Abstract
Chronic inflammation in the kidneys and vascular wall is a major contributor to hypertension. However, the stimuli and cellular mechanisms responsible for such inflammatory responses remain poorly defined. Inflammasomes are crucial initiators of sterile inflammation in other diseases such as rheumatoid arthritis and gout. These pattern recognition receptors detect host-derived danger-associated molecular patterns (DAMPs), such as microcrystals and reactive oxygen species, and respond by inducing activation of caspase-1. Caspase-1 then processes the cytokines pro-IL-1β and pro-IL-18 into their active forms thus triggering inflammation. While IL-1β and IL-18 are known to be elevated in hypertensive patients, no studies have examined whether this occurs downstream of inflammasome activation or whether inhibition of inflammasome and/or IL-1β/IL-18 signalling prevents hypertension. In this review, we will discuss some known actions of IL-1β and IL-18 on leukocyte and vessel wall function that could potentially underlie a prohypertensive role for these cytokines. We will describe the major classes of inflammasome-activating DAMPs and present evidence that at least some of these are elevated in the setting of hypertension. Finally, we will provide information on drugs that are currently used to inhibit inflammasome/IL-1β/IL-18 signalling and how these might ultimately be used as therapeutic agents for the clinical management of hypertension.
Tables of Links
| TARGETS | |
|---|---|
| Catalytic receptorsa2013a | Enzymesd2013a |
| IL-1 receptor | Caspase-1 |
| IL-1 decoy receptor (IL-1RII) | HMG CoA reductase |
| IL-18 receptor | Endothelial NOS |
| GPCRsb2013a | Inducible NOS |
| Angiotensin AT1 receptor | |
| CCR2 | |
| Ligand-gated ion channelsc2013a | |
| P2X7 receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,d Alexander et al., 2013a,b,c,d,,,).
| LIGANDS |
|---|
| A-438079 |
| Anakinra |
| Angiotensin II |
| Canakinumab |
| IL-1Ra |
| IL-18 |
| IL-33 |
| Simvastatin |
| TNF-α |
Introduction
Hypertension is associated with chronic inflammation in key tissues and organs involved in the regulation of BP such as the kidneys and blood vessels. Renal inflammation results in glomerular injury and impaired sodium urinary excretion, while inflammation in the vasculature can contribute to impaired endothelial function, resistance and stiffening, all of which are key factors involved in the development of hypertension (Ross, 1999; Pauletto and Rattazzi, 2006; Rodriguez-Iturbe et al., 2012). The signalling platforms known as inflammasomes have emerged as crucial initiators of inflammation in response to diverse pathogen- and host-derived danger signals. The primary function of inflammasomes is to activate the cysteine protease, caspase-1, which in turns processes the proinflammatory IL-1 family cytokines IL-1β and IL-18 from their inactive to active forms (Schroder and Tschopp, 2010a). While it is clear that circulating levels of IL-1β and IL-18 are increased in hypertension (Dalekos et al., 1997; Rabkin, 2009), to date, no studies have examined whether this occurs downstream of inflammasome and caspase-1 activation. It is also not known whether inhibition of the production or actions of IL-1β and/or IL-18 reduces renal and vascular inflammation and thereby affords protection in hypertension. This review will highlight the role that IL-1β and IL-18 play as early initiators of inflammation. Furthermore, we will describe what inflammasomes are and present evidence for why they might be considered as important mediators of renal and vascular inflammation in hypertension, and thus potential targets for future antihypertensive therapies.
Renal and vascular inflammation in hypertension
Hypertension is a major risk factor for the two leading causes of death worldwide, ischaemic heart disease and stroke (WHO, 2013). It is widely accepted that chronic overactivation of the renin-angiotensin-aldosterone system is a major contributor to hypertension (Weir and Dzau, 1999). The actions of angiotensin II on AT1 receptors expressed on resident cells of blood vessels, kidneys and the CNS are responsible for its ‘classical’ prohypertensive actions, including vasoconstriction, increased vascular superoxide production, enhanced sodium reabsorption and elevated sympathetic activity (Palatini, 2001; Levy, 2004; Probstfield and O'Brien, 2010). However, it has recently been shown that angiotensin II may contribute to renal and vascular inflammation by inducing the activation and accumulation of leukocytes in the kidneys and artery wall respectively (Johnson et al., 1992; Haller et al., 1997; Suzuki et al., 2003; Rodriguez-Iturbe et al., 2004; Guzik et al., 2007).
Chronic low-grade inflammation appears to play an important role in the pathogenesis of hypertension. In hypertensive patients and in animal models, there is increased activity of the prototypic transcription factor, NF-κB (Ruiz-Ortega et al., 2001; Zhou et al., 2010a), which leads to increased tissue and/or circulating levels of proinflammatory mediators including the acute phase protein, C-reactive protein (CRP) (Bautista, 2003), adhesion molecules including intercellular adhesion molecule 1 and vascular cell adhesion molecule 1, chemokines, such as CCL2 (MCP-1) and CCL5 (RANTES) (Mervaala et al., 1999; Dorfmuller et al., 2003; Boulbou et al., 2005; Madej et al., 2005; Chan et al., 2012), and proinflammatory cytokines such as IL-6, TNF-α (Gu et al., 2006; Zhang et al., 2012) and, of direct relevance to the current review, IL-1β and IL-18 (Dalekos et al., 1997; Rabkin, 2009). Furthermore, numerous studies have shown that by blocking the actions of several of the above mediators either by genetic deletion or pharmacological inhibition, it is possible to reduce disease parameters in hypertension. For example, mice lacking IL-6 display a blunted increase in systolic BP and a reduction in renal damage and fibrosis compared with wild-type mice following induction of hypertension by acute stress or the infusion of angiotensin II (Lee et al., 2004; 2006,; Zhang et al., 2012). Chemokine receptor antagonists prevent the accumulation of immune cells in target tissues by blocking chemokine-dependent chemotaxis of these cells. A selective antagonist of the chemokine receptor CCR2 was shown to reduce macrophage infiltration into the aorta, and consequently to reduce systolic BP in deoxycorticosterone acetate/salt-treated mice (Chan et al., 2012). Similar protective actions have been reported following inhibition of TNF-α and NF-κB in various experimental models of hypertension (Muller et al., 2000; Zhou et al., 2010a; Sriramula et al., 2013; Wang et al., 2013).
IL-1β and IL-18 are elevated in hypertension and are potential mediators of renal and vascular inflammation
IL-1β and IL-18 are members of the proinflammatory IL-1 cytokine superfamily (Dinarello, 2002). The major cellular sources of IL-1β and IL-18 are monocytes and macrophages (Kahlenberg and Dubyak, 2004; Dinarello et al., 2013), however other cell types, such as vascular endothelial cells and renal tubular epithelial cells, may also generate these cytokines under certain conditions (Ala et al., 1992; Dewberry et al., 2000; Striz et al., 2005). The proinflammatory actions of IL-1β and IL-18 are achieved by stimulation of their specific cell surface receptors, namely the IL-1 type 1 receptor (IL-1RI) and the IL-18 receptor α chain (IL-18Rα) respectively (Dinarello, 2002). These receptors are found on several leukocyte subsets relevant to renal and vascular inflammation in hypertension. These include immune cells, such as lymphocytes, monocytes and macrophages, constitutive cell types of the vessel wall, such as vascular endothelial cells and vascular smooth muscle cells (VSMCs), as well as cells in the kidney such as renal endothelial cells and tubular epithelial cells (Nakamura et al., 2000; Gerdes et al., 2002; Miyauchi et al., 2009). Both receptors are members of the immunoglobulin superfamily and display remarkable similarities in terms of their amino acid sequences, overall architecture and the signal transduction mechanisms they utilize (O'Neill, 2002; Sims, 2002).
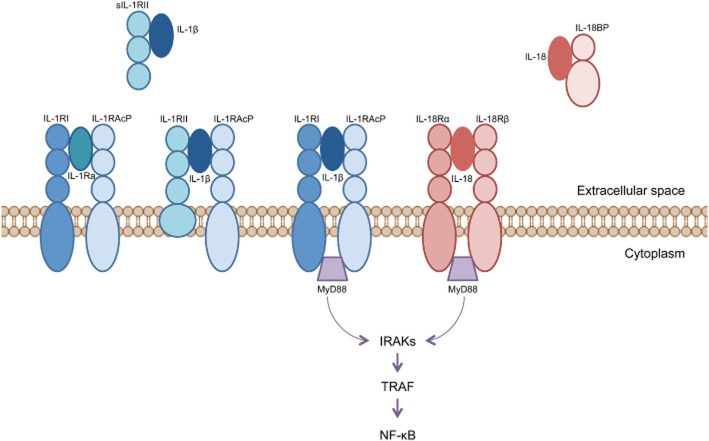
The binding of IL-1β and IL-18 to their receptors causes the recruitment of distinct yet highly homologous accessory proteins, which facilitate high-affinity binding between the ligand-receptor complex. For the IL-1β/IL-1RI complex, the relevant accessory protein is termed IL-1RAcP, whereas that for the IL-18/IL-18Rα complex is IL-18Rβ (Figure 1) (Sims, 2002; Arend et al., 2008). The binding of accessory proteins to IL-1RI and IL-18R also initiates the recruitment of several adapter molecules to the cytoplasmic domains of the receptors. Such adapter molecules include myeloid differentiation factor 88, IL-1R-associated kinase and TNF receptor-associated factor 6 (Figure 1) (Thomassen et al., 1998; Arend et al., 2008). These in turn activate signal transduction pathways involving the kinases, JNK and p38 MAPK, as well as transcription factors such as NF-κB and activator protein-1 (AP-1) (Thomassen et al., 1998; Arend et al., 2008) which are renowned for inducing a proinflammatory gene expression profile in various cell types.
Figure 1.
Signalling pathway and endogenous antagonists of IL-1β and IL-18. Binding of IL-1β to IL-1R1 and IL-18 to IL-18Rα is facilitated by the accessory proteins IL-1RAcP and IL-18Rβ, respectively, resulting in recruitment of the adapter proteins myeloid differentiation factor 88 (MyD88), IL-1 receptor-associated kinase (IRAK) and TNF receptor-associated factor (TRAF), which then causes NF-κB activation. Endogenous inhibitory molecules also exist for both cytokines. For IL-1β, these include an IL-1R antagonist (IL-1Ra), which competes with the IL-1RI for IL-1β binding, as well as a second IL-1β receptor, IL-1RII. The membrane-bound form of IL-1RII receptor contains a short cytosolic signalling domain whereas the soluble form of IL-1RII contains only the extracellular portion of the receptor. Thus, while they bind IL-1β, they fail to support the activation of intracellular signal transduction pathways. Similarly, the actions of IL-18 are negatively regulated by a binding protein known as IL-18BP.
IL-33 is a more recently identified member of the IL-1 family (Arend et al., 2008). In contrast to IL-1β and IL-18, it is the uncleaved form of IL-33 that is active. Moreover, IL-33 triggers an anti-inflammatory type 2 immune response when it binds to its receptor, ST2, which results in the release of cytokines such as IL-5 and IL-13 (Pei et al., 2014). While recent studies have suggested a possible protective role of IL-33/ST2 signalling in other cardiovascular diseases such as heart failure and atherosclerosis (Miller and Liew, 2011; Januzzi, 2013), to date no studies have investigated the role of IL-33 in hypertension.
It is important to note that the actions of IL-1β and IL-18 in a given tissue are governed not only by their concentrations within that tissue and the expression profile of their respective receptors, but also by the presence of several inhibitor molecules that exist for each cytokine (Figure 1). For IL-1β, these include the decoy receptor, IL-1R type II (IL-1RII), which is similar in structure to the extracellular domain of the IL-1RI, however, it has a very short cytoplasmic tail and thus lacks the ability to stimulate intracellular transduction mechanisms (Dinarello, 1996; Schroder and Tschopp, 2010a). Furthermore the IL-1 receptor antagonist (IL-1Ra) is another endogenous inhibitor of IL-1β. IL-1Ra occurs in two forms, one that is secreted from circulating leukocytes and another that is retained intracellularly, especially in monocytes and epithelial cells (Arend et al., 1998). Similarly, there exists an endogenous antagonist of IL-18 known as the IL-18 binding protein (IL-18BP). IL-18BP is constitutively secreted and binds to IL-18 with high affinity (400 pM), thereby neutralizing the actions of this cytokine (Dinarello et al., 2013). The experimental and clinical use of these inhibitors in inflammatory disease models are discussed in further detail later in this review.
IL-1 family cytokines are considered to be ‘early-response’ cytokines. This means that they are released in the earliest stage of an immune response and act as a trigger for a subsequent cascade of proinflammatory cytokines. IL-1β stimulates the release of IL-6 and IL-17a, while IL-18 promotes the production of IFN-γ, IL-2 and IL-12 (Labow et al., 1997; Dinarello, 2002; Cahill and Rogers, 2008; Mills et al., 2013). These downstream cytokines are associated with highly proinflammatory T-helper 1 (Th1)- and T-helper 17 (Th17)-type immune responses and there is evidence to suggest that Th1 and Th17 cells play a major role in hypertension (Shao et al., 2003; Platten et al., 2009; Madhur et al., 2010). In addition to these well-described actions on immune cells, IL-1β and IL-18 have also been shown to have direct effects on the vascular wall that might be consistent with a prohypertensive role. For example, large and resistance-like rat arteries that had undergone ex vivo incubation with IL-1β displayed impaired endothelium-dependent relaxation responses to ACh compared with vessels that were incubated with vehicle (Loughrey et al., 2003; Jimenez-Altayo et al., 2006). This effect appeared to be due to increased vascular reactive oxygen species (ROS) production, as IL-1β-treated vessels expressed higher levels of the pro-oxidant enzymes, inducible NOS and xanthine oxidase, and generated more superoxide than controls (Briones et al., 2005; Jimenez-Altayo et al., 2006). Moreover, treatment of the vessels with superoxide dismutase partially reversed the impaired relaxation response to ACh (Jimenez-Altayo et al., 2006). In a separate study on isolated aortas from spontaneously hypertensive rats, IL-1β directly evoked contractile responses and augmented those to the α1-adrenoceptor agonist, phenylephrine (Dorrance, 2007). Together with the IL-1β-mediated impairment of endothelium-dependent vasodilatation, such increases in contractile activity could conceivably contribute to increased total peripheral vascular resistance, which is a major determinant of BP.
Although no studies have examined the effects of IL-18 on vascular tone, this cytokine has been shown in several studies to promote the proliferation and migration of VSMCs (Chandrasekar et al., 2006; Valente et al., 2012); processes that are critical to the vascular remodelling associated with and contributing to hypertension. Again these effects appeared to result from increases in NADPH oxidase (NOX)-derived ROS production and the subsequent activation of NF-κB- and AP-1-dependent signalling pathways (Valente et al., 2012). Furthermore, the proliferative response of cultured VSMCs to angiotensin II was blocked following siRNA-mediated knockdown of IL-18 (Valente et al., 2012), indicating that IL-18 may be a crucial intermediate in the pathway by which angiotensin II promotes vascular remodelling. The actions of IL-1β and/or IL-18 in mediating inflammation associated with hypertension are summarized in Figure 2.
Figure 2.
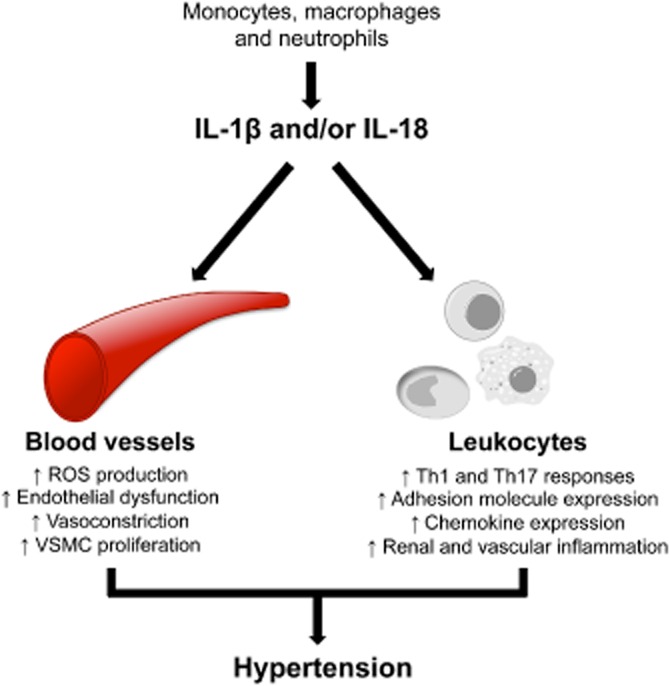
Actions of IL-1β and/or IL-18 in mediating hypertension. IL-1β and IL-18 are mainly secreted by monocytes, macrophages and neutrophils. These proinflammatory cytokines can act on immune cells such as macrophages, dendritic cells and neutrophils as well as non-immune cell types, including vascular endothelial and smooth muscle cells, to induce inflammation and other prohypertensive effects.
As mentioned, there is evidence that circulating and vascular levels of IL-1β and IL-18 are elevated in hypertension. For instance, patients with essential hypertension had higher serum levels of IL-1β than normotensive controls (Dalekos et al., 1997). Furthermore, monocytes isolated from peripheral blood of hypertensive individuals generated higher amounts of IL-1β in response to ex vivo stimulation with either angiotensin II or LPS than monocytes from normotensive controls (Dörffel et al., 1999; Li et al., 2005). These findings not only suggest that monocytes from hypertensive individuals are primed for the production of IL-1β, but they also indicate that angiotensin II may directly act on monocytes to initiate the production and/or release of the cytokine. Consistent with this latter concept, angiotensin AT1 receptor antagonists inhibited IL-1β production by monocytes taken from hypertensive individuals, either when administered to patients in vivo or when pre-incubated ex vivo with cells following their isolation (Dörffel et al., 1999; Li et al., 2005). Given that monocytes accumulate in the vessel wall and interstitium of the kidneys during hypertension (Haller et al., 1997; Boos and Lip, 2006), these cells could represent a significant source of vascular and renal IL-1β. There is also evidence of enhanced responsiveness to IL-1β in hypertension. Specifically, ex vivo treatment with IL-1β caused a greater vasoconstrictive response in aortas from hypertensive rats compared with normotensive rats, and this involved activation of COX (Dorrance, 2007). Whether this increased vascular responsiveness was due to up-regulation of IL-1R1 or downstream signalling elements remains to be determined. Finally, levels of IL-1Ra were found to be elevated in patients with essential hypertension compared with normotensive individuals (Peeters et al., 2001), and this might be indicative of a compensatory response to offset elevated concentrations of IL-1β.
Regarding IL-18, a meta-analysis investigating the association between IL-18 and hypertension identified a significant positive correlation between BP and circulating IL-18 levels (Rabkin, 2009). IL-18 levels are also positively correlated with intima-media thickness of the carotid artery (Yamagami et al., 2005), which is a downstream consequence of hypertension and a marker of future cardiovascular risk in patients (Van Bortel, 2005). Taken together, the points raised earlier highlight the role of IL-1 family cytokines as early mediators of inflammation and as potential contributors to the pathogenesis of hypertension.
Release of IL-1β and IL-18 occurs as a consequence of caspase-1 and ‘inflammasome’ activation
Caspases are cysteine proteases that are best known for their role in regulating apoptosis. However, it is now known that the primary function of some members of the caspase family is to regulate inflammation (Wolf and Green, 1999). Collectively, these proinflammatory caspases are termed group I caspases (Martinon and Tschopp, 2007). Of the 13 mammalian caspases identified, five are thought to regulate inflammation (caspases 1, 4 and 5 in humans and caspases 1, 11 and 12 in mice) (Martinon and Tschopp, 2004; 2007,), with caspase-1 being the best characterized proinflammatory caspase in humans and mice. The major role of caspase-1 in inflammation is to catalyze the intracellular processing of the proinflammatory cytokines, pro-IL-1β (31 kDa) and pro-IL-18 (24 kDa) into their mature and biologically active forms, IL-1β (17.5 kDa) and IL-18 (18 kDa) respectively (Dinarello, 2002). This step is essential as it allows the cytokines to be released from the cytosol into the extracellular space where they can act in a paracrine fashion on receptors on neighbouring cells to exert their proinflammatory influence. There is some evidence that IL-1β can be activated independently of caspase-1 by neutrophil-derived serine proteases such as elastase, cathepsin G and proteinase 3. However, these pathways are likely to play a role in the maturation of the cytokine only in disease conditions associated with an increase in neutrophil infiltration (Guma et al., 2009).
Caspases are themselves synthesized as zymogens and must be cleaved in order to be activated. This is achieved by the multi-protein enzyme complexes known as inflammasomes (Petrilli et al., 2007; Schroder and Tschopp, 2010a). Inflammasomes are comprised of upstream NOD-like receptors (NLRs), which are part of the pattern recognition receptor (PRR) superfamily (Lamkanfi and Dixit, 2014). PRRs are known to play an integral role in the innate immune response (Gordon, 2002; Kanneganti et al., 2007). NLRs are auto-activated when they detect ‘pathogen-associated molecular patterns’ (PAMPs) such as conserved motifs on microbes such as LPS and flagellin (Jha and Ting, 2009). Furthermore, host-derived stress signals otherwise known as danger-associated molecular patterns (DAMPs) have also been shown to induce activation of NLRs. DAMPs that have been shown to activate NLRs include ROS such as superoxide and hydrogen peroxide (Davis and Ting, 2010; Latz, 2010; Zhou et al., 2010b), high concentrations of extracellular ATP (Mariathasan et al., 2006), hyaluronan, which is released from the extracellular matrix in response to injury (Yamasaki et al., 2009), β amyloid, the major peptide present in amyloid plaques characteristic of Alzheimer's disease (Halle et al., 2008) and crystalline substances such as uric acid (Martinon et al., 2006), cholesterol (Duewell et al., 2010) and silica (Hornung et al., 2008), which are thought to mediate the chronic inflammatory responses in gout, atherosclerosis and silicosis respectively.
While several NLRs have been identified, information on functional significance is only available for a few of these receptors. This includes the NLRP3-, NLRP1- and IPAF-containing inflammasomes, all of which respond to a diverse range of stimuli (Schroder et al., 2010b). To date, the NLRP3-containing inflammasome (also known as NALP3) is the best characterized and the isoform that is reported to link inflammation to several metabolic diseases, including diabetes and atherosclerosis (De Nardo and Latz, 2011; Wen et al., 2012; Lu and Kakkar, 2014). There are three basic subunits that make up the NLRP3 inflammasome: (i) the NLRP3 protein, which consists of the basic NLR structure [leucine-rich repeats at the C-terminus, a central nucleotide-binding and oligomerization domain (NACHT) and a pyrin-domain at the N-terminus]; (ii) ASC, a heterodimeric adapter protein also consisting of a pyrin domain as well as a caspase activation and recruitment domain (CARD); and (iii) pro-caspase-1 (Jha and Ting, 2009; Schroder et al., 2010b).
Inflammasome activity and production of IL-1β and IL-18 in monocytes and macrophages are tightly regulated via a two-step signal process. Signal I involves NF-κB- and/or AP-1-dependent up-regulation of the genes that encode for the various signalling components including NLRP3, pro-caspase-1, pro-IL-1β and pro-IL-18. Signal II involves the detection of PAMPS or DAMPs by NLRP3, and this in turn promotes the recruitment of ASC and pro-caspase-1 to the complex (Figure 3). The clustering of pro-caspase-1 at the inflammasome complex initiates its autocleavage into two subunits, p10 (10 kDa) and p20 (20 kDa), which heterodimerize to form the active caspase-1 enzyme (Schroder and Tschopp, 2010a).
Figure 3.
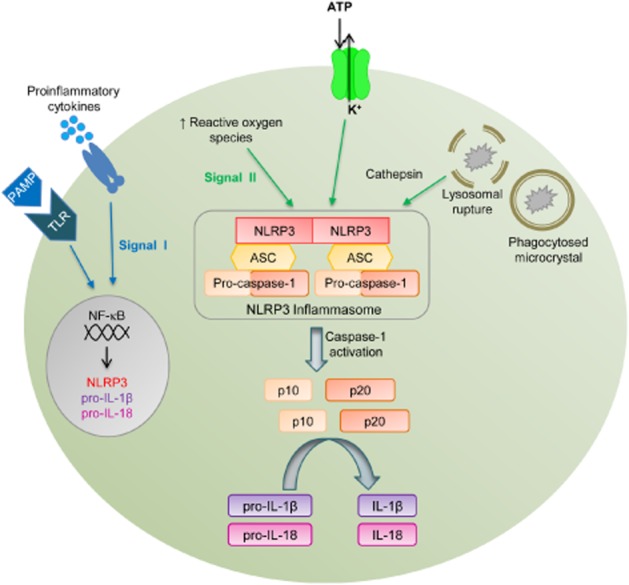
Schematic representation of activators and effectors of the NLRP3 inflammasome. The NLRP3 inflammasome consists of the pattern recognition receptor, NLRP3, the adaptor protein, ASC, and pro-caspase-1. Activation of the NLRP3 inflammasome occurs in two steps. Signal I occurs downstream of Toll-like receptors (TLR) and receptors for cytokines such as TNF, and involves NF-κB-mediated up-regulation of NLRP3, pro-IL-1β and pro-IL-18 gene expression. Signal II occurs when danger-associated molecular patterns (DAMPs) including ATP, microcrystals and ROS, all of which have been shown to be elevated in hypertension, are detected by NLRP3. This leads to oligomerization of NLRP3 subunits and recruitment of ASC and pro-caspase-1. Pro-caspase-1 then undergoes autocleavage into two subunits p10 and p20, which heterodimerize to form the fully active caspase-1. Caspase-1 then processes pro-IL-1β and pro-IL-18 into their active, proinflammatory forms.
Evidence of a role for inflammasome activation in hypertension
The consistent observation that levels of IL-1β and IL-18 are elevated in hypertension (Dalekos et al., 1997; Rabkin, 2009) might be taken as circumstantial evidence that the condition is associated with an increase in inflammasome-dependent caspase-1 activation. However, apart from a single study describing an increase in mRNA expression of pro-caspase-1 in the aorta and renal artery of spontaneously hypertensive rats compared with normotensive Wistar Kyoto rats (Chen et al., 1997), no studies have directly investigated whether hypertension is associated with inflammasome activation. In a genotype association analysis, Omi et al. (2006) showed that the incidence of a specific gain-in-function polymorphism of the NLRP3 gene was significantly higher in hypertensive than normotensive individuals. Furthermore, these authors described a gene–dose relationship whereby homozygotes for the polymorphism displayed higher BPs than heterozygotes (by 3 mmHg), who in turn displayed higher BPs than wild-type individuals (by 2 mmHg) (Omi et al., 2006).
If inflammasome activity is indeed a crucial determinant of hypertension, we are left with the question: what stimuli are responsible for inflammasome activation in the setting of hypertension? While we can presently only speculate on the nature of such stimuli, it is worth noting that hypertension is associated with increased levels of certain DAMPs that are often regarded as ‘classical’ activators of the NLRP3 inflammasome. These stimuli, which include microcrystals, high levels of extracellular ATP and ROS (Schroder and Tschopp, 2010a), are described in the succeeding paragraphs.
Microcrystals
There is a growing body of evidence that microcrystals can induce inflammasome activation, and may be implicated in the pathogenesis of various inflammatory diseases, including atherosclerosis and inflammatory lung diseases (Dostert et al., 2008; Duewell et al., 2010). Microcrystals, which range in size from 0.5 to 3.0 nm, form as a result of high concentrations of relatively insoluble solutes in the circulation and tissues. Microcrystals are detected by phagocytes and engulfed into the phagolysosome within the cell. However, the shard-like structures of many microcrystals rupture the lysosomal membrane, releasing its contents, including cathepsins and other proteolytic enzymes, into the cytosol. These lysosomal enzymes are thought to act as the triggers of inflammasome activation (Schroder et al., 2010b).
Monosodium urate crystals are known to trigger NLRP3 inflammasome activation, and thereby mediate inflammation associated with gout and pseudo-gout (Martinon et al., 2006). Importantly, a high serum level of urate (hyperuricaemia) is considered a risk factor for the development of hypertension (Ward, 1998; Bos et al., 2006). Studies dating back to the 19th century have reported a strong association between hyperuricaemia and hypertension (Haig, 1889; Bos et al., 2006). In support of a causal link between the two conditions, induction of mild hyperuricaemia in rats resulted in a marked increase in BP (Mazzali et al., 2001). Furthermore, clinical trials have shown that allopurinol, a drug used for the treatment of hyperuricaemia and gout, was highly effective at reducing BP in hypertensive adolescents, but less so in older individuals, suggesting that hyperuricaemia may have an especially important role early in the pathogenesis of hypertension (Feig et al., 2008). It remains to be determined whether microcrystal-induced inflammasome activation represents the mechanistic link between hyperuricaemia and elevated BP.
Extracellular ATP
Extracellular ATP acting at the P2X7 receptor is a well-described stimulus for NLRP3 inflammasome activation and there is evidence that this receptor might play a role in hypertension. In general, high levels of extracellular ATP occur as a consequence of cellular damage and a loss of plasma membrane integrity and thereby serve as a danger signal to the immune system (Trautmann, 2009). There is some controversy surrounding how ATP/P2X7 signalling actually leads to inflammasome assembly. The P2X7 receptor is a ligand-gated ion channel and initially it was thought that the K+ efflux that followed activation of this receptor represented the signal for inflammasome activation (Mariathasan et al., 2006). It has also been suggested that P2X7-dependent activation of inflammasomes may involve the recruitment of the pore-forming protein, pannexin-1, to the plasma membrane, in turn allowing the entry of DAMPs into the cell which are ultimately the stimuli for inflammasome activation (Schroder and Tschopp, 2010a). However, more recently, it was suggested that DAMPs, including microcrystals, are able to directly stimulate the release of endogenous ATP to cause IL-1β production in a P2X7-dependent mechanism (Riteau et al., 2012). Regardless, all of these possibilities involve a central role for the P2X7 receptor in inflammasome activation. In addition, expression of this receptor was elevated in various models of hypertension in rodents, and its deletion (i.e. in P2X7 receptor-knockout mice) is associated with lower BP and less renal fibrosis and inflammation (Vonend et al., 2004; Ji et al., 2012a,b,).
ROS
It is clear that ROS and NF-κB play important roles in priming of the inflammasome (i.e. Signal I) to cause transcriptional up-regulation of NLRP3, pro-IL-1β and pro-IL-18 (Bauernfeind et al., 2009; 2011,). However, the role of ROS in NLRP3 and caspase-1 activation (i.e. Signal II) still remains controversial. On the one hand, high levels of ROS have been shown to oxidatively modify caspase-1 protein resulting in a reduction in its catalytic activity (Meissner et al., 2008). Conversely, various DAMPs that are known to activate the NLRP3 inflammasome induce the production of ROS (Cruz et al., 2007; Dostert et al., 2008; Tschopp and Schroder, 2010). Furthermore, several studies have shown that inhibition of ROS can prevent ATP- and microcrystal-induced inflammasome activation (Dostert et al., 2008; Liao et al., 2013; Kojima et al., 2014) and it has thus been proposed that ROS (rather than microcrystals and extracellular ATP) are the actual triggers for assembly of the NLRP3 inflammasome (Schroder and Tschopp, 2010a).
It is well established that hypertensive stimuli, such as angiotensin II, aldosterone and endothelin-1, increase the expression and activity of a family of enzymes called NOX in both immune and non-immune cell types (Drummond et al., 2011; Touyz and Briones, 2011). NOX enzymes are considered primary sources of ROS and play key roles in physiological redox signalling and in the host-defense response to invading pathogens (Drummond et al., 2011). However, in the setting of hypertension, elevated NOX expression may lead to excessive ROS production, which can in turn result in oxidative modifications to other enzymes including endothelial NOS (eNOS), xanthine dehydrogenase and the subunits of the mitochondrial electron transport chain (Touyz and Schiffrin, 2004; Touyz and Briones, 2011). Such modifications uncouple these enzymes from their normal catalytic function and render them as additional enzymatic sources of ROS. In summary, hypertension is associated with elevated ROS production by a range of enzymic sources. Furthermore, inhibition of ROS production reduces BP and renal and vascular dysfunction in hypertension (Touyz and Schiffrin, 2004; Chan et al., 2007; Araujo and Wilcox, 2014). Thus, it will be interesting to determine the role of ROS-dependent inflammasome priming and activation in the pathogenesis of hypertension.
Therapeutic opportunities
The data discussed above provide evidence for an association between hypertension and inflammasome and/or caspase-1-dependent IL-1β/IL-18 production. However, it remains to be established conclusively whether there exists a causal relationship between inflammasome activation and vascular and renal inflammation. Testing for such an association will involve studies examining the effects of strategies that either inhibit inflammasome activation, or block the actions of IL-1β and IL-18, on renal and vascular inflammation and other disease parameters in experimental models of hypertension. Transgenic mice with selective deficiencies in various components of the inflammasome/IL-1 signalling cascade are available and could be readily used to examine the role of this system in hypertension. Several studies have demonstrated that mice with deficiencies in either caspase-1, IL-1β, IL-1R or IL-18 have a dampened immune response and an impaired ability to produce cytokines such as IL-6, TNF and/or IFN-γ, and that this is associated with protection against chronic inflammatory diseases such as arthritis, atherosclerosis and inflammatory bowel disease (Siegmund et al., 2001; Dinarello, 2002; Chamberlain et al., 2009; Joosten et al., 2009; Duewell et al., 2010). However, to our knowledge, the only study to have utilized such transgenic models for the study of hypertension is that by Chamberlain et al. (2009). In this study, it was shown that deficiency of the IL-1R in atherosclerosis-prone apolipoprotein E knockout mice (i.e. IL-1R−/−/ApoE−/− double knockouts) was associated with a blunted hypertensive response to high fat diet-feeding compared with the ApoE−/− single knockout strain, as well as reductions in vascular oxidative stress and endothelial dysfunction (Chamberlain et al., 2009). Further studies like this are expected to provide the necessary proof-of-concept that the inflammasome and its cytokine products are promising targets for future antihypertensive therapies. The following section will review the broad classes of pharmacological agents that have been identified as inhibitors of inflammasome-dependent signalling and which could therefore represent future therapeutic agents for the treatment of hypertension.
Inhibitors of IL-1β signalling
The IL-1Ra is an endogenous antagonist that specifically inhibits the actions of IL-1α and IL-1β, but not IL-18 (Dinarello, 2002). Anakinra is a recombinantly synthesized IL-1Ra consisting of the same structure as endogenous human IL-1Ra except for an additional methionine residue at the N-terminus to confer stability (Muller et al., 2000). It is currently used for the clinical treatment of the auto-inflammatory disease, rheumatoid arthritis (Mertens and Singh, 2009). Despite its short half-life and poor oral bioavailability (it must be administered subcutaneously), anakinra has been shown in clinical trials to be effective at reducing monocyte infiltration and inflammation in the synovial joints of patients with rheumatoid arthritis (Fleischmann et al., 2004).
Canakinumab is a high-affinity human monoclonal antibody against IL-1β (Kuemmerle-Deschner and Haug, 2013). It has a longer plasma half-life and more favourable safety profile than anakinra and is currently approved for clinical use in the treatment of cryopyrin-associated periodic syndrome – a rare inflammatory condition caused by a mutation in the NLRP3 gene (Kuemmerle-Deschner and Haug, 2013). In a Phase IIb trial on men and women with well-controlled diabetes and a high cardiovascular risk profile, canakinumab treatment for 4 months was shown to reduce circulating markers of inflammation including CRP, IL-6 and fibrinogen, without altering plasma lipid profiles (Ridker et al., 2012). Based on these promising findings, canakinumab was taken into a large multinational Phase III clinical trial [Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS)], to investigate its effects on recurrent cardiovascular events such as myocardial infarction and stroke in patients with coronary artery disease and elevated levels of high-sensitivity CRP (Ridker et al., 2011). Results from this study are expected to be released in 2017 and it will be interesting to see what effects (if any) canakinumab treatment has on BP in these high risk patients.
Caspase-1 inhibitors
Caspase-1 has been a prime target for several inflammatory diseases including arthritis and inflammatory bowel disease (Randle et al., 2001). Because caspase-1 inhibition should block the production of both IL-1β and IL-18, it is reasonable to expect that inhibitors of this enzyme will be more efficacious than IL-1R antagonists at reducing inflammation. However, it is also conceivable that caspase-1 inhibitors might have more off-target effects than drugs that selectively target either IL-1β or IL-18 alone.
Ac-YVAD-cmk and ac-YVAD-CHO are tetrapeptides that specifically and irreversibly inhibit caspase-1. These inhibitors are highly selective for caspase-1 (Ki ∼ 1 nM) over other caspase isoforms (Ki = 163 to more than 10 000 nM) (Rabuffetti et al., 2000). Moreover, several studies have shown that ac-YVAD inhibits capsase-1 activity in vivo, thereby reducing inflammation in experimental models of spinal cord injury and cerebral haemorrhage (Karaoglan et al., 2008; Suzuki et al., 2009; Wu et al., 2010). Several low MW caspase-1 inhibitors have also been developed and tested in clinical trials for the treatment of inflammatory conditions including rheumatoid arthritis, psoriasis and hepatitis C (Cornelis et al., 2007; MacKenzie et al., 2010). However, each of these trials were terminated either because of toxicity, especially with regard to liver function, or as a result of poor efficacy (MacKenzie et al., 2010). A clinical trial is currently underway to assess the effects of another caspase-1 inhibitor, VX-765, for the treatment of epilepsy (Kaminski et al., 2014). Post hoc analysis of data from this trial suggests that VX-765 decreased seizure frequency and that this effect was sustained for >2 weeks after treatment was discontinued (Kaminski et al., 2014). Epilepsy is a condition that is not classically associated with inflammation. Rather, IL-1β is thought to contribute to epilepsy through directly enhancing NMDA receptor activity via a Src kinase-dependent mechanism (Viviani et al., 2003; Kaminski et al., 2014). It is unclear what effect this action of caspase-1 inhibition would have in terms of treatment of hypertension. On one hand, Src kinase activity is enhanced in VSMCs of spontaneously hypertensive rats and is thought to contribute to vascular remodelling associated with hypertension (Touyz et al., 2002). On the other hand, activation of NMDA receptors in the nucleus tractus solitarius (NTS) has been associated with a reduction in BP (Kubo and Kihara, 1988), and thus caspase-1-mediated inhibition of these receptors might be expected to worsen hypertension. Clearly, these issues, as well as those relating to toxicity, need to be resolved before caspase-1 inhibition can be considered as a therapeutic option for the treatment of hypertension.
P2X7 receptor antagonists
The P2X7 receptor is an ATP-gated ion channel that allows the passage of cations such as Na+, Ca2+ and K+ (Volonté et al., 2012; Alexander et al., 2013c). It displays a restricted expression profile found primarily in macrophages, certain lymphocytes and fibroblasts (Carroll et al., 2009). As mentioned, activation of P2X7 receptors is thought to induce inflammasome activation by facilitating K+ efflux and/or recruitment of the hemi-channel pannexin-1 and subsequent entry of DAMPs into the cell. A-438079 is a competitive reversible inhibitor of the P2X7 receptor that is at least 100-fold more selective for this receptor than other members of the P2 receptor family (Donnelly-Roberts and Jarvis, 2007). Of direct relevance to the present discussion, A-438079, as well as a structurally distinct inhibitor of P2X7 receptors, Brilliant Blue G, were shown to reduce urinary albumin excretion, macrophage infiltration and BP in a rat model of salt-sensitive hypertension (Ji et al., 2012a). These findings highlight the potential of P2X7 receptor antagonists as novel therapies for the treatment of hypertension.
Pleiotropic actions of statins
Statins (3-hydroxy-3-methylglutaryl-coenzyme A [HMG-CoA] reductase inhibitors) are widely used in the clinic to reduce serum cholesterol levels – and thus cardiovascular risk – in patients with hypercholesterolaemia (Sirtori, 2014). However, in addition to cholesterol lowering, statins display pleiotropic effects that likely contribute to their beneficial effects on the cardiovascular system. Thus, statins have been shown to have modest antihypertensive effects, especially in patients with resistant hypertension (Borghi et al., 2000; Wassmann et al., 2001; Strazzullo et al., 2007; Briasoulis et al., 2013), and the ability to enhance endothelial function (Tsunekawa et al., 2001; de Jongh et al., 2002; Landmesser et al., 2005) and inhibit ROS production (Wassmann et al., 2001; Delbosc et al., 2002). In addition, statins possess anti-inflammatory properties such as reducing circulating levels of proinflammatory cytokines and suppressing adhesion molecule expression on vascular endothelial and smooth muscle cells (Albert et al., 2001; Chung et al., 2002; Rezaie-Majd et al., 2002). In a recent study, it was shown that treatment of bone marrow-derived macrophages from mice with statins interfered with the processing of pro-IL-1β (Davaro et al., 2014). Specifically, statin treatment was associated with the formation of a 28 kDa intermediate form of IL-1β, which came at the expense of production of the mature 17 kDa form. The partly processed form of IL-1β failed to induce IL-6 production in HEK 293T cells, indicating that it had no intrinsic agonistic activity. Furthermore, pretreatment of cells with the 28 kDa variant blocked the ability of mature IL-1β to stimulate cytokine production in the same assay, suggesting that it may be a novel IL-1RI antagonist. While these findings need to be confirmed in vivo in humans, it is tempting to speculate that inhibition of IL-1β processing may explain at least some of the pleiotropic actions of statins in reducing cardiovascular risk.
Conclusion
In summary, there is a growing body of evidence to suggest that hypertension is associated with elevated production of the IL-1 family cytokines, IL-1β and IL-18. At this stage, it is not known whether elevated levels of IL-1β and IL-18 are causes or mere consequences of chronically elevated BP and/or its disease sequelae such as vascular remodelling, atherosclerosis and renal dysfunction. It also remains to be determined whether inflammasome activation is involved and, if so, which stimuli are responsible. Several drugs that are currently in clinical use or undergoing trials for the treatment of other inflammatory disorders act by targeting different components of the inflammasome/IL-1 signalling pathway. Therefore, a better understanding of the activation mechanisms and role of inflammasome-derived IL-1 family cytokines in hypertension has a high potential to improve the way we manage the condition in the clinic.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia (NHMRC; APP1062721) and the Group-of-Eight Australia (Go8)/German Academic Exchange Service (DAAD) Joint Research-Cooperation Scheme. G. R. D. and C. G. S. are supported by Senior Research Fellowships from the NHMRC of Australia (ID Nos. APP1006017 and 350327 respectively). S. M. K. is supported by a Monash Graduate Scholarship (ID No. 5129593). None of these funding sources had any role in the writing of the report or in the decision to submit the article for publication.
Glossary
- AP-1
activator protein-1
- CRP
C-reactive protein
- DAMP
danger-associated molecular pattern
- eNOS
endothelial NOS
- IL-18BP
IL-18 binding protein
- IL-18Rα
IL-18 receptor α chain
- IL-1Ra
IL-1 receptor antagonist
- IL-1RI
IL-1 receptor type I
- IL-1RII
IL-1 receptor type II
- NLR
NOD-like receptor
- NOX
NADPH oxidase
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- ROS
reactive oxygen species
- Th1
T-helper 1
- Th17
T-helper 17
- VSMC
vascular smooth muscle cell
Conflict of interest
None.
References
- Ala Y, Palluy O, Favero J, Bonne C, Modat G, Dornand J. Hypoxia/reoxygenation stimulates endothelial cells to promote interleukin-1 and interleukin-6 production. Effects of free radical scavengers. Agents Actions. 1992;37:134–139. doi: 10.1007/BF01987902. [DOI] [PubMed] [Google Scholar]
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic Receptors. Br J Pharmacol. 2013a;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: ligand-gated ion channels. Br J Pharmacol. 2013c;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013d;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal. 2014;20:74–101. doi: 10.1089/ars.2013.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F, Bartok E, Rieger A, Franchi L, Núñez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006;12:1623–1635. doi: 10.2174/138161206776843313. [DOI] [PubMed] [Google Scholar]
- Borghi C, Prandin MG, Costa FV, Bacchelli S, Degli Esposti D, Ambrosioni E. Use of statins and blood pressure control in treated hypertensive patients with hypercholesterolemia. J Cardiovasc Pharmacol. 2000;35:549–555. doi: 10.1097/00005344-200004000-00006. [DOI] [PubMed] [Google Scholar]
- Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- Boulbou MS, Koukoulis GN, Makri ED, Petinaki EA, Gourgoulianis KI, Germenis AE. Circulating adhesion molecules levels in type 2 diabetes mellitus and hypertension. Int J Cardiol. 2005;98:39–44. doi: 10.1016/j.ijcard.2003.07.037. [DOI] [PubMed] [Google Scholar]
- Briasoulis A, Agarwal V, Valachis A, Messerli FH. Antihypertensive effects of statins: a meta-analysis of prospective controlled studies. J Clin Hypertens (Greenwich) 2013;15:310–320. doi: 10.1111/jch.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones A, Salaices M, Vila E. Ageing alters the production of nitric oxide and prostanoids after IL-1beta exposure in mesenteric resistance arteries. Mech Ageing Dev. 2005;126:710–721. doi: 10.1016/j.mad.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283:25900–25912. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll WA, Donnelly-Roberts D, Jarvis MF. Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signal. 2009;5:63–73. doi: 10.1007/s11302-008-9110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J, Francis S, Brookes Z, Shaw G, Graham D, Alp NJ, et al. Interleukin-1 regulates multiple atherogenic mechanisms in response to fat feeding. PLoS ONE. 2009;4:e5073. doi: 10.1371/journal.pone.0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Moore J, Budzyn K, Guida E, Diep H, Vinh A, et al. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension. 2012;60:1207–1212. doi: 10.1161/HYPERTENSIONAHA.112.201251. [DOI] [PubMed] [Google Scholar]
- Chan EC, Datla SR, Dilley R, Hickey H, Drummond GR, Dusting GJ. Adventitial application of the NADPH oxidase inhibitor apocynin in vivo reduces neointima formation and endothelial dysfunction in rabbits. Cardiovasc Res. 2007;75:710–718. doi: 10.1016/j.cardiores.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Mummidi S, Mahimainathan L, Patel DN, Bailey SR, Imam SZ, et al. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J Biol Chem. 2006;281:15099–15109. doi: 10.1074/jbc.M600200200. [DOI] [PubMed] [Google Scholar]
- Chen H, Lu ZZ, Wei H, Han C. Induction of ICE and inhibition of c-fos, jun D and zif 268 in 12-month old spontaneously hypertensive rats. Life Sci. 1997;61:PL27–PL31. [PubMed] [Google Scholar]
- Chung HK, Lee IK, Kang H, Suh JM, Kim H, Park KC, et al. Statin inhibits interferon-gamma-induced expression of intercellular adhesion molecule-1 (ICAM-1) in vascular endothelial and smooth muscle cells. Exp Mol Med. 2002;34:451–461. doi: 10.1038/emm.2002.63. [DOI] [PubMed] [Google Scholar]
- Cornelis S, Kersse K, Festjens N, Lamkanfi M, Vandenabeele P. Inflammatory caspases: targets for novel therapies. Curr Pharm Des. 2007;13:367–385. doi: 10.2174/138161207780163006. [DOI] [PubMed] [Google Scholar]
- Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129:300–308. doi: 10.1016/s0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- Davaro F, Forde SD, Garfield M, Jiang Z, Halmen K, Tamburro ND, et al. 3-Hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor (statin)-induced 28-kDa interleukin-1beta interferes with mature IL-1beta signaling. J Biol Chem. 2014;289:16214–16222. doi: 10.1074/jbc.M114.571505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BK, Ting JP. NLRP3 has a sweet tooth. Nat Immunol. 2010;11:105–106. doi: 10.1038/ni0210-105. [DOI] [PubMed] [Google Scholar]
- De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B, Cristol JP. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J Cardiovasc Pharmacol. 2002;40:611–617. doi: 10.1097/00005344-200210000-00015. [DOI] [PubMed] [Google Scholar]
- Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2394–2400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- Dinarello C, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20(5 Suppl. 27):S1–S13. [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörffel Y, Lätsch C, Stuhlmuller B, Schreiber S, Scholze S, Burmester GR, et al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117. doi: 10.1161/01.hyp.34.1.113. [DOI] [PubMed] [Google Scholar]
- Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- Dorrance A. Interleukin 1-beta (IL-1beta) enhances contractile responses in endothelium-denuded aorta from hypertensive, but not normotensive, rats. Vascul Pharmacol. 2007;47:160–165. doi: 10.1016/j.vph.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R, Stern R, Iqbal I. Anakinra: an inhibitor of IL-1 for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2004;4:1333–1344. doi: 10.1517/14712598.4.8.1333. [DOI] [PubMed] [Google Scholar]
- Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Gu J-W, Tian N, Shparago M, Tan W, Bailey A, Manning R. Renal NF-kappaB activation and TNF-alpha upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1817–R1824. doi: 10.1152/ajpregu.00153.2006. [DOI] [PubMed] [Google Scholar]
- Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig A. On uric acid and arterial tension. Br Med J. 1889;1:288–291. doi: 10.1136/bmj.1.1467.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller H, Park JK, Dragun D, Lippoldt A, Luft FC. Leukocyte infiltration and ICAM-1 expression in two-kidney one-clip hypertension. Nephrol Dial Transplant. 1997;12:899–903. doi: 10.1093/ndt/12.5.899. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januzzi J. ST2 as a cardiovascular risk biomarker: from the bench to the bedside. J Cardiovasc Transl Res. 2013;6:493–500. doi: 10.1007/s12265-013-9459-y. [DOI] [PubMed] [Google Scholar]
- Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol. 2009;183:7623–7629. doi: 10.4049/jimmunol.0902425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Naito Y, Hirokawa G, Weng H, Hiura Y, Takahashi R, et al. P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res. 2012a;35:173–179. doi: 10.1038/hr.2011.153. [DOI] [PubMed] [Google Scholar]
- Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N. P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol. 2012b;303:F1207–F1215. doi: 10.1152/ajprenal.00051.2012. [DOI] [PubMed] [Google Scholar]
- Jimenez-Altayo F, Briones AM, Giraldo J, Planas AM, Salaices M, Vila E. Increased superoxide anion production by interleukin-1beta impairs nitric oxide-mediated relaxation in resistance arteries. J Pharmacol Exp Ther. 2006;316:42–52. doi: 10.1124/jpet.105.088435. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, et al. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- de Jongh S, Lilien MR, op't Roodt J, Stroes ES, Bakker HD, Kastelein JJ. Early statin therapy restores endothelial function in children with familial hypercholesterolemia. J Am Coll Cardiol. 2002;40:2117–2121. doi: 10.1016/s0735-1097(02)02593-7. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg JM, Dubyak GR. Differing caspase-1 activation states in monocyte versus macrophage models of IL-1beta processing and release. J Leukoc Biol. 2004;76:676–684. doi: 10.1189/jlb.0404221. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Rogawski MA, Klitgaard H. The potential of antiseizure drugs and agents that act on novel molecular targets as antiepileptogenic treatments. Neurother. 2014;11:385–400. doi: 10.1007/s13311-014-0266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Karaoglan A, Kaya E, Akdemir O, Sagmanligil A, Bilguvar K, Cirakoglu B, et al. Neuroprotective effects of Ac.YVAD.cmk on experimental spinal cord injury in rats. Surg Neurol. 2008;69:561–567. doi: 10.1016/j.surneu.2007.03.041. [DOI] [PubMed] [Google Scholar]
- Kojima S, Negishi Y, Tsukimoto M, Takenouchi T, Kitani H, Takeda K. Purinergic signaling via P2X receptor mediates IL-1beta production in Kupffer cells exposed to silica nanoparticle. Toxicology. 2014;321C:13–20. doi: 10.1016/j.tox.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kihara M. Evidence of N-methyl-D-aspartate receptor-mediated modulation of the aortic baroreceptor reflex in the rat nucleus tractus solitarii. Neurosci Lett. 1988;87:69–74. doi: 10.1016/0304-3940(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Kuemmerle-Deschner JB, Haug I. Canakinumab in patients with cryopyrin-associated periodic syndrome: an update for clinicians. Ther Adv Musculoskelet Dis. 2013;5:315–329. doi: 10.1177/1759720X13502629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;59:2452–2461. [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–2363. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- Latz E. NOX-free inflammasome activation. Blood. 2010;116:1393–1394. doi: 10.1182/blood-2010-06-287342. [DOI] [PubMed] [Google Scholar]
- Lee D, Leite R, Fleming C, Pollock J, Webb R, Brands M. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension. 2004;44:259–263. doi: 10.1161/01.HYP.0000139913.56461.fb. [DOI] [PubMed] [Google Scholar]
- Lee D, Sturgis L, Labazi H, Osborne J, Fleming C, Pollock J, et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- Levy BI. Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin-angiotensin system. Circulation. 2004;109:8–13. doi: 10.1161/01.CIR.0000096609.73772.C5. [DOI] [PubMed] [Google Scholar]
- Li QZ, Deng Q, Li JQ, Yi GH, Zhao SP. Valsartan reduces interleukin-1beta secretion by peripheral blood mononuclear cells in patients with essential hypertension. Clin Chim Acta. 2005;355:131–136. doi: 10.1016/j.cccn.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Liao PC, Chao LK, Chou JC, Dong WC, Lin CN, Lin CY, et al. Lipopolysaccharide/adenosine triphosphate-mediated signal transduction in the regulation of NLRP3 protein expression and caspase-1-mediated interleukin-1beta secretion. Inflamm Res. 2013;62:89–96. doi: 10.1007/s00011-012-0555-2. [DOI] [PubMed] [Google Scholar]
- Loughrey JP, Laffey JG, Moore BJ, Lynch F, Boylan JF, McLoughlin P. Interleukin-1 beta rapidly inhibits aortic endothelium-dependent relaxation by a DNA transcription-dependent mechanism. Crit Care Med. 2003;31:910–915. doi: 10.1097/01.CCM.0000053516.15727.E5. [DOI] [PubMed] [Google Scholar]
- Lu X, Kakkar V. Inflammasome and atherogenesis. Curr Pharm Des. 2014;20:108–124. doi: 10.2174/13816128113199990586. [DOI] [PubMed] [Google Scholar]
- MacKenzie SH, Schipper JL, Clark AC. The potential for caspases in drug discovery. Curr Opin Drug Discov Devel. 2010;13:568–576. [PMC free article] [PubMed] [Google Scholar]
- Madej A, Okopien B, Kowalski J, Haberka M, Herman ZS. Plasma concentrations of adhesion molecules and chemokines in patients with essential hypertension. Pharmacol Rep. 2005;57:878–881. [PubMed] [Google Scholar]
- Madhur M, Lob H, McCann L, Iwakura Y, Blinder Y, Guzik T, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36:1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- Mervaala EM, Müller DN, Park JK, Schmidt F, Lohn M, Breu V, et al. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. 1999;33(1 Pt 2):389–395. doi: 10.1161/01.hyp.33.1.389. [DOI] [PubMed] [Google Scholar]
- Miller A, Liew F. The IL-33/ST2 pathway – a new therapeutic target in cardiovascular disease. Pharmacol Ther. 2011;131:179–186. doi: 10.1016/j.pharmthera.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Mills K, Dungan L, Jones S, Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J Leukoc Biol. 2013;93:489–497. doi: 10.1189/jlb.1012543. [DOI] [PubMed] [Google Scholar]
- Miyauchi K, Takiyama Y, Honjyo J, Tateno M, Haneda M. Upregulated IL-18 expression in type 2 diabetic subjects with nephropathy: TGF-beta1 enhanced IL-18 expression in human renal proximal tubular epithelial cells. Diabetes Res Clin Pract. 2009;83:190–199. doi: 10.1016/j.diabres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, et al. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35(1 Pt 2):193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Otani T, Okura R, Ijiri Y, Motoda R, Kurimoto M, et al. Expression and responsiveness of human interleukin-18 receptor (IL-18R) on hematopoietic cell lines. Leukemia. 2000;14:1052–1059. doi: 10.1038/sj.leu.2401789. [DOI] [PubMed] [Google Scholar]
- Omi T, Kumada M, Kamesaki T, Okuda H, Munkhtulga L, Yanagisawa Y, et al. An intronic variable number of tandem repeat polymorphisms of the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies gene expression and is associated with essential hypertension. Eur J Hum Genet. 2006;14:1295–1305. doi: 10.1038/sj.ejhg.5201698. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr Top Microbiol Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- Palatini P. Sympathetic overactivity in hypertension: a risk factor for cardiovascular disease. Curr Hypertens Rep. 2001;3(Suppl. 1):S3–S9. doi: 10.1007/s11906-001-0065-z. [DOI] [PubMed] [Google Scholar]
- Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant. 2006;21:850–853. doi: 10.1093/ndt/gfl019. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters AC, Netea MG, Janssen MC, Kullberg BJ, Van der Meer JW, Thien T. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Invest. 2001;31:31–36. doi: 10.1046/j.1365-2362.2001.00743.x. [DOI] [PubMed] [Google Scholar]
- Pei C, Barbour M, Fairlie-Clarke K, Allan D, Mu R, Jiang H-R. Emerging role of interleukin-33 in autoimmune diseases. Immunology. 2014;141:9–17. doi: 10.1111/imm.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci U S A. 2009;106:14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probstfield JL, O'Brien KD. Progression of cardiovascular damage: the role of renin-angiotensin system blockade. Am J Cardiol. 2010;105(1 Suppl):10A–20A. doi: 10.1016/j.amjcard.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Rabkin SW. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat Clin Pract Cardiovasc Med. 2009;6:192–199. doi: 10.1038/ncpcardio1453. [DOI] [PubMed] [Google Scholar]
- Rabuffetti M, Sciorati C, Tarozzo G, Clementi E, Manfredi AA, Beltramo M. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J Neurosci. 2000;20:4398–4404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle JC, Harding MW, Ku G, Schonharting M, Kurrle R. ICE/Caspase-1 inhibitors as novel anti-inflammatory drugs. Expert Opin Investig Drugs. 2001;10:1207–1209. doi: 10.1517/13543784.10.7.1207. [DOI] [PubMed] [Google Scholar]
- Rezaie-Majd A, Maca T, Bucek RA, Valent P, Muller MR, Husslein P, et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22:1194–1199. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, et al. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012;3:e403. doi: 10.1038/cddis.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol. 2004;286:F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2012;39:96–103. doi: 10.1111/j.1440-1681.2011.05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT(1) and AT(2) receptors. Am J Pathol. 2001;158:1743–1756. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010a;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010b;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, et al. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension. 2003;42:31–38. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci U S A. 2001;98:13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE. IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol. 2002;14:117–122. doi: 10.1016/s0952-7915(01)00306-5. [DOI] [PubMed] [Google Scholar]
- Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88C:3–11. doi: 10.1016/j.phrs.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Sriramula S, Cardinale J, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS ONE. 2013;8:e63847. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzullo P, Kerry SM, Barbato A, Versiero M, D'Elia L, Cappuccio FP. Do statins reduce blood pressure?: a meta-analysis of randomized, controlled trials. Hypertension. 2007;49:792–798. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- Striz I, Krasna E, Honsova E, Lacha J, Petrickova K, Jaresova M, et al. Interleukin 18 (IL-18) upregulation in acute rejection of kidney allograft. Immunol Lett. 2005;99:30–35. doi: 10.1016/j.imlet.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sozen T, Hasegawa Y, Chen W, Zhang JH. Caspase-1 inhibitor prevents neurogenic pulmonary edema after subarachnoid hemorrhage in mice. Stroke. 2009;40:3872–3875. doi: 10.1161/STROKEAHA.109.566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Thomassen E, Bird TA, Renshaw BR, Kennedy MK, Sims JE. Binding of interleukin-18 to the interleukin-1 receptor homologous receptor IL-1Rrp1 leads to activation of signaling pathways similar to those used by interleukin-1. J Interferon Cytokine Res. 1998;18:1077–1088. doi: 10.1089/jir.1998.18.1077. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Wu XH, He G, Salomon S, Schiffrin EL. Increased angiotensin II-mediated Src signaling via epidermal growth factor receptor transactivation is associated with decreased C-terminal Src kinase activity in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2002;39(2 Pt 2):479–485. doi: 10.1161/hy02t2.102909. [DOI] [PubMed] [Google Scholar]
- Trautmann A. Extracellular ATP in the immune system: more than just a ‘danger signal’. Sci Signal. 2009;2:e6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Tsunekawa T, Hayashi T, Kano H, Sumi D, Matsui-Hirai H, Thakur NK, et al. Cerivastatin, a hydroxymethylglutaryl coenzyme a reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days. Circulation. 2001;104:376–379. doi: 10.1161/hc2901.094094. [DOI] [PubMed] [Google Scholar]
- Valente AJ, Yoshida T, Murthy SN, Sakamuri SS, Katsuyama M, Clark RA, et al. Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18. Am J Physiol Heart Circ Physiol. 2012;303:H282–H296. doi: 10.1152/ajpheart.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bortel LM. What does intima-media thickness tell us? J Hypertens. 2005;23:37–39. doi: 10.1097/00004872-200501000-00009. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonté C, Apolloni S, Skaper SD, Burnstock G. P2X7 receptors: channels, pores and more. CNS Neurol Disord Drug Targets. 2012;11:705–721. doi: 10.2174/187152712803581137. [DOI] [PubMed] [Google Scholar]
- Vonend O, Turner C, Chan C, Loesch A, Dell'Anna G, Srai K, et al. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int. 2004;66:157–166. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zuo X-R, Wang Y-Y, Xie W-P, Wang H, Zhang M. Monocrotaline-induced pulmonary arterial hypertension is attenuated by TNF-α antagonists via the suppression of TNF-α expression and NF-κB pathway in rats. Vascul Pharmacol. 2013;58:71–77. doi: 10.1016/j.vph.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Ward HJ. Uric acid as an independent risk factor in the treatment of hypertension. Lancet. 1998;352:670–671. doi: 10.1016/S0140-6736(05)60816-1. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens. 1999;12(12 Pt 3):205S–213S. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- Wen H, Ting J, O'Neill L. A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2013. The top 10 causes of death Vol. 2014: World Health Organization. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed 2/3/2014)
- Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- Wu B, Ma Q, Khatibi N, Chen W, Sozen T, Cheng O, et al. Ac-YVAD-CMK decreases blood-brain barrier degradation by inhibiting caspase-1 activation of interleukin-1beta in intracerebral hemorrhage mouse model. Transl Stroke Res. 2010;1:57–64. doi: 10.1007/s12975-009-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami H, Kitagawa K, Hoshi T, Furukado S, Hougaku H, Nagai Y, et al. Associations of serum IL-18 levels with carotid intima-media thickness. Arterioscler Thromb Vasc Biol. 2005;25:1458–1462. doi: 10.1161/01.ATV.0000168417.52486.56. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Muto J, Taylor KR, Cogen AL, Audish D, Bertin J, et al. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284:12762–12771. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension. 2012;59:136–144. doi: 10.1161/HYPERTENSIONAHA.111.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens. 2010a;28:527–535. doi: 10.1097/HJH.0b013e3283340da8. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010b;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]