Abstract
Background and Purpose
Impaired function of spinal strychnine-sensitive glycine receptors gives rise to chronic pain states and movement disorders. Therefore, increased activity of glycine receptors should help to treat such disorders. Although compounds targeting glycine receptors with a high selectivity are lacking, halogenated analogues of propofol have recently been considered as potential candidates. Therefore we asked whether 4-bromopropofol attenuated the excitability of spinal neurons by promoting glycine receptor-dependent inhibition.
Experimental Approach
The actions of sub-anaesthetic concentrations of propofol and 4-bromopropofol were investigated in spinal tissue cultures prepared from mice. Drug-induced alterations in action potential firing were monitored by extracellular multi-unit recordings. The effects on GABAA and glycine receptor-mediated inhibition were quantified by whole-cell voltage-clamp recordings.
Key Results
Low concentrations of 4-bromopropofol (50 nM) reduced action potential activity of ventral horn neurons by about 30%, compared with sham-treated slices. This effect was completely abolished by strychnine (1 μM). In voltage-clamped neurons, 4-bromopropofol activated glycine receptors, generating a tonic current of 65 ± 10 pA, while GABAA- and glycine receptor-mediated synaptic transmission remained unaffected.
Conclusions and Implications
The highest glycine levels in the CNS are found in the ventral horn of the spinal cord, a region mediating pain-induced motor reflexes and participating in the control of muscle tone. 4-Bromopropofol may serve as a starting point for the development of non-sedative, non-addictive, muscle relaxants and analgesics to be used to treat low back pain.
Tables of Links
| TARGETS |
|---|
| Ligand-gated ion channelsa |
| GABAA receptors |
| Glycine receptors |
| α1 subunits, glycine receptors |
| α2 subunits, glycine receptors |
| Transportersb |
| Glycine transporter 2 (GlyT2/SLC6A5) |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b,).
| LIGANDS |
|---|
| Bicuculline |
| Propofol |
| Sevoflurane |
| Strychnine |
| TTX, tetrodotoxin |
Introduction
Strychnine-sensitive glycine receptors play a central role in mediating inhibitory neurotransmission in the spinal cord (Zeilhofer, 2005; Callister and Graham, 2010; Zeilhofer et al., 2012b). Hence, it is not surprising that loss-of-function mutations in glycine receptors translate into spasticity, ataxia and hypertonia (Laube et al., 2002; Chung et al., 2010; Davies et al., 2010). Moreover, there is evidence that inhibition of spinal glycine receptors by PGE2 contributes to the genesis of inflammatory pain (Harvey et al., 2004). These observations suggest glycine receptors as promising therapeutic targets (Laube et al., 2002; Gilbert et al., 2009). Glycine and GABAA receptors are both members of the cysteine-loop family of ligand-gated ion channels, thus sharing many similarities in structure and function (Olsen and Sieghart, 2008). The GABAA receptors are known to be targeted by many anaesthetic agents, including propofol, isoflurane and sevoflurane (Belelli et al., 1999). In patients undergoing surgical interventions, these drugs are used for providing sedation, muscle relaxation and immobility (Grasshoff et al., 2006). In contrast to GABAA receptors, clinically applicable compounds that preferably act via glycine receptors remain to be identified (Gilbert et al., 2009; Yevenes and Zeilhofer, 2011). Interestingly, the i.v. anaesthetic propofol does not only activate GABAA receptors (Orser et al., 1994), but also promotes the opening of glycine receptors (Ahrens et al., 2008). However, these two types of receptor differ greatly in their sensitivity to propofol (Belelli et al., 1999). Concurrent evidence from in vitro and in vivo investigations indicates that propofol anaesthesia is predominantly based on an altered function of GABAA receptors, whereas glycine receptors are of minor importance (Rudolph and Antkowiak, 2004). Unlike propofol, the immobilizing properties of the halogenated ether anaesthetics isoflurane and sevoflurane also involve, besides GABAA receptors, glycine receptors and further molecular targets (Campagna et al., 2003). Moreover, comparative studies on the effects of isoflurane and sevoflurane on glycinergic and GABAergic synaptic transmission showed that spinal GABAA receptors and glycine receptors do not differ in their sensitivity to halogenated ether anaesthetics (Eckle and Antkowiak, 2013; Eckle et al., 2013). Along these lines it has been hypothesized that halogenation of propofol might provide compounds that bind with a higher affinity to glycine receptors and potentiate chloride currents (de la Roche et al., 2012). Therefore we explored the actions of propofol and its analogue, 4-bromopropofol, on GABAergic and glycinergic transmission in organotypic tissue slices derived from the mouse spinal cord. These investigations were carried out in the ventral horn area. The spinal ventral horn comprises pattern-generating motor networks, mediates painful stimuli-induced withdrawal reflexes and participates in the control of muscle tone (Talpalar et al., 2011; Arber, 2012). In this region, the highest glycine levels within the CNS were detected (Aprison et al., 1969; Boehme et al., 1973; Patrick et al., 1983). In the present work, propofol and 4-bromopropofol were administered at sub-anaesthetic concentrations. Characterization of drug actions at small concentrations seems to be an essential requirement for addressing the question, whether halogenated propofol analogues are potential compounds that can be administered in awake patients without causing sedation. Here we report that 4-bromopropofol reduced the discharge rate of ventral horn interneurons by selectively enhancing glycine receptor-mediated inhibition, suggesting the potential use of halogenated propofol analogues as non-sedative, non-addictive muscle relaxants and analgesics in the future.
Methods
Organotypic spinal cultures
All animal care and experimental procedures were in accordance with institutional and federal guidelines, including the German law on animal experimentation, and were approved by the Animal Care Committee of Eberhard-Karls-University (Tübingen, Germany).
Organotypic spinal cultures were prepared as first described by Braschler et al. (1989). Pregnant C57/BL6 mice (Charles River Laboratories, Sulzfeld, Germany) were anaesthetized and decapitated. Embryonic tissue (day E 14–15) was removed and placed into Gey's balanced salt solution containing (in mM) 1.5 CaCl2, 5 KCl, 0.22 KH2PO4, 11 MgCl2, 0.3 MgSO4, 137 NaCl, 0.7 NaHCO3 and 33 D-glucose (all from Sigma, Taufkirchen, Germany). Spinal columns were separated from inner organs, limbs and transversely cut into 300 μm thick slices using a microslicer (NVSLM1, World Precision Instruments, Sarasota, FL, USA). Slices were put onto coverslips and embedded in heparin-treated chicken plasma (Sigma). In a second step, these slices were fixed on the coverslips by thrombin (Sigma). Tissue slices were put into plastic tubes containing nutrient fluid (0.75 mL) and neuronal growth factor (10 nM, Sigma). Nutrient fluid (100 mL) consisted of 25 mL horse serum (Invitrogen, Karlsruhe, Germany), 25 mL Hanks' balanced salt solution (Sigma) and 50 mL Eagle's basal medium (Sigma). The roller tube technique was used to culture the tissue as first described by Gähwiler (1981). After 1 day in culture (DIC), antimitotics [5-fluoro-2-deoxyuridine (10 μM), cytosine-β-D-arabino-furanoside (10 μM), and uridine (10 μM); all from Sigma] were added to reduce proliferation of glial cells. Slices were typically used after 3 weeks in culture for extracellular recordings, and after 2 weeks in culture for whole-cell voltage-clamp recordings.
Extracellular recordings
Spontaneous action potential activity was recorded as reported previously (Grasshoff et al., 2007a). In brief, slices were perfused with artificial CSF (ACSF) consisting of (in mM) 120 NaCl, 3.5 KCl, 1.13 NaH2PO4, 1 MgCl2, 26 NaHCO3, 1.2 CaCl2 and 11 D-glucose. The ACSF was equilibrated with 95% oxygen and 5% carbon dioxide. Glass electrodes with a resistance of approximately 2–5 MΩ were filled with ACSF and introduced into the ventral horn area until single-unit or multi-unit action potential activity could be clearly identified. All experiments were performed at 34°C–36°C. Signals were bandpass-filtered (passband 200–5000 Hz) and digitized at 10 kHz via a Digidata 1200 interface and Axoscope 9.0 software (Molecular Devices, Sunnyvale, CA, USA). The recording chamber was continuously perfused with ACSF at a flow rate of 1 mL/min. After reaching stable recording conditions, 4-bromopropofol or propofol was applied for at least 12 minutes. In a subset of experiments, the effects of 4-bromopropofol and propofol were investigated in the presence of bicuculline (80 μM) or strychnine (1 μM) for selectively blocking GABAA receptors or glycine receptors respectively. For minimizing bias related to the recording procedure, sham applications were performed and were set as control conditions for analysis purposes.
Whole-cell voltage-clamp recordings
Whole-cell voltage-clamp experiments were performed on videomicroscopically identified neurons in lamina VIII of the spinal cord, which is predominantly populated by commissural interneurons (Zeilhofer et al., 2012b). These cells displayed a typical cell capacitance around 35 pF as previously reported (Eckle and Antkowiak, 2013; Eckle et al., 2013). In the recording chamber, cultures were continuously perfused with ACSF as specified earlier. 6-Cyano-7-nitroquinoxaline-2.3-dione (CNQX; 50 μM) and DL-2-amino-5-phosphonopentanoic acid (AP5, 50 μM) were added for blocking excitatory synaptic transmission. To isolate GABAA receptor-mediated currents, strychnine (1 μM) was added and for glycine receptor-mediated currents, bicuculline (80 μM), respectively, as previously reported (Eckle and Antkowiak, 2013; Eckle et al., 2013). Signals were acquired with a Multiclamp 700B patch-clamp amplifier (Molecular Devices, Foster City, CA, USA) equipped with a CV-7B headstage, low-pass-filtered at 2.2 kHz, and digitized at 10 kHz via a Digidata 1440 A interface and Clampex 10.1 (Molecular Devices). Patch pipettes were pulled from thin-wall borosilicate capillaries (World Precision Instruments). Pipette resistances ranged between 3 and 5 MΩ. The pipette solution contained (in mM) 121 CsCl, 24 CsOH, 10 HEPES, 5 EGTA, 1 MgCl2 and 2 ATP, adjusted to pH 7.2 with 1 M HCl (all from Sigma). Cells displayed typically a capacitance of 35 pF, (Eckle and Antkowiak, 2013; Eckle et al., 2013) and were clamped at a holding potential of −70 mV. To record tonic glycinergic currents, tetrodotoxin (TTX, 1 μM) was added for blocking action potential-driven synaptic transmission. The change in holding current upon drug treatment was defined as tonic conductance (Eckle and Antkowiak, 2013).
mRNA expression of glycine receptor α1 and α2 subunits
Spinal ventral horn tissue (n = 5) from embryonic, adult mice and organotypic spinal slices after 10 and 20 days DIC was homogenized, RNA isolated and transcribed into cDNA (Köhler et al., 2014). Semiquantitative analysis of mRNA was performed using real-time PCR (iCycler; Bio-Rad Laboratories GmbH, München, Germany) to detect glycine receptor α1 and α2 mRNA levels. The following primers were used: forward primer for glycine receptor α1 5′-TCACAAGAGCCCCATGCTAA-3′, and 5′-CATGGGGAAACCGATGCGAG-3′ as reverse primer; forward primer for glycine receptor α2 5′–TGTGTTTGCTGCCTTACTGG-3′; and 5′–GACAGCTGTGCCATCTTTCA-3′ as reverse primer. Samples were normalized to the 18s housekeeping gene using sense 5′-GTAACCCGTTGAACCCCA-3′ and antisense 5′-CCATCCAATCGGTAGTAGCG-3′ primers. For each data point, two replicates were performed.
Data analysis
Data were analysed with in-house software written in OriginPro version 7 (OriginLab Corp., Northampton, MA, USA) and MATLAB version 6.5 (The MathWorks, Inc., Natick, MA, USA). Inhibitory postsynaptic current (IPSC) decays were fitted with mono-exponential functions. Data analysis of extracellular recordings was performed as described previously (Grasshoff et al., 2007a). After close inspection of the raw data, action potentials were detected by setting a threshold well above baseline noise. The mean firing rate was obtained from single- or multi-unit activity. The spontaneous action potential activity was normalized to control conditions.
Data are presented as mean ± SEM. Comparative statistics were performed using a t-test or one-way anova combined with a Bonferroni's multiple comparison post hoc analysis, as appropriate. Data are presented as mean ± S.E.M. All point histograms were plotted and fitted to a Gaussian curve as described elsewhere (Takazawa and MacDermott, 2010). The mean of the fitted Gaussian curve was set as the mean holding current. The SD of the holding current was considered as current noise.
Materials
4-Bromopropofol and 4-chloropropofol was kindly provided by Paul M O'Neill and Neil Berry (University of Liverpool, UK) as a light-protected 1 M solution in ethanol and stored at −20°C. 4-Bromopropofol, 4-chloropropofol and propofol (Fresenius Kabi, Bad Homburg, Germany) were dissolved in distilled water to a 1 mM stock solution. The 1 mM stock solution was used for preparation of the final test solutions in ACSF. All other chemicals were obtained from Sigma (Taufkirchen, Germany).
Clinically relevant propofol concentrations at the spinal cord are reported between 1.0 and 1.5 μM (Franks and Lieb, 1998; Rehberg and Duch, 1999). Thus, sub-anaesthetic propofol concentrations (50, 200 nM) were investigated here. Under stable recording conditions, 4-bromopropofol, 4-chloropropofol or propofol was applied for at least 12 minutes via bath perfusion using a gastight syringe pump system (ZAK Medicine Technique, Marktheidenfeld, Germany), which was connected to the recording chamber via Teflon tubing (Lee, Sulzbach/Taunus, Germany). The sham conditions consisted of additions of ACSF only. The calibration of the recording system was performed as previously reported (Antkowiak and Heck, 1997).
Results
4-bromopropofol inhibits action potential generation in ventral horn neurons
Motor networks in the ventral horn of the spinal cord are activated by nociceptive stimulation. In the absence of anaesthetic agents, this activity gives rise to movements. There is mounting evidence that anaesthetics cause muscle relaxation and immobility by depressing the excitability of ventral horn neurons (Antognini and Carstens, 1999; Kim et al., 2007). These cells express GABAA and glycine receptors. Accordingly, drugs acting as agonists or positive modulators at one or both of these receptors are expected to attenuate neuronal excitability in the ventral horn. A recent study reported that, at sub-anaesthetic concentrations, the halogenated propofol analogue 4-chloropropofol enhanced currents across glycine receptors expressed in HEK cells (de la Roche et al., 2012). This observation prompted us to hypothesize that halogenated propofol analogues should depress action potential activity in spinal motor networks. In order to test this idea, we explored the effects of 4-bromopropofol in cultured slices prepared from the mouse spinal cord. The chemical structure of 4-bromopropofol differs from propofol by the presence of a bromide substituent in the para-position to the hydroxyl group (Figure 1A). In Figure 1B, a typical recording is presented. Action potential activity was monitored in the ventral horn area by an extracellular electrode. The neuron under observation spontaneously generated action potentials at a rate of 1.6 Hz. After exposing this cell to 4-bromopropofol, its discharge rate dropped to 1.1 Hz. On average, 4-bromopropofol (50 nM) reduced the firing rates of ventral horn interneurons by about 30% (n = 31). In comparison with the sham group, this effect was statistically significant (P < 0.01, tested by anova). Interestingly, 4-chloropropofol (50 nM) did not reduce the spontaneous firing rate (sham: 90 ± 5%, n = 57; 4-chloropropofol: 90 ± 4%, n = 92).
Figure 1.
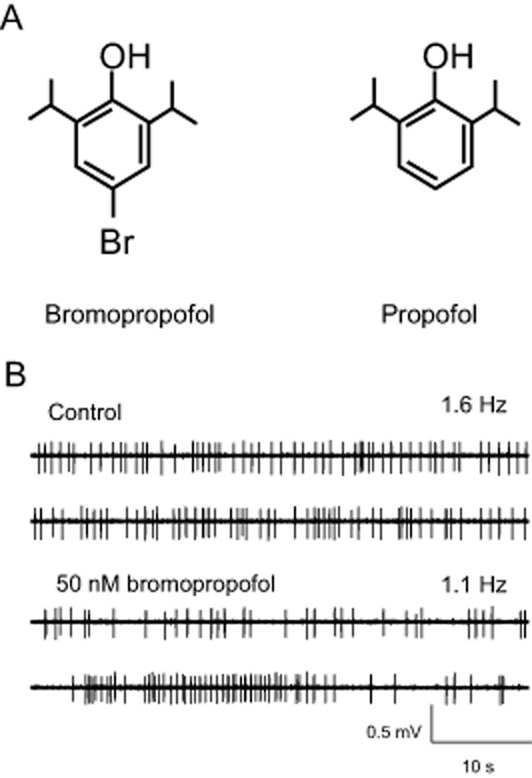
Representative recording of the effect of 4-bromopropofol (50 nM) on spontaneous action potential firing of ventral horn neurons. (A) Chemical structures of 4-bromopropofol and propofol differ with respect to the presence of a bromide atom in para-position to the hydroxyl group. (B) Action potentials, visible as the vertical deflections in the voltage traces, were monitored with an extracellular electrode. Under drug-free conditions the neuron generated action potentials at a frequency of 1.6 Hz. Application of 4-bromopropofol reduced the discharge frequency to 1.1 Hz.
4-bromopropofol acts via glycine receptors, not GABAA receptors
In further experiments, we quantified the effects of the anaesthetic propofol on the discharge rates of ventral horn interneurons (Figure 2). At 50 nM, propofol (n = 30) failed to depress action potential firing suggesting that the decreased activity of spinal neurons following 4-bromopropofol was critically related to the presence of the bromide atom.
Figure 2.
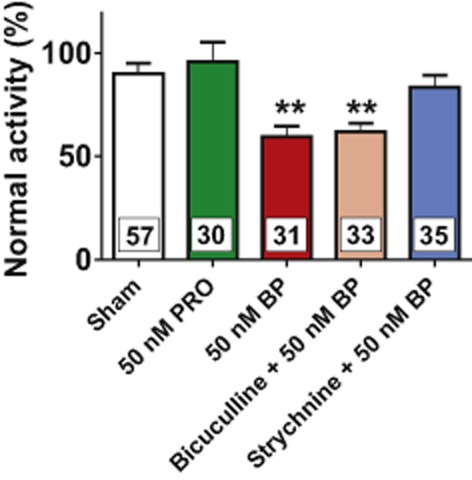
4-bromopropofol, but not propofol, significantly decreased the discharge rate of ventral horn neurons by opening glycine receptors. In each individual experiment, action potential activity was normalized to the firing rate observed under drug-free control conditions. A normalized activity of 100% indicates that the tested drugs did not alter the discharge rate as compared with the pre-drug condition. A normalized activity of 0% stands for the total depression of action potential firing. The graph shows that 50 nM propofol (PRO; n = 30) did not decrease action potential firing as compared with sham-treated slices (n = 57). Contrastingly, 4-bromopropofol (BP) reduced neuronal activity by about 30% (n = 31). 4-bromopropofol was similarly effective in the presence and absence of the specific GABAA receptor antagonist bicuculline (80 μM, n = 33), indicating that 4-bromopropofol did not act via GABAA receptors. In the presence of the specific glycine antagonist strychnine (1 μM, n = 35), 4-bromopropofol failed to reduce action potential firing, indicating that the drug acted via glycine receptors. **P < 0.01, significantly different from sham; anova. Numbers of experiments are shown as insets in the columns.
Next, classical antagonism experiments were undertaken in order to identify the molecular targets by which 4-bromopropofol attenuates the excitability of ventral horn neurons. Our basic assumption was that glycine receptors or GABAA receptors did not mediate the effect of 4-bromopropofol on neuronal firing rates if this agent was equally effective in the presence and absence of the corresponding receptor antagonist, strychnine or bicuculline respectively. We first considered the possibility that 4-bromopropofol reduced neuronal excitability by enhancing the function of GABAA receptors. However, these studies revealed that the drug was equally effective in the absence and presence of bicuculline (n = 33), prompting the conclusion that GABAA receptors were not involved. In contrast to bicuculline, strychnine completely abolished the effects of 4-bromopropofol on action potential firing of ventral horn neurons, indicating that the drug predominantly acted via glycine receptors (n = 35).
4-bromopropofol does not alter synaptically mediated inhibitory currents
In a following step, we characterized the interactions between 4-bromopropofol and glycine receptors in greater detail, making use of whole-cell voltage-clamp recordings. We previously showed that the halogenated ether derivative sevoflurane potentiated glycinergic synaptic inhibition of ventral horn interneurons by increasing the decay time and frequency of IPSCs (Eckle and Antkowiak, 2013; Eckle et al., 2013), raising the question whether 4-bromopropofol acts by a similar mechanism. Remarkably, 4-bromopropofol neither altered the decay time, nor the frequency nor the amplitude of glycinergic IPSCs as compared with sham-treated slices (Figures 3A and 4A). Similar to 4-bromopropofol, propofol was not effective in modulating the decay time and amplitude of glycine receptor-mediated synaptic currents (Figures 3A and 4A). However, in our experiments, the anaesthetic did reduce the frequency of events.
Figure 3.
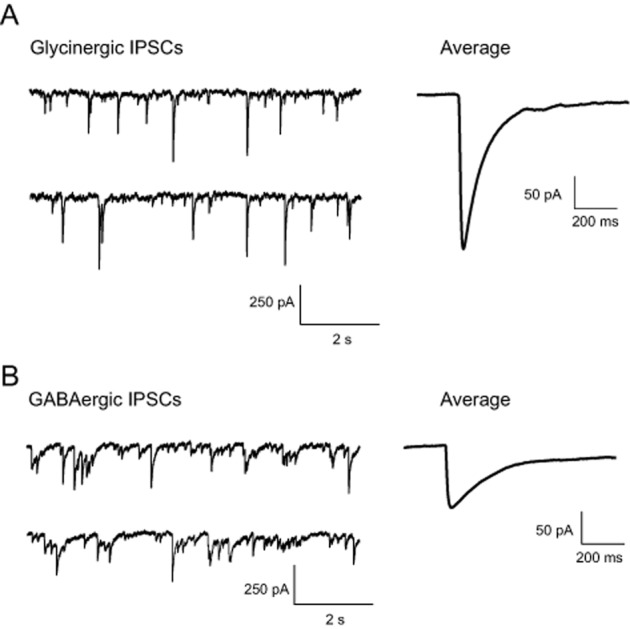
Typical recording of inhibitory postsynaptic currents mediated via glycine receptors (A) or GABAA receptors (B). On the right-hand side, averaged synaptic events are displayed. GABAergic synaptic events (amplitude: 62 ± 5 pA, n = 30; decay time: 25 ± 7 ms, n = 30; frequency: 14 ± 2 Hz, n = 23) had a smaller amplitude and slower decay time as compared with glycinergic synaptic currents (amplitude: 117 ± 14 pA, n = 32; decay time: 11 ± 1 ms, n = 32; frequency: 18 ± 3 Hz, n = 26).
Figure 4.

Effects of propofol and 4-bromopropofol on glycine receptor- and GABAA receptor-mediated synaptic events. (A) The frequency, decay time and amplitude of glycine receptor-mediated synaptic currents were not significantly altered by 4-bromopropofol (BP; tested by anova). However, propofol (PRO) decreased the amplitude of glycinergic currents. The latter action may explain that propofol slightly increased action potential firing of ventral horn neurons as indicated in Figure 2. (B) Neither 4-bromopropofol nor propofol altered the frequency, decay time and amplitude of GABAA receptor-mediated events (tested by anova). Numbers of experiments are shown as insets in the columns.
The observation that the action of 4-bromopropofol in decreasing action potential firing of ventral horn interneurons was not diminished by the selective GABAA receptor antagonist bicuculline already suggested that the drug did not modulate GABAergic transmission (Figure 2). To confirm this conclusion, GABAA receptor-mediated synaptic currents were quantified in the absence and presence of 4-bromopropofol. The results displayed in Figures 3B and 4B demonstrate that, consistent with our expectation, 4-bromopropofol did not alter GABAA receptor-mediated synaptic currents. The same result was obtained with propofol. In summary, we found that neither 4-bromopropofol nor propofol was effective in modulating glycinergic or GABAergic synaptic transmission of ventral horn neurons when administered in the nanomolar concentration range.
4-bromopropofol but not propofol modulates a tonic glycinergic current
Besides synaptic-mediated inhibition, tonic inhibition has been shown to regulate the inhibitory tone in several regions of the CNS (Mody and Pearce, 2004; Muller et al., 2008; Takazawa and MacDermott, 2010). Therefore, it seemed possible that 4-bromopropofol either induced or enhanced a tonic current via activating glycine receptors, thereby attenuating the action potential activity of ventral horn interneurons. To clarify this point, the effects of 4-bromopropofol and propofol on strychnine-sensitive tonic currents were evaluated. At the onset of these recordings, glutamatergic synaptic transmission was blocked. Thus, synaptic events that can be seen at the beginning of the trace displayed in Figure 5A, visible as large downward deflections, were mediated by GABAA receptors and glycine receptors. Consistent with our previous findings, synaptic activity dramatically decreased after the action potential activity was blocked by TTX (Grasshoff et al., 2007b). Furthermore, bicuculline was added to the bathing solution to block GABAA receptor-mediated chloride currents. When the neuron was exposed to 4-bromopropofol with TTX and bicuculline still present, a large tonic current of about 60 pA was induced and baseline noise increased (Figures 5B and 5C). To test the hypothesis that this current was carried by glycine receptors, the antagonist strychnine was administered (Figure 6). We compared strychnine-induced changes in the tonic current as observed in sham-treated preparations with those changes evoked in the presence of 4-bromopropofol or propofol. In sham-treated slices, application of strychnine to the bathing solution reduced the tonic current by only 22 ± 5 pA (n = 6). Almost the same effect of strychnine was evident in slices treated with propofol (20 ± 10 pA, n = 8), indicating that this anaesthetic was unable to activate glycine receptors at a sub-anaesthetic concentration of 200 nM. However, in the presence of 4-bromopropofol, strychnine on average altered the baseline current by 65 ± 10 pA (n = 7). This current was significantly different from the effects of strychnine in sham-treated slices and in slices exposed to propofol (P < 0.01 by anova). These findings provide evidence that 4-bromopropofol either opened glycine receptors or potentiated glycine-induced activation of glycine receptors.
Figure 5.
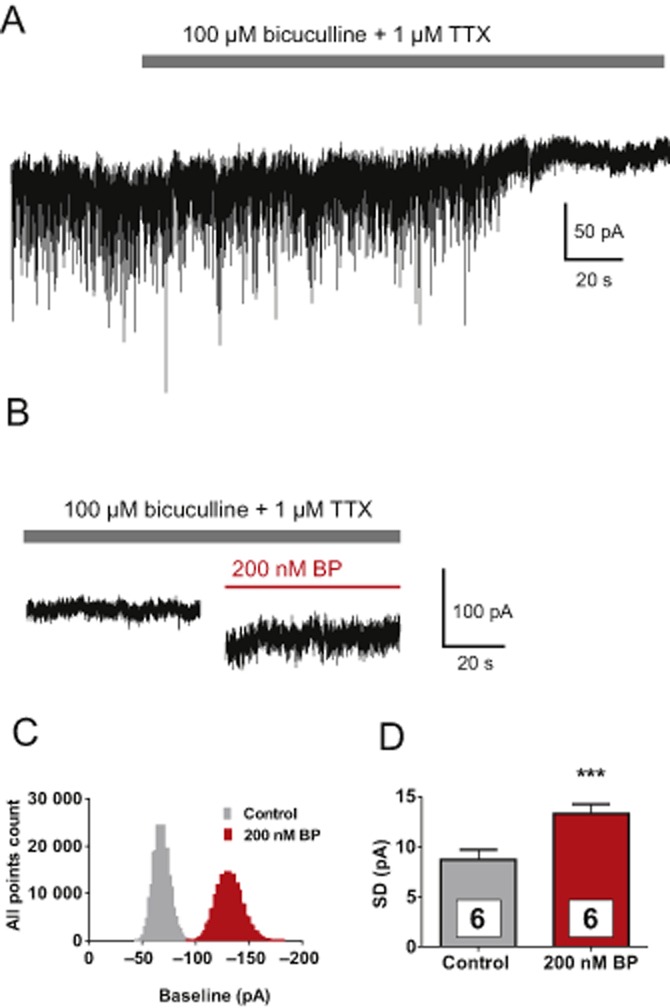
4-bromopropofol induced a tonic current and increased current fluctuations in voltage-clamped ventral horn neurons. (A) During a whole-cell voltage-clamp recording, the membrane potential was clamped to −70 mV. Action potential-dependent synaptic events were abolished by adding TTX (1 μM) to the perfusate. In addition, GABAA receptor-dependent conductance was blocked by bicuculline (80 μM). (B) With TTX and bicuculline still present, application of 4-bromopropofol (BP) caused a large negative shift in the baseline current and increased the noise level. (C) The corresponding all point histograms of the currents indicate that the baseline was shifted to more negative values by 4-bromopropofol. (D) The distribution of the baseline fluctuations (membrane noise) as observed before and after exposing the cells to 4-bromopropofol was well fitted with a Gaussian function. 4-bromopropofol significantly increased the SD of the fitted curves [control 8.7 ± 0.96 pA (n = 6) vs. 4-bromopropofol (n = 6) 13.3 ± 0.91 pA. ***P < 0.001, significantly different from control; t-test].
Figure 6.
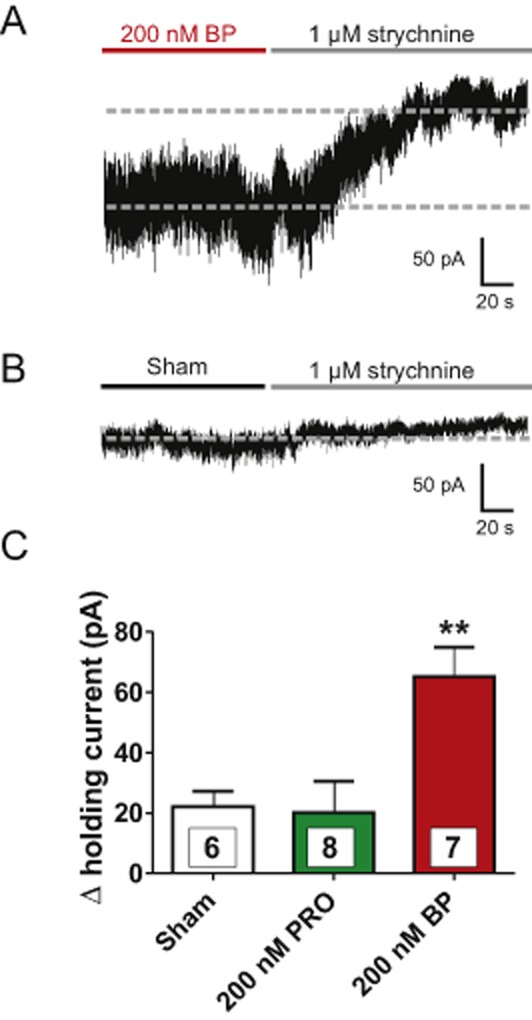
4-bromopropofol, but not propofol induced a large strychnine-sensitive tonic conductance. (A) A typical recording demonstrating the change in tonic current caused by strychnine upon 4-bromopropofol (BP) treatment. Note that strychnine not only shifted the baseline of the holding current, but also decreased the current noise. (B) In sham-treated slices, the strychnine-induced change in tonic current was small as compared with the change observed with 4-bromopropofol. (C) Quantification of the effects of strychnine in sham-treated slices and in slices treated either with propofol (PRO) or 4-bromopropofol. Note that the changes observed in sham-treated slices and in propofol-treated slices were almost identical, indicating that propofol did not alter the strychnine-sensitive conductance. **P < 0.01, significantly different from sham; anova. Numbers of experiments are shown as insets in the columns.
Glycine receptors α1 and α2 subunit expression in the spinal ventral horn
The observation that 4-bromopropofol enhanced a tonic conductance raised the issue whether this action involves α2 subunit containing receptors, possibly expressed at artificially high levels in our cultures. Thus, we performed real-time PCR analysis of spinal ventral horn tissue from embryonic, adult mice and organotypic slices after 10 and 20 DIC (n = 5 each sample). These experiments revealed a similar expression of the α1 subunit in all samples, while the α2 subunit was highly expressed after 10 DIC and not detectable in adult tissue. Interestingly, there was a marked contrast in the expression of α2 subunits, between 10 and 20 DIC (Figure 7).
Figure 7.
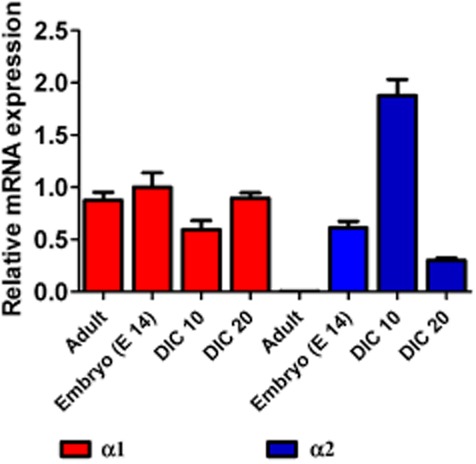
The expression of α1 subunits of glycine receptors did not substantially alter in samples from spinal ventral horn tissue obtained from adult, embryonic (E 14) mice, and from organotypic cultures after 10 and 20 DIC. Contrastingly, the α2 glycine receptor subunits were not detectable in adult spinal ventral horn tissue, highly expressed organotypic cultures after 10 DIC and only in small amounts in organotypic cultures after 20 DIC.
Discussion
The therapeutic option to enhance the activity of glycine receptors may open new ways into the treatment of patients suffering from chronic pain or movement disorders (Laube et al., 2002; Zeilhofer et al., 2012a). Thus, spinal glycine receptors have been proposed as interesting molecular targets for novel drugs. The function of these receptors is indeed augmented by a number of compounds including cannabinoid ligands, general anaesthetics, alcohols, neuroactive steroids and ivermectin (Webb and Lynch, 2007; Yevenes and Zeilhofer, 2011). But unfortunately, all these agents lack selectivity. There are two reasons why we consider halogenated propofol analogues as promising compounds for selectively modulating the activity of glycine receptors. First, a recent study has shown that low concentrations of 4-chloropropofol enhanced the function of homopentameric α1 glycine receptors expressed in HEK cells (de la Roche et al., 2012). Second, anaesthetists have a pronounced expertise in the handling of propofol and halogenated ether anaesthetics, thereby limiting the risk of inadmissible side effects (Green, 2007). However, the effects of halogenated propofols in complex neuronal networks remain to be characterized. Most importantly, their efficacy and selectivity has to be determined. The present study, carried out in organotypic slice cultures derived from the mouse spinal cord, showed that low nanomolar concentrations of 4-bromopropofol promoted the opening of glycine receptors, thereby causing ventral horn neurons to discharge at lower rates. As the latter effect was completely antagonized by strychnine, it is concluded that 4-bromopropofol acted predominantly via glycine receptors. Moreover, at the tested nanomolar concentrations, 4-bromopropofol did not alter the function of GABAA receptor-mediated transmission. The latter result emphasizes that 4-bromopropofol, unlike halogenated ether anaesthetics and ethanol, clearly discriminates between the structurally related glycine receptors and GABAA receptors. Taken together, these findings support the idea that selective therapeutics, acting by enhancing the function of glycine receptors, can be developed from halogenated propofol analogues.
There is good evidence that, at micromolar concentrations, 4-bromopropofol not only binds to glycine receptors, but also affects several other molecular targets in the CNS. For example, the drug potentiated agonist-induced responses of GABAA receptors (Trapani et al., 1998). In another study, 4-bromopropofol blocked voltage-gated sodium channels (Haeseler et al., 2008). Therefore, the selectivity for glycine receptors is most likely restricted to the low nanomolar concentration range. Interestingly, the widely used benzodiazepines exhibit a very similar selectivity profile. At nanomolar concentrations, these compounds act via specific binding sites that reside on a subpopulation of GABAA receptors (Möhler et al., 2002). However, at higher, micromolar concentrations, benzodiazepines produce anaesthesia via additional molecular targets (Walters et al., 2000; Drexler et al., 2010). Also, it seems likely that, in analogy to the benzodiazepines, micromolar concentrations of halogenated propofol analogues may act as CNS depressants by affecting multiple molecular targets.
In marked contrast to sevoflurane (Eckle and Antkowiak, 2013; Eckle et al., 2013), 4-bromopropofol failed to alter the decay times of glycine receptor-mediated synaptic currents. Unexpectedly, this agent induced a strychnine-sensitive inhibitory chloride conductance. What molecular mechanism of action may explain this experimental finding? It seems possible that 4-bromopropofol directly opens glycine receptors, thus acting in a manner very much like the natural agonist glycine. In their classical studies, Barker et al. (1982) and Katz and Miledi (1972) reported that upon applying GABA and ACh onto spinal neurons and muscle cells, tonic currents were induced and noise levels considerably increased. These effects are very similar to the actions of 4-bromopropofol seen in the present study. However, another important criterion for an agonist-like mode of action relates to the associated changes in synaptic transmission. A direct activation of glycine receptors reduces the number of postsynaptic receptors that can be activated upon presynaptic neurotransmitter release. As a consequence, application of agonists decreases the amplitude of glycine receptor-mediated synaptic events in a concentration-dependent manner. However, although the tonic current that was induced by 4-bromopropofol was prominent, only a minor and statistically insignificant change in the amplitudes of IPSCs was observed. The latter finding is inconsistent with the hypothesis that 4-bromopropofol acts like an agonist.
An alternative explanation for the finding that 4-bromopropofol induces tonic, strychnine-sensitive currents while leaving glycinergic IPSCs unaffected, assumes that this agent only interacts with a subpopulation of glycine receptors, namely the ones that are present at extrasynaptic sites. Experiments in oocytes suggest that subtypes of the glycine receptors lacking a β-protein subunit, such as homopentameric α2 glycine receptors, predominantly reside at extrasynaptic sites (Grudzinska et al., 2005). However, GABAA receptors are known to switch between synaptic and extrasynaptic sites during receptor trafficking (Jacob et al., 2008). A similar dynamic modulation may be suggested for glycine receptors. In the spinal cord, the latter receptor-subtype is heavily expressed during early development and is later replaced, although not completely, by receptors incorporating a β subunit (Lynch, 2009). But the physiological function of these receptors is unclear as knockout of α2 subunits has no obvious effect on development (Young-Pearse et al., 2006). The possible existence of α2 receptors in ventral horn neurons raises the possibility that 4-bromopropofol binds to and activates such receptors. The presence of α2 receptors in ventral horn neurons that are activated by ambient glycine might also explain the result that a strychnine-sensitive tonic current was already abundant under drug-free conditions. However, the involvement of α2 glycine receptors in mediating this tonic current is questioned by several observations. The concentration of glycine in the extracellular space has been estimated to be close to 5 μM (Whitehead et al., 2001). In our preparation, it should be smaller, because the extracellular space is oversized and glycine was not added to the bathing solution. This is of relevance because α2 homopentameric glycine receptors proved to be rather insensitive to glycine. A concentration required to produce half-maximal receptor activation is as high as 200 μM (Mangin et al., 2003). Thus, a potential role of α2 assemblies in mediating tonic glycinergic inhibition in spinal neurons is controversial. Interestingly, the study of Meier and coworkers demonstrated that RNA editing may render glycine receptors highly sensitive to glycine (Meier et al., 2005). Thus, such a mechanism could also be involved in mediating the strychnine-sensitive tonic current, observed in our preparation (Legendre et al., 2009). A major role of α2 glycine receptors is unlikely as α2 mRNA expression strongly declined between DIC 10 and DIC 20, whereas the efficacy of 4-bromopropofol slightly increased in older cultures (data not shown). Although the latter effect did not reach statistical significance, this observation strongly argues against a major role of α2 glycine receptors in mediating the effects of 4-bromopropofol.
However, for uncovering those glycine receptor-subtypes that are targeted by 4-bromopropofol, additional research is clearly required. Further, our results do not exclude the possibility that 4-bromopropofol may indirectly modulate glycine receptors by blocking glycine transporter 2, which is highly expressed in the spinal ventral horn (Zafra et al., 1995). Such a mechanism would increase ambient glycine and enhance tonic conductance, as reported for the glycine transporter 2 inhibition by ALX 1393 (Eckle and Antkowiak, 2013).
Glycine receptor-dependent inhibition of ventral horn neurons is modified by the volatile anaesthetic sevoflurane (Eckle and Antkowiak, 2013; Eckle et al., 2013) and 4-bromopropofol in qualitatively distinct ways. Of these two agents, the former prolongs the decay time of synaptic currents, while the latter enhances a tonic conductance. Interestingly, there is mounting evidence in the literature that phasic and tonic inhibition can serve different functions (Mitchell and Silver, 2003; Prescott and De Koninck, 2003; Pavlov et al., 2009). It is well established that the elementary components of voluntary movements and motor reflexes are to a large part generated by neuronal circuits in the ventral horn of the spinal cord (Talpalar et al., 2011; Arber, 2012). Pattern generation involves precisely timed synaptic-mediated activation of GABAA receptors, glycine receptors and glutamate receptors. Therefore, pharmacological interventions that modify synaptic-mediated phasic transmission in the ventral horn are expected to alter motor performance. This is not necessarily the case if tonic inhibition is changed. Drug-induced enhancement of tonic currents should preferably decrease basal action potential activity of ventral horn neurons and may translate into a reduced muscle tone. However, as synaptic transmission remains unimpaired and the relative impact of tonic inhibition decreases during episodes of movement-associated synaptic transmission, we speculate that drugs like 4-bromopropofol might reduce muscle tone without seriously impairing motor coordination.
Clinical implications
What are potential therapeutic applications for drugs that enhance the function of synaptic or, alternatively, extrasynaptic glycine receptors? In those cases in which a movement disorder is caused by a deficiency in glycinergic synaptic transmission, it is evident that pharmacological potentiation of synaptic receptors is required. But in other circumstances, a selective decrease in muscle tone may be more appropriate. For example, clinical studies have provided evidence that in patients suffering from low back pain, the administration of muscle relaxants is of significant benefit (van Tulder et al., 2003). However, agents that are currently administered to produce muscle relaxation exhibit a small effect size and a number of serious side effects including sedation and addiction, challenging their therapeutic advantage in general (Cohen, 2010). Thus, 4-bromopropofol may be regarded as a model compound that opens new approaches to the acute therapy of low back pain. In the future, novel halogenated propofol derivatives may display analgesic and muscle relaxant properties without causing sedation and minimal impairment of motor coordination.
Acknowledgments
The authors thank Claudia Holt, Claudia Bernardo de Oliveira Franz and Ina Pappe for excellent technical assistance, and Harald Hentschke for providing analysis software tools. The present study was supported by the Medical Research Council (MR/J014826/1).
Glossary
- ACSF
artificial CSF
- DIC
days in culture
- TTX
tetrodotoxin
Author contributions
V.-S. E. helped conceive and design the experiments, performed the experiments, analysed the data and approved the final manuscript. C. G. helped conceive and design the experiments, and approved the final manuscript. V. M. helped conceive and design the experiments, and approved the final manuscript. P. M. O. helped contribute reagents/materials/analysis tools and approved the final manuscript. N. B. helped contribute reagents/materials/analysis tools and approved the final manuscript. M. L. helped conceive and design the experiments, contributed reagents/materials/analysis tools and approved the final manuscript. B. A. conceived and designed the experiments, helped analyse the data, wrote the paper and approved the final manuscript.
Conflict of interest
M. L. is one of the named inventors of the patent AU 2006328198. All other authors have no conflict of interest to declare.
References
- Ahrens J, Leuwer M, Stachura S, Krampfl K, Belelli D, Lambert J, et al. A transmembrane residue influences the interaction of propofol with the strychnine-sensitive glycine alpha1 and alpha1 beta receptor. Anesth Analg. 2008;107:1875–1883. doi: 10.1213/ane.0b013e3181875a31. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013a;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013b;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S, Mathie A, Peters J. Guide to receptors and channels (GRAC) Brit J Pharmacol. 2011;164:S1–S2. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkowiak B, Heck D. Effects of the volatile anesthetic enflurane on spontaneous discharge rate and GABA(A)-mediated inhibition of Purkinje cells in rat cerebellar slices. J Neurophysiol. 1997;77:2525–2538. doi: 10.1152/jn.1997.77.5.2525. [DOI] [PubMed] [Google Scholar]
- Antognini JF, Carstens E. Increasing isoflurane from 0.9 to 1.1 minimum alveolar concentration minimally affects dorsal horn cell responses to noxious stimulation. Anesthesiology. 1999;90:208–214. doi: 10.1097/00000542-199901000-00027. [DOI] [PubMed] [Google Scholar]
- Aprison M, Shank R, Davidoff R. A comparison of the concentration of glycine, a transmitter suspect, in different areas of the brain and spinal cord in seven different vertebrates. Comp Biochem Physiol. 1969;28:1345–1355. doi: 10.1016/0010-406x(69)90571-4. [DOI] [PubMed] [Google Scholar]
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron. 2012;74:975–989. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Barker JL, McBurney RN, MacDonald JF. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Pistis I, Peters JA, Lambert JJ. General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends Pharmacol Sci. 1999;20:496–502. doi: 10.1016/s0165-6147(99)01405-4. [DOI] [PubMed] [Google Scholar]
- Boehme DH, Fordice MW, Marks N, Vogel W. Distribution of glycine in human spinal cord and selected regions of brain. Brain Res. 1973;50:353–359. doi: 10.1016/0006-8993(73)90736-1. [DOI] [PubMed] [Google Scholar]
- Braschler UF, Iannone A, Spenger C, Streit J, Lüscher HR. A modified roller tube technique for organotypic cocultures of embryonic rat spinal cord, sensory ganglia and skeletal muscle. J Neurosci Methods. 1989;29:121–129. doi: 10.1016/0165-0270(89)90023-x. [DOI] [PubMed] [Google Scholar]
- Callister R, Graham B. Early history of glycine receptor biology in mammalian spinal cord circuits. Front Mol Neurosci. 2010;3:13. doi: 10.3389/fnmol.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- Chung S-K, Vanbellinghen J-F, Mullins JGL, Robinson A, Hantke J, Hammond CL, et al. Pathophysiological mechanisms of dominant and recessive GLRA1 mutations in hyperekplexia. J Neurosci. 2010;30:9612–9620. doi: 10.1523/JNEUROSCI.1763-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SP. Benzodiazepines for neuropathic back pain: when the cure is worse than the disease. Pain. 2010;149:424–425. doi: 10.1016/j.pain.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Davies J, Chung S, Thomas R, Robinson A, Hammond C, Mullins J, et al. The glycinergic system in human startle disease: a genetic screening approach. Front Mol Neurosci. 2010;3:8. doi: 10.3389/fnmol.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler B, Zinser S, Hentschke H, Antkowiak B. Diazepam decreases action potential firing of neocortical neurons via two distinct mechanisms. Anesth Analg. 2010;111:1394–1399. doi: 10.1213/ANE.0b013e3181f9c035. [DOI] [PubMed] [Google Scholar]
- Eckle V-S, Antkowiak B. ALX 1393 inhibits spontaneous network activity by inducing glycinergic tonic currents in the spinal ventral horn. Neuroscience. 2013;253:165–171. doi: 10.1016/j.neuroscience.2013.08.042. [DOI] [PubMed] [Google Scholar]
- Eckle V-S, Hauser S, Drexler B, Antkowiak B, Grasshoff C. Opposing actions of sevoflurane on GABAergic and glycinergic synaptic inhibition in the spinal ventral horn. PLoS ONE. 2013;8:e60286. doi: 10.1371/journal.pone.0060286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Which molecular targets are most relevant to general anaesthesia? Toxicol Lett. 1998;100–101:1–8. doi: 10.1016/s0378-4274(98)00158-1. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Islam R, Lynagh T, Lynch J, Webb T. High throughput techniques for discovering new glycine receptor modulators and their binding sites. Front Mol Neurosci. 2009;2:17. doi: 10.3389/neuro.02.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasshoff C, Drexler B, Rudolph U, Antkowiak B. Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des. 2006;12:3665–3679. doi: 10.2174/138161206778522038. [DOI] [PubMed] [Google Scholar]
- Grasshoff C, Drexler B, Hentschke H, Thiermann H, Antkowiak B. Cholinergic modulation of sevoflurane potency in cortical and spinal networks in vitro. Anesthesiology. 2007a;106:1147–1155. doi: 10.1097/01.anes.0000267598.65120.f2. [DOI] [PubMed] [Google Scholar]
- Grasshoff C, Jurd R, Rudolph U, Antkowiak B. Modulation of presynaptic {beta}3-containing GABAA receptors limits the immobilizing actions of GABAergic anesthetics. Mol Pharmacol. 2007b;72:780–787. doi: 10.1124/mol.107.037648. [DOI] [PubMed] [Google Scholar]
- Green SM. Propofol in emergency medicine: further evidence of safety. Emerg Med Australas. 2007;19:389–393. doi: 10.1111/j.1742-6723.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, et al. The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Haeseler G, Karst M, Foadi N, Gudehus S, Roeder A, Hecker H, et al. High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues. Br J Pharmacol. 2008;155:265–275. doi: 10.1038/bjp.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wässle H, Ahmadi S, Heindl C, Reinold H, et al. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The statistical nature of the acetylcholine potential and its molecular components. J Physiol. 1972;224:665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yao A, Atherley R, Carstens E, Jinks SL, Antognini JF. Neurons in the ventral spinal cord are more depressed by isoflurane, halothane, and propofol than are neurons in the dorsal spinal cord. Anesth Analg. 2007;105:1020–1026. doi: 10.1213/01.ane.0000280483.17854.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler D, Devanathan V, Bernardo de Oliveira Franz C, Eldh T, Novakovic A, Roth J, et al. Gαi2- and gαi3-deficient mice display opposite severity of myocardial ischemia reperfusion injury. PLoS ONE. 2014;9:e98325. doi: 10.1371/journal.pone.0098325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Maksay G, Schemm R, Betz H. Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol Sci. 2002;23:519–527. doi: 10.1016/s0165-6147(02)02138-7. [DOI] [PubMed] [Google Scholar]
- Legendre P, Förstera B, Jüttner R, Meier J. Glycine receptors caught between genome and proteome – functional implications of RNA editing and splicing. Front Mol Neurosci. 2009;2:23. doi: 10.3389/neuro.02.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Mangin JM, Baloul M, Prado de Carvalho L, Rogister B, Rigo JM, Legendre P. Kinetic properties of the α2 homo-oligomeric glycine receptor impairs a proper synaptic functioning. J Physiol. 2003;553:369–386. doi: 10.1113/jphysiol.2003.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JC, Henneberger C, Melnick I, Racca C, Harvey RJ, Heinemann U, et al. RNA editing produces glycine receptor [alpha]3P185L, resulting in high agonist potency. Nat Neurosci. 2005;8:736–744. doi: 10.1038/nn1467. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Muller E, Le-Corronc H, Legendre P. Extrasynaptic and postsynaptic receptors in glycinergic and GABAergic neurotransmission: a division of labor? Front Mol Neurosci. 2008;1:3. doi: 10.3389/neuro.02.003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orser BA, Wang LY, Pennefather PS, MacDonald JF. Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neurons. J Neurosci. 1994;14:7747–7760. doi: 10.1523/JNEUROSCI.14-12-07747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J, McBride W, Felten D. Distribution of glycine, GABA, aspartate and glutamate in the rat spinal cord. Brain Res Bull. 1983;10:415–418. doi: 10.1016/0361-9230(83)90115-6. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci. 2009;29:15341–15350. doi: 10.1523/JNEUROSCI.2747-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc Natl Acad Sci U S A. 2003;100:2076–2081. doi: 10.1073/pnas.0337591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg B, Duch DS. Suppression of central nervous system sodium channels by propofol. Anesthesiology. 1999;91:512–520. doi: 10.1097/00000542-199908000-00026. [DOI] [PubMed] [Google Scholar]
- de la Roche J, Leuwer M, Krampfl K, Haeseler G, Dengler R, Buchholz V, et al. 4-chloropropofol enhances chloride currents in human hyperekplexic and artificial mutated glycine receptors. BMC Neurol. 2012;12:104. doi: 10.1186/1471-2377-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Takazawa T, MacDermott AB. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J Physiol. 2010;588:2571–2587. doi: 10.1113/jphysiol.2010.188292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpalar AE, Endo T, Löw P, Borgius L, Hägglund M, Dougherty KJ, et al. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron. 2011;71:1071–1084. doi: 10.1016/j.neuron.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Trapani G, Latrofa A, Franco M, Altomare C, Sanna E, Usala M, et al. Propofol analogues. synthesis, relationships between structure and affinity at GABAA receptor in rat brain, and differential electrophysiological profile at recombinant human GABAA receptors. J Med Chem. 1998;41:1846–1854. doi: 10.1021/jm970681h. [DOI] [PubMed] [Google Scholar]
- van Tulder M, Touray T, Furlan A, Solway S, Bouter L Cochrane Back Review Group. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the Cochrane collaboration. Spine (Phila Pa 1976) 2003;28:1978–1992. doi: 10.1097/01.BRS.0000090503.38830.AD. [DOI] [PubMed] [Google Scholar]
- Walters R, Hadley S, Morris K, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci. 2000;3:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- Webb T, Lynch J. Molecular pharmacology of the glycine receptor chloride channel. Curr Pharm Des. 2007;13:2350–2367. doi: 10.2174/138161207781368693. [DOI] [PubMed] [Google Scholar]
- Whitehead KJ, Manning J-P, Smith CGS, Bowery NG. Determination of the extracellular concentration of glycine in the rat spinal cord dorsal horn by quantitative microdialysis. Brain Res. 2001;910:192–194. doi: 10.1016/s0006-8993(01)02684-1. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Zeilhofer HU. Allosteric modulation of glycine receptors. Brit J Pharmacol. 2011;164:224–236. doi: 10.1111/j.1476-5381.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Ivic L, Kriegstein AR, Cepko CL. Characterization of mice with targeted deletion of glycine receptor alpha 2. Mol Cell Biol. 2006;26:5728–5734. doi: 10.1128/MCB.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU. The glycinergic control of spinal pain processing. Cell Mol Life Sci. 2005;62:2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Benke D, Yevenes GE. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol. 2012a;52:111–133. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Wildner H, Yévenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012b;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


