Abstract
Background and Purpose
We assessed whether endothelin-1 (ET-1) inhibits NO and contributes to endothelial dysfunction in penile arteries in a model of insulin resistance-associated erectile dysfunction (ED).
Experimental Approach
Vascular function was assessed in penile arteries, from obese (OZR) and lean (LZR) Zucker rats, mounted in microvascular myographs. Changes in basal and stimulated levels of superoxide (O2−) were detected by lucigenin-enhanced chemiluminescence and ET receptor expression was determined by immunohistochemistry.
Key Results
ET-1 stimulated acute O2− production that was blunted by tempol and the NADPH oxidase inhibitor, apocynin, but markedly enhanced in obese animals. ET-1 inhibited the vasorelaxant effects of ACh and of the NO donor S-nitroso-N-acetyl-DL-penicillamine in arteries from both LZR and OZR. Selective ETA (BQ123) or ETB receptor (BQ788) antagonists reduced both basal and ET-1-stimulated superoxide generation and reversed ET-1-induced inhibition of NO-mediated relaxations in OZR, while only BQ-123 antagonized ET-1 actions in LZR. ET-1-induced vasoconstriction was markedly enhanced by NO synthase blockade and reduced by endothelium removal and apocynin. In endothelium-denuded penile arteries, apocynin blunted augmented ET-1-induced contractions in OZR. Both ETA and ETB receptors were expressed in smooth muscle and the endothelial layer and up-regulated in arteries from OZR.
Conclusions and Implications
ET-1 stimulates ETA-mediated NADPH oxidase-dependent ROS generation, which inhibits endothelial NO bioavailability and contributes to ET-1-induced contraction in healthy penile arteries. Enhanced vascular expression of ETB receptors contributes to augmented ROS production, endothelial dysfunction and increased vasoconstriction in erectile tissue from insulin-resistant obese rats. Hence, antagonism of ETB receptors might improve the ED associated with insulin-resistant states.
Tables of Links
| TARGETS | LIGANDS | |||
|---|---|---|---|---|
| ETA receptor | Nitric oxide synthase (NOS) | Acetylcholine (ACh) | Endothelin-1 (ET-1) | Phenylephrine |
| ETB receptor | Angiotensin II | Methacholine | Prostacyclin | |
| BQ123 | NADPH | TNF-α | ||
| BQ788 | Nitric oxide (NO) |
These Tables list key protein targets and ligands in this document, which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b,).
Introduction
Endothelial dysfunction is an early pathogenic event in the vascular complications associated with the insulin-resistant states of diabetes and obesity and has traditionally been ascribed to the reduced bioavailability of vasodilators such as NO and prostacyclin (Galili et al., 2007; Vanhoutte et al., 2009; Prieto et al., 2014). However, earlier evidence established the importance of enhanced endogenous activity of the vasoconstrictor, pro-inflammatory and mitogenic endothelial peptide endothelin-1 (ET-1) in humans who are overweight, obese and/or have type 2 diabetes. ET-1-induced vasoconstrictor tone was augmented and blockade of ETA receptors improved basal and blunted methacholine-elicited increases in blood flow in obese adults and patients with type 2 diabetes, thus suggesting that ET-1 contributes to endothelial dysfunction (Mather et al., 2002; 2004,; Weil et al., 2011).
Dysregulation of reactive oxygen species (ROS) signalling and oxidative stress seriously interfere with the synthesis and actions of NO and prostacyclin and reduce endothelium-dependent vasodilatation (Montezano and Touyz, 2012). NADPH oxidase is one of the main enzymatic sources of superoxide radical (O2−) generation in the vascular wall and ROS production can be augmented via activation of NADPH oxidase by some inducing factors such as ET-1, angiotensin II and TNF-α (Münzel et al., 2010; Montezano and Touyz, 2012). ET-1 has been demonstrated to significantly increase O2− production in human arteries (Cerrato et al., 2012) and animal vessels (Elmarakby et al., 2005; Loomis et al., 2005; Romero et al., 2009; 2010,), and ET-1 infusion in the forearm of healthy individuals (Böhm et al., 2007) or in vitro exposure of intact arteries to ET-1 have been shown to produce endothelial dysfunction (Romero et al., 2009; 2010,). Furthermore, ET receptor blockade improves endothelial function in human coronary arteries (Verma et al., 2001) and endothelium-dependent vasodilatation in patients with insulin resistance (Mather et al., 2002; Shemyakin et al., 2006; Rafnsson et al., 2012) and experimental models of atherosclerosis (Barton et al., 1998) and type 2 diabetes (Abdelsaid et al., 2014). ET-1 vascular actions are mediated by two G-protein coupled membrane receptors, ETA and ETB, and both receptor types have been suggested to contribute to ET-1-induced vascular ROS generation (Duerrschmidt et al., 2000; Li et al., 2003; Dai et al., 2004; Dong et al., 2005; Fellner and Arendshorst, 2007; Just et al., 2008; Cerrato et al., 2012).
Erectile dysfunction (ED) is considered to be an early manifestation of endothelial dysfunction and vascular disease and it is a highly prevalent condition in diabetic men and patients with cardiovascular risk factors (Vlachopoulos et al., 2013). We have recently demonstrated that both changes in the NO signalling (Villalba et al., 2009; Contreras et al., 2010) and impaired release of vasodilator prostanoids (Sánchez et al., 2010) contribute to the pathogenesis of endothelial dysfunction in penile arteries from the obese Zucker rat (OZR), an established model of genetic obesity and prediabetes-associated ED (Kovanecz et al., 2006). High levels of oxidative stress in these arteries lead to neuronal (n) NOS uncoupling and nitrergic dysfunction thus also being involved in the pathogenesis of impaired erectile function (Sánchez et al., 2012). On the other hand, ET-1 levels are augmented in diabetic men with ED and up-regulation of both ETA and ETB receptors has been demonstrated in erectile tissue in experimental models of diabetes and insulin resistance (Bell et al., 1995; Francavilla et al., 1997; Sullivan et al., 1997; Ritchie and Sullivan, 2011; Contreras et al., 2013).
Although ET-1-NO interactions have been suggested to be key factors in the endothelial dysfunction of obesity and diabetes (Böhm et al., 2002; 2007,; Mather et al., 2002; 2004,), the exact nature of these interactions is not completely understood and the ET receptors and sources of oxidative stress involved have not yet been investigated in penile erectile tissue. Therefore, the purpose of the present study was to assess whether ET-1 can inhibit endothelial NO bioavailability through its ability to stimulate ROS generation and, if so, determine the ET receptors and vascular sources involved. Furthermore, we sought to investigate whether ET-1–NO interactions may underlie penile endothelial dysfunction in the OZR, a well-established model of obesity/insulin resistance-associated ED.
Methods
Animal model
All animal care and experimental protocols conformed to the European Union Guidelines for the Care and the Use of Laboratory Animals (European Union Directive 2010/63/EU) and were approved by the Institutional Animal Care and Use Committee of the Madrid Complutense University. Male OZR (fa/fa, n = 42) and their control strain, lean Zucker rats (LZR) (fa/–, n = 42), were purchased from Charles River Laboratories (Barcelona, Spain) at 8–10 weeks of age. Animals were housed at the Pharmacy School animal care facility and maintained on standard chow and water ad libitum, until they were used for study, at 17–18 weeks of age. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Dissection of microvessels, mounting and force measurement
After the animals were killed, the penis was quickly removed and placed in cold physiological saline solution (PSS). The penile arteries, first- or second-order branches of the rat dorsal penile artery from LZR and OZR rats, were carefully dissected by removing the connective and fat tissue, as described previously (Sánchez et al., 2012) and mounted in parallel in double microvascular myographs (Danish Myotechnology, Aarhus, Denmark) by inserting two 40 μm tungsten wires into the vessel lumen. After being mounted, the arteries were equilibrated for 30 min in PSS maintained at 37°C of the following composition (mM): NaCl 119, NaHCO3 25, KCl 4.7, KH2PO4 1.17, MgSO4 1.18, CaCl2 1.5, EDTA 0.027 and glucose 11, continuously gassed with a mixture of 5% CO2/95% O2 to maintain pH at 7.4. The relationship between passive wall tension and internal circumference was determined for each individual artery and from this, the internal diameter, l1, that yielded a circumference equivalent to 90% of that given by an internal pressure of 100 mmHg was calculated, and arteries were set to an l1 at which all experiments were performed (Sánchez et al., 2012).
Experimental procedure for the functional experiments
To assess a possible influence of ET-1 on the endothelium-dependent and endothelium-independent relaxing responses of penile arteries, the effect of a threshold concentration of ET-1 (0.3 nM) was tested on the relaxant responses induced by ACh, the NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP) and the adenylate cyclase activator forskolin in arteries precontracted with phenylephrine (1 μM) from LZR and OZR, by incubating the arteries with ET-1 for 30 min before cumulative addition of these agents.
The involvement of ETA and/or ETB receptors in the ET-1 effects on the relaxant responses to ACh and SNAP was assessed by incubation with the selective antagonists of the ETA receptor (BQ123, 1 μM) or the ETB receptor (BQ788, 0.1 μM). These drugs were introduced 30 min before a second concentration-response curve for either ACh or SNAP in the presence of 0.3 nM ET-1 was constructed. ACh and SNAP relaxant responses were reproducible in a second stimulation and two concentration-response curves for these agonists were performed in each artery. Due to tachyphylaxis to ET-1, arteries were exposed to the peptide only once during the experiment. Phenylephrine concentration was adjusted to match the contraction during the first control curve. Experiments with the NO donor, SNAP, were performed under conditions of NOS blockade with NG-nitro-L-arginine (L-NOARG; 100 μM).
Cumulative concentration-response curves to ET-1 (0.01 nM–0.1 μM) were performed in the presence and absence of the NOS inhibitor L-NOARG (100 μM) or the NADPH oxidase inhibitor apocynin (30 μM). The role of the vascular endothelium was assessed in arteries where the endothelium was mechanically removed by passing a human hair through the vessel lumen. The absence of functional endothelium was confirmed by the lack of relaxation to ACh (10 μM).
Measurement of superoxide production by lucigenin-enhanced chemiluminescence
Changes in basal or ET-1-stimulated levels of superoxide were detected in the corpus cavernosum by lucigenin-enhanced chemiluminescence, as previously described in erectile tissue (Prieto et al., 2010; Sánchez et al., 2012). Corpora cavernosa (4–5 mm long strips) from LZR and OZR were dissected and equilibrated in Krebs buffer for 30 min at room temperature and then incubated in the absence (controls) and presence of ET-1 (1 nM), the specific antagonists of the ETA receptors, BQ123 (1 μM), and ETB receptors, BQ788 (0.1 μM), the superoxide scavenger tempol (30 μM), the NADPH oxidase inhibitors apocynin (30 μM) and diphenylene iodonium (DPI) (10 μM), or the PKC activator 12,13-dibutyrate (PDBu, 10 μM) for 30 min at 37°C. The corpus cavernosum was then transferred to microtitre plate wells containing 5 μM lucigenin (bis-N-methylacridinium nitrate) in air-equilibrated Krebs solution buffered with 10 mM HEPES-NaOH. Chemiluminescence was measured in a luminometer (BMG Fluostar Optima, BMG LABTECH, Ortenberg, Germany), and for calculation baseline values were subtracted from the counting values under the different experimental conditions and superoxide production was normalized to tissue weight.
Immunohistochemistry
Tissue samples from the penis containing the dorsal penile artery were immersion-fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB), cryoprotected in 30% sucrose in PB and snap frozen in liquid nitrogen and stored at −80°C. Transverse sections of 10 μm were obtained by means of a cryostat and pre-incubated in 10% normal goat serum in PB containing 0.3% Triton-X-100 for 2–3 h. Then, sections were incubated with either a rabbit anti-ETA receptor antibody (Alomone Labs, Jerusalem, Israel) diluted at 1:100 or a rabbit anti-ETB receptor antibody (Alomone Labs) diluted at 1:100 for 48 h at 4°C. Location of ET receptors in perivascular nerve fibres was visualized by coimmunostaining with a mouse anti-eNOS (Chemicon International Inc., Millipore Corporation, MA USA; 1:500 dilution). Sections were then washed and reacted with the second antibodies for 2 h at room temperature. Secondary antibodies used were Alexa Fluor 594 (red) goat anti-rabbit (Invitrogen, Life Technologies, Madrid, Spain; 1:200 dilution) and Alexa Fluor 488 (green) goat anti-mouse (Invitrogen, Life Technologies; 1:200 dilution). The slides were covered with a specific mounting medium with the nuclear stain DAPI (Invitrogen, Life Technologies). No immunoreactivity could be detected in sections incubated in the absence of the primary antisera. Pre-adsorption with ETA and ETB receptors showed no cross-reactivity for the antibodies.
Drugs and materials
ACh, apocynin (acetovanillone), DPI, L-NOARG, noradrenaline (arterenol), phenylephrine and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (tempol) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). ET-1, forskolin, BQ123 and BQ788 sodium salt, SNAP, PDBu were from Tocris Cookson (Bristol, UK). For in vitro experiments, drugs were dissolved in distilled water, except SNAP, apocynin, PDBu and BQ788 which were dissolved at 10 mM concentration in DMSO. The subsequent dilutions were made in distilled water.
Data presentation and statistical analysis
Results are expressed as either Nm−1 of tension or as a % of the responses to either phenylephrine or high K+-physiological saline solution (KPSS) in each artery, as means ± SEM of n number of animals (one to two arteries from each animal were used). Statistically significant differences between means were analysed by one-way anova or by using Student's paired or unpaired t-test when appropriate. Probability levels of P < 0.05 were considered statistically significant.
Results
General parameters
At the time of the experiment (17–18 weeks of age), OZR were significantly heavier than LZR (473 ± 7 g vs. 361 ± 6 g, P < 0.001; n = 42). We have reported that animals from the OZR group exhibit mild non-fasting hyperglycaemia, hyperinsulinaemia and dyslipidaemia with elevated total cholesterol and triglycerides levels (Villalba et al., 2009). The normalized internal lumen diameters, l1, were significantly smaller in penile arteries from OZR (127 ± 3 μm, n = 32) compared with LZR (142 ± 3 μm, P < 0.01; n = 32) indicating vascular remodelling. Contractions to KPSS were also reduced in the OZR group (1.6 ± 0.2 Nm−1 vs. 2.1 ± 0.1 Nm−1 in LZR, P < 0.01; n = 32), as reported previously (Villalba et al., 2009).
ET-1 stimulates superoxide production in erectile tissue and inhibits NO-mediated relaxations in penile arteries
In order to determine whether ET-1 stimulates ROS generation in penile erectile tissue, strips of corpus cavernosum were acutely stimulated with 10 nM ET-1 for 30 min and superoxide generation was measured by lucigenin-enhanced chemiluminescence. Because basal NO release has been reported to counteract basal superoxide release in healthy penile arteries (Prieto et al., 2010), these experiments were performed in the absence and the presence of the NOS inhibitor L-NOARG (100 μM). While ET-1-induced increase in superoxide production was negligible in erectile tissue from LZR, this effect was magnified and blunted by tempol treatment under conditions of NOS blockade in both LZR and OZR, indicating that ET-1-stimulated superoxide is counteracted by NO release. Both basal and basal plus ET-1-stimulated ROS levels were significantly augmented in OZR compared with LZR (Figure 1), which suggests that ET-1 contributes to the higher levels of oxidative stress in obese animals.
Figure 1.
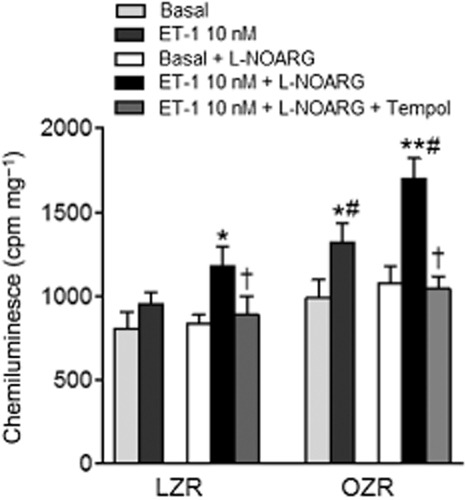
Basal superoxide production in corpus cavernosum tissue from LZR and OZR detected by lucigenin-enhanced chemiluminescence. Effect of ET-1 (10 nM) on basal superoxide production, and of tempol (100 μM) on the ET-1-induced superoxide production, in the absence and presence of the NOS inhibitor L-NOARG (100 μM). Superoxide production is expressed in counts per minute (cpm) mg−1 of tissue. Data are shown as the means ± SEM of n = 4 animals (two corpus cavernosum samples from each animal). *P < 0.05, **P < 0.01 versus basal, †P < 0.05 versus ET-1-treated, #P < 0.05 versus LZR. Student's t-test for unpaired observations.
The acute effects of a threshold concentration ET-1 (0.3 nM) on the endothelium-dependent and endothelium-independent relaxant responses of penile arteries were investigated next. Incubation with ET-1 for 30 min markedly reduced the relaxations elicited by ACh in arteries from LZR (Figure 2A, Table 1) and showed a trend for inhibition in arteries from OZR (Figure 2B, Table 1), in which ACh control responses were already blunted compared with LZR indicating endothelial dysfunction, as earlier reported (Villalba et al., 2009).
Figure 2.
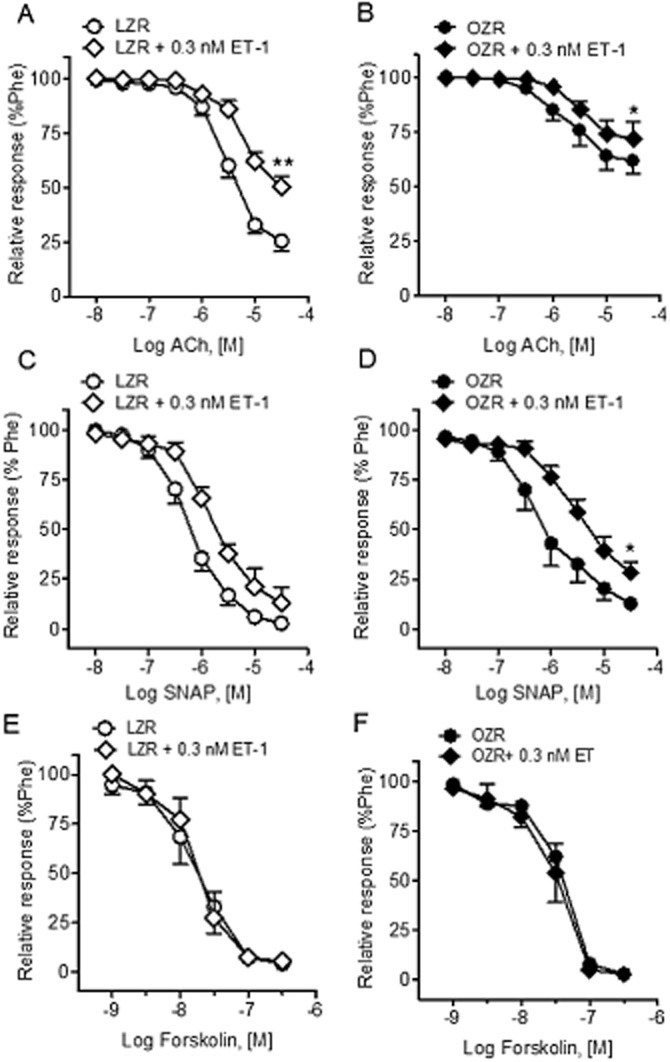
Threshold concentrations of ET-1 inhibited NO-mediated relaxant responses in penile arteries from LZR and OZR. Effect of ET-1 (0.3 nM) on (A, B) the average contractions response curves to ACh, (C, D) the concentration-response curves to the NO donor SNAP and (E, F) the concentration-response curves to the AC activator forskolin in penile arteries precontrated with phenylephrine (Phe; 1 μM) from LZR (A, C, E) and OZR (B, D, F). Results are means ± SEM of n = 3–5 animals (one to two arteries from each animal). *P < 0.05, **P < 0.01 versus control Emax; Student's t-test for paired observations.
Table 1.
Effect of ET-1 (0.3 nM) and selective antagonism of ETA (BQ123, 1 μM) and ETB (BQ788, 0.1 μM) receptors on the concentration-relaxation curves to ACh and to the NO donor in penile arteries from LZR and OZR
| ACh | ||||||||
|---|---|---|---|---|---|---|---|---|
| LZR | OZR | |||||||
| Phe | pEC50 | Emax | n | Phe | pEC50 | Emax | n | |
| Control | 2.4 ± 0.5 | 5.56 ± 0.08 | 75 ± 5 | 7 | 1.1 ± 0.2 | 5.61 ± 0.15 | 38 ± 6 | 7 |
| +ET-1 | 2.5 ± 0.6 | 5.27 ± 0.10b | 49 ± 5b | 4 | 1.3 ± 0.2 | 5.54 ± 0.07 | 28 ± 7b | 4 |
| +ET-1 + BQ123 | 2.6 ± 0.5 | 5.72 ± 0.19c | 65 ± 6c | 3 | 1.3 ± 0.1 | 5.57 ± 0.13 | 40 ± 4c | 5 |
| +ET-1 + BQ788 | 2.2 ± 0.3 | 5.52 ± 0.07 | 55 ± 8a | 4 | 1.5 ± 0.3 | 5.43 ± 0.11 | 38 ± 7 | 4 |
| Control | 2.4 ± 0.3 | 5.74 ± 0.12 | 83 ± 4 | 7 | 1.8 ± 0.2 | 5.28 ± 0.24 | 54 ± 6 | 9 |
| +BQ123 | 2.2 ± 0.2 | 5.82 ± 0.13 | 79 ± 9 | 3 | 1.7 ± 0.3 | 5.52 ± 0.10 | 58 ± 11 | 4 |
| +BQ788 | 2.7 ± 0.3 | 5.88 ± 0.24 | 82 ± 4 | 4 | 1.5 ± 0.3 | 4.76 ± 0.12 | 63 ± 8b | 4 |
| +BQ123 + BQ788 | 2.1 ± 0.3 | 5.88 ± 0.16 | 87 ± 6 | 3 | 2.0 ± 0.2 | 5.06 ± 0.13 | 82 ± 6a | 4 |
| SNAP | ||||||||
| LZR | OZR | |||||||
| Phe | pEC50 | Emax | n | Phe | pEC50 | Emax | n | |
| Control | 1.6 ± 0.4 | 6.23 ± 0.12 | 97 ± 2 | 8 | 1.7 ± 0.6 | 6.19 ± 0.19 | 89 ± 3 | 8 |
| +ET-1 | 1.3 ± 0.4 | 5.79 ± 0.08b | 86 ± 8 | 4 | 1.5 ± 0.2 | 5.57 ± 0.11b | 75 ± 5a | 4 |
| +ET-1 + BQ123 | 1.3 ± 0.3 | 6.28 ± 0.17c | 96 ± 2 | 4 | 1.3 ± 0.1 | 5.90 ± 0.19 | 90 ± 3d | 4 |
| +ET-1 + BQ788 | 1.7 ± 0.3 | 5.86 ± 0.20 | 87 ± 6 | 4 | 1.1 ± 0.4 | 6.20 ± 0.12d | 77 ± 8 | 4 |
Values represent mean ± SEM of the number n of animals (one to two individual arteries were used from each animal). Significant differences from controls were analysed by paired Student's t-test and one-way anova followed by Bonferroni a posterio test. Phe precontraction (Nm−1).
P < 0.05;
P < 0.01 versus control.
cP < 0.05;
P < 0.01; versus ET-1-treated.
Pretreatment with 0.3 nM ET-1 blunted the NO-mediated vasodilator responses to the exogenous NO donor SNAP in penile arteries from both OZR and LZR, this inhibition being more pronounced in OZR (Figure 2C and D, Table 1), suggesting that ET-1 interferes with the relaxant action of NO. In contrast, ET-1 (0.3 nM) did not alter the relaxant responses elicited by the adenylate cyclase activator forskolin in either LZR (pD2 7.76 ± 0.16 and 7.86 ± 0.22, n = 3 before and after ET-1 respectively) or OZR (pD2 7.40 ± 0.04 and 7.50 ± 0.11, n = 3 before and after ET-1 respectively) (Figure 2E and F).
Effect of NADPH inhibition on the ET-1-induced superoxide formation in erectile tissue
To determine the mechanisms of the ET-1-induced superoxide production in erectile tissue, the effects of NADPH inhibition and NADPH stimulation were assessed. Treatment with the NADPH oxidase inhibitors apocynin (30 μM) or DPI (10 μM) significantly reduced superoxide production induced by ET-1 in corpus cavernosum from LZR and OZR, but apocynin did not change basal superoxide generation (Figure 3). Because PKC is known to activate NADPH oxidase, the effect of non-receptor activation of PKC with PDBu (10 μM) was investigated next. PDBu markedly increased superoxide generation in both LZR and OZR, this augmentation being higher in OZR (Figure 3).
Figure 3.
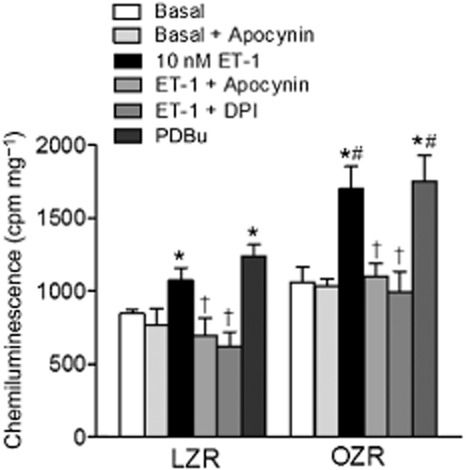
Basal superoxide production in corpus cavernosum tissue from LZR and OZR detected by lucigenin-enhanced chemiluminescence. Effect of the inhibitors of NADPH oxidase, apocynin (30 μM) and diphenylene iodonium (DPI) (10 μM) on both basal and ET-1 (10 nM)-stimulated superoxide production, and of the activator of PKC PDBu (10 μM) on the basal superoxide generation in corpus cavernosum tissue from LZR and OZR detected by lucigenin-enhanced chemiluminescence and expressed in counts per minute (cpm) mg−1 of tissue. Experiments were performed in the presence of the NOS inhibitor L-NOARG (100 μM). Data are shown as the means ± SEM of n = 3–4 animals (two corpus cavernosum samples from each animal). *P < 0.05 versus basal, †P < 0.05 versus ET-1-treated, #P < 0.05 versus LZR; Student's t-test for unpaired observations.
Effect of NOS and NADPH oxidase inhibition and role of endothelium in the ET-1-induced vasoconstriction of penile arteries
To determine the interactions between endothelial-derived NO and superoxide generation stimulated by ET-1, the vasoconstrictor effect of ET-1 was assessed under conditions of NOS or NADPH oxidase blockade in the absence and the presence of endothelium. Treatment with L-NOARG (100 μM) markedly enhanced the constriction induced by the lower concentrations of ET-1 in penile arteries from both LZR and to a lesser extent those in OZR in which ET-1 contractions were already enhanced compared with LZR (Figure 4A and B, Table 2). Mechanical removal of the endothelium suppressed the augmentation induced by NOS blockade of the ET-1 contractions in arteries from both control and obese rats, although this procedure significantly enhanced Emax for ET-1 in obese rats (Figure 4C and D, Table 2). On the other hand, inhibition of NADPH oxidase with apocynin (30 μM) largely reduced the augmented vasoconstriction induced by ET-1 under conditions of NOS blockade in both LZR and OZR (Figure 4C and D, Table 2). In endothelium-denuded arteries, apocynin did not reduce any longer ET-1-induced contractions in LZR, while markedly inhibited ET-1 vasoconstriction in OZR (Figure 4E and F, Table 2), suggesting that vascular smooth muscle (VSM) superoxide contributes to the augmented ET-1 contractile responses in obese animals.
Figure 4.
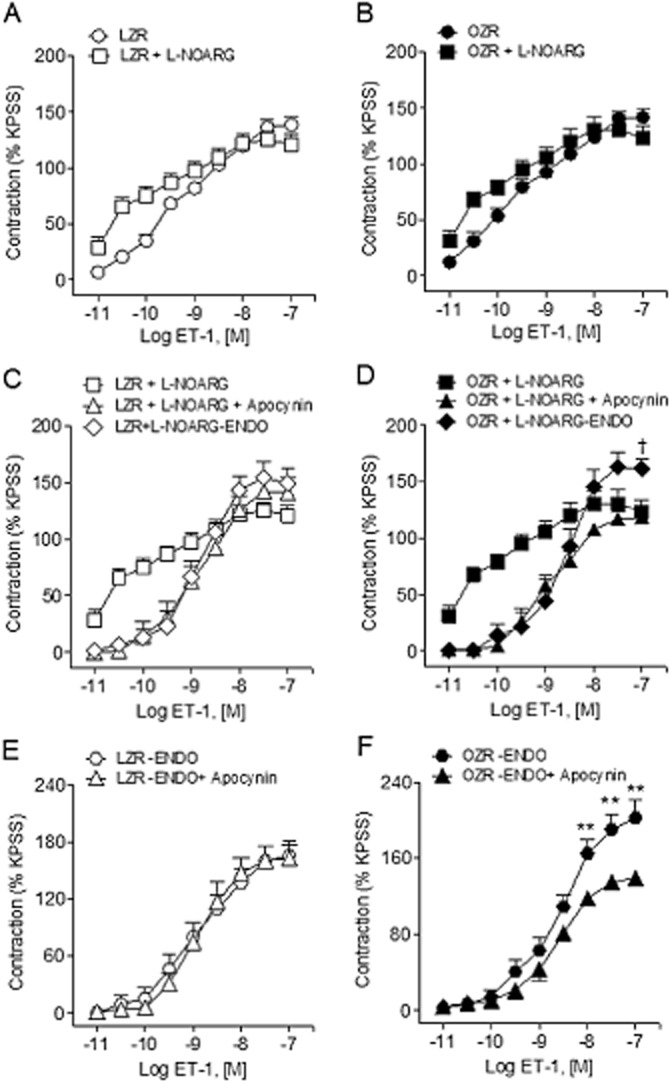
Effect of NOS inhibition, endothelium removal and NADPH blockade on the ET-1-induced vasoconstriction of penile arteries. (A, B) Effect of L-NOARG (100 μM) treatment on the contractile response to ET-1 in penile arteries from LZR (A) and OZR (B). (C, D) Average effect of apocynin (30 μM) and endothelium removal in the presence of L-NOARG on the vasoconstriction induced by ET-1 in penile arteries from LZR and OZR. (E, F) Average effects of apocynin (30 μM) on the ET-1-induced contractions in endothelium-denuded (-ENDO) penile arteries from LZR (E) and OZR (F). Results are means ± SEM of n = 3–5 animals (one to two arteries from each animal). †P < 0.05 versus L-NOARG-treated Emax; one-way anova followed by Bonferroni as a posterio test. **P < 0.01 versus -ENDO; Student's t-test for unpaired observations.
Table 2.
Effect of inhibition of NOS (L-NOARG, 100 μM), endothelium removal (-ENDO) and inhibition of NADPH oxidase (apocynin, 30 μM) on the vasoconstriction elicited by ET-1 in penile arteries from LZR and OZR
| ET-1 | ||||||
|---|---|---|---|---|---|---|
| LZR | OZR | |||||
| pEC50 | Emax | n | pEC50 | Emax | n | |
| Control | 9.22 ± 0.12 | 138 ± 7 | 6 | 9.56 ± 0.18f | 142 ± 7 | 7 |
| +L-NOARG | 10.14 ± 0.18b | 121 ± 9 | 5 | 10.44 ± 0.22a | 123 ± 10 | 5 |
| +L-NOARG -ENDO | 8.95 ± 0.15d | 150 ± 13 | 3 | 8.70 ± 0.18ad | 163 ± 9c | 4 |
| +L-NOARG + apocynin | 8.92 ± 0.25d | 141 ± 14 | 4 | 8.92 ± 0.14d | 119 ± 7 | 4 |
| Control-ENDO | 9.04 ± 0.20a | 165 ± 15a | 5 | 8.68 ± 0.16b | 203 ± 19b | 5 |
| -ENDO + apocynin | 8.91 ± 0.16 | 156 ± 14 | 4 | 8.78 ± 0.18 | 139 ± 7e | 4 |
Values represent mean ± SEM of the number n of animals (one to two individual arteries from each animal were used). Significant differences from controls were analysed by one-way anova followed by Bonferroni as a posterio test or Student's t-test for unpaired observations.
P < 0.05;
P < 0.01 versus control.
P < 0.05;
P < 0.01 versus L-NOARG-treated.
P < 0.01 versus control-ENDO.
P < 0.05 versus LZR.
Role of ETA and ETB receptors in ET-1-induced oxidative stress
Contribution of ET receptors to the higher superoxide levels in erectile tissue from obese animals was assessed in strips of corpus cavernosum incubated with BQ123 or BQ788 under basal conditions and before acute exposure to ET-1. Both the ETA receptor antagonist BQ123 (1 μM) and the ETB receptor antagonist BQ788 (0.1 μM) significantly reduced basal and ET-1-induced enhancement of superoxide levels in OZR (Figure 5), indicating that both ETA and ETB receptors mediate the elevated levels of oxidative stress induced by ET-1 in obese rats. In erectile tissue from LZR, only the ETA receptor antagonist BQ123 reduced the ET-1-elicited generation of superoxide.
Figure 5.
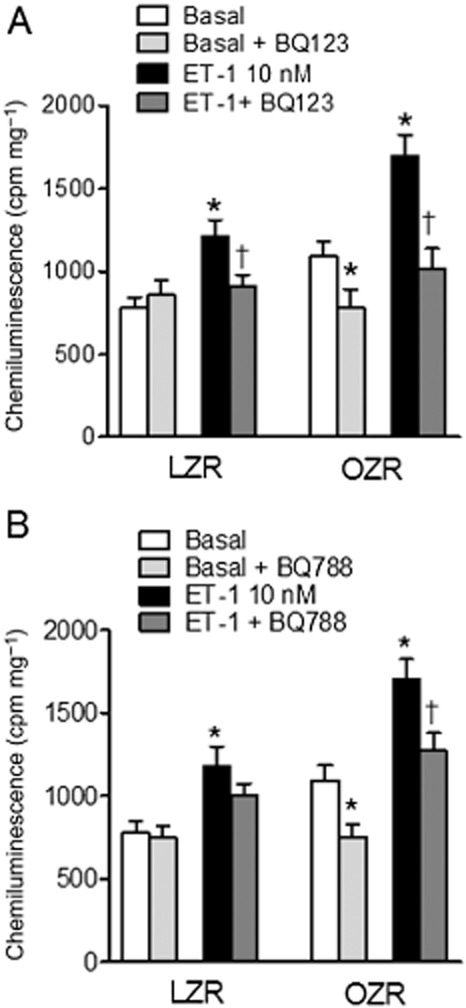
Effect of ET receptor antagonists on the ET-1-induced superoxide production in corpus cavernosum from LZR and OZR detected by lucigenin-enhanced chemiluminescence. Average effects of (A) the ETA receptor antagonist BQ123 (1 μM) and (B) the ETB receptor antagonist BQ788 (0.1 μM) on basal and on the ET-1 (10 nM)-stimulated superoxide generation in LZR and OZR. Superoxide production is expressed as counts per minute (cpm) mg−1 of tissue. Experiments were performed in the presence of the NOS inhibitor L-NOARG (100 μM). Data are shown as the means ± SEM of n = 3–5 animals (two corpus cavernosum samples from each animal). Five to 10 corpus cavernosum samples. *P < 0.05 versus basal, †P < 0.05 versus ET-1-treated; Student's t-test for unpaired observations.
Role of ETA and ETB receptors in the ET-1 induced impairment of NO-mediated relaxations
To determine the ET receptor/s involved in the acute inhibitory effect of ET-1 on the NO-mediated relaxant responses of penile arteries, the action of selective antagonists of ETA and ETB receptors was assessed on the relaxant responses to ACh and SNAP in the presence of ET-1 (10 nM). Figure 6A shows that the antagonist of ETA receptors BQ123 (1 μM) reversed the inhibitory effect elicited by ET-1 (0.3 nM) on the relaxations induced by ACh in penile arteries from LZR and also in arteries from OZR (Figure 6A and B, Table 1). In contrast, the antagonist of ETB receptors BQ788 (0.1 μM) failed to significantly change the ET-1-induced inhibition of ACh relaxant responses in arteries from LZR, but reversed the modest inhibition in arteries from OZR (Figure 6C and D, Table 1).
Figure 6.
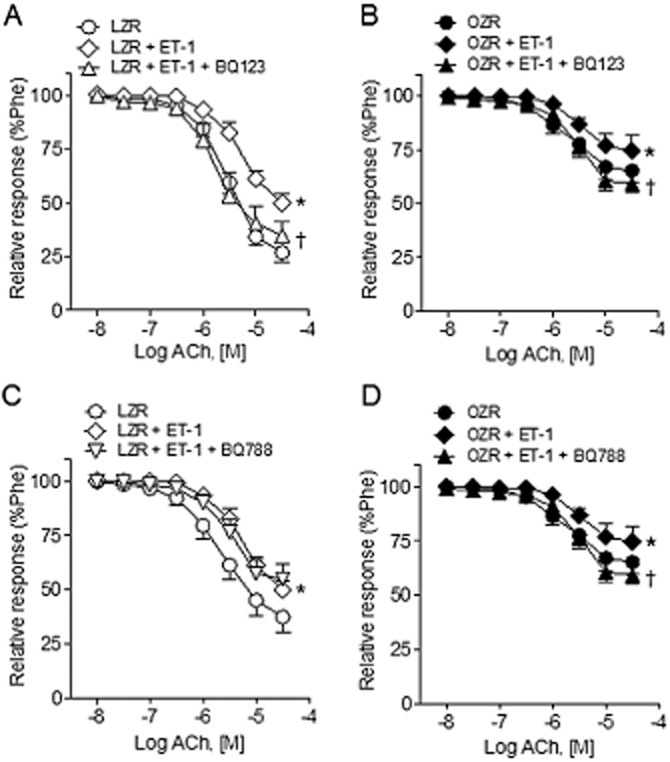
Effects of ETA and ETB receptor antagonists on the ET-1-elicited blunting of ACh-induced relaxation of penile arteries from LZR and OZR. (A, B) Effects of ET-1 (0.3 nM) and the ETA receptor antagonist BQ123 (1 μM) on the relaxant responses elicited by ACh in penile arteries from LZR (A) and OZR (B). (C, D) Average effects of ET-1 and of the ETB receptor antagonist BQ788 (0.1 μM) on the relaxant responses to ACh in penile arteries from LZR (C) and OZR (D). Results are means ± SEM of n = 3–5 animals (one to two arteries from each animal). *P < 0.05 versus control Emax, †P < 0.05 versus ET-1-treated Emax; Student's t-test for unpaired observations.
Incubation with the selective antagonist of ETA receptors BQ123 restored the blunted concentration-response curves to the NO donor SNAP elicited by ET-1 in penile arteries from both LZR and OZR (Figure 7A and B, Table 1). On the other hand, treatment with the ETB receptor antagonist BQ788 restored SNAP relaxant responses to values before inhibition with ET-1 only in arteries from OZR (Figure 7D) but not in LZR (Figure 7C, Table 1).
Figure 7.
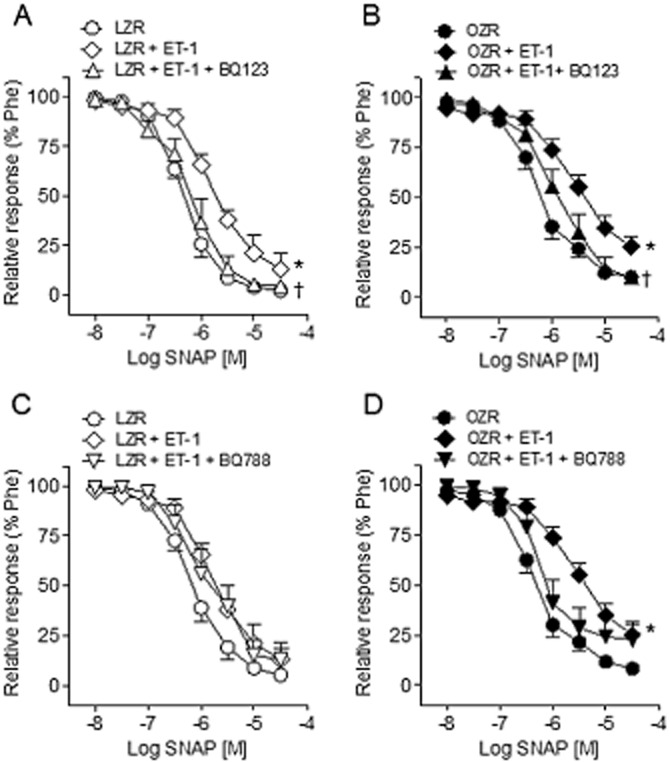
Effects of ETA and ETB receptor antagonists on the ET-1-elicited blunting of the relaxations induced by the NO donor SNAP in penile arteries of LZR and OZR. (A, B) Effects of ET-1 (0.3 nM) and of the ETA receptor antagonist BQ123 (1 μM) on the relaxant responses elicited by SNAP in penile arteries from LZR (A) and OZR (B). (C, D) Average effects of ET-1 and of the ETB receptor antagonist BQ788 (0.1 μM) on the relaxant responses to SNAP in penile arteries from LZR (C) and OZR (D). Results are means ± SEM of n = 4–8 animals (one to two arteries from each animal). *P < 0.05 versus control Emax, †P < 0.05 versus ET-1-treated Emax; Student's t-test for unpaired observations.
In order to investigate whether ET endogenous activity may interfere with the endothelium-dependent relaxations in penile arteries, the effect of selective ETA and ETB receptor blockade was assessed on the ACh relaxant responses. Figure 8 shows that while the ETA receptor antagonist BQ123 did not alter the ACh-induced relaxations in either LZR or OZR (Figure 8A and B), treatment with the antagonist of ETB receptors BQ788 or combined blockade of ETA and ETB receptors improved relaxant responses to ACh in arteries from obese animals (Figure 8C–F, Table 1).
Figure 8.
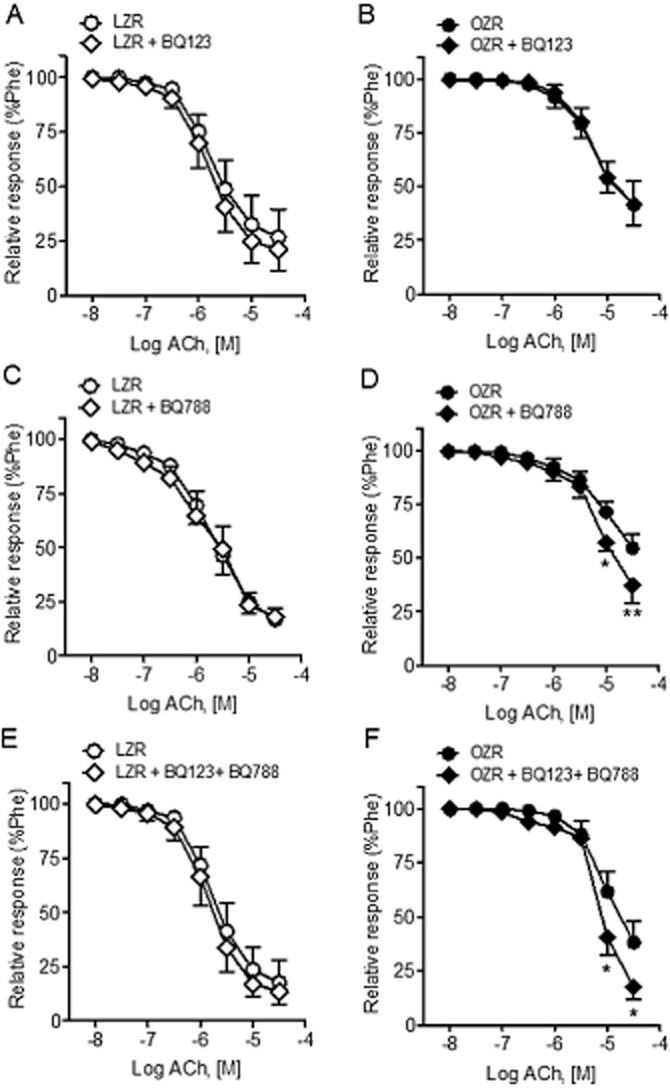
Effects of ETA and ETB receptor antagonists on the ACh-induced relaxations of penile arteries of LZR and OZR. Average effects of (A, B) the ETA receptor antagonist BQ123 (1 μM), (C, D) the ETB receptor antagonist BQ788 (0.1 μM) or (E,F) combined blockade of ETA receptors (BQ123, 1 μM) and ETB receptor (BQ788, 0.1 μM) on the relaxant responses elicited by ACh in penile arteries from LZR (A, C, E) and OZR (B, D, F). Results are means ± SEM of 6–8 arteries. *P < 0.05, **P < 0.01 versus control; Student's t-test for paired observations.
Localization of ET receptors in the endothelium of penile arteries
To further investigate the interactions between endothelial NO and ET-1-derived superoxide, colocalization of ET receptors and eNOS was assessed in penile arteries. Immunoreactivity for ETA receptors was found in VSM and in the endothelial layer of penile arteries from both LZR and OZR (Figure 9A and D), where it was colocalized with eNOS (Figure 9B, C, E and F). Intensity of immunoreactivity for the ETA receptor was increased in obese animals, as reported earlier (Contreras et al., 2013). The ETB receptor was found to be primarily expressed in the endothelium of penile arteries from LZR colocalized with eNOS (Figure 9G–I), while it was expressed in both endothelium and smooth muscle layer in arteries from OZR (Figure 9J–L).
Figure 9.
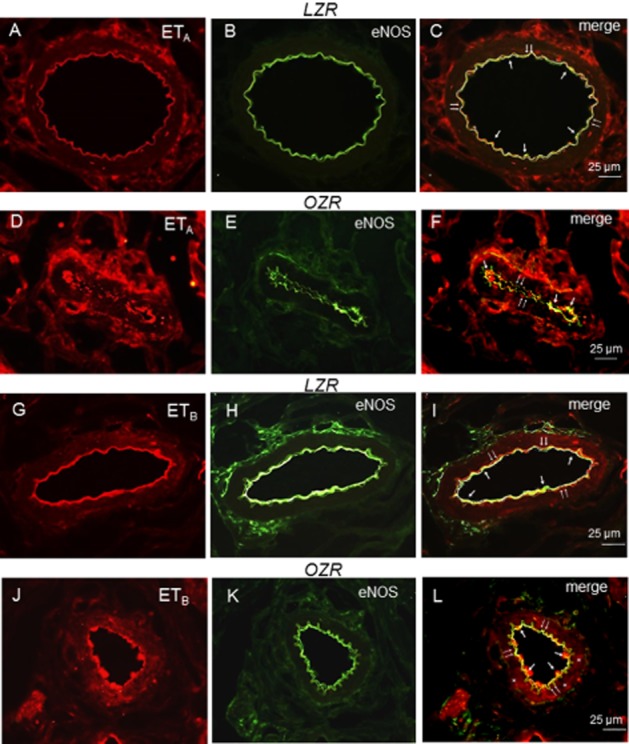
Representative immunohistochemical staining of ETA and ETB receptor expression in the endothelium and smooth muscle layer of penile arteries from LZR and OZR. (A, D) Immunofluorescence for ETA receptors (red areas) is distributed in the endothelial and media layers of the arterial wall in LZR and OZR. (B, E) Endothelial cell layer was visualized with the anti-eNOS marker (green). (C, F) Immunofluorescence double labelling for eNOS marker and ETA receptor expression in endothelial cell layer demonstrates colocalization in endothelium (yellow areas) in LZR and OZR penile arteries. (G, J) Immunofluorescence for ETB receptors (red areas) is distributed in the endothelial layer of the arterial wall in LZR and OZR penile arteries and in the smooth muscle layer in OZR (asterisks). (H, K) Endothelial cell layer was visualized with the anti-eNOS marker (green). (I, L) Immunofluorescence double labelling for eNOS marker and ETB receptor expression in endothelial cell layer demonstrates colocalization in endothelium (yellow areas, arrows) in LZR and OZR. Scale bars indicate 25 μm. Sections are representative of n = 3 LZR animals and OZR animals. Double arrows: internal elastic layer.
Discussion
Enhanced activity and/or levels of ET-1 have been associated to endothelial dysfunction in the insulin-resistant states of obesity and type 2 diabetes (Mather et al., 2002; 2004,; Pernow et al., 2012). In the present study, we demonstrate that ET-1 stimulates NADPH oxidase-derived ROS production which inhibits NO-mediated endothelial relaxations and contributes to contraction through superoxide–NO interactions in penile small arteries. ROS generation by ET-1 was mediated by ETA receptors in healthy arteries, while in arteries from obese animals both ETA and ETB receptors significantly contributed to the augmented ET-1-induced superoxide vascular production and to the blunting of the NO relaxant responses. Acute treatment with the selective ETB receptor antagonist or combined ETA and ETB receptor blockade improved penile endothelial dysfunction while both endothelium- and VSM-derived superoxide contributed to the enhanced vasoconstriction under conditions of obesity-associated insulin resistance.
Enhanced ET-1 activity reported in insulin-resistant states has been ascribed to the concurrent hyperinsulinaemia resulting in overstimulation of the MAPK pathway and increased production of ET (Potenza et al., 2009), but also to the augmented expression of ET receptors in the vascular wall (Mundy et al., 2007; Kobayashi et al., 2008; Kelly-Cobbs et al., 2011; Contreras et al., 2013), all this leading to enhanced vasoconstriction, inflammation and oxidative stress. We have recently demonstrated up-regulation of ETA and ETB receptors in penile arteries from insulin-resistant OZR linked to both increased contraction and augmented VSM intracellular Ca2+ mobilization (Contreras et al., 2013). Our current data show that enhanced expression of ET receptors in penile arteries is additionally coupled to increased ROS production, oxidative stress and endothelial dysfunction in obese animals.
ET-1 can stimulate superoxide generation in both endothelial and VSM cells (Duerrschmidt et al., 2000; Dong et al., 2005; Loomis et al., 2005; Fellner and Arendshorst, 2007; Just et al., 2008; Matsuo et al., 2009; Romero et al., 2009; 2010,) through a mechanism involving up-regulation of the main NADPH oxidase cytosolic subunit p47phox (Romero et al., 2009; 2010,). This contributes to vasoconstriction (Loomis et al., 2005; Fellner and Arendshorst, 2007; Matsuo et al., 2009) and endothelial dysfunction (Böhm et al., 2007; Romero et al., 2009; 2010,) in healthy vessels, and promotes proliferation and reduces apoptosis in human endothelial cells (Dong et al., 2005). Accordingly, the current data demonstrate that acute exposure to ET-1 induces NADPH oxidase-mediated superoxide generation, inhibits relaxations to both ACh and to the NO donor SNAP and causes endothelium-dependent vasoconstriction involving ROS in penile small arteries. Superoxide acts as a vasoconstrictor by directly acting on VSM or by quenching NO. In penile arteries, blockade of NOS greatly enhanced ET-1-induced ROS generation and contractions, and both endothelial removal and NADPH oxidase inhibition with apocynin blunted enhanced constriction which suggests that endothelial NO is buffering the constrictor influence of superoxide released by ET-1 from the endothelium in healthy arteries. These findings are consistent with our recent data demonstrating that ET-1 releases a contractile factor from the penile endothelium (Contreras et al., 2013), and likewise support that reported in both large arteries (Loomis et al., 2005) and in renal and retinal microvessels (Fellner and Arendshorst, 2007; Just et al., 2008), where NADPH oxidase-derived superoxide importantly contributes to the acute vasoconstriction upon ET receptor stimulation.
Oxidative stress is a key pathogenic factor in vascular diseases, such as hypertension and atherosclerosis, and also in the vascular complications of obesity and insulin-resistant states (Sonta et al., 2004; Montezano and Touyz, 2012). Up-regulation of the ET-1 precursor has been found to be associated with enhanced NADPH activity, oxidative stress and expression of the NF-κB in endothelial cells from obese individuals suggesting that ET-1 may induce endothelial dysfunction through NADPH-derived ROS production (Silver et al., 2007). The current data demonstrate that ET-1 is a considerable source of oxidative stress and might contribute to endothelial dysfunction in penile arteries from insulin-resistant rats and confirm the key contribution of NADPH oxidase to vascular ROS generation in obesity-associated insulin resistance (Sonta et al., 2004; Silver et al., 2007; Sánchez et al., 2012). Thus, both basal and basal plus ET-1-stimulated superoxide levels were markedly increased in penile erectile tissue from OZR, blunted by NADPH oxidase inhibition with apocynin and DPI and mimicked by PKC activation with PDBu, the latter being also markedly enhanced in obese animals, which suggests that ET-1 contributes to oxidative stress in arteries from obese animals through NADPH oxidase PKC-dependent ROS generation.
Impaired NO availability as a result of enhanced ET activity was earlier suggested to contribute to endothelial dysfunction in human obesity although the underlying mechanisms were unclear (Mather et al., 2004). In the present study, we found that ET-1 inhibited the relaxations induced by the NO donor SNAP in arteries from OZR, consistent with its contribution to the higher ROS production in erectile tissue, and in agreement with recent clinical studies showing that acute infusion of ET-1 did not only reduce basal forearm blood blow but also impaired endothelium-dependent and endothelium-independent vasodilatations both in healthy individuals (Böhm et al., 2007) and in patients with insulin resistance (Shemyakin et al., 2011). In our study, ET-1 exhibited a lesser inhibitory effect on the endothelium-dependent relaxations to ACh that were already blunted in penile arteries from OZR (Villalba et al., 2009). This suggests that other mechanisms probably dependent on chronic oxidative stress, such as eNOS uncoupling (Münzel et al., 2005; 2010,; Sánchez et al., 2012) or prostacyclin synthase oxidation (Du et al., 2006; Sánchez et al., 2010), might additionally contribute to penile endothelial dysfunction in obese animals.
Enhanced ET levels and ROS vascular generation have been linked to increased ROS-mediated ET-1-induced venoconstriction in mineralocorticoid hypertension, (Li et al., 2003). Here, we also demonstrate that dysregulated ROS production in erectile tissue from OZR results in a higher constrictor influence of ROS released by ET-1 not only from the endothelium but also from VSM. Thus, enhanced contractions to lower concentrations of ET-1 under conditions of NOS inhibition were largely reduced by endothelial removal or apocynin confirming the constrictor influence of ET-1-stimulated endothelial ROS in arteries from OZR, this influence exhibiting a trend to be higher than in lean rats. Interestingly, removal of the endothelium unmasked an augmented vasoconstriction to the highest concentrations of ET-1 in OZR (Contreras et al., 2013) that was blunted by inhibition of NADPH oxidase with apocynin. These findings suggest an increased ROS release by ET-1 from VSM counterbalanced by an increased NO relaxing influence from the endothelium in obese rats, as earlier observed in obese individuals where ET antagonism unmasked an augmented NO synthesis capacity that counterbalanced enhanced ET vasoconstrictor activity (Mather et al., 2004).
ET receptor blockade has been reported to improve endothelial function in obese and type 2 diabetic individuals (Mather et al., 2002; Weil et al., 2011; Rafnsson et al., 2012) and experimental studies have confirmed the involvement of ET-1 through ETA receptors in the blunted NO-mediated endothelium-dependent relaxations of arteries from diet-induced obese mice (Traupe et al., 2002) and mouse models of atherosclerosis (Barton et al., 1998; Böhm et al., 2002). ETA receptors have likewise been involved in the enhanced vascular oxidative stress in experimental hypertension (Callera et al., 2003; Li et al., 2003; Laplante et al., 2005; Viel et al., 2008).
In the present study, ETA receptor-mediated effects were functionally comparable in erectile tissue from LZR and OZR, although up-regulation of these receptors in penile arteries of OZR (Contreras et al., 2013) along with the ability of BQ123 to restore augmented basal and ET-1-stimulated superoxide to levels similar to those in LZR suggest a role for ETA receptors in oxidative stress and endothelial dysfunction in obese rats. Interestingly, we also demonstrate here that while ETB receptors do not significantly contribute to superoxide generation in healthy arteries, up-regulation of ETB receptors is associated to enhanced oxidative stress, blunting of NO-mediated relaxant responses and endothelial dysfunction in penile arteries from insulin-resistant OZR. ETB receptors have earlier been shown to stimulate superoxide release in human umbilical endothelial cells (Duerrschmidt et al., 2000; Dong et al., 2005) through a mechanism involving enhanced expression of the NADPH oxidase subunits gp91phox (Duerrschmidt et al., 2000) and gp47phox (Dong et al., 2005) and subsequent increased NADPH oxidase activity with submaximal effects after 30 min (Duerrschmidt et al., 2000). This might explain the acute effects of ET-1 on endothelial dysfunction observed here and in clinical studies (Böhm et al., 2007; Shemyakin et al., 2011), and also the beneficial effect of acute treatment with the ETB receptor antagonist on the endothelium-dependent relaxations and on basal and ET-1-stimulated O2− production in penile arteries from OZR. The contribution of ETB along with ETA receptors to the ET-1-induced superoxide generation, as shown in the present study, has also been demonstrated in healthy aorta (Loomis et al., 2005) and renal microvessels (Fellner and Arendshorst, 2007; Just et al., 2008) in which these receptors mediate vasoconstriction, and in coronary artery bypass grafts (Cerrato et al., 2012). Furthermore, up-regulation of ETB receptors coupled to increased superoxide production has been reported in sympathetic neurons of deoxycorticosterone acetate and high-salt diet (DOCA-salt) hypertensive rats (Dai et al., 2004). Enhanced expression of both ETA and ETB receptors colocalized with eNOS in the penile endothelium of obese rats, as shown in the present study, would favour superoxide–NO interactions and endothelial dysfunction under conditions of dysregulated ROS signalling and enhanced superoxide production in insulin resistance.
ETB receptors minimally contribute to vasoconstriction in healthy penile arteries, but their up-regulation in both endothelium and VSM of penile arteries from OZR was associated to augmented ET-1 endothelium-dependent and endothelium-independent contractions respectively (Contreras et al., 2013). ETB receptor antagonism significantly reduced VSM calcium mobilization and endothelium-independent contractions to ET-1 only in OZR (Contreras et al., 2013) to a degree similar to that obtained with the NADPH oxidase inhibitor apocynin in the present study. Therefore, ETB receptors might be involved in the ET-1-induced ROS release from VSM in obese rats. Accordingly, augmented expression of VSM ETB receptors has recently been associated to an attenuation of the endothelial ETB receptor-mediated prevention of vascular remodelling in arteries from type 2 diabetic animals (Kelly-Cobbs et al., 2011) and ET-1 stimulates proliferation via an ETB-NADPH oxidase-dependent pathway (Dong et al., 2005).
Taken together, the current findings would be consistent with clinical studies showing that dual ETA/ETB receptor blockade, but not selective ETA blockade, improved endothelium-dependent vasodilatation and peripheral endothelial function in subjects with insulin resistance and type 2 diabetes (Shemyakin et al., 2006; Rafnsson et al., 2012), and restored endothelial cerebral vasodilatation in experimental models of type 2 diabetes (Abdelsaid et al., 2014), thus supporting the concept that ETB receptor-mediated activation of NADPH oxidase importantly contributes to oxidative stress and endothelial dysfunction under conditions of insulin resistance. Likewise, our results showing that ETB receptor antagonism reduced oxidative stress and endothelial dysfunction induced by ET-1 in erectile tissue from insulin-resistant obese rats might explain why the use of ETA receptor antagonists alone has been reported to render variable results in earlier clinical studies, failing to improve erectile function in men with mild to moderate ED of unstated aetiology while enhancing erectile responses in animal models (Kim et al., 2002). Thus, while selective ETA receptor blockade improved erectile function in experimental models of hypertension (Carneiro et al., 2008), our findings suggest that antagonism of ETB receptors might be beneficial for endothelial dysfunction in the ED associated with insulin-resistant states.
Acknowledgments
This work was supported by grants no. SAF2009-10448 from MICINN and SAF 2012-31631 from MINECO (Spain). We thank Francisco Puente and Manuel Perales for expert technical assistance.
Glossary
- DPI
diphenylene iodonium
- ED
erectile dysfunction
- ET-1
endothelin-1
- KPSS
high K+-physiological saline solution
- LZR
lean Zucker rat
- O2−
superoxide radical
- OZR
obese Zucker rat
- PDBu
phorbol 12,13-dibutyrate
- ROS
reactive oxygen species
- SNAP
S-nitroso-N-acetyl-DL-penicillamine
- VSM
vascular smooth muscle
Author contributions
A. S. was responsible for acquisition of data, analysis and interpretation of data and drafting of the article. P. M. was responsible for acquisition of data and analysis and interpretation of data. M. M. was responsible for acquisition of data and analysis and interpretation of data. S. B. was responsible for analysis and interpretation of data and revising the article for intellectual content. A. G.-S. was responsible for revising the article for intellectual content. M. H. was responsible for analysis and interpretation of data and revising the article for intellectual content. D. P. was responsible for drafting the article, revising the article for intellectual content and final approval of the completed article.
Conflict of interest
None of the authors have any conflict of interests.
References
- Abdelsaid M, Ma H, Coucha M, Ergul A. Late dual endothelin receptor blockade with bosentan restores impaired cerebrovascular function in diabetes. Life Sci. 2014 doi: 10.1016/j.lfs.2013.12.231. pii: S0024-3205(14)00051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M, Haudenschild CC, d'Uscio LV, Shaw S, Münter K, Lüscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CR, Sullivan ME, Dashwood MR, Muddle JR, Morgan RJ. The density and distribution of endothelin 1 and endothelin receptor subtypes in normal and diabetic rat corpus cavernosum. Br J Urol. 1995;76:203–207. doi: 10.1111/j.1464-410x.1995.tb07675.x. [DOI] [PubMed] [Google Scholar]
- Böhm F, Ahlborg G, Pernow J. Endothelin-1 inhibits endothelium dependent vasodilatation in the human forearm: reversal by ETA receptor blockade in patients with atherosclerosis. Clin Sci (Lond) 2002;102:321–327. [PubMed] [Google Scholar]
- Böhm F, Settergren M, Pernow J. Vitamin C blocks vascular dysfunction and release of interleukin-6 induced by endothelin-1 in humans in vivo. Atherosclerosis. 2007;190:408–415. doi: 10.1016/j.atherosclerosis.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, et al. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003;42:811–817. doi: 10.1161/01.HYP.0000088363.65943.6C. [DOI] [PubMed] [Google Scholar]
- Carneiro FS, Nunes KP, Giachini FR, Lima VV, Carneiro ZN, Nogueira EF, et al. Activation of the ET-1/ETA pathway contributes to erectile dysfunction associated with mineralocorticoid hypertension. J Sex Med. 2008;5:2793–2807. doi: 10.1111/j.1743-6109.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- Cerrato R, Cunnington C, Crabtree MJ, Antoniades C, Pernow J, Channon KM, et al. Endothelin-1 increases superoxide production in human coronary artery bypass grafts. Life Sci. 2012;91:723–728. doi: 10.1016/j.lfs.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Contreras C, Sánchez A, Martínez P, Raposo R, Climent B, García-Sacristán A, et al. Insulin resistance in penile arteries from a rat model of metabolic syndrome. Br J Pharmacol. 2010;161:350–364. doi: 10.1111/j.1476-5381.2010.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras C, Sánchez A, Martínez P, Climent B, Benedito S, García-Sacristán A, et al. Impaired endothelin calcium signaling coupled to endothelin Type B receptors in penile arteries from insulin-resistant obese Zucker rats. J Sex Med. 2013;10:2141–2153. doi: 10.1111/jsm.12234. [DOI] [PubMed] [Google Scholar]
- Dai X, Galligan JJ, Watts SW, Fink GD, Kreulen DL. Increased O2+-production and upregulation of ETB receptors by sympathetic neurons in DOCA-salt hypertensive rats. Hypertension. 2004;43:1048–1054. doi: 10.1161/01.HYP.0000126068.27125.42. [DOI] [PubMed] [Google Scholar]
- Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol. 2005;145:323–333. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerrschmidt N, Wippich N, Goettsch W, Broemme HJ, Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun. 2000;269:713–717. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension. 2005;45:283–287. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]
- Fellner SK, Arendshorst W. Endothelin-A and -B receptors, superoxide, and Ca2+ signalling in afferent arterioles. Am J Physiol Renal Physiol. 2007;292:175–184. doi: 10.1152/ajprenal.00050.2006. [DOI] [PubMed] [Google Scholar]
- Francavilla S, Properzi G, Bellini C, Marino G, Ferri C, Santucci A. Endothelin-1 in diabetic and nondiabetic men with erectile dysfunction. J Urol. 1997;158:1770–1774. doi: 10.1016/s0022-5347(01)64125-9. [DOI] [PubMed] [Google Scholar]
- Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, et al. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:904–911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- Just A, Whitten CL, Arendshorst WJ. Reactive oxygen species participate in acute renal vasoconstrictor responses induced by ETA and ETB receptors. Am J Physiol Renal Physiol. 2008;294:719–728. doi: 10.1152/ajprenal.00506.2007. [DOI] [PubMed] [Google Scholar]
- Kelly-Cobbs AI, Harris AK, Elgebaly MM, Li W, Sachidanandam K, Portik-Dobos V, et al. Endothelial endothelin B receptor-mediated prevention of cerebrovascular remodeling is attenuated in diabetes because of up-regulation of smooth muscle endothelin receptors. J Pharmacol Exp Ther. 2011;337:9–15. doi: 10.1124/jpet.110.175380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NN, Dhir V, Azadzoi KM, Traish AM, Flaherty E, Goldstein I. Pilot study of the endothelin-A receptor selective antagonist BMS-193884 for the treatment of erectile dysfunction. J Androl. 2002;23:76–83. doi: 10.1002/jand.2002.23.1.76. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nogami T, Taguchi K, Matsumoto T, Kamata K. Diabetic state, high plasma insulin and angiotensin II combine to augment endothelin-1-induced vasoconstriction via ETA receptors and ERK. Br J Pharmacol. 2008;155:974–983. doi: 10.1038/bjp.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Pioglitazone prevents corporal veno-occlusive dysfunction in a rat model of type 2 diabetes mellitus. BJU Int. 2006;98:116–124. doi: 10.1111/j.1464-410X.2006.06268.x. [DOI] [PubMed] [Google Scholar]
- Laplante MA, Wu R, Moreau P, Champlain J. Endothelin mediates superoxide production in angiotensin II-induced hypertension in rats. Free Radic Biol Med. 2005;38:589–596. doi: 10.1016/j.freeradbiomed.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Li L, Watts SW, Banes AK, Galligan JJ, Fink GD, Chen AF. NADPH oxidase-derived superoxide augments endothelin-1-induced venoconstriction in mineralocorticoid hypertension. Hypertension. 2003;42:316–321. doi: 10.1161/01.HYP.0000084853.47326.F2. [DOI] [PubMed] [Google Scholar]
- Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther. 2005;315:1058–1064. doi: 10.1124/jpet.105.091728. [DOI] [PubMed] [Google Scholar]
- Mather KJ, Mirzamohammadi B, Ltief A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–2066. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- Matsuo J, Oku H, Kanbara Y, Kobayashi T, Sugiyama T, Ikeda T. Involvement of NADPH oxidase and protein C in endothelin-1-induced superoxide production in retinal microvessels. Exp Eye Res. 2009;89:693–699. doi: 10.1016/j.exer.2009.06.012. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezano AC, Touyz RM. Reactive oxygen species and endothelial function – role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol. 2012;110:87–94. doi: 10.1111/j.1742-7843.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Hofmann-Lehmann R, et al. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: implications for diet-induced obesity. Cardiovasc Res. 2007;73:368–375. doi: 10.1016/j.cardiores.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Münzel T, Daiber A, Ullrich V, Mülsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- Münzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue) doi: 10.1093/nar/gkt1143. D1098-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernow J, Shemyakin A, Böhm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci. 2012;91:507–516. doi: 10.1016/j.lfs.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Potenza MA, Addabbo F, Montagnani M. Vascular actions of insulin with implications for endothelial dysfunction. Am J Physiol Endocrinol Metab. 2009;297:568–577. doi: 10.1152/ajpendo.00297.2009. [DOI] [PubMed] [Google Scholar]
- Prieto D, Kaminski PM, Bagi Z, Ahmad M, Wolin MS. Hypoxic relaxation of penile arteries: involvement of endothelial nitric oxide and modulation by reactive oxygen species. Am J Physiol Heart Circ Physiol. 2010;299:915–924. doi: 10.1152/ajpheart.00382.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto D, Contreras C, Sánchez A. Endothelial dysfunction, obesity and insuline resistance. Curr Vasc Pharmacol. 2014;12:412–426. doi: 10.2174/1570161112666140423221008. [DOI] [PubMed] [Google Scholar]
- Rafnsson A, Böhm F, Settergren M, Gonon A, Brismar K, Pernow J. The endothelin receptor antagonist bosentan improves peripheral endothelial function in patients with type 2 diabetes mellitus and microalbuminuria: a randomised trial. Diabetologia. 2012;55:600–607. doi: 10.1007/s00125-011-2415-y. [DOI] [PubMed] [Google Scholar]
- Ritchie R, Sullivan M. Endothelins and erectile dysfunction. Pharmacol Res. 2011;63:496–501. doi: 10.1016/j.phrs.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Romero M, Jiménez R, Sánchez M, López-Sepúlveda R, Zarzuelo MJ, O'Valle F, et al. Quercetin inhibits vascular superoxide production induced by endothelin-1: role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis. 2009;202:58–67. doi: 10.1016/j.atherosclerosis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Romero M, Jiménez R, Sánchez M, López-Sepúlveda R, Zarzuelo A, Tamargo J, et al. Vascular superoxide production by endothelin-1 requires Src non-receptor protein tyrosine kinase and MAPK activation. Atherosclerosis. 2010;212:78–85. doi: 10.1016/j.atherosclerosis.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Sánchez A, Contreras C, Villalba N, Martínez P, Martínez AC, Bríones A, et al. Altered arachidonic acid metabolism via COX-1 and COX-2 contributes to the endothelial dysfunction of penile arteries from obese Zucker rats. Br J Pharmacol. 2010;159:604–616. doi: 10.1111/j.1476-5381.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A, Contreras C, Martínez P, Climent B, Benedito S, García-Sacristán A, et al. Role of neural NO synthase (nNOS) uncoupling in the dysfunctional nitrergic vasorelaxation of penile arteries from insulin-resistant obese Zucker rats. PLoS ONE. 2012;7:36027. doi: 10.1371/journal.pone.0036027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemyakin A, Böhm F, Wagner H, Efendic S, Båvenholm P, Pernow J. Enhanced endothelium-dependent vasodilatation by dual endothelin receptor blockade in individuals with insulin resistance. J Cardiovasc Pharmacol. 2006;47:385–390. doi: 10.1097/01.fjc.0000210070.47205.16. [DOI] [PubMed] [Google Scholar]
- Shemyakin A, Salehzadeh F, Esteves Duque-Guimaraes D, Böhm F, Rullman E, Gustafsson T, et al. Endothelin-1 reduces glucose uptake in human skeletal muscle in vivo and in vitro. Diabetes. 2011;60:2061–2067. doi: 10.2337/db10-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, et al. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, et al. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med. 2004;37:115–123. doi: 10.1016/j.freeradbiomed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Sullivan ME, Dashwood MR, Thompson CS, Muddle JR, Mikhailidis DP, Morgan RJ. Alterations in endothelin B receptor sites in cavernosal tissue of diabetic rabbits: potential relevance to the pathogenesis of erectile dysfunction. J Urol. 1997;158:1966–1972. doi: 10.1016/s0022-5347(01)64195-8. [DOI] [PubMed] [Google Scholar]
- Traupe T, d'Uscio LV, Muenter K, Morawietz H, Vetter W, Barton M. Effects of obesity on endothelium-dependent reactivity during acute nitric oxide synthase inhibition: modulatory role of endothelin. Clin Sci (Lond) 2002;103:13–15. doi: 10.1042/CS103S013S. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Verma S, Lovren F, Dumont AS, Mather KJ, Maitland A, Kieser TM, et al. Endothelin receptor blockade improves endothelial function in human internal mammary arteries. Cardiovas Res. 2001;49:146–151. doi: 10.1016/s0008-6363(00)00244-3. [DOI] [PubMed] [Google Scholar]
- Viel EC, Benkirane K, Javeshghani D, Touyz RM, Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:281–288. doi: 10.1152/ajpheart.00304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba N, Martínez P, Bríones A, Sánchez A, Salaíces M, García-Sacristán A, et al. Differential structural and functional changes in penile and coronary arteries from obese Zucker rats. Am J Physiol Heart Circ Physiol. 2009;297:696–707. doi: 10.1152/ajpheart.01308.2008. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Jackson G, Stefanadis C, Montorsi P. Erectile dysfunction in the cardiovascular patient. Eur Heart J. 2013;34:2034–2046. doi: 10.1093/eurheartj/eht112. [DOI] [PubMed] [Google Scholar]
- Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Circ Physiol. 2011;301:689–695. doi: 10.1152/ajpheart.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


