Abstract
Background and Purpose
Uveitis is a prevalent intraocular inflammatory disease and one of the most damaging ocular conditions. Pretreatment with melatonin prevented ocular inflammation induced by an intravitreal injection of bacterial LPS in the Syrian hamster. Here, we have assessed the anti-inflammatory effects of melatonin administered after the onset of ocular inflammation.
Experimental Approach
The eyes of male Syrian hamsters were intravitreally injected with vehicle or LPS. Melatonin was injected i.p. every 24 h, starting 12 or 24 h after the LPS injection. A clinical evaluation (with a score index based on clinical symptoms), the number of infiltrating cells, protein concentration and PGE2 and PGF2α levels in the aqueous humour, as well as retinal NOS activity, lipid peroxidation and TNF-α levels were assessed. Retinal function was assessed by scotopic electroretinography, and light microscopy and immunohistochemistry were used to evaluate the state of the retinal structure.
Key Results
Both treatment regimens with melatonin decreased clinical symptoms, reduced the leakage of cells and proteins, and decreased PG levels in aqueous humour from eyes injected with LPS. In addition, melatonin treatment blocked the decrease in scotopic electroretinogram a- and b-wave amplitude, protected the retinal structure and reduced the increase in NOS activity, lipid peroxidation and TNF-α levels, induced by LPS.
Conclusions and Implications
These results indicate that treatment with melatonin, starting after the onset of uveitis, attenuated ocular inflammation induced by LPS in the Syrian hamster and support the use of melatonin as a therapeutic resource for uveitis treatment.
Tables of Links
| Targets | Ligands | |
|---|---|---|
| NO synthase | LPS, bacterial lipopolysaccharide | PGE2 |
| Melatonin | PGF2α |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Uveitis is an ocular condition characterized by intraocular inflammation caused by several different aetiologies and is a significant cause of visual loss (Dick, 2000; Durrani et al., 2004), with high prevalence worldwide (London et al., 2010). A variety of mediators play key roles in ocular inflammation, including NF-κB, pro-inflammatory cytokines (Commodaro et al., 2010), oxidative and nitrosative stress (Wu et al., 1997), and PGs (Sand and Krogh, 1991).
Unravelling which are the most critical mechanisms is unlikely to be achieved in studies that are limited to the clinically observable ocular changes that are seen in human uveitis. Far more detailed and invasive studies are required, preferably in a readily available animal model. Endotoxin-induced uveitis (EIU), a widely recognized experimental model of acute ocular inflammation (Rosenbaum et al., 1980) that mimics the central features of human uveitis, is induced by systemic or local injection LPS, a cell wall component of Gram-negative bacteria. LPS induces the breakdown of the blood–ocular barrier (BOB), leukocyte infiltration, and enhances the expression of various inflammatory mediators, such as PGs (Bellot et al., 1996), NO (Mandai et al., 1994; Bellot et al., 1996), TNF-α and IL-6 (De Vos et al., 1994), all of which contribute to the development and progression of the disease. Moreover, oxidative biomarkers are shown to be elevated in EIU (Wu et al., 1997), suggesting that inflammation and oxidative stress cooperatively contribute to its pathogenesis. Even though EIU was generally considered as a model of anterior uveitis, a number of studies show that it also involves the inflammation of the retina (Miyamoto et al., 1996). We have demonstrated that, in the Syrian hamster, an intravitreal injection of LPS provokes a significant decrease in the scotopic electroretinogram (ERG), which correlates with focal destruction of photoreceptors, alterations in Müller cells and retinal infiltration (Sande et al., 2008), suggesting that, at least in the Syrian hamster, EIU is a model of panuveitis.
Present pharmacological treatment for uveitis primarily includes corticosteroids and non-steroidal anti-inflammatory drugs. However, these drugs do not completely control the disease in many patients and, with long-term application, they may result in many adverse effects such as cataract, glaucoma, susceptibility to microbial infection and nephrotoxicity (McGhee et al., 2002; Nguyen et al., 2006; El Afrit et al., 2007). Hence, there is a need for therapeutic agents with safer modes of action. In that context, much attention has been paid to a variety of candidates as ocular anti-inflammatory agents. Most of these treatments administered before or immediately after the injection of LPS were effective in the prevention of the ocular inflammation induced by LPS. For instance, oral supplementation with resveratrol for 5 days until LPS injection (Kubota et al., 2009), ginkgo biloba extract (Ilieva et al., 2004) or fucoxanthin administered immediately after LPS injection (Shiratori et al., 2005), as well as lutein or dexamethasone i.v. administered at 30 min before, at the same time and at 30 min after LPS treatment (Jin et al., 2006) prevented ocular inflammation. However, the utilization of these treatments in a clinical setting is mostly limited because the onset of uveitis is largely unpredictable. In contrast, therapeutic strategies that are effective when administered after the onset of the inflammatory process may have more translational relevance as therapeutic resources for uveitis treatment.
Melatonin is an endogenous neuromodulator in the retina of vertebrates (Tosini et al., 2012), including Syrian hamsters (Faillace et al., 1995). Several lines of evidence support that melatonin may act as a protective agent in ocular conditions such as photokeratitis, cataract, retinopathy of prematurity and ischaemia/reperfusion injury (Siu et al., 2006; Rosenstein et al., 2010). Moreover, melatonin prevented retinal glaucomatous (Belforte et al., 2010) and diabetic (Salido et al., 2013) damage. Melatonin also prevented the biochemical, clinical, histological and functional alterations induced by EIU in Syrian hamsters (Sande et al., 2008) and cats (Del Sole et al., 2012). The above observations prompted us to evaluate, in the work described here, the anti-inflammatory effect of melatonin administered after the onset of ocular inflammation induced by a single intravitreal injection of LPS in Syrian hamsters. Our results indicated that treatment with melatonin after the onset of the uveitis significantly attenuated ocular inflammation induced by LPS in the Syrian hamster, and support the use of melatonin as a therapeutic option for the treatment of uveitis.
Methods
Test systems used
All animal care and experimental procedures reported here were approved by the Animal Care Committee of the Center for Pharmacological and Botanical Studies, National Research Council, and by the Institutional Committee for the Care and Use of Laboratory Animals from the School of Medicine, University of Buenos Aires. Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 240 animals were used in the experiments described here.
Male Syrian hamsters (average weight 120 ± 20 g), derived from a stock supplied by Charles River Breeding Laboratories (Wilmington, MA, USA), were kept under a 14 h light:10 h dark lighting schedule (lights on at 06:00 h), with free access to food and water. The experimental groups were made up as follows: 14 animals per group for assessment of the clinical score, ERG and retinal histology; 12 animals per group for the assessment of infiltrated cell number, protein concentration and PG level in aqueous humour; four animals per group for the immunohistochemical study; 10 animals per group for NOS activity assessment; 10 animals per group for lipid peroxidation assessment; and 10 animals per group for TNF-α level assessment.
Experimental design
Syrian hamsters were anaesthetized with ketamine hydrochloride (150 mg·kg−1) and xylazine hydrochloride (0.5 mg·kg−1) administered i.p. A drop of proparacaine (2%) was administered in each eye for local anaesthesia. With a syringe (Hamilton, Reno, NV, USA) and a 30-gauge needle, 2 μL of LPS (1.5 mg·mL−1 in sterile pyrogen-free saline) from Salmonella typhimurium was injected into one eye of anaesthetized hamsters. Another group of hamsters was intravitreally injected with vehicle (in sterile pyrogen-free saline) into one eye, as previously described (Sande et al., 2008). Injections were applied at 1 mm near the limbus, and the needle was left in the eye for 60 s to allow aqueous humour to flow out; this small volume prevented the increase of intraocular pressure and the volume loss. Intravitreal injections of vehicle or LPS were performed at 08:00 h. Melatonin (10 mg·kg−1) or vehicle (9% ethanol in saline solution) was injected i.p. for 8 days, every 24 h, starting 12 h (Mel 12 h) or 24 h (Mel 24 h) after intravitreal injections. The dose of melatonin was selected on the basis of previous reports (Crespo et al., 1999; Ortiz et al., 1999; Chen et al., 2006).
Animals were randomized into four experimental groups: group 1 (control): animals received an intravitreal injection of vehicle (sterile saline solution) and daily i.p. injections of vehicle (9% ethanol in normal saline), starting 12 h after the intravitreal injection; group 2 (vehicle): animals received an intravitreal injection of LPS and daily i.p. injections of vehicle (9% ethanol in normal saline), starting 12 h after the intravitreal injection; group 3 (Mel 12 h): animals received an intravitreal injection of LPS and daily i.p. injections of melatonin, starting 12 h after LPS injection; and group 4 (Mel 24 h): animals received an intravitreal injection of LPS and daily i.p. injections of melatonin, starting 24 h after LPS injection. For group 3, melatonin was injected daily at 20:00 h, while for group 4, melatonin was injected daily at 08:00 h. In the present study, eyes intravitreally injected with vehicle served as the control group because in preliminary studies we found that in comparison with intact animals, the injection of vehicle did not affect retinal function and histology (data not shown).
Measurements made
Clinical score
A range of signs were observed when examining hamster eyes after the intravitreal injection of vehicle or LPS, as previously described (Sande et al., 2008). Clinical severity of these signs was graded on a scale from 0 to 14, as follows: degree of conjunctival (0–3) and episcleral hyperaemia (0–3), degree of cornea inflammation (0–3), degree of alteration of iris and pupil (vasodilatation, synechia, presence of exudates at the pupil rim and degree of miosis) (0–3). The absence (0) or presence of a cataract was scored 1 or 2 (less or more than 50% of the lens surface respectively). The animals were examined by an observer unaware of the treatments and in a random order at 24, 36, 48 and 72 h after the injection of vehicle or LPS, before the next injection of melatonin.
Integrity of the blood ocular barrier
Animals were killed by decapitation, and the aqueous humour was collected immediately from each eye by anterior chamber puncture using a 30-gauge needle under a surgical microscope. Infiltrated cell number and protein concentration in aqueous humour samples obtained at 36 h after the injection of vehicle or LPS were assessed as previously described (Sande et al., 2008). For cell counting, aqueous humour samples were suspended in four volumes of Türk solution, and cells were counted with a haemocytometer under an optical microscope. The number of cells per field was manually counted, and the number of cells per μL was obtained by averaging the results of four fields from each sample. Protein concentration in the aqueous humour was determined by the method of Lowry et al. (1951), using BSA as the standard.
Assessment of PG levels
Levels of PGE2 or PGF2α were determined by radioimmunoassay, as previously described (de Zavalía et al., 2010). Briefly, aliquots of aqueous humour (1 μL) were preincubated with a specific antiserum (1:20 for both anti-PGE2 and anti-PGF2α) for 45 min at 4°C. Then, [3H]PGE2 or [3H]PGF2α (20 000 dpm) were added and incubated for 1 h at 4°C. PG levels were obtained from a standard curve, with an assay limit sensitivity of 1 pg per tube.
Electroretinography
ERGs were recorded as previously described (Sande et al., 2008). Eight days after intravitreal injections, full-field ERGs were recorded in anaesthetized hamsters following 6 h dark adaptation. Pupils were dilated with 1% tropicamide and 2.5% phenylephrine (Alcon Laboratories, Buenos Aires, Argentina), and the cornea was irrigated with balanced salt solution (Alcon Laboratories) to prevent keratopathy. Hamsters were placed in a Ganzfeld light stimulator, and recordings were completed within 20 min of the induction of anaesthesia. A gold ring-shaped electrode was placed in contact with the central cornea; a reference needle electrode was placed through the ear, and a ground electrode was attached to the back of the head. Electrode placement was performed under a 15 W red light, which did not affect dark adaptation and was switched off during the recordings. Both eyes were recorded simultaneously and the response to one flash of unattenuated white light (4 ms) from a photic stimulator set at maximum brightness (5 cd s/m2 without filter) was amplified (gain set at 100), filtered (1.5 Hz low-pass filter, 1000 Hz high-pass filter), notch activated and registered with an Akonic BIO-PC device (Akonic, Buenos Aires, Argentina). Each recording started simultaneously with the application of the stimulus. Amplitudes and latencies of a- and b-waves were then measured and analysed, as previously described (Sande et al., 2008).
Histological examination
Hamsters were killed at 8 days after intravitreal injections. Eyes were immediately enucleated and stored in PBS with 4% formaldehyde for 24–48 h. Eyes were then dehydrated, embedded in paraffin and sectioned with a microtome at 5 μm thickness. Each section was cut along the vertical meridian of the eye near the optic nerve head. The retinal layer thickness (in μm) for each eye was measured in the central and peripheral retina. The ganglion cell layer number (cells per 200 μm) was assessed, without distinction of cell types. Measurements were obtained at 0.5 mm dorsal and ventral from the optic disc (central retina), and at 0.5 mm from the ora serrata (peripheral retina). Results obtained from five separate sections for each eye were averaged, and the mean of five eyes was recorded as the representative value for each group. Microscopic images were digitally captured with a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan; illumination: 6 V halogen lamp, 20 W, equipped with a stabilized light source) via a Sony SSC-DC50 camera. Sections were stained with haematoxylin and eosin and analysed by an observer, unaware of the treatments.
Immunohistochemical study
Immunodetection of retinal glial cells was performed as previously described (Sande et al., 2008; Dorfman et al., 2013). Briefly, heat-induced antigen retrieval in sections, by heating at 90 °C for half an hour was carried out and then the sections were preincubated with 2% normal horse serum followed by 0.4% Triton X-100 in 0.01 M PBS for 1 h. Afterwards, sections were incubated overnight at 4°C with a mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody conjugated to Cy3 (1:1200; Sigma Chemical Co., St. Louis, MO, USA). An Olympus BX50 microscope was used for microscopic observations.
Measurement of thiobarbituric acid reactive substances (TBARS) levels
Retinal lipid peroxidation was evaluated by measuring malondialdehyde bis-dimethyl acetal (MDA) levels, as previously described (Moreno et al., 2004). Retinas were homogenized in 300 μL of 15 mM potassium buffer plus 60 mM KCl (pH 7.2). Homogenates were mixed with 75 μL 10% SDS and 1.4 mL 0.8% thiobarbituric acid in 10% acetic acid (pH 3.5), and heated to 100°C for 1 h. After cooling, samples were centrifuged at 3200× g for 10 min and the flocculent precipitate was removed. After addition of 1.0 mL of water and 5.0 mL of n-butanol-pyridine mixture (15:1, v/v), the mixture was shaken and centrifuged at 2000× g for 15 min. Absorbance of the organic phase was measured using a Jasco FP 770 fluorescence spectrophotometer (Japan Spectroscopic Co., Ltd. Tokyo, Japan), with at an emission λ of 553 nm and an excitation λ of 515 nm. The amount of TBARS was determined according to a standard calibration curve generated from MDA.
NOS activity assessment
Animals were killed by decapitation at 36 h after vehicle or LPS injections, and NOS activity was assessed as previously described (Sáenz et al., 2002). Retinas were homogenized in buffer (100 μL) containing 0.32 mol L−1 sucrose and 0.1 mmol L−1 EDTA (pH 7.4). Reaction mixtures contained 50 μL of the enzyme source and 50 μL of a buffer stock solution (final concentrations: 10 mM HEPES, 3 mM CaCl2, 1 mM NADPH, 5 μM FAD, 1 mM β-mercaptoethanol, L-[3H]-arginine (5 μCi·mL−1) and 1 μM L-arginine). The reaction was stopped after incubation at 37°C for 30 min, by adding buffer containing 50 mM HEPES, 10 mM EDTA and 10 mM EGTA, pH 5.5 and cooling the tubes for 5 min. The solution was mixed with 600 μL of resin Dowex AG50W-X8 (Na+ form) to remove L-arginine, and centrifuged at 10 000× g for 5 min. L-[3H]-citrulline in the supernatant was quantified by liquid scintillation counting. Non-enzymic conversion of L-[3H]-arginine to L-[3H]-citrulline was tested by adding buffer instead of the enzyme source.
Assay for TNF-α assessment
Retinal TNF-α levels were assessed as previously described (Salido et al., 2013). Retinas were homogenized in buffer containing 20 mM imidazole hydrochloride, 100 mM KCl, 1 mM MgCl2, 1 mM EGTA, 1% Triton, 1 mM sodium molybdynate, 10 mM NaF, 1 mM EDTA and a cocktail of protease inhibitors. Homogenates were centrifuged for 10 min at 13 000 rpm, and TNF-α levels were assessed in the supernatant using an elisa kit [TNF (Mono/Mono) elisa set of BD Biosciences Pharmingen, San Diego, CA, USA], according to the manufacturer's instructions, and measured in an elisa reader at 450 nm.
Data analysis
Data are shown as means SEM. Results were analysed with two-way anova followed by a Tukey's test, or by a Mann–Whitney U-test, as shown.
Materials
LPS, melatonin, BSA, PGE2, PGF2α, arginine, anti-GFAP antibody and MDA were obtained from Sigma Chemical Co. [3H]-PGE2, [3H]-PGF2α and L-[3H]-arginine were purchased from New England Nuclear Corp. (Boston, MA, USA), while Dowex was from Bio-Rad Laboratories (Richmond, CA, USA).
Results
Clinical score
The time course (24–72 h) of the clinical severity scores in eyes injected with vehicle or LPS from animals untreated or treated with melatonin is shown in Figure 1. At 36, 48 and 72 h (but not 24) after the injection of LPS, both treatments with melatonin (Mel 12 h and Mel 24 h) significantly decreased the average clinical score induced by LPS, in a similar manner. In eyes intravitreally injected with vehicle from animals untreated with melatonin (control), no significant differences in the clinical score were observed along the study. In vehicle-injected eyes, melatonin did not affect the clinical score (data not shown). Representative images of Syrian hamster eyes submitted to these treatments are shown in the lower panel of Figure 1. At 36 h after the intravitreal injection of LPS, signs of uveitis, such as episcleral vessel congestion, miosis, iris swelling and synequiae, were observed in animals untreated with melatonin, whereas both treatments with melatonin decreased the occurrence of inflammatory signs.
Figure 1.
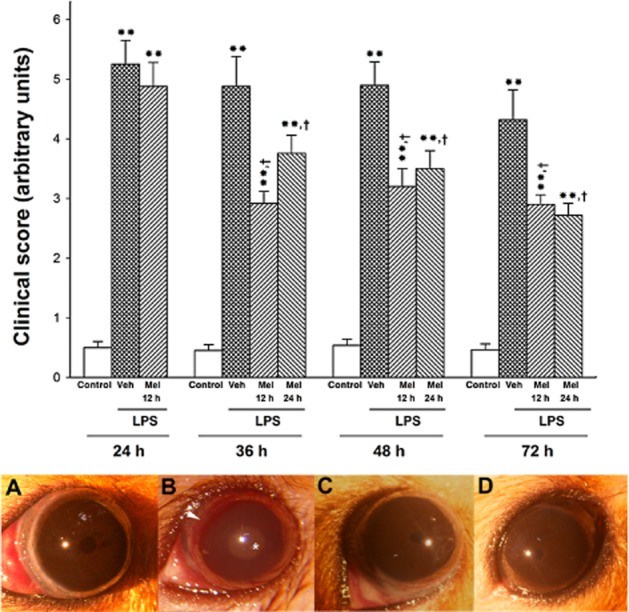
Upper panel: average clinical score of hamster eyes injected with vehicle (control) or LPS from animals untreated (vehicle) or treated with melatonin (Mel 12 or Mel 24 h). At 24, 36, 48 and 72 h after the injection of LPS, the clinical score was significantly increased. Both treatments with melatonin (starting at 12 or 24 h post-injection) significantly decreased the clinical score at 36, 48 and 72 h (but not 24) post-LPS. Data are mean ± SEM (n = 14 eyes per group). **P < 0.01 versus vehicle-injected eyes; †P < 0.01 versus LPS-injected eyes, by Mann–Whitney U-test. Lower panel: representative photographs of clinical signs observed at 36 h after the intravitreal injections, in eyes submitted to the following treatments: control (A), LPS (B), LPS + Mel 12 h (C) and LPS + Mel 24 h (D). Note episcleral congestion (arrow), synechiae and cataract (*) in one eye injected with LPS from an animal untreated with melatonin. In eyes from animals treated with melatonin (Mel 12 h and Mel 24 h), the occurrence of inflammatory signs was lower. Note the miosis in the animal receiving Mel 12 or Mel 24 h.
Integrity of the BOB and PG level assessment
The integrity of the BOB was examined by the assessment of cell count and protein concentration in the aqueous humour at 36 h after the injection of vehicle or LPS. LPS induced a significant increase in these variables (Figure 2A and B, respectively), whereas both treatments with melatonin significantly reduced cell number and protein content in aqueous humour from hamster eyes injected with LPS, with a similar efficacy. Both treatments with melatonin significantly reduced the increase in PGE2 and PGF2α levels in aqueous humour induced by LPS, as shown in Figure 3A and B respectively.
Figure 2.
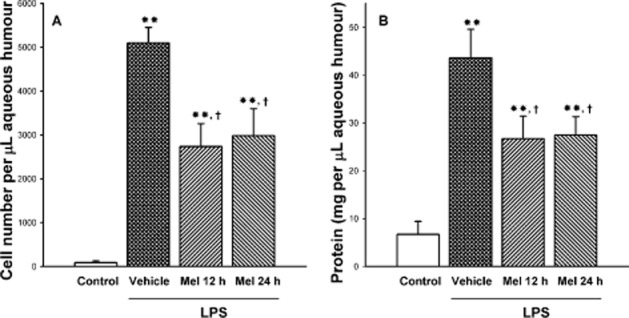
Effect of LPS on cell number and protein concentration in aqueous humour at 36 h after intravitreal injection of vehicle or LPS. LPS induced and increased in both parameters, whereas both treatments with melatonin significantly reduced the increase in cell number (panel A) and protein concentration (panel B) in the aqueous humour induced by LPS. Data are mean ± SEM (n = 12 eyes per group). **P < 0.01 versus vehicle-injected eyes; †P < 0.01 versus LPS-injected eyes, by Tukey's test.
Figure 3.
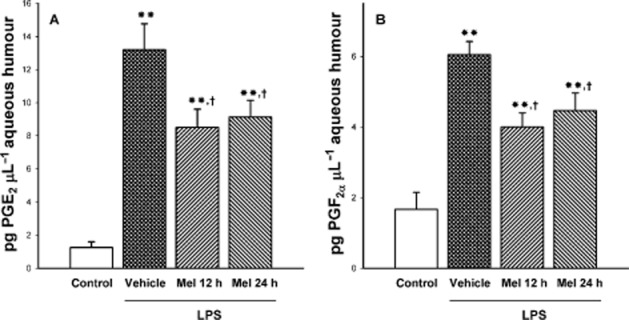
Effect of treatment after onset of uveitis with melatonin on PGE2 and PGF2α levels in the aqueous humour from eyes injected with vehicle or LPS. At 36 h post-injection, LPS induced a significant increase in PG levels, whereas both treatments with melatonin (i.e. Mel 12 h and Mel 24 h) significantly decreased PGE2 and PGF2α content. Data are mean ± SEM (n = 12 eyes per group). **P < 0.01 versus vehicle-injected eyes; †P < 0.01 versus LPS-injected eyes, by Tukey's test.
Retinal function
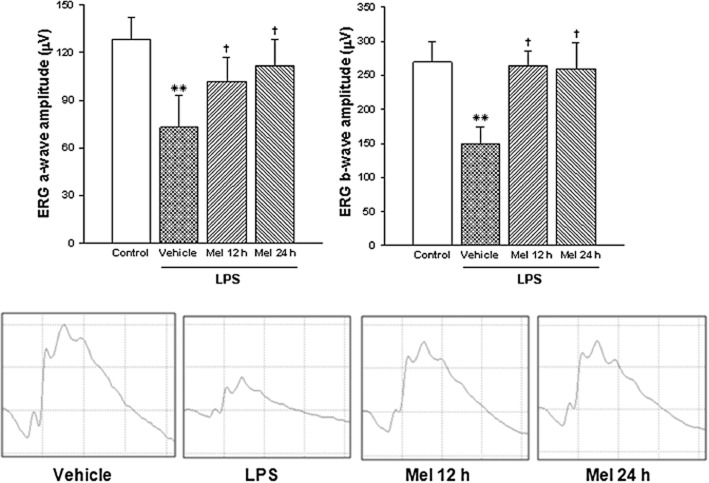
The average amplitudes of scotopic ERG a- and b-waves, as well as representative scotopic ERG traces from Syrian hamster eyes, are shown in Figure 4. At 8 days after the injection, LPS decreased scotopic ERG a- and b-wave amplitude, whereas both treatments with melatonin significantly reversed the effect of LPS. The ERG a- and b-wave latency did not differ among groups, as shown in Table 1.
Figure 4.
Upper panel: ERG a- and b-wave amplitude in hamster eyes injected with vehicle or LPS from animals untreated or treated with melatonin at day 8 after intravitreal injections. LPS induced a significant decrease in the scotopic ERG a- and b-wave amplitude, whereas both treatments with melatonin (starting at 12 or 24 h post-intravitreal injections) blocked the effect of LPS. Data are mean ± SEM (n = 10 eyes per group). **P < 0.01 versus control; †P < 0.01 versus LPS, by Tukey's test. Lower panel: representative scotopic ERG traces.
Table 1.
Effect of LPS and melatonin on scotopic ERG a-wave and b-wave latency Average latency (ms)
| Treatment | a-wave | b-wave |
|---|---|---|
| Control | 24.4 ± 0.1 | 54.9 ± 0.5 |
| LPS | 23.8 ± 0.2 | 56.3 ± 0.7 |
| LPS + Mel 12 h | 24.8 ± 0.2 | 52.7 ± 0.3 |
| LPS + Mel 24 h | 27.0 ± 0.3 | 55.1 ± 0.4 |
Scotopic ERG a- and b-wave latency in hamster eyes injected with vehicle or LPS from animals untreated or treated with melatonin at day 8 after intravitreal injections. No significant differences in these parameters were observed among groups. Data are mean ± SEM (n = 10 eyes per group).
Retinal histology
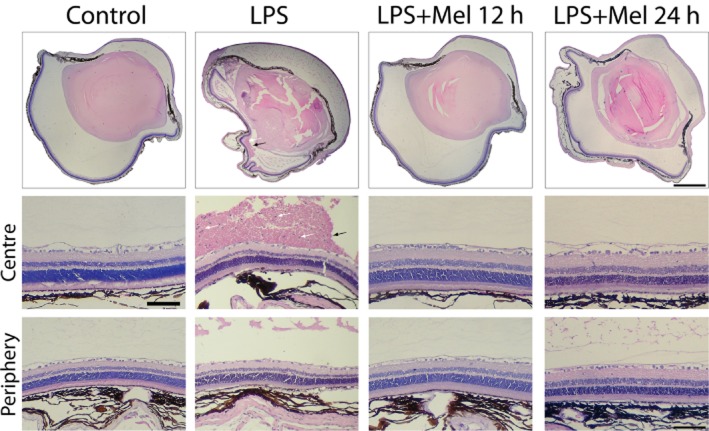
Figure 5 shows the histological analysis performed at 8 days after the intravitreal injection of LPS. Retinal cell infiltration, haemorrhage and structural disorganization were observed in animals untreated with melatonin, as shown in Figure 5, whereas the daily treatments with melatonin starting 12 or 24 h after the injection of LPS preserved the retinal structure and decreased cell infiltration to a similar extent. In the absence of melatonin, LPS induced a decrease in retinal layer thickness at the centre and periphery, whereas both treatments with melatonin (Mel 12 h and Mel 24 h) decreased the effect of LPS on these values, as shown in Table 2. Retinal immunoreactivity for GFAP was analysed at 8 days after the intravitreal injection of vehicle or LPS in animals untreated or treated with melatonin. The injection of LPS induced an increase in retinal GFAP levels that was reduced by both treatments with melatonin (Figure 6).
Figure 5.
Upper panel: representative photomicrographs of transverse sections of eyes injected with LPS in the absence or presence of melatonin. At 8 days after the intravitreal injection, LPS induced vitreal haemorrhage (black arrow), inflammatory cell infiltration (white arrow) and retinal alterations. Middle and lower panel: photomicrographs of central and peripheral retina. LPS induced marked structural alterations at both eccentricities. Note retinal haemorrhage (black arrow) and inflammatory cell infiltration (white arrow). Both treatments with melatonin preserved the retinal structure and reduced haemorrhage and cell infiltration. H&E staining. Scale bar: 600 μm (upper panel) and 100 μm (middle and lower panels).
Table 2.
Histological analysis of retinas in the absence or presence of LPS
| PS | ONL | OPL | INL | IPL | Cell number in GCL/200 μm | ||
|---|---|---|---|---|---|---|---|
| Control | Centre | 24.1 ± 0.9 | 34.6 ± 0.7 | 11.3 ± 1.4 | 17.4 ± 2.6 | 34.5 ± 1.2 | 15.3 ± 0.3 |
| Periphery | 22.4 ± 1.2 | 26.5 ± 0.2 | 9.4 ± 0.6 | 10.8 ± 1.5 | 19.0 ± 0.9 | 9.0 ± 0.4 | |
| LPS | Centre | 14.3 ± 0.6** | 26.1 ± 0.9** | 6.4 ± 0.9* | 9.6 ± 0.5* | 36.0 ± 0.6** | 8.3 ± 0.5** |
| Periphery | 7.3 ± 0.8** | 15.8 ± 0.5* | 6.8 ± 1.7 | 6.8 ± 0.8 | 9.2 ± 0.3** | 5.3 ± 0.7** | |
| LPS + Mel 12 h | Centre | 21.49 ± 1.3†† | 28.5 ± 1.8** | 8.5 ± 0.47 | 14.9 ± 1.2 | 32.8 ± 2.6†† | 13.3 ± 1.1†† |
| Periphery | 19.7 ± 2.2†† | 25.6 ± 1.1† | 9.6 ± 0.5 | 11.8 ± 1.7 | 22.5 ± 2.7†† | 8.7 ± 0.3†† | |
| LPS + Mel 24 h | Centre | 22.3 ± 0.9†† | 28.3 ± 0.3** | 7.9 ± 0.8 | 20.1 ± 1.4†† | 34.6 ± 1.2†† | 13.7 ± 0.9†† |
| Periphery | 23.2 ± 1.7†† | 25.7 ± 4.22† | 6.9 ± 0.9 | 10.3 ± 1.9 | 22.8 ± 1.9†† | 9.3 ± 0.9†† | |
Retinal layer thicknesses (in μm) from eyes injected with vehicle or LPS in the presence or absence of melatonin. At 8 days post-injection, the intravitreal injection of LPS induced a significant decrease in all retinal layer thickness in the central retina, and in most of retinal layer thickness at the periphery, whereas both treatments with melatonin (Mel 12 h and Mel 24 h) significantly reduced the effect of LPS on most of these parameters. A similar profile was observed for GCL cell number. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PS, photoreceptor outer and inner segment. Data are mean ± SEM (n = 5 eyes per group).
P < 0.05,
P < 0.01 versus vehicle-injected eyes;
P < 0.01,
P < 0.05, versus LPS-injected eyes in the absence of melatonin, by Tukey's test.
Figure 6.
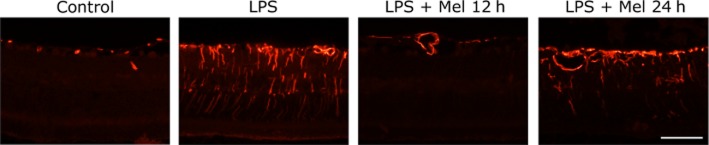
Effect of LPS in the absence or presence of melatonin on retinal GFAP immunoreactivity. At 8 days after the injection, LPS increased GFAP (+) immunoreactivity in Müller cell bodies and processes, whereas both treatments with melatonin reduced GFAP immunoreactivity, showing only few positive Müller cells in the Mel 24 h group. Shown are photomicrographs representative of four animals per group. Scale bar: 50 μm.
Lipid peroxidation, NOS activity and TNF-α levels
Retinal lipid peroxidation, NOS activity and TNF-α levels were assessed at 36 h after vehicle or LPS injection in animals untreated or treated with melatonin. LPS increased these variables, whereas both treatments with melatonin inhibited the effect of LPS on retinal TBARS levels (Figure 7A), NOS activity (Figure 7B) and TNF-α levels (Figure 7C).
Figure 7.
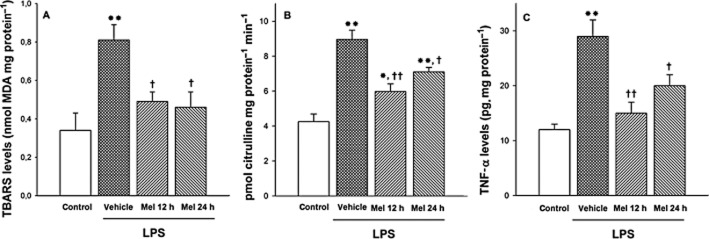
Retinal TBARS, NOS activity and TNF-α levels at 36 h after intravitreal injection of vehicle or LPS. LPS significantly increased lipid peroxidation (panel A), NOS activity (panel B) and TNF-α levels (panel C), whereas both treatments with melatonin decreased the effect of LPS on these parameters. Data are mean ± SEM (n = 10 eyes per group). **P < 0.01, *P < 0.05 versus vehicle-injected eyes; ††P < 0.01, †P < 0.05, versus LPS-injected eyes, by Tukey's test.
Discussion
The present results indicated that daily treatment with melatonin after the intravitreal injection of LPS attenuated ocular inflammation in Syrian hamsters. Although more studies are needed to establish the optimal dose of melatonin, a daily treatment with 10 mg·kg−1 melatonin, starting 12 or 24 h after LPS significantly reduced anterior segment (clinical signs, inflammatory cells, protein concentration and PG levels in aqueous humour), and posterior segment alterations (ERG, retinal structure, Müller cell GFAP levels, retinal lipid peroxidation, NOS activity and TNF-α levels). Further work will be performed in order to analyse the dose–response relationship for the ocular anti-inflammatory effect of melatonin. In agreement with previous reports (Sande et al., 2008; Del Sole et al., 2012), the present results confirm the action of melatonin as an ocular anti-inflammatory agent, in this case, by showing that, even when administered after the challenge with LPS, melatonin was effective in counteracting the uveitis induced by LPS. These findings are entirely consistent with the fact that melatonin is also a potential therapeutic agent in neurological diseases, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and ischaemic stroke, that have an inflammatory pathogenesis (see Wang, 2009).
Melatonin was administered under two different daily regimens: one starting at an early phase (i.e. at 12 h post-LPS), a time point at which clinical signs of inflammation were incipient, and the other one starting at a late phase (i.e. at 24 h after LPS), at which a maximal clinical score was already evident. Both treatments with melatonin significantly reduced the clinical signs, and preserved the integrity of the BOB (as shown by its effect on protein concentration and the number of infiltrated cells in the aqueous humour). PGs are inflammatory mediators that induce breakdown of the BOB during EIU. In fact, COX-2 inhibitors have been reported to inhibit inflammatory reactions in various animal models of ocular anterior segment inflammation (Kulkarni, 1991; Barañano et al., 2009). The present results suggest that the effect of melatonin on the anterior segment inflammation and on preservation of BOB integrity, could be mediated by blocking the LPS-induced increase of PGs in aqueous humour. In agreement, several reports support that melatonin inhibits COX-2 activity and expression (Franchi et al., 1987; Lim et al., 2012). In addition to its effect on the anterior segment inflammatory process, melatonin also protected the function and histology of the retina from the deleterious effect of LPS-induced inflammation. Müller cells that do not express GFAP under physiological conditions are known to express GFAP in several pathological situations, such as retinal ischaemia and diabetic retinopathy (Dorfman et al., 2013; Salido et al., 2013). The present results indicate that the injection of LPS provoked a significant alteration in retinal Müller cells, as shown by an increase in GFAP immunoreactivity that was reduced by both treatments with melatonin.
Electroretinography is one of the most reliable methods for objectively evaluating retinal function. As shown herein, both treatments with melatonin avoided the decrease in scotopic ERG a- and b-wave amplitude observed at 8 days post-injection of LPS. Because the a-wave of the flash ERG is classically thought to represent photoreceptor activity, whereas the b-wave reflects inner retinal (bipolar and Müller cells) functions, the protection induced by melatonin could be a panretinal phenomenon. In fact, both treatments with melatonin preserved the retinal structure and reduced GFAP up-regulation in Müller cells induced by LPS in the Syrian hamster retina.
Oxidative stress, increased NO and cytokine production induced by LPS are major triggering factors for ocular inflammation and tissue damage during inflammatory processes (Wang et al., 1996; Yadav et al., 2011). Accordingly, antioxidants (Sasaki et al., 2009), selective iNOS inhibitors (Goureau et al., 1995) and etanercept (a soluble TNF-α receptor) (Avunduk et al., 2004) protect visual function during retinal inflammation. Melatonin is one of the most powerful endogenous free radical scavengers (Galano et al., 2011) and stimulates antioxidant enzymes in several tissues including the retina (Rodriguez et al., 2004; Rosenstein et al., 2010). In addition to the potent antioxidant property of melatonin by itself, some of its metabolites are themselves direct free radical scavengers,thereby increasing melatonin's antioxidant capacity (Hardeland et al., 2009). We have previously shown that melatonin significantly decreases retinal NOS activity and L-arginine uptake in the Syrian hamster (Sáenz et al., 2002), and it is able to directly scavenge NO (Turjanski et al., 2001). Moreover, as shown here, melatonin decreased the effect of LPS on TNF-α, a key signal involved in inflammatory processes. Thus, the available evidence supports the possibility that manipulation of intracellular redox status using antioxidants, decreasing NO levels, inhibiting TNF-α effect or preferably the combination of these treatments could protect the retina against uveitic damage. In this context, melatonin would have promise in the management of ocular inflammation as, by itself, it exhibits antioxidant and anti-NO properties and reduces TNF-α levels, particularly at retinal level.
As shown here, delaying the start of the treatment with melatonin to 24 h after LPS resulted in a similar protection to that obtained when the treatment with melatonin started at 12 h after the injection of LPS. We do not have any clear explanation for these results; however, these observations support that melatonin could be potentially effective in patients with fully established uveitis, as it was capable of suppressing actively ongoing inflammatory responses. In contrast, in spite of the well-known efficacy of steroidal therapy in uveitis patients, dexamethasone did not ameliorate the clinical course of EIU in rats, when administered 12 or 16 h after LPS (Mangano et al., 2008).
Like melatonin, various therapeutic agents have been reported to prevent the inflammatory response in EIU. However, prescription-based use of many of these agents, such as guggulsterone (Kalariya et al., 2010), ethyl pyruvate (Kalariya et al., 2011), lutein (Jin et al., 2006) and astaxanthin (Suzuki et al., 2006) in humans for the treatment of uveitis has not materialized for various reasons. Major factors limiting their use in humans are lack of toxicity studies, lack of knowledge on safe dosage range and the potential toxicity of higher therapeutic dosage. Moreover, the conventional treatments of intraocular inflammation may have a wide range of significant side effects. Against this background, treatment with melatonin could overcome these issues, as it has been extensively studied and found to be safe for human use (Jan et al., 2000; Malow et al., 2012). The efficacy, clinical safety and low cost of melatonin could make it an ideal candidate as a pharmacological agent for the clinical treatment of uveitis.
Acknowledgments
This research was supported by grants from the University of Buenos Aires, the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and CONICET, Argentina.
Glossary
- BOB
blood–ocular barrier
- EIU
endotoxin-induced uveitis
- ERG
electroretinogram
- GFAP
glial fibrillary acidic protein
- MDA
malondialdehyde bis-dimethyl acetal
- TBARS
thiobarbituric acid reactive substances
Author contributions
P. H. S. performed intravitreal injections, clinical score, electroretinography, animal manipulation, aqueous humour measurements and made contributions to concept/design. D. D. conducted histological studies, immunohistochemistry and TNF-α assessment. D. C. F. performed histological studies. M. C. carried out aqueous humour measurements, NOS activity and lipid peroxidation. A. P. D. R. and A. M. F. were responsible for TNF-α assessment. D. M. S. administered NOS activity and lipid peroxidation. R. E. R. made contributions to concept/design, discussion of results and drafting of the manuscript. D. A. S. contributed clinical score, contributions to concept/design, discussion of results and approval of the PAPER.
Conflict of interest
None.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avunduk MC, Avunduk AM, Oztekin E, Baltaci AK, Ozyazgan Y, Mogolkoc R. Etanercept treatment in the endotoxin-induced uveitis of rats. Exp Eye Res. 2004;79:357–365. doi: 10.1016/j.exer.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Barañano DE, Kim SJ, Edelhauser HF, Durairaj C, Kompella UB, Handa JT. Efficacy and pharmacokinetics of intravitreal non-steroidal anti-inflammatory drugs for intraocular inflammation. Br J Ophthalmol. 2009;93:1387–1390. doi: 10.1136/bjo.2009.157297. [DOI] [PubMed] [Google Scholar]
- Belforte NA, Moreno MC, de Zavalía N, Sande PH, Chianelli MS, Keller Sarmiento MI, et al. Melatonin: a novel neuroprotectant for the treatment of glaucoma. J Pineal Res. 2010;48:353–364. doi: 10.1111/j.1600-079X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- Bellot JL, Palmero M, Garcia-Cabanes C, Espí R, Hariton C, Orts A. Additive effect of nitric oxide and prostaglandin-E2 synthesis inhibitors in endotoxin-induced uveitis in the rabbit. Inflamm Res. 1996;45:203–208. doi: 10.1007/BF02285162. [DOI] [PubMed] [Google Scholar]
- Chen YH, Xu DX, Wang JP, Wang H, Wei LZ, Sun MF, et al. Melatonin protects against lipopolysaccharide-induced intra-uterine fetal death and growth retardation in mice. J Pineal Res. 2006;40:40–47. doi: 10.1111/j.1600-079X.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- Commodaro AG, Peron JP, Genre J, Arslanian C, Sanches L, Muccioli C, et al. IL-10 and TGF-beta immunoregulatory cytokines rather than natural regulatory T cells are associated with the resolution phase of Vogt–Koyanagi–Harada (VKH) syndrome. Scand J Immunol. 2010;72:31–37. doi: 10.1111/j.1365-3083.2010.02401.x. [DOI] [PubMed] [Google Scholar]
- Crespo E, Macías M, Pozo D, Escames G, Martín M, Vives F, et al. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999;13:1537–1546. [PubMed] [Google Scholar]
- De Vos AF, Klaren VN, Kijlstra A. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci. 1994;35:3873–3883. [PubMed] [Google Scholar]
- Del Sole MJ, Sande PH, Fernandez DC, Sarmiento MI, Aba MA, Rosenstein RE. Therapeutic benefit of melatonin in experimental feline uveitis. J Pineal Res. 2012;52:29–37. doi: 10.1111/j.1600-079X.2011.00913.x. [DOI] [PubMed] [Google Scholar]
- Dick AD. Immune mechanisms of uveitis: insights into disease pathogenesis and treatment. Int Ophthalmol Clin. 2000;40:1–18. doi: 10.1097/00004397-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Dorfman D, Fernandez DC, Chianelli M, Miranda M, Aranda ML, Rosenstein RE. Post-ischemic environmental enrichment protects the retina from ischemic damage in adult rats. Exp Neurol. 2013;240:146–156. doi: 10.1016/j.expneurol.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–236. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- El Afrit MA, Mazlout H, Trojet S, Larguech L, Megaieth K, Belhaj S, et al. Cortisone glaucoma: epidemiological, clinical, and therapeutic study. J Fr Ophtalmol. 2007;30:49–52. doi: 10.1016/s0181-5512(07)89550-7. [DOI] [PubMed] [Google Scholar]
- Faillace MP, Cutrera R, Sarmiento MI, Rosenstein RE. Evidence for local synthesis of melatonin in golden hamster retina. Neuroreport. 1995;6:2093–2095. doi: 10.1097/00001756-199510010-00033. [DOI] [PubMed] [Google Scholar]
- Franchi AM, Gimeno MF, Cardinali DP, Vacas MI. Melatonin, 5-methoxytryptamine and some of their analogs as cyclo-oxygenase inhibitors in rat medial basal hypothalamus. Brain Res. 1987;405:384–388. doi: 10.1016/0006-8993(87)90311-8. [DOI] [PubMed] [Google Scholar]
- Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- Goureau O, Bellot J, Thillaye B, Courtois Y, de Kozak Y. Increased nitric oxide production in endotoxin-induced uveitis. Reduction of uveitis by an inhibitor of nitric oxide synthase. J Immunol. 1995;154:6518–6523. [PubMed] [Google Scholar]
- Hardeland R, Tan DX, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res. 2009;47:109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- Ilieva I, Ohgami K, Shiratori K, Koyama Y, Yoshida K, Kase S, et al. The effects of Ginkgo biloba extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2004;79:181–187. doi: 10.1016/j.exer.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon M. Clinical trials of controlled-release melatonin in children with sleep–wake cycle disorders. J Pineal Res. 2000;29:34–39. doi: 10.1034/j.1600-079x.2000.290105.x. [DOI] [PubMed] [Google Scholar]
- Jin XH, Ohgami K, Shiratori K, Suzuki Y, Hirano T, Koyama Y, et al. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2006;47:2562–2568. doi: 10.1167/iovs.05-1429. [DOI] [PubMed] [Google Scholar]
- Kalariya NM, Shoeb M, Reddy AB, Zhang M, van Kuijk FJ, Ramana KV. Prevention of endotoxin-induced uveitis in rats by plant sterol guggulsterone. Invest Ophthalmol Vis Sci. 2010;51:5105–5113. doi: 10.1167/iovs.09-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalariya NM, Reddy AB, Ansari NH, VanKuijk FJ, Ramana KV. Preventive effects of ethyl pyruvate on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2011;52:5144–5152. doi: 10.1167/iovs.10-7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Kurihara T, Mochimaru H, Satofuka S, Noda K, Ozawa Y, et al. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50:3512–3519. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- Kulkarni PS. The role of endogenous eicosanoids in rabbit-intraocular inflammation. J Ocul Pharmacol. 1991;7:227–241. [PubMed] [Google Scholar]
- Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG, Chun YH, et al. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J Pineal Res. 2012;53:225–237. doi: 10.1111/j.1600-079X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- London NJ, Rathinam SR, Cunningham ET., Jr The epidemiology of uveitis in developing countries. Int Ophthalmol Clin. 2010;50:1–17. doi: 10.1097/IIO.0b013e3181d2cc6b. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Malow B, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, et al. Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J Autism Dev Disord. 2012;42:1729–1737. doi: 10.1007/s10803-011-1418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Yoshimura N, Yoshida M, Iwaki M, Honda Y. The role of nitric oxide synthase in endotoxin-induced uveitis: effects of NG-nitro L-arginine. Invest Ophthalmol Vis Sci. 1994;35:3673–3680. [PubMed] [Google Scholar]
- Mangano K, Sardesai NY, Quattrocchi C, Mazzon E, Cuzzocrea S, Bendtzen K, et al. Effects of the immunomodulator, VGX-1027, in endotoxin-induced uveitis in Lewis rats. Br J Pharmacol. 2008;155:722–730. doi: 10.1038/bjp.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25:33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Ogura Y, Hamada M, Nishiwaki H, Hiroshiba N, Honda Y. In vivo quantification of leukocyte behavior in the retina during endotoxin induced uveitis. Invest Ophthalmol Vis Sci. 1996;37:2708–2715. [PubMed] [Google Scholar]
- Moreno MC, Campanelli J, Sande P, Sánez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Callanan D, Dugel P, Godfrey DG, Goldstein DA, Wilensky JT. Treating chronic noninfectious posterior segment uveitis: the impact of cumulative damage. Proceedings of an expert panel roundtable discussion. Retina. 2006;Suppl:1–16. doi: 10.1097/01.iae.0000250601.15893.5f. [DOI] [PubMed] [Google Scholar]
- Ortiz GG, Coto-Montes A, Bitzer-Quintero OK, Falcón-Franco MA, Ruiz-Rizo L, Bravo-Cuellar A, et al. Effects of melatonin on the Harderian gland of lipopolysaccharide-treated rats: morphological observations. Biomed Pharmacother. 1999;53:432–437. doi: 10.1016/S0753-3322(99)80123-1. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- Rosenstein RE, Pandi-Perumal SR, Srinivasan V, Spence DW, Brown GM, Cardinali DP. Melatonin as a therapeutic tool in ophthalmology: implications for glaucoma and uveitis. J Pineal Res. 2010;49:1–13. doi: 10.1111/j.1600-079X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- Sáenz DA, Turjanski AG, Sacca GB, Marti M, Doctorovich F, Sarmiento MI, et al. Physiological concentrations of melatonin inhibit the nitridergic pathway in the Syrian hamster retina. J Pineal Res. 2002;33:31–36. doi: 10.1034/j.1600-079x.2002.01880.x. [DOI] [PubMed] [Google Scholar]
- Salido EM, Bordone M, De Laurentiis A, Chianelli M, Keller Sarmiento MI, Dorfman D, et al. Therapeutic efficacy of melatonin in reducing retinal damage in an experimental model of early type 2 diabetes in rats. J Pineal Res. 2013;54:179–189. doi: 10.1111/jpi.12008. [DOI] [PubMed] [Google Scholar]
- Sand BB, Krogh E. Topical indometacin, a prostaglandin inhibitor, in acute anterior uveitis. A controlled clinical trial of non-steroid versus steroid anti-inflammatory treatment. Acta Ophthalmol (Copenh) 1991;69:145–148. doi: 10.1111/j.1755-3768.1991.tb02703.x. [DOI] [PubMed] [Google Scholar]
- Sande PH, Fernandez DC, Aldana Marcos HJ, Chianelli MS, Aisemberg J, Silberman DM, et al. Therapeutic effect of melatonin in experimental uveitis. Am J Pathol. 2008;173:1702–1713. doi: 10.2353/ajpath.2008.080518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Ozawa Y, Kurihara T, Noda K, Imamura Y, Kobayashi S, et al. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:1433–1439. doi: 10.1167/iovs.08-2493. [DOI] [PubMed] [Google Scholar]
- Shiratori K, Ohgami K, Ilieva I, Jin XH, Koyama Y, Miyashita K, et al. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2005;81:422–428. doi: 10.1016/j.exer.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Siu AW, Maldonado M, Sanchez-Hidalgo M, Tan DX, Reiter RJ. Protective effects of melatonin in experimental free radical-related ocular diseases. J Pineal Res. 2006;40:101–109. doi: 10.1111/j.1600-079X.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ohgami K, Shiratori K, Jin XH, Ilieva I, Koyama Y, et al. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp Eye Res. 2006;82:275–281. doi: 10.1016/j.exer.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski AG, Sáenz DA, Doctorovich F, Estrin DA, Rosenstein RE. Nitrosation of melatonin by nitric oxide: a computational study. J Pineal Res. 2001;31:97–101. doi: 10.1034/j.1600-079x.2001.310201.x. [DOI] [PubMed] [Google Scholar]
- Wang X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Ther. 2009;15:345–357. doi: 10.1111/j.1755-5949.2009.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Alm P, Håkanson R. The contribution of nitric oxide to endotoxin-induced ocular inflammation: interaction with sensory nerve fibres. Br J Pharmacol. 1996;118:1537–1543. doi: 10.1111/j.1476-5381.1996.tb15571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GS, Zhang J, Rao NA. Peroxynitrite and oxidative damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 1997;38:1333–1339. [PubMed] [Google Scholar]
- Yadav UC, Kalariya NM, Ramana KV. Emerging role of antioxidants in the protection of uveitis complications. Curr Med Chem. 2011;18:931–942. doi: 10.2174/092986711794927694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zavalía N, Fernandez DC, Sande PH, Keller Sarmiento MI, Golombek DA, Rosenstein RE, et al. Circadian variations of prostaglandin E2 and F2 alpha release in the golden hamster retina. J Neurochem. 2010;112:972–979. doi: 10.1111/j.1471-4159.2009.06517.x. [DOI] [PubMed] [Google Scholar]