V, Deak AT, Malli R, et al. N-arachidonoyl glycine suppresses Na+/Ca2+ exchanger-mediated Ca2+ entry into endothelial cells and activates BK channels independently of G-protein coupled receptors. Br J Pharmacol. 2013;169:933–948. doi: 10.1111/bph.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booz GW. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med. 2011;51:1054–1061. doi: 10.1016/j.freeradbiomed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80:1–12. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Popp R, Ott G, Bredenkotter D, Wallner C, Busse R, et al. The extracellular regulated kinases (ERK) 1/2 mediate cannabinoid-induced inhibition of gap junctional communication in endothelial cells. Br J Pharmacol. 2002;136:709–716. doi: 10.1038/sj.bjp.0704776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Daniels DA, Kassim M, Brown S, Haslam CP, Terrell VR, et al. Pharmacology of GPR55 in yeast and identification of GSK494581A as a mixed-activity glycine transporter subtype 1 inhibitor and GPR55 agonist. J Pharmacol Exp Ther. 2011;337:236–246. doi: 10.1124/jpet.110.172650. [DOI] [PubMed] [Google Scholar]
- Bukoski RD, Batkai S, Jarai Z, Wang Y, Offertaler L, Jackson WF, et al. CB1 receptor antagonist SR141716A inhibits Ca2+-induced relaxation in CB1 receptor-deficient mice. Hypertension. 2002;39:251–257. doi: 10.1161/hy0202.102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MD, Hu SS, Viswanathan S, Bradshaw H, Kelly ME, Straiker A. A GPR18-based signaling system regulates IOP in murine eye. Br J Pharmacol. 2013;169:834–843. doi: 10.1111/bph.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Martin PE, Evans WH, Randall MD, Griffith TM. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J Physiol. 1999;520(Pt 2):539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME. Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol. 2014;171:3908–3917. doi: 10.1111/bph.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak AT, Groschner LN, Alam MR, Seles E, Bondarenko AI, Graier WF, et al. The endocannabinoid N-arachidonoyl glycine (NAGly) inhibits store-operated Ca2+ entry by preventing STIM1-Orai1 interaction. J Cell Sci. 2013;126:879–888. doi: 10.1242/jcs.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Schermer B, Popp R, Busse R. Inhibition of the production of endothelium-derived hyperpolarizing factor by cannabinoid receptor agonists. Br J Pharmacol. 1999;126:949–960. doi: 10.1038/sj.bjp.0702381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad AA, Albuali WH, Al-Mulhim AS, Jresat I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ Toxicol Pharmacol. 2013;36:347–357. doi: 10.1016/j.etap.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Wang P, Liu F, Hershenson MB, Borbiev T, Verin AD. Pertussis toxin directly activates endothelial cell p42/p44 MAP kinases via a novel signaling pathway. Am J Physiol Cell Physiol. 2001;280:C1233–C1241. doi: 10.1152/ajpcell.2001.280.5.C1233. [DOI] [PubMed] [Google Scholar]
- Gasperi V, Dainese E, Oddi S, Sabatucci A, Maccarrone M. GPR55 and its interaction with membrane lipids: comparison with other endocannabinoid-binding receptors. Curr Med Chem. 2012;20:64–78. [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Osei-Hyiaman D, Mo FM, Harvey-White J, Liu J, et al. The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J Pharmacol Exp Ther. 2009;328:351–361. doi: 10.1124/jpet.108.144717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, Eldeeb K, Millns P, Bennett A, Alexander S, Kendall D. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br J Pharmacol. 2014;171:2426–2439. doi: 10.1111/bph.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23:183–193. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- Herradon E, Martin MI, Lopez-Miranda V. Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. Br J Pharmacol. 2007;152:699–708. doi: 10.1038/sj.bjp.0707404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR, Hoi PM. Oleamide: a fatty acid amide signaling molecule in the cardiovascular system? Cardiovasc Drug Rev. 2007;25:46–60. doi: 10.1111/j.1527-3466.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Jones NA, Smith I, Hill CL, Williams CM, Stephens GJ, et al. Voltage-gated sodium (Na) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci Lett. 2014;566:269–274. doi: 10.1016/j.neulet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003a;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx. Br J Pharmacol. 2003b;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasorelaxant activities of the putative endocannabinoid virodhamine in rat isolated small mesenteric artery. J Pharm Pharmacol. 2004;56:869–875. doi: 10.1211/0022357023682. [DOI] [PubMed] [Google Scholar]
- Ho WS, Yeung SYM. Novel G-protein-coupled receptors in rat arteries: potential targets for N-arachidonoyl glycine? Proc Br Pharmacol Soc. 2009;7:060P. [Google Scholar]
- Hoi PM, Hiley CR. Vasorelaxant effects of oleamide in rat small mesenteric artery indicate action at a novel cannabinoid receptor. Br J Pharmacol. 2006;147:560–568. doi: 10.1038/sj.bjp.0706643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi PM, Visintin C, Okuyama M, Gardiner SM, Kaup SS, Bennett T, et al. Vascular pharmacology of a novel cannabinoid-like compound, 3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16) in the rat. Br J Pharmacol. 2007;152:751–764. doi: 10.1038/sj.bjp.0707470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Vandenberg RJ, Vaughan CW. N-arachidonyl-glycine modulates synaptic transmission in superficial dorsal horn. Br J Pharmacol. 2010;161:925–935. doi: 10.1111/j.1476-5381.2010.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargl J, Balenga N, Parzmair GP, Brown AJ, Heinemann A, Waldhoer M. The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55. J Biol Chem. 2012;287:44234–44248. doi: 10.1074/jbc.M112.364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HI, Kim TH, Shin YK, Lee CS, Park M, Song JH. Anandamide suppression of Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2005;1062:39–47. doi: 10.1016/j.brainres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Kohler R, Brakemeier S, Kuhn M, Degenhardt C, Buhr H, Pries A, et al. Expression of ryanodine receptor type 3 and TRP channels in endothelial cells: comparison of in situ and cultured human endothelial cells. Cardiovasc Res. 2001;51:160–168. doi: 10.1016/s0008-6363(01)00281-4. [DOI] [PubMed] [Google Scholar]
- Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, et al. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347:827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- Kozlowska H, Baranowska M, Schlicker E, Kozlowski M, Laudanski J, Malinowska B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J Hypertens. 2007;25:2240–2248. doi: 10.1097/HJH.0b013e3282ef7a0a. [DOI] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Song S, Jones PM, Persaud SJ. GPR55: from orphan to metabolic regulator? Pharmacol Ther. 2014 doi: 10.1016/j.pharmthera.2014.06.007. doi: 10.1016/j.pharmthera.2014.06.007. http://dx.doi.org/10.1016/j.pharmthera.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, et al. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346(Pt 3):835–840. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Miranda V, Dannert MT, Herradon E, Alsasua A, Martin MI. Cytochrome P450 pathway contributes to methanandamide-induced vasorelaxation in rat aorta. Cardiovasc Drugs Ther. 2010;24:379–389. doi: 10.1007/s10557-010-6261-9. [DOI] [PubMed] [Google Scholar]
- Lu VB, Puhl HL, 3rd, Ikeda SR. N-arachidonyl glycine (NAGly) does not activate G protein-coupled receptor 18 (GPR18) signaling via canonical pathways. Mol Pharmacol. 2012;83:267–282. doi: 10.1124/mol.112.081182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre J, Dong A, Straiker A, Zhu J, Howlett SE, Bagher A, et al. Cannabinoid and lipid-mediated vasorelaxation in retinal microvasculature. Eur J Pharmacol. 2014;735C:105–114. doi: 10.1016/j.ejphar.2014.03.055. [DOI] [PubMed] [Google Scholar]
- Mangmool S, Kurose H. G(i/o) protein-dependent and -independent actions of Pertussis Toxin (PTX) Toxins (Basel) 2011;3:884–899. doi: 10.3390/toxins3070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko SM, Sage SO. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko SM, Sage SO. Calcium-activated potassium channels in the endothelium of intact rat aorta. J Physiol. 1996;492(Pt 1):53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pinilla E, Reyes-Resina I, Onatibia-Astibia A, Zamarbide M, Ricobaraza A, Navarro G, et al. CB and GPR55 receptors are co-expressed and form heteromers in rat and monkey striatum. Exp Neurol. 2014;261C:44–52. doi: 10.1016/j.expneurol.2014.06.017. [DOI] [PubMed] [Google Scholar]
- McCollum L, Howlett AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor–independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther. 2007;321:930–937. doi: 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Page J, Dunn E, Bradshaw HB. Delta(9)-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol. 2012;165:2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo FM, Offertaler L, Kunos G. Atypical cannabinoid stimulates endothelial cell migration via a Gi/Go-coupled receptor distinct from CB1, CB2 or EDG. Eur J Pharmacol. 2004;489:21–27. doi: 10.1016/j.ejphar.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- Nicholson RA, Liao C, Zheng J, David LS, Coyne L, Errington AC, et al. Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Res. 2003;978:194–204. doi: 10.1016/s0006-8993(03)02808-7. [DOI] [PubMed] [Google Scholar]
- Nishida M, Suda R, Nagamatsu Y, Tanabe S, Onohara N, Nakaya M, et al. Pertussis toxin up-regulates angiotensin type 1 receptors through Toll-like receptor 4-mediated Rac activation. J Biol Chem. 2010;285:15268–15277. doi: 10.1074/jbc.M109.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br J Pharmacol. 2004;142:435–442. doi: 10.1038/sj.bjp.0705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. The effects of Delta9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br J Pharmacol. 2005a;145:514–526. doi: 10.1038/sj.bjp.0706218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Vascular effects of delta 9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. Eur J Pharmacol. 2005b;507:211–221. doi: 10.1016/j.ejphar.2004.11.056. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Sun Y, Bennett AJ, Randall MD, Kendall DA. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur J Pharmacol. 2009;612:61–68. doi: 10.1016/j.ejphar.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Overton HA, Fyfe MC, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol. 2008;153(Suppl. 1):S76–S81. doi: 10.1038/sj.bjp.0707529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M. Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol Ther. 2006;111:114–144. doi: 10.1016/j.pharmthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Parmar N, Ho WS. N-arachidonoyl glycine, an endogenous lipid that acts as a vasorelaxant via nitric oxide and large conductance calcium-activated potassium channels. Br J Pharmacol. 2010;160:594–603. doi: 10.1111/j.1476-5381.2009.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem. 2010;17:1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA, Craib SJ, Thomas A. (-)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol. 2002;456:99–106. doi: 10.1016/s0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA. Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. Eur J Pharmacol. 1997;335:205–209. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, et al. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- Randall MD, Harris D, Kendall DA, Ralevic V. Cardiovascular effects of cannabinoids. Pharmacol Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Robson PJ. Therapeutic potential of cannabinoid medicines. Drug Test Anal. 2013;6:24–30. doi: 10.1002/dta.1529. [DOI] [PubMed] [Google Scholar]
- Ross HR, Gilmore AJ, Connor M. Inhibition of human recombinant T-type calcium channels by the endocannabinoid N-arachidonoyl dopamine. Br J Pharmacol. 2009;156:740–750. doi: 10.1111/j.1476-5381.2008.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol. 2009;297:H1–H7. doi: 10.1152/ajpheart.00042.2009. [DOI] [PubMed] [Google Scholar]
- Shapira M, Vogel Z, Sarne Y. Opioid and cannabinoid receptors share a common pool of GTP-binding proteins in cotransfected cells, but not in cells which endogenously coexpress the receptors. Cell Mol Neurobiol. 2000;20:291–304. doi: 10.1023/A:1007058008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JY, Vo AC. 2-Arachidonylglyceryl ether and abnormal cannabidiol-induced vascular smooth muscle relaxation in rabbit pulmonary arteries via receptor-pertussis toxin sensitive G proteins-ERK1/2 signaling. Eur J Pharmacol. 2007;559:189–195. doi: 10.1016/j.ejphar.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Takenouchi R, Inoue K, Kambe Y, Miyata A. N-arachidonoyl glycine induces macrophage apoptosis via GPR18. Biochem Biophys Res Commun. 2012;418:366–371. doi: 10.1016/j.bbrc.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Vang A, Mazer J, Casserly B, Choudhary G. Activation of endothelial BKCa channels causes pulmonary vasodilation. Vascul Pharmacol. 2010;53:122–129. doi: 10.1016/j.vph.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, et al. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K, Khakpour S, Tran A, Sheehan K, Schumacher M, Xu F, et al. The endocannabinoid/endovanilloid N-arachidonoyl dopamine (NADA) and synthetic cannabinoid WIN55,212-2 abate the inflammatory activation of human endothelial cells. J Biol Chem. 2014;289:13079–13100. doi: 10.1074/jbc.M113.536953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Kohler R. Endothelial small-conductance and intermediate-conductance KCa channels: an update on their pharmacology and usefulness as cardiovascular targets. J Cardiovasc Pharmacol. 2013;61:102–112. doi: 10.1097/FJC.0b013e318279ba20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KH, Galadari S, Isaev D, Petroianu G, Shippenberg TS, Oz M. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J Pharmacol Exp Ther. 2010;333:547–554. doi: 10.1124/jpet.109.162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YY, Lin HC, Huang YT, Lee TY, Hou MC, Wang YW, et al. Role of Ca2+-dependent potassium channels in in vitro anandamide-mediated mesenteric vasorelaxation in rats with biliary cirrhosis. Liver Int. 2007;27:1045–1055. doi: 10.1111/j.1478-3231.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, et al. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009;284:12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzeska A, Schlicker E, Baranowska M, Kozlowska H, Kwolek G, Malinowska B. A cannabinoid receptor, sensitive to O-1918, is involved in the delayed hypotension induced by anandamide in anaesthetized rats. Br J Pharmacol. 2010;160:574–584. doi: 10.1111/j.1476-5381.2009.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Maor Y, Wang JF, Kunos G, Groopman JE. Endocannabinoid-like N-arachidonoyl serine is a novel pro-angiogenic mediator. Br J Pharmacol. 2010;160:1583–1594. doi: 10.1111/j.1476-5381.2010.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


