Abstract
Background and Purpose
Tamoxifen is a prodrug that is metabolically activated by 4-hydroxylation to the potent primary metabolite 4-hydroxytamoxifen (4OHT) or via another primary metabolite N-desmethyltamoxifen (NDMTAM) to a biologically active secondary metabolite endoxifen through a cytochrome P450 2D6 variant system (CYP2D6). To elucidate the mechanism of action of tamoxifen and the importance of endoxifen for its effect, we determined the anti-oestrogenic efficacy of tamoxifen and its metabolites, including endoxifen, at concentrations corresponding to serum levels measured in breast cancer patients with various CYP2D6 genotypes (simulating tamoxifen treatment).
Experimental Approach
The biological effects of tamoxifen and its metabolites on cell growth and oestrogen-responsive gene modulation were evaluated in a panel of oestrogen receptor-positive breast cancer cell lines. Actual clinical levels of tamoxifen metabolites in breast cancer patients were used in vitro along with actual levels of oestrogens observed in premenopausal patients taking tamoxifen.
Key Results
Tamoxifen and its primary metabolites (4OHT and NDMTAM) only partially inhibited the stimulant effects of oestrogen on cells. The addition of endoxifen at concentrations corresponding to different CYP2D6 genotypes was found to enhance the anti-oestrogenic effect of tamoxifen and its metabolites with an efficacy that correlated with the concentration of endoxifen; at concentrations corresponding to the extensive metabolizer genotype it further inhibited the actions of oestrogen. In contrast, lower concentrations of endoxifen (intermediate and poor metabolizers) had little or no anti-oestrogenic effects.
Conclusions and Implications
Endoxifen may be a clinically relevant metabolite in premenopausal patients as it provides additional anti-oestrogenic actions during tamoxifen treatment.
Introduction
The development of anti-oestrogenic strategies (Jordan and Brodie, 2007) for the adjuvant treatment of oestrogen receptor (ER)-positive breast cancer has revolutionized the prospects for patient survival (Dowsett et al., 2010; Davies et al., 2011). There are two targeted approaches to anti-oestrogenic therapy: tamoxifen and its metabolites block the tumour ER and prevent oestrogen-stimulated growth whereas an aromatase inhibitor (AI) blocks the small but relevant background production of oestrogen in postmenopausal patients. The AIs are now considered to be the adjuvant treatment of choice for postmenopausal breast cancer patients; however, tamoxifen remains the antihormone adjuvant therapy of choice for premenopausal patients with an ER-positive node positive or negative breast cancer. Longer therapy (up to 5 years) is currently recommended as standard therapy as shorter therapy (<5years) does not as effectively control recurrence or enhance survival (Davies et al., 2011). Recent data demonstrate that 10 years of adjuvant tamoxifen is superior to 5 years of adjuvant tamoxifen (Davies et al., 2013).
Tamoxifen itself is a prodrug that is metabolized by cytochrome P450 isoforms into potent metabolites (Allen et al., 1980) (Figure 1). Two biologically relevant metabolites of tamoxifen are 4-hydroxytamoxifen (4OHT) and 4-hydroxy-N-desmethyltamoxifen (endoxifen) formed from the primary metabolite N-desmethyltamoxifen (NDMTAM). Hydroxylation of tamoxifen or NDMTAM at the 4 position increases the compound's affinity for the ER a 100-fold when compared with tamoxifen (Jordan et al., 1977; Johnson et al., 2004), or the major primary metabolite of tamoxifen NDMTAM. The cytochrome P450 2D6 variant (CYP2D6) enzyme was first implicated in the hydroxylation of tamoxifen in human liver in 1997 (Dehal and Kupfer, 1997). Subsequently, it was found that selective 5-HT (serotonin) reuptake inhibitors used to treat hot flashes in breast cancer patients taking tamoxifen-blocked CYP2D6 and reduced endoxifen levels (Stearns et al., 2003). The CYP2D6 enzyme was subsequently identified as responsible for endoxifen synthesis (Desta et al., 2004). A later hypothesis connected aberrant CYP2D6 genotypes with clinical outcome of tamoxifen therapy (Goetz et al., 2005), but this hypothesis remains controversial, and the relevance in postmenopausal patients remains unresolved (Schroth et al., 2009). Attempts to improve tamoxifen's effectiveness unequivocally by selecting out poor metabolizers (PM) of tamoxifen based on an absent CYP2D6 genotype have been unsuccessful in postmenopausal patients (Rae et al., 2012; Regan et al., 2012); although an overview meta-analysis of studies demonstrates a weak association with 5 years of tamoxifen treatment (Province et al., 2014).
Figure 1.
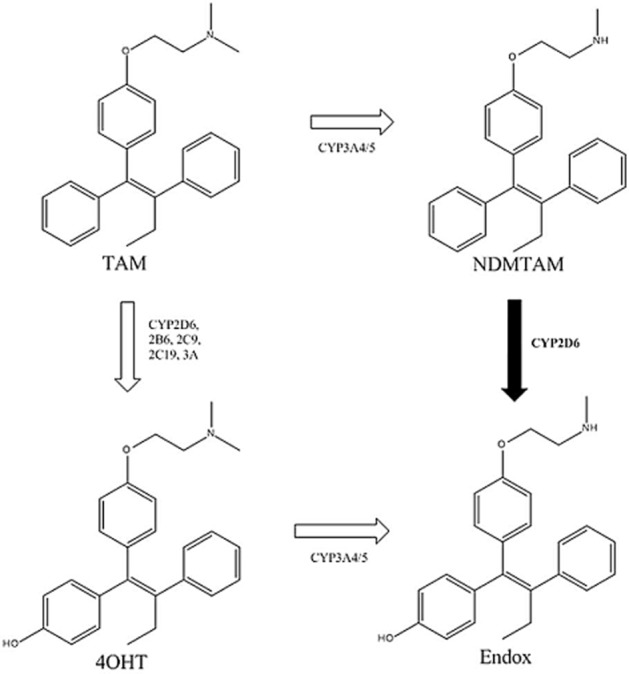
Metabolism of tamoxifen by isoforms of cytochrome P450 to NDMTAM and hydroxylated metabolites 4OHT and 4-hydroxy-N-desmthyltamoxifen (endoxifen) with high affinity for ER. CYP2D6 plays a major role in the metabolism of NDMTAM into endoxifen.
As tamoxifen is the standard of care for premenopausal ER-positive breast cancer patients, we now address the hypothesis that the conversion of tamoxifen to endoxifen is of value for the antitumour actions of tamoxifen in the average oestrogen environment during the menstrual cycle, that is oestrone (E1) plus oestradiol (E2), observed in premenopausal patients taking adjuvant tamoxifen as a monotherapy. There are no extensive clinical studies that have addressed this issue. The complicating factor with tamoxifen therapy in premenopausal women is the increase in circulating oestrogen caused by an anti-oestrogenic blockade of the feedback loop in the hypothalamic pituitary axis (Jordan et al., 1991). Our study is the first to demonstrate a potential pharmacological effect of tamoxifen and its metabolites, including endoxifen, on ER-positive breast cancer cells in vitro, using the same concentrations of tamoxifen and its metabolites as circulating levels found in breast cancer patients that were CYP2D6 genotyped [extensive metabolizers (EM), intermediate metabolizers (IM) and PM], which were provided by Mürdter and Flockhart from previous studies (Irvin et al., 2011; Mürdter et al., 2011). We evaluated the proposal that doubling the dose of tamoxifen (40 mg daily) could be employed to treat patients (Irvin et al., 2011), thereby increasing the overall mix of ‘anti-oestrogenic metabolites’ to block the replication of breast cancer. Lastly, we addressed the effectiveness of tamoxifen and its metabolites to control oestrogenic events in breast cancer cells exposed to perimenopausal levels of oestrogens, that is for women with intermittent and low oestrogen levels but not a cessation of ovarian function at menopause. In general, endoxifen appears to be a clinically relevant metabolite and necessary to control breast cancer cell growth in a high-oestrogen environment.
Methods
Cells and culture conditions
A panel of ER-positive human breast cancer cell lines MCF7, T47D, BT474 and ZR-75-1 were used in this study. All cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were maintained in phenol red RPMI 1640 medium, containing 10% FBS (HyClone Laboratories, Logan, UT, USA), 2 mM glutamine, penicillin at 100 units·mL−1, streptomycin at 100 μg·mL−1, 1× non-essential amino acids (all from Life Technologies, Grand Island, NY, USA), and bovine insulin at 6 ng·mL−1 (Sigma-Aldrich, St. Louis, MO, USA). All cells were cultured in T185 culture flasks (Thermo Scientific, Pittsburgh, PA, USA) and passaged twice a week in 1:3 ratio. All cultures were grown in 5% CO2 at 37°C.
Reagents for treatments
E1, 17β-E2, tamoxifen, 4OHT, NDMTAM were all purchased from Sigma-Aldrich. Endoxifen was a generous gift from Dr James Ingle (Mayo Clinic, Rochester, MN, USA) and was used for Western blot experiments. Endoxifen was also synthesized by Mürdter and used for all other experiments in this study. All compounds were dissolved in ethanol and were stored at −20°C and protected from light.
Cell proliferation assays
All cells were cultured in oestrogen-free medium [phenol red-free RPMI 1640 media supplemented with 10% charcoal-stripped FBS (SFS) ] for 3 days before the start of the proliferation assay. On day 0 of the experiment, cells were seeded in oestrogen-free RPMI media containing 10% SFS at a density of 10 000 cells per well, respectively, in a 24-well cell culture plates (Corning, Tewksbury, MA, USA). After 24 h, cells were treated with combinations of oestrogens, tamoxifen and its metabolites in different concentrations (Tables 1–3) prepared in oestrogen-free RPMI. All treatments were performed in triplicate. The medium containing the test compounds was changed on days 4 and 7, and the experiment was stopped on day 8. Cells were washed with cold PBS (Life Technologies) at least twice and analysed with a fluorescent DNA quantification kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions, and samples were read on a Mithras LB540 fluorometer/luminometer (Berthold Technologies, Oak Ridge, TN, USA) in black wall 96-well plates (Thermo Scientific).
Table 1.
Circulating levels of tamoxifen and its metabolites measured in breast cancer patients who were genotyped for CYP2D6 and categorized into EM, IM and PM
| Drug and metabolite | EMs | IMs | PMs |
|---|---|---|---|
| Tamoxifen | 383 nM | 413 nM | 459 nM |
| NDMTAM | 558 nM | 776 nM | 952 nM |
| 4OHT | 6.3 nM | 5.3 nM | 5.1 nM |
| Endoxifen | 35.6 nM | 24.7 nM | 9.0 nM |
Concentrations provided by Mürdter and were acquired during a previous study (Mürdter et al., 2011).
Table 3.
Calculated concentrations of circulating tamoxifen and its metabolites in breast cancer patients with IM and PM genotype based on concentrations provided by Dr Mürdter
| Drug and metabolite | IMs | PMs |
|---|---|---|
| Tamoxifen | 520 nM | 706 nM |
| NDMTAM | 1132 nM | 1580 nM |
| 4OHT | 6.7 nM | 6.3 nM |
| Endoxifen | 28.9 nM | 27 nM |
Ratios for concentrations increase during 20–40 mg daily tamoxifen dosage increase for IM and PM genotype provided by Flockhart were applied to IM and PM genotype concentrations from Dr. Mürdter. Concentration increase ratios from clinical concentrations provided by Flockhart (Irvin et al., 2011) were applied to concentrations provided by Mürdter et al. (2011) for IM and PM patients to obtain hypothetical concentrations for tamoxifen when thde dose was increase to 40 mg·day−1.
Table 2.
Circulating levels of tamoxifen and its metabolites measured in breast cancer patients who were genotyped for CYP2D6 and categorized as EM, IM and PM (Irvin et al., 2011)
| Drug and metabolite | EMs | IMs | PMs | ||
|---|---|---|---|---|---|
| 20 mg·day−1 | 40 mg·day−1 | 20 mg·day−1 | 40 mg·day−1 | ||
| Tamoxifen | 228.7 nM | 270.9 nM | 342.7 nM | 265.7 nM | 425.3 nM |
| NDMTAM | 409.6 nM | 573.1 nM | 763.4 nM | 748.2 nM | 1198.3 nM |
| 4OHT | 4 nM | 3 nM | 3.8 nM | 3.1 nM | 3.9 nM |
| Endoxifen | 78.4 nM | 49.7 nM | 58.6 nM | 11.3 nM | 34.6 nM |
Tamoxifen dosage during treatment in patients in IM and PM categories was increased from 20 to 40 mg·day−1. Concentrations were provided by Flockhart and were acquired during a previously published study (Irvin et al., 2011). Original concentrations were measured in ng·mL−1 and are available in the Supporting Information Table S1.
Real-time PCR
MCF-7 cells were cultured in oestrogen-free medium for 3 days before seeding and treatment. Cells were seeded after oestrogen deprivation in 6-well cell culture plates (Corning) at a density of 300 000 cells per well. Cells were treated with test compounds for 48 h. Total RNA was isolated using TRIzol reagent (Life Technologies) and an RNeasy RNA purification kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's instructions. Real-time PCR was performed by first synthesizing cDNA by reverse transcribing 1 μg of total RNA using a high-capacity cDNA reverse transcription kit (Life Technologies) as per the manufacturer's instructions and subsequently diluted to 500 μL with nuclease-free water. The real-time PCR was performed in a 20 μL reaction, which included 1× SYBR green PCR master mix (Life Technologies), 125 nM each of forward and reverse primers and 5 μL of diluted cDNA using an ABI Prism 7900 HT Sequence Detection System (Life Technologies). The fold change in expression of transcripts was calculated using the ΔΔCt method, with the ribosomal protein RPLP0 mRNA as the internal control. Primers' sequences that were used for human TFF1 cDNA amplification are: 5′-CATCGACGTCCCTCCAGAAGA-3′ sense, and 5′-CTCTGGGACTAATCACCGTGCTG-3′ anti-sense; human GREB1 gene: 5′-CAAAGAATAACCTGTTGGCCCTGC-3'sense,5′-GACATGCCTGCGCTCTCATACTTA-3′ anti-sense; the reference gene RPLP0: 5′-GTGTTCGACAATGGCAGCAT-3′ sense, 5′-GACACCCTCCAGGAAGCGA-3′ anti-sense. All primers were obtained from Integrated DNA Technologies Inc. (IDT, Coralville, IA, USA).
Immunoblotting
Cells were kept in oestrogen-free media (oestrogen-starved) for 3 days before seeding. Cells were seeded on 10 cm Petri dishes (Corning) at a density of 3 million cells per plate and were incubated overnight. The cells were treated for 24 h with the test compounds. Subsequently cells were washed with cold PBS (Life Techologies) twice and were lysed using a RIPA lysis buffer (Sigma-Aldrich), that contained 1× Complete Mini Protease Inhibitor Cocktail (Roche Diagnostics, Indianapolis, IN, USA) and 1× phosphatase inhibitors (Calbiochem, Gibbstown, NJ, USA). The cells were lysed for 60 min on rotation at 4°C and then centrifuged at 8000× g for 20 min. Supernatants were transferred into fresh tubes and stored on ice. The concentrations of proteins were measured by use of a BCA assay (Pierce, Rockford, IL, USA). Twenty micrograms of each protein sample diluted in a NuPAGE LDS loading dye were loaded and separated on NuPAGE 4–12% Bis-Tris Gel (Life Technologies). After the electrophoresis the samples were transferred onto Hybond-ECL Nitrocellulose Membranes (Amersham Biosciences, Piscataway, NJ, USA), which were then blocked using 5% skimmed milk in TBS-T (50 nM Tris-HCl pH 7.5, 150 nM NaCl, 0.1% Tween-20) for 1 h at room temperature. The membranes were subsequently probed with primary antibodies anti-ERα (clone G-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and with anti-β-actin (clone AC-15; Sigma-Aldrich) diluted in blocking buffer at ratios recommended by the suppliers at 4°C overnight. The membranes were washed three times (10 min each) the next day with the TBS-T buffer and subsequently incubated with the appropriate HRP-linked secondary antibodies (anti-mouse or anti-rabbit from Cell Signaling Technology, Danvers, MA, USA) diluted in blocking buffer for 1 h at room temperature. The membranes were washed again as described earlier with TBS-T buffer, and the signal was visualized using Western Lightning Plus-ECL Reagents (Perkin Elmer, Waltham, MA, USA). The immunoblot was analysed by densitometry using Image J software (National Institutes of Health, Bethesda, MD, USA) measuring the pixel intensity of the lanes, normalized to their corresponding β-actin lane pixel intensity and then normalized to vehicle control as 100%.
Statistical analysis
To test the effects and possible interactions between treatment and genotype in the proliferation assays, we used anova with a balanced two-factor design, followed by Tukey's pair-wise comparison of treatments and genotypes. A P-value less than 0.05 was considered significant. To test the effects of treatment alone, including control, we used one-way anova, followed by Tukey's pair-wise comparisons of treatment doses. To test the effects and possible interactions of treatment and dose, we used anova with a balanced two-factor design. All computations were carried out using R, Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria). For testing the significance of treatment in RT-PCR experiments Student's t-test was used.
Nomenclature
All drug/molecular nomenclature conforms to British Journal of Pharmacology's Concise Guide to PHARMACOLOGY (Alexander et al., 2013a,b,).
Results
Proliferation assays
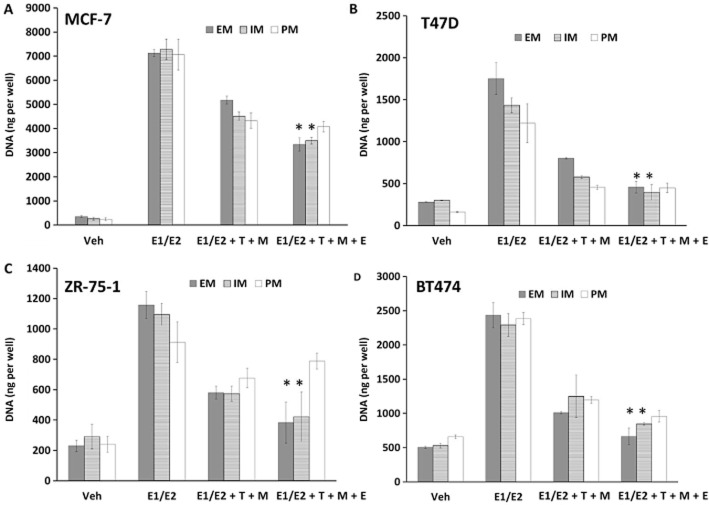
To assess the biological effect of the different treatments on the panel of ER-positive breast cancer cell lines (MCF-7, T47D, ZR-75-1, BT474), we used a DNA quantification-based assay as described in the Methods section. To simulate the premenopausal environment we used E1 and E2 concentrations at average circulating levels measured in premenopausal women taking tamoxifen (Jordan et al., 1991). The calculated concentrations for E1 and E2 were 4 and 2 nM, respectively, for luteal phase, which corresponds to the average levels of oestrogens throughout the 30 day menstrual cycle in patients taking tamoxifen. The concentrations of tamoxifen and its metabolites grouped by CYP2D6 genotypes were acquired during a previously published study (Mürdter et al., 2011) (Table 1). Oestrogens were able to stimulate the growth of all cells lines (P < 0.05 by one-way anova with Tukey's pair-wise comparisons) (Figure 2) and the addition of tamoxifen (labelled as T in the figures) and the combined primary metabolites 4OHT and NDMTAM (labelled as M in the figures) were able to only partially but significantly inhibit the oestrogen action in all the cell lines (P < 0.05 by one-way anova with Tukey's pair-wise comparisons) (Figure 2). Endoxifen (labelled as E in the figures) in EM concentration in combination with tamoxifen, 4OHT and NDMTAM was able to further inhibit oestrogen action and reduce proliferation further (P < 0.05 by one-way anova with Tukey's pair-wise comparisons) (Figure 2). Addition of endoxifen at the IM concentration produced less of an anti-oestrogenic effect when compared with EM concentration, but still significant in MCF-7, T47D and BT474 cells with P < 0.05 (two-way anova), but not in ZR-75-1 cell lines when compared with treatment with no endoxifen in that genotype group with P > 0.05 with two-way anova (Figure 2). Lastly, endoxifen added at the PM concentration did not result in statistically significant reduction of the oestrogenic effect on cell growth in any of the tested cell lines (P > 0.05 by two-way anova for all cell lines) (Figure 2).
Tables of Links
| TARGETS | LIGANDS |
|---|---|
| Cytochrome P450 | Fulvestrant |
| ERα | Oestrone |
| Tamoxifen |
This Table lists key protein targets and ligands in this document, which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b,).
Figure 2.
Results of ER-positive breast cancer cell proliferation assays: (A) MCF-7, (B) T47D, (C) ZR-75-1 and (D) BT474. Treatments were made as follows: Veh, vehicle control; E1/E2 premen, premenopausal oestrogens (E1 4 nM, E2 2 nM); E1/E2 premen + T + M, oestrogens at premenopausal levels with tamoxifen (T) and its primary metabolites (NDMTAM and 4OHT) (M) at different CYP2D6 genotype concentrations (Table 1); E1/E2 premen + T + M + E, oestrogens at premenopausal levels; tamoxifen (T), primary metabolites (M) and endoxifen (E) at different CYP2D6 genotype concentrations (Table 1). Asterisk indicates statistically significant change in treatment from addition of endoxifen.
It should be noted that the addition of endoxifen to tamoxifen and its primary metabolites was not able to completely inhibit the effects of oestrogens to vehicle control levels in any of the cell lines, in any of the genotype groups (P < 0.05 by one-way anova with Tukey's pair-wise comparisons), except ZR-75-1 cells in EM and IM genotype groups only (P > 0.05 by two-way anova) (Figure 2). This is most obvious in the most oestrogen-responsive cell line MCF-7. There is a significant difference in cell numbers between vehicle and endoxifen with tamoxifen and primary metabolites treatment (P < 0.05 by one-way anova with Tukey's pair-wise comparisons) (Figure 2). We decided to focus in our further experiments on the MCF-7 cell line, in particular, since it is the most oestrogen-responsive and most difficult to block growth.
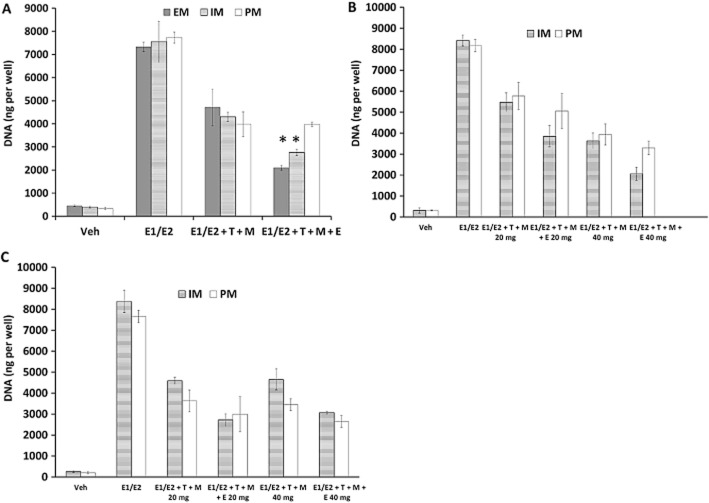
We assessed the hypothesis that increasing the administered dose of tamoxifen from the standard 20 mg·day−1 to a higher dose (40 mg·day−1) would be beneficial by subsequently increasing endoxifen levels for patients with IM and PM genotypes and further inhibit the oestrogenic effect. We used the circulating concentrations of tamoxifen and its metabolites provided by Flockhart (Table 2) based on a study (Irvin et al., 2011) in breast cancer patients treated with tamoxifen. These patients were genotyped for CYP2D6 as IM and PM and treated with both standard dose of tamoxifen (20 mg·day−1) and 40 mg·day−1 (Irvin et al., 2011). Treatments of MCF-7 cells with concentrations for 20 mg·day−1 of tamoxifen and its metabolites in the study (Irvin et al., 2011) showed that the EM concentration of endoxifen was able to further inhibit the oestrogenic action in cells than tamoxifen with 4OHT and NDMTAM alone (P < 0.05 by one-way anova with Tukey's pair-wise comparisons) (Figure 3A). The IM concentration of endoxifen was less potent than EM concentration; however, this was still able to enhance the anti-oestrogenic effect of tamoxifen and mixture of its primary metabolites (P < 0.05 by one-way anova with Tukey's pair-wise comparisons) (Figure 3A). The PM concentration of endoxifen was not able to produce any additional anti-oestrogenic effect in these cells (P > 0.05 by one-way anova with Tukey's pair-wise comparisons) (Figure 3A). To further test the anti-oestrogenic potency tamoxifen and its metabolites during treatment with an increased dose of 40 mg·day−1, we used the corresponding concentrations shown in Table 2. The two-way anova analysis shows that there are no statistically significant interactions between the treatment and dose for the anti-oestrogenic efficacy of endoxifen and tamoxifen with primary metabolites at concentrations corresponding to 40 mg·day−1 when compared with 20 mg·day−1 concentrations in both IM and PM genotype scenarios in MCF-7 cells (P > 0.05) (Figure 3B). Although it should be noted, that in both genotype scenarios, increased concentrations corresponding to 40 mg·day−1 dose did reduce cell proliferation compared with 20 mg·day−1.
Figure 3.
(A) Cell proliferation assessment in MCF-7 cell line using premenopausal levels of oestrogens (E1/E2) and anti-oestrogens tamoxifen, and its primary metabolites (NDMTAM and 4OHT) alone or in combination with endoxifen corresponding to concentrations obtained by Flockhart in breast cancer patients with EMs, IMs and PMs CYP2D6 genotype (Irvin et al., 2011). Treatments were made as follows: Veh, vehicle control; E1/E2, the premenopausal average oestrogen concentrations; E1/E2 + T + M, oestrogens with tamoxifen (T) and its primary metabolites (NDMTAM and 4OHT) (M) (Table 2); E1/E2 + T + M + E, oestrogens; tamoxifen, primary metabolites and endoxifen (E) (Table 2). Asterisk indicates statistically significant change from treatment before the addition of endoxifen. (B) Cell proliferation assessment in MCF-7 cells after treatment with premenopausal levels of oestrogens (E1/E2) and with tamoxifen (T) and its primary metabolites (M) without or with endoxifen (E) corresponding to IM and PM genotypes during treatment with 20 or 40 mg daily; measured by Flockhart (Irvin et al., 2011) (Table 2). Treatments were made as follows: Veh, vehicle control; E1/E2, the premenopausal average oestrogen concentrations; E1/E2 + T + M 20 mg, premenopausal oestrogens with tamoxifen and its primary metabolites (NDMTAM and 4OHT) corresponding to 20 mg·day−1 treatments corresponding to IM and PM genotypes (Table 2); E1/E2 + T + M + E 20 mg, oestrogens; tamoxifen, primary metabolites and endoxifen corresponding to 20 mg·day−1 treatment corresponding to IM and PM genotypes (Table 2); E1/E2 + T + M 40 mg, oestrogens with tamoxifen and its primary metabolites corresponding to 40 mg·day−1 treatments (Table 2); E1/E2 + T + M + E 40 mg, oestrogens; tamoxifen, primary metabolites and endoxifen corresponding to 40 mg·day−1 treatment (Table 2). (C) Cell proliferation assessment in MCF-7 cells after treatment with premenopausal levels of oestrogens (E1/E2) and with tamoxifen (T) and its primary metabolites (M) with or without endoxifen (E) corresponding to IM and PM genotypes during treatment with 20 mg·day−1 measured by Mürdter et al. (2011) and calculated 40 mg·day−1 concentrations based on the metabolite concentration increase ratios from concentrations provided by Flockhart (Irvin et al., 2011). Treatments were made as follows: Veh, vehicle control; E1/E2, the premenopausal average oestrogen concentrations; E1/E2 + T + M 20 mg, premenopausal oestrogens with tamoxifen (T) and its primary metabolites (NDMTAM and 4OHT) (M) corresponding to 20 mg·day−1 treatments corresponding to IM and PM genotypes (Table 1); E1/E2 + T + M + E 20 mg, oestrogens; tamoxifen (T), primary metabolites (M) and endoxifen (E) corresponding to 20 mg·day−1 treatment corresponding to IM and PM genotypes (Table 1); E1/E2 + T + M 40 mg, oestrogens with tamoxifen (T) and its primary metabolites (M) corresponding to 40 mg·day−1 treatments (Table 3); E1/E2 + T + M + E 40 mg, oestrogens; tamoxifen (T), primary metabolites (M) and endoxifen (E) corresponding to 40 mg·day−1 treatment (Table 1).
Using the ratios of increase for concentrations of each metabolite in both IM and PM genotypes with 40 mg·day−1 dose from 20 mg·day−1, provided by Flockhart (Irvin et al., 2011), we calculated the metabolite concentrations obtained with the 40 mg·day−1 dose using the 20 mg·day−1 results obtained by Mürdter et al. (2011). The resulting concentrations are presented in Table 3 and were used in the treatments of MCF-7 cells. The two-way anova analysis of the results again showed that there are no significant interactions between treatment and dose, without taking genotype as a factor (P > 0.05) (Figure 3C), indicating that the anti-oestrogenic efficacy of tamoxifen and its metabolites in concentrations corresponding to 40 and 20 mg·day−1 of tamoxifen with primary metabolites and endoxifen in both IM and PM concentrations had no significant biological improvement on inhibiting the effects of average premenopausal concentrations of oestrogens in MCF-7 cells.
To assess why the anti-oestrogens were not able to fully inhibit the growth in the MCF-7 cell line, we used premenopausal levels of oestrogens in combination with tamoxifen and its primary metabolites at EM concentrations (Table 1) with increasing concentration of endoxifen (Figure 4). The results demonstrate that addition of increasing concentrations of endoxifen is able to inhibit the proliferation effects of oestrogens. The inhibition results in this experiment are consistent with the inhibition results in previous experiments in MCF-7 cells (Figures 2 and 3). However, to fully inhibit oestrogen-stimulated growth a much higher concentration of endoxifen is needed, which is outside the range of concentrations observed in patients (Tables 1 and 2013b) (Figure 4, solid line). We also tested the anti-oestrogenic properties of increasing concentrations of endoxifen alone against constant premenopausal levels of oestrogens. The results show that endoxifen alone (Figure 4, dashed line) is not as effective as an anti-oestrogen as when it is administered in combination with tamoxifen and its primary metabolites (Figure 4, solid line).
Figure 4.
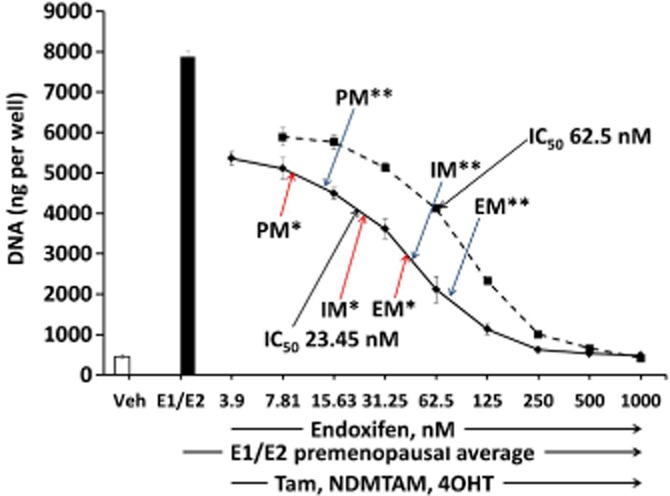
Assessment of MCF-7 cell line growth after treatment with increasing concentrations of endoxifen alone (broken line) or in combination with tamoxifen (Tam) and its primary metabolites (NDMTAM and 4OHT) at EM levels (solid line) (Mürdter et al., 2011) (Table 1). Single asterisk indicates concentrations provided by Mürdter; double asterisk indicates concentrations provided by Flockhart.
As complete inhibition of oestrogen action in MCF-7 cells occurred only with very high concentrations of endoxifen, we also assessed the effect of the different levels of tamoxifen metabolites in perimenopausal women (49–55 years). Wedefined the level as perimenopausal when tamoxifen and its primary metabolites inhibited oestrogen-induced growth of MCF-7 cells by 50%. We used constant tamoxifen and primary metabolites at EM concentrations (Mürdter et al., 2011) (Table 1) and serially diluted the average premenopausal concentrations of oestrogens down 32-fold (Figure 5A). The concentration of oestrogens that was inhibited by tamoxifen and primary metabolites by 50% was four times lower the average premenopausal levels of oestrogens (Figure 5A), however, this still produced the same level of cell proliferation as in previous experiments (Figure 5B). Addition of endoxifen at concentrations corresponding to EM, IM and PM genotypes to that perimenopausal level of oestrogens showed that the anti-oestrogens almost completely inhibit cell proliferation with no differences between genotype groups (Figure 5B).
Figure 5.
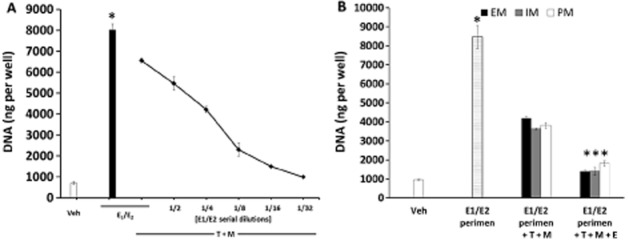
(A) Determination of the average putative ‘perimenopausal’ concentrations of oestrogens. MCF-7 cells were treated with tamoxifen (T) and primary metabolites (NDMTAM and 4OHT) (M) at EM genotype concentrations (Table 1) in combination with titrated premenopausal concentrations of oestrogens (E1/E2). Asterisk indicates statistically significant change after addition of premenopausal levels of oestrogens alone when compared with vehicle control. (B) Cell proliferation assay in MCF-7 cells showing the effect of endoxifen at different levels of oestrogen corresponding to different CYP2D6 genotypes in ‘perimenopausal women’. Treatments were made as follows: Veh, vehicle control; E1/E2 perimen, calculated perimenopausal oestrogens (E1 1 nM, E2 0.5 nM); E1/E2 perimen + T + M, oestrogens at ‘perimenopausal’ levels with tamoxifen (T) and its primary metabolites (NDMTAM and 4OHT) (M) at different CYP2D6 genotype concentrations (Table 1); E1/E2 perimen + T + M + E, oestrogens at ‘perimenopausal’ levels; tamoxifen (T), primary metabolites (M) and endoxifen (E) at different CYP2D6 genotype concentrations (Table 1). Asterisk indicates statistically significant change after addition of endoxifen or addition of oestrogens when compared with vehicle control.
Real-time PCR
To assess the anti-oestrogenic properties of endoxifen and tamoxifen with primary metabolites on regulating oestrogen-responsive genes in MCF-7 we employed real-time PCR using primers for GREB1 and TFF1 cDNAs. The cells were treated for 48 h. The results show that average premenopausal levels of oestrogens are able to induce both of the selected oestrogen-responsive genes (Figure 6) (P < 0.05 by Student's t-test). Results demonstrate that tamoxifen and its primary metabolites in EM concentrations are not able to significantly reduce the oestrogen-induced RNA production (Figure 6A) (P > 0.05 by Student's t-test), the same result was observed for TFF1 gene (Figure 6B) (P > 0.05 by Student's t-test). However, addition of endoxifen in EM concentration was able to significantly reduce the oestrogen-induced GREB1 and TFF1 mRNA expression by an average of 50% (P < 0.05 by Student's t-test for both genes) (Figure 6).
Figure 6.
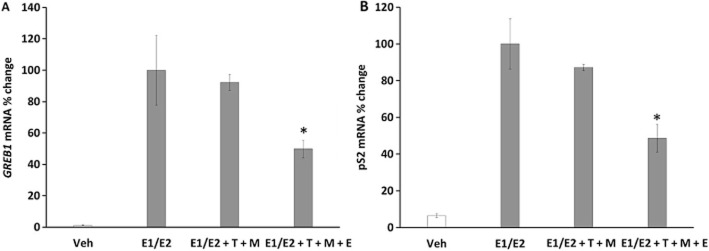
Pharmacological effect of tamoxifen and its metabolites with or without endoxifen at concentrations corresponding to EM genotype on oestrogen-responsive gene expression. GREB1 (A), and TFF1 (B) genes mRNA expression measurement by real-time PCR were chosen. The results show that endoxifen is crucial for inhibition of premenopausal oestrogen-stimulated gene expression (GREB1 and TFF1) by at least 50%. Treatments were made as follows: Veh, vehicle control; E1/E2, the premenopausal average oestrogen concentrations; E1/E2 + T + M, oestrogens with tamoxifen (T) and its primary metabolites (NDMTAM and 4OHT) (M) at EM genotype concentrations (Table 1); E1/E2 + T + M + E, oestrogens; tamoxifen (T), primary metabolites (M) and endoxifen (E) at EM genotype concentrations (Table 1). Asterisk indicates statistically significant change after addition of endoxifen.
Immunoblotting
To investigate the effect of tamoxifen and its metabolites in an average premenopausal environment on ERα protein we performed treatments in MCF-7 cells for 24 h and investigated total cell lysates by Western blotting. The results show that the E1/E2 treatment is able to reduce the levels of ERα protein (Figure 7). Addition of tamoxifen and its primary metabolites (T + M) and EM concentrations partially reversed the oestrogen action; however, the addition of endoxifen at EM concentration to the anti-oestrogenic mix (T + M + E), not just completely reversed the oestrogen action, but also increased the ERα protein levels, enhancing the anti-oestrogenic effect. The pure anti-oestrogen fulvestrant (ICI) was used as a positive control for ERα protein degradation.
Figure 7.
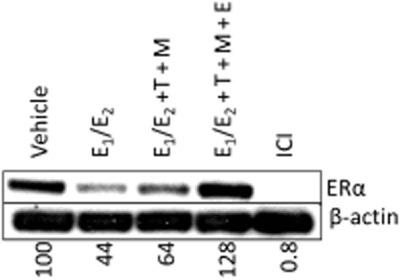
Western blotting in MCF-7 cells to show the effects of different 24 h treatments on ERα protein levels. Treatments were made as follows: Veh, vehicle control; E1/E2, the premenopausal average oestrogen concentrations; E1/E2 + T + M, oestrogens with tamoxifen (T) and its primary metabolites (NDMTAM and 4OHT) (M) at EM genotype concentrations (Table 1); E1/E2 + T + M + E, oestrogens; tamoxifen (T), primary metabolites (M) and endoxifen (E) at EM genotype concentrations (Table 1). Fulvestrant (ICI) was used as a positive control for ERα protein degradation.
Discussion and conclusions
Tamoxifen and its metabolites have always been classified (Jordan, 1984) as competitive inhibitors of oestrogen action at the ER. Overall, our findings confirm this classification in a simulation of the anti-oestrogenic therapeutic environment of women with functioning ovaries being treated with tamoxifen to control the growth of ER-positive breast cancer. We demonstrate the competitive and reversible relationship between tamoxifen and its metabolites with major oestrogens (E1/E2) measured in premenopausal women taking tamoxifen. We successfully demonstrated the positive relationship between the serum concentrations of endoxifen and the PM, IM and EM genotypes that control the successful conversion of NDMTAM to its active metabolite endoxifen (Figure 1). In practical terms, tamoxifen is not really a classical prodrug; tamoxifen does not need to be metabolically activated, it is an advantage but not a requirement for anti-oestrogenic efficacy (Allen et al., 1980). The medicine accumulates in patients and achieves steady state within 4 weeks, and both tamoxifen and NDMTAM, which are capable of blocking oestrogen action, have a circulating half-life of 7 and 14 days respectively (Furr and Jordan, 1984). Clinical and laboratory data concerning the role and actions of endoxifen remain inconsistent and contradictory so it is perhaps appropriate to place our observations into perspective with other reports and models.
The traditional approach to study different anti-oestrogens to prevent tumour growth would be to employ the athymic mouse animal model that is used ubiquitously (Wardell et al., 2013). However, this approach cannot be employed to address the current question of specific mixes of human metabolites of tamoxifen linked to genotypes. Published results are instructive because of known differences in metabolism, excretion and human/animal differences in pharmacokinetics. Oestrogen administration is required for athymic mice to sustain MCF-7 tumour growth (Soule and McGrath, 1980) as there is a hypothalamopituitary lesion that prevents oestrous cycles from occurring (Weinstein, 1978). A silastic sustained E2 release capsule may be used and the circulating oestrogen levels to stimulate tumour cell growth track well from patients, to the athymic animal model to cell culture, but this is not true for the xenobiotic tamoxifen and its metabolites in humans (Robinson et al., 1989; 1991,; Langan-Fahey et al., 1990).
Published human circulating levels at steady state of tamoxifen and metabolites during adjuvant therapy (20 mg·day−1 – 465 ± 54 days) are: tamoxifen 108 ± 23 ng·mL−1, 4OHT 2.6 ± 0.5 ng·mL−1 and NDMTAM 238 ± 58 mg·mL−1 with endoxifen detected only in 6 out of 10 samples (Robinson et al., 1989). These are almost exactly the concentrations of tamoxifen and primary metabolites used by Hawse et al. (2013) for the 24 h gene array studies in vitro (competing against 10 nM E2), that are used to show new unique differences for actions of endoxifen. By contrast, reported levels of tamoxifen and metabolites are completely different in athymic mice treated to inhibit oestrogen-stimulated growth (Robinson et al., 1989). There is clear evidence that the antitumour actions of a xenobiotic tamoxifen in an animal tumour model, does not correlate with human serum levels; therefore, the present cell culture approach is the appropriate experimental strategy to simulate the premenopausal patient. Another approach in vivo is to administer endoxifen alone. Gong et al. (2013) calculate the precise concentration of endoxifen (53 nM) to prevent tumour growth of ER-positive MCF-7 tumours following a dose-ranging study of 0.1–100 mg·kg−1 endoxifen p.o. alone. However, this approach is focused on the possible use of endoxifen as a therapy alone (Goetz et al., 2013).
If the ER complex is the key element in oestrogen-regulated breast cancer cell growth, it is particularly interesting that Wu et al. (2009) report that endoxifen alone causes the rapid destruction of the ER complex. The pure anti-oestrogen fulvestrant is classified as an ER down-regulator as the fulvestrant–ER complex adopts an alien conformation targeting it for rapid ubiquitination and proteosomal lysis. Fulvestrant alone was used as a positive control for ER down-regulation in the current study (Figure 7). Endoxifen reversed the down-regulation of ER noted with E1/E2 and caused an accumulation of ER when compared with treatment with tamoxifen and its primary metabolites without endoxifen (Figure 7). We recently demonstrated that endoxifen alone does not produce down-regulation of ER (Maximov et al., 2014). Wu et al. (2009) also noted a profound down-regulation of ER complex with endoxifen in T47D cells, but these cells have a unique form of ER regulation; oestrogen is necessary to increase ER synthesis so it would be anticipated that a potent nonsteroid anti-oestrogen such as endoxifen would switch off ER synthesis (Pink and Jordan, 1996).
Two further studies support the data presented in Figure 7 for the accumulation of ER with endoxifen in MCF-7 cells. Firstly, the same pattern of ER complex accumulation (Figure 7) is noted with ER and endoxifen alone in multiple cellular context and compared with 4OHT (Obiorah et al., 2014). Secondly, it is possible that different proportions of geometric isomers of endoxifen occur in different preparations. If the isomers have different oestrogenic/anti-oestrogenic pharmacology at the ER then this could lead to complex biochemical changes in structure and function. This is an explanation offered by Hawse et al. (2013) to explain why Lim et al. (2006) noted no significant changes in gene array profile between 4OHT and endoxifen; the Lim endoxifen (Lim et al., 2006) was apparently a 3:1 mixture of Z and E isomers, but the Hawse endoxifen was 98% Z isomer. In our studies, we used the endoxifen provided by the same group at the Mayo Clinic, MN. To address the pharmacology of the E and Z isomers of endoxifen and 4OHT, we synthesized the individual isomers as fixed ring derivatives. We examined each compound for oestrogenic/anti-oestrogenic activity in MCF-7 cells and the regulation of prolactin gene expression in GH3 rat pituitary tumour cells (Maximov et al., 2014). The E fixed ring isomers are weak oestrogens with anti-oestrogenic properties, whereas the Z fixed ring isomers are anti-oestrogens. However, the Z isomers of 4OHT and endoxifen cause accumulation of the ER complex just like the commercially available Z-endoxifen or 4OHT and the E isomers, though weak oestrogens, do not down-regulate ER like E2. It is the shape of the oestrogen and the conformation that cause ER complex degradation not the fact it is an oestrogen. Although a mixture of endoxifen isomers was found clinically in patient serum by Mürdter et al. (2011), the proportion found was predominantly anti-oestrogenic on breast cancer cell growth (Maximov et al., 2014). Molecular modelling has demonstrated that Z-endoxifen and 4OHT have a very similar fit in the ER complex whereas the SERMs raloxifene and bazedoxifene with their larger side chain rings can cause down-regulation of ER (Wardell et al., 2013) [but not as dramatic as the pure anti-oestrogen fulvestrant (Nicholson et al., 1995; Osborne et al., 1995)], and have the capacity to completely neutralize and shield amino acid D351 thereby preventing helix 12 from closing.
We created a simulation of genotypes of tamoxifen metabolism in vitro because it is not possible to replicate these genotypes in vivo in tumours being grown in athymic mice or rats used in other antitumour studies (Robinson et al., 1989; 1991,). Nevertheless, many important lessons are learnt by the development of a precise database in vitro because they are instructive to interpret current results with endoxifen in vivo. The models in vitro create an understanding of the circulating ratios of tamoxifen metabolites that can effectively control precise levels of oestrogen-stimulated ER-positive tumour growth. There is, however, one final caveat. tamoxifen is equally efficacious as a therapy in pre- and postmenopausal patients despite large increases in circulating oestrogen in premenopausal patients (EBCTCG, 2005). However, the simulation using reported concentrations of oestrogens and optimal reported mixtures of tamoxifen and metabolites do not completely block oestrogen-induced replication or gene activation except in a ‘perimenopausal’ scenario (Figure 5). Nevertheless, tamoxifen + the mix of anti-oestrogenic metabolites do predictably reverse the down-regulation of ER with oestrogens alone (Figure 7). This is yet another dimension of complexity when addressing the pharmacology of tamoxifen in the laboratory. We are ignorant about the actual concentrations of tamoxifen and metabolites in the tumour cell and these may be log concentrations higher than circulating levels once steady state is achieved. Thus, circulating levels of tamoxifen, a lipophilic and highly protein-bound drug may only be a rough guide to reality at the receptor. The animal studies reported in the literature already teach us that lesson.
Based on the results of cell growth assays, gene expression regulation and ER protein level regulation, we can conclude that endoxifen has a major anti-oestrogenic role. At concentrations corresponding to different genotypes of CYP2D6 endoxifen in combination with tamoxifen and its metabolites can cause inhibition of oestrogen-induced growth of breast cancer cells at higher concentrations, but not at concentrations corresponding to the PM genotype of CYP2D6. Endoxifen was also shown to block the action of oestrogens on oestrogen-responsive genes and ER protein. These results indicate that higher concentrations of endoxifen are important for producing more anti-oestrogenic effects during adjuvant therapy.
Acknowledgments
This work was supported by the Department of Defense Breast Program Center of Excellence (award number W81XWH-06-1-0590), the Susan G. Komen for the Cure Foundation (award number SAC100009), the Lombardi Comprehensive Cancer Center Support Grant (NIH P30 CA051008) and the German Research Foundation (DFG, MU1727/2-1; T. E. M.). The views and opinions of the authors do not reflect those of the US Army or the Department of Defense.
Glossary
- 4OHT
4-hydroxytamoxifen
- CYP2D6
cytochrome P450 2D6 variant
- E1
oestrone
- E2
oestradiol
- EM
extensive metabolizer
- ER
oestrogen receptor
- IM
intermediate metabolizer
- NDMTAM
N-desmethyltamoxifen
- PM
poor metabolizer
Author contributions
P. Y. M. did the experimental design and execution, and manuscript preparation. R. E. M. and P. B. provided technical support and D. J. F. technical support; V. R. K. did the statistical analysis; T. E. M. provided clinical concentrations of tamoxifen and metabolites; D. A. F. provided clinical concentrations of tamoxifen and metabolites; and V. C. J. did the experimental design and was the project leader.
Conflict of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site: http://dx.doi.org/10.1111/bph.12864
Clinical concentrations of tamoxifen and its metabolites measured in breast cancer patients by Flockhart. Concentrations were obtained during a previous study (Irvin et al., 2011).
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear hormone receptors. Br J Pharmacol. 2013a;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KE, Clark ER, Jordan VC. Evidence for the metabolic activation of non-steroidal antioestrogens: a study of structure–activity relationships. Br J Pharmacol. 1980;71:83–91. doi: 10.1111/j.1476-5381.1980.tb10912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehal SS, Kupfer D. CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res. 1997;57:3402–3406. [PubMed] [Google Scholar]
- Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Furr BJ, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25:127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- Goetz MP, Suman VA, Reid JR, Northfelt DW, Mahr MA, Dockter T, et al. San Antonio, TX: San Antonio Breast Cancer Symposium; 2013. A first-in-human phase I study of the tamoxifen (TAM) metabolite, Z-endoxifen hydrochloride (Z-Endx) in women with aromatase inhibitor (AI) refractory metastatic breast cancer (MBC) ( NCT01327781). Abstract PD3-4. [Google Scholar]
- Gong IY, Teft WA, Ly J, Chen YH, Alicke B, Kim RB, et al. Determination of clinically therapeutic endoxifen concentrations based on efficacy from human MCF7 breast cancer xenografts. Breast Cancer Res Treat. 2013;139:61–69. doi: 10.1007/s10549-013-2530-1. [DOI] [PubMed] [Google Scholar]
- Hawse JR, Subramaniam M, Cicek M, Wu X, Gingery A, Grygo SB, et al. Endoxifen's molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS ONE. 2013;8:e54613. doi: 10.1371/journal.pone.0054613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin WJ, Jr, Walko CM, Weck KE, Ibrahim JG, Chiu WK, Dees EC, et al. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: a multicenter study. J Clin Oncol. 2011;29:3232–3239. doi: 10.1200/JCO.2010.31.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984;36:245–276. [PubMed] [Google Scholar]
- Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75:305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Fritz NF, Langan-Fahey S, Thompson M, Tormey DC. Alteration of endocrine parameters in premenopausal women with breast cancer during long-term adjuvant therapy with tamoxifen as the single agent. J Natl Cancer Inst. 1991;83:1488–1491. doi: 10.1093/jnci/83.20.1488. [DOI] [PubMed] [Google Scholar]
- Langan-Fahey SM, Tormey DC, Jordan VC. Tamoxifen metabolites in patients on long-term adjuvant therapy for breast cancer. Eur J Cancer. 1990;26:883–888. doi: 10.1016/0277-5379(90)90191-u. [DOI] [PubMed] [Google Scholar]
- Lim YC, Li L, Desta Z, Zhao Q, Rae JM, Flockhart DA, et al. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006;318:503–512. doi: 10.1124/jpet.105.100511. [DOI] [PubMed] [Google Scholar]
- Maximov PY, Fernandes DJ, McDaniel RE, Myers CB, Curpan RF, Jordan VC. Influence of the length and positioning of the antiestrogenic side chain of endoxifen and 4-hydroxytamoxifen on gene activation and growth of estrogen receptor positive cancer cells. J Med Chem. 2014;57:4569–4583. doi: 10.1021/jm500569h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mürdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Gee JM, Manning DL, Wakeling AE, Montano MM, Katzenellenbogen BS. Responses to pure antiestrogens (ICI 164384, ICI 182780) in estrogen-sensitive and -resistant experimental and clinical breast cancer. Ann N Y Acad Sci. 1995;761:148–163. doi: 10.1111/j.1749-6632.1995.tb31376.x. [DOI] [PubMed] [Google Scholar]
- Obiorah IE, Sengupta S, Curpan R, Jordan VC. Defining the conformation of the estrogen receptor complex that controls estrogen induced apoptosis in breast cancer. Mol Pharmacol. 2014;85:789–799. doi: 10.1124/mol.113.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA, et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink JJ, Jordan VC. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res. 1996;56:2321–2330. [PubMed] [Google Scholar]
- Province MA, Goetz MP, Brauch H, Flockhart DA, Hebert JM, Whaley R, et al. CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations. Clin Pharmacol Ther. 2014;95:216–227. doi: 10.1038/clpt.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104:452–460. doi: 10.1093/jnci/djs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1–98 Trial. J Natl Cancer Inst. 2012;104:441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SP, Langan-Fahey SM, Jordan VC. Implications of tamoxifen metabolism in the athymic mouse for the study of antitumor effects upon human breast cancer xenografts. Eur J Cancer Clin Oncol. 1989;25:1769–1776. doi: 10.1016/0277-5379(89)90347-7. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab Dispos. 1991;19:36–43. [PubMed] [Google Scholar]
- Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule HD, McGrath CM. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980;10:177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- Wardell SE, Nelson ER, Chao CA, McDonnell DP. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res. 2013;19:2420–2431. doi: 10.1158/1078-0432.CCR-12-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y. Impairment of the hypothalamo–pituitary–ovarian axis of the athymic ‘nude’ mouse. Mech Ageing Dev. 1978;8:63–68. doi: 10.1016/0047-6374(78)90007-6. [DOI] [PubMed] [Google Scholar]
- Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69:1722–1727. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical concentrations of tamoxifen and its metabolites measured in breast cancer patients by Flockhart. Concentrations were obtained during a previous study (Irvin et al., 2011).