Abstract
Background and Purpose
Caffeine is one of the most commonly used psychoactive substances. Circadian rhythms consist of the main suprachiasmatic nucleus (SCN) clocks and peripheral clocks. Although caffeine lengthens circadian rhythms and modifies phase changes in SCN-operated rhythms, the effects on caffeine on the phase, period and amplitude of peripheral organ clocks are not known. In addition, the role of cAMP/Ca2+ signalling in effects of caffeine on rhythm has not been fully elucidated.
Experimental Approach
We examined whether chronic or transient application of caffeine affects circadian period/amplitude and phase by evaluating bioluminescence rhythm in PER2::LUCIFERASE knock-in mice. Circadian rhythms were monitored in vitro using fibroblasts and ex vivo and in vivo for monitoring of peripheral clocks.
Key Results
Chronic application of caffeine (0.1–10 mM) increased period and amplitude in vitro. Transient application of caffeine (10 mM) near the bottom of the decreasing phase of bioluminescence rhythm caused phase advance in vitro. Caffeine (0.1%) intake caused a phase delay under light–dark or constant dark conditions, suggesting a period-lengthening effect in vivo. Caffeine (20 mg·kg−1) at daytime or at late night-time caused phase advance or delay in bioluminescence rhythm in the liver and kidney respectively. The complicated roles of cAMP/Ca2+ signalling may be involved in the caffeine-induced increase of period and amplitude in vitro.
Conclusions and Implications
Caffeine affects circadian rhythm in mice by lengthening the period and causing a phase shift of peripheral clocks. These results suggest that caffeine intake with food/drink may help with food-induced resetting of peripheral circadian clocks.
Tables of Links
| TARGETS |
|---|
| Enzymes |
| AC, adenylyl cyclase |
| CLOCK |
| Epac, exchange protein activated by cAMP |
| PDE, phosphodiesterase |
| LIGANDS |
|---|
| Caffeine |
| cAMP |
| H-89 |
| IBMX |
| Ryanodine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Caffeine, one of the most commonly used psychoactive substances, promotes behavioural functions such as vigilance, attention, mood and arousal (Fisone et al., 2004; Butt and Sultan, 2011). The actions of caffeine are mediated via antagonism of adenosine receptor signalling, increase in the second messenger cAMP by inhibition of PDE activity and activation/inhibition of ryanodine receptors (Francis et al., 2011; Guerreiro et al., 2011; Müller and Jacobson, 2011). Interestingly, these mechanisms are all also involved in the circadian signalling pathways (Hastings et al., 2008). Circadian clocks drive daily physiological and behavioural rhythms that are adapted to the 24 h solar cycle. In mammals, the principal circadian oscillator is located in the suprachiasmatic nucleus (SCN) in the brain, whereas additional oscillators are found in other regions of the brain and peripheral organs (Lowrey and Takahashi, 2011). Through genetic and molecular approaches, the mechanisms underlying circadian oscillations have been identified as comprising interconnected transcriptional and post-translational delayed feedback loops (Lowrey and Takahashi, 2011; Buhr and Takahashi, 2013). The heterodimer composed of the two proteins, circadian locomotor output cycles kaput (CLOCK) and brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1), initiates transcription by binding to specific DNA elements (E- and E′-boxes) in the promoters of target genes Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2), both of which affect negative feedback (Gekakis et al., 1998; Hogenesch et al., 1998; Kume et al., 1999; Yoo et al., 2005). PER and CRY proteins dimerize and inhibit CLOCK/BMAL1 transcriptional activity, leading to the depletion of PER and CRY and the initiation of a new cycle of transcription (Sangoram et al., 1998; Griffin et al., 1999; Sato et al., 2006). Recent studies have focused on the role of oscillatory cytosolic mechanisms in addition to transcriptional/post-translational delayed feedback loops, because human red blood cells, which are devoid of nuclei, showed circadian cycling of metabolic status in the absence of this feedback system (O'Neill et al., 2013). In particular, cAMP- and Ca2+-dependent signalling are important core components of the clock. Signal transduction pathways are activated by elevated cAMP/Ca2+ levels, leading to transcriptional activation via cAMP response elements (CREs) in the Per1/2 promoter. Thus, if second messenger signalling provides input into and transmits rhythmic output from a hypothetical clock such as cytosolic rhythms in small signalling molecules, then dynamic cAMP/Ca2+ signalling becomes indistinguishable from the core circadian mechanism (Hastings et al., 2008), and manipulation of cAMP and/or Ca2+ signalling would be expected to determine key properties of cellular rhythms in vitro and also physiological rhythms in vivo, that is, the amplitude, phase and period.
Because caffeine can elevate cytosolic Ca2+ and cAMP via ryanodine activation and PDE inhibition, respectively, it is possible that caffeine can affect circadian rhythm amplitude, phase and/or period through elevation of cAMP/Ca2+. Several reports have described the effects of caffeine on circadian rhythm at both the cellular and behaviour levels in various plant and animal species. Caffeine has been shown to lengthen circadian period in Neurospora, Chlamydomonas, Drosophila and mice (Feldman, 1975; Goodenough and Bruce, 1980; Wu et al., 2009; Oike et al., 2011). Caffeine causes a phase delay in firing rhythm in the rat SCN, and blocks light-induced phase shifts in mouse circadian locomotor rhythm (Ding et al., 1998; Vivanco et al., 2013). However, none of these studies have systematically examined the effects of caffeine on the phase, amplitude and periodicity of circadian rhythm in peripheral organs. The present investigation addressed this question by monitoring the circadian oscillations in peripheral organs both ex vivo and in vivo (Tahara et al., 2012), using Period2-driven luciferase (PER2::LUC) knock-in mice. As cAMP/Ca2+ signalling may be involved in the effects of caffeine on circadian rhythm, we examined this possibility by in vitro experiments using mouse embryonic fibroblasts (MEFs) derived from these mice.
Methods
Animals
All animal care and experimental procedures were in accordance with the guidelines of the Committee for Animal Experimentation of the School of Science and Engineering at Waseda University and in compliance with the law (No. 105) passed by and notification (No. 6) of the Japanese government. These studies were approved by the School of Science and Engineering at Waseda University (permission 2013-A058, 2013-A061). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 64 animals were used in the experiments described here.
Heterozygous PER2::LUC knock-in mice, from a mixed background of C57/BL6J and imprinting control region (ICR; albino), were backcrossed more than five times with PER2::LUC C57/BL6J mice (courtesy of Dr Joseph Takahashi, Southwestern Medical Center, Texas University, TX, USA; Yoo et al., 2004) and ICR mice (Tokyo Laboratory Animals, Tokyo, Japan). As albino mice can facilitate the monitoring of bioluminescence from peripheral organs, we used ICR backcrossed mice (Tahara et al., 2012). Mice were kept in a room maintained on a 12:12 h light–dark cycle (with lights on at 08:00) at a temperature of 23 ± 1°C, 60 ± 5% humidity and light intensity of 100–150 lux at cage level, and were provided with a standard mouse chow ( MF, Oriental Yeast Co. Ltd., Tokyo, Japan) and water ad libitum. Individually housed female mice (age: 8–12 weeks) were used in the experiments.
Measurement of cAMP concentration in the MEFs
The MEFs were incubated with DMEM containing caffeine (1 mM) at 37°C in 5% CO2 for 1 h. The cells were washed with PBS and incubated with the sample diluent solution of the cAMP chemiluminescent immunoassay kit (Arbor Assays, Ann Arbor, MI, USA) at room temperature for 10 min. Following the manufacturer's instructions, cells were scraped from the dish and lysed. Supernatants were collected after centrifugation at 600× g for 15 min at 4°C. The cAMP concentrations in MEFs were measured using the cAMP chemiluminescent immunoassay kit and are expressed as pM per 106 cells.
Caffeine treatment and assessment of bioluminescence in PER2::LUC MEFs
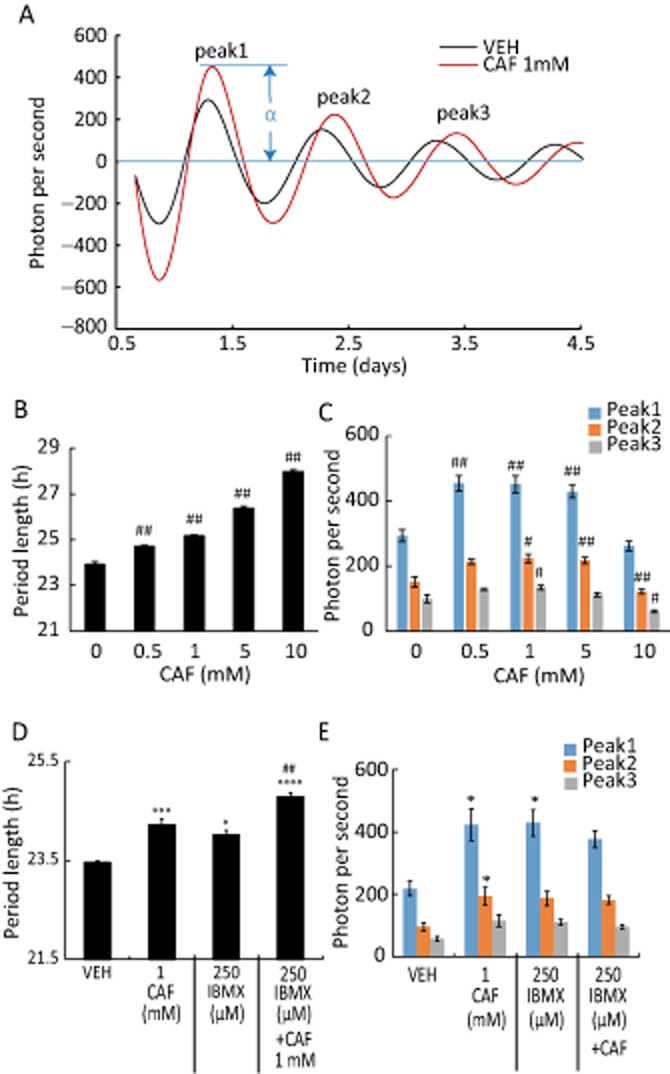
The rhythmic expression of Per2 was measured using a real-time LUC assay in MEFs derived from PER2::LUC knock-in mice (Tahara et al., 2011). Bioluminescence was monitored once per minute over 10 min intervals with a dish-type luminometer (LumiCycle; Actimetrics, Wilmette, IL, USA). Cell lines were stimulated with 200 nM dexamethasone for 2 h to synchronize circadian rhythms. The medium was replaced with DMEM containing 0.1 mM d-luciferin potassium salt (Promega, Madison, WI, USA), 10% FBS (Bio West, Kansas City, MO, USA) and the specific drug before measurements were begun. The period length was measured as half the distance between peaks 1 and 3 of the waveform, whereas the length α in representative detrended data (Figure 1A) was a measure of amplitude of rhythmic PER2::LUC expression. For transient caffeine treatment, MEFs were treated with vehicle (water) or caffeine (1 mM) for 30 min at 36 or 43 h after dexamethasone stimulation. After caffeine treatment, the caffeine-containing culture medium was replaced with fresh medium, and bioluminescence was measured over 4 days. The phase and amplitude of the first peak after treatment was recorded for analysis.
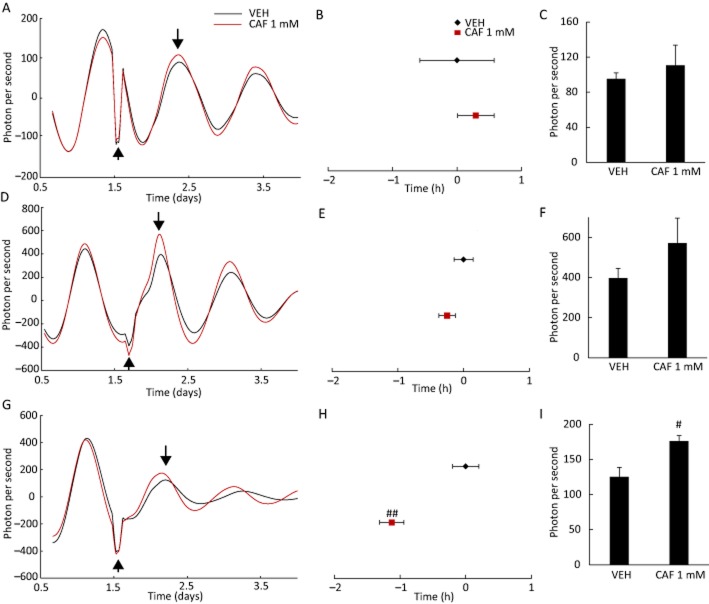
Figure 1.
Caffeine and PDE1 inhibitor IBMX lengthen circadian period in MEFs. (A) Representative detrended data for cells treated with water as vehicle (VEH) or 1 mM caffeine (CAF). (B) Effect of caffeine on period of rhythmic PER2::LUC expression in MEFs. The distance between peaks 1 and 3 was multiplied by 0.5 to obtain period length. (C) Effect of caffeine on amplitude of oscillatory PER2::LUC bioluminescence. (D) The period was increased by treatment with IBMX, caffeine or both compared with cells treated with the VEH dimethyl sulfoxide. (E) Amplitude of PER2::LUC bioluminescence oscillations was increased by treatment with IBMX. #P < 0.05, ##P < 0.01 versus VEH or caffeine 0 mM; *P < 0.05 versus 1 mM caffeine; Tukey's test. All values are expressed as mean + SEM (n = 4 per group). The length α in (A) represents the amplitude of PER2::LUC expression.
Measurement of bioluminescence in ex vivo cultures of liver and SCN from PER2::LUC mice
After daily injections of saline or caffeine at Zeitgeber time (ZT) 5 (ZT 0, lights on) for 3 days, PER2::LUC mice were killed by cervical dislocation at ZT 7 for the evaluation of bioluminescence rhythmicity in the liver or SCN. Livers were rapidly dissected and placed in ice-cold HBSS (pH 7.2). Livers were cut with scissors into small pieces (3 × 1 mm) in the plate. Two randomly selected pieces were taken from each of the two lobes of the liver. For SCN sections, 300 μm thick SCN slices were cut with a microtome (Dohan Co., Osaka, Japan), and dissected into small pieces containing SCN. Each tissue explant was placed on a membrane (0.4 μm, 30 mm in diameter, Millicell cell culture inserts; Millipore, Billerica, MA, USA) in a 35 mm Petri dish (Iwaki, Tokyo, Japan), sealed with parafilm and cultured in 1.3 mL DMEM supplemented with NaHCO3 (2.7 mM), HEPES (10 mM), kanamycin (20 mg·L−1), insulin (5 μg·mL−1), putrescine (100 μM), human transferrin (100 μg·mL−1), progesterone (20 nM), sodium selenite (30 nM) and d-luciferin potassium salt (0.1 mM). Cultures were incubated at 37°C and bioluminescence was monitored once per minute over 10 min intervals with a dish-type luminometer.
Assessment of circadian rhythm in MEFs and tissues
Raw data (1 min bins) were smoothed by an adjusting–averaging method with 2 h running means as previously described (Hayasaka et al., 2007; Ohta et al., 2008). The data were then detrended by subtracting the 24 h running average from the raw data using R software (R development Core Team; http://www.r-project.org/), created by Mr Tsuyoshi Yaita and Dr Shigenobu Shibata (Waseda University, Tokyo, Japan). Details of this assessment have been reported previously (Tahara et al., 2012). Peaks were defined as the point at which bioluminescence was higher compared with adjacent points and confirmed by wave form.
In vivo monitoring of PER2::LUC bioluminescence
Caffeine (20 mg·kg−1) was administered to mice by i.p. injection (0.01 mL·g−1), while control mice were injected with saline. In vivo monitoring of PER2::LUC bioluminescence was performed as previously described (Tahara et al., 2012) using an in vivo imaging system (IVIS) kinetics system (Caliper Life Sciences, Hopkinton, MA, USA). Mice were anaesthetized with isoflurane (Mylan Inc., Tokyo, Japan) and concentrated oxygen (SO-005B; Sanyo Electronic Industries Co. Ltd, Okayama, Japan) in a black box using a gas anaesthesia system (XGI-8; Caliper Life Sciences). Anaesthetized mice were injected with d-luciferin potassium salt s.c. (15 mg·kg−1). Images were acquired with a 1 min exposure time at 6 and 8 min after luciferin injection in the prone position for the kidney, and at 10 and 12 min after injection in the supine position for the liver and submandibular gland. Each bioluminescence image was merged with the corresponding greyscale image. Images were obtained six times per day (ZT 9, 13, 17, 21, 1 and 5). Mice were returned to their home cages after each imaging session where they recovered quickly from anaesthesia. The total time under anaesthesia was approximately 20 min per session. A previous study has shown that LUC activity in the peripheral tissues and behaviour are unaffected by four hourly anaesthesia and bioluminescence analysis per day (Tahara et al., 2012).
Analysis of in vivo monitoring data
In vivo monitoring data were analysed as described previously (Tahara et al., 2012). The bioluminescence emitted from each organ (kidney, liver and submandibular gland) was calculated automatically using Living Image 3.2 software (Caliper Life Sciences). For each organ, the region of interest was set to the same shape and size in all images. In the case of the kidney, data from the right and left kidneys were combined for the analysis. The average photon per second value from the set of six time points for each day was taken as 100%, and the bioluminescence rhythm for the entire day was expressed as a percentage of each set for each organ. The peak phase and amplitude of these normalized data were determined using the single cosinor procedure (Acro.exe version 3.5; Refinetti et al., 2007).
Analysis of locomotor activity rhythm
General locomotor activity was recorded with an infrared radiation sensor (F5B; Omron, Tokyo, Japan) according to our previous method (Tahara et al., 2012). Double-plotted actograms of locomotor activity are shown with 6 min epochs, and circadian rhythmicity of activity was evaluated by χ2 periodogram analysis with P = 0.01 as the significance threshold using CLOCKLAB software (Actimetrics).
Data analysis
All values are expressed as means ± SEM. Statistical analysis was performed using the GraphPad Prism version 6.03 (GraphPad software, San Diego, CA, USA). We checked whether data showed the normal or non-normal distribution, and equal or biased variation assessed by D'Agostino–Pearson test/Kolmogorov–Smirnov test and F-value test/Bartlett's test respectively. Parametric analysis was conducted using a one-way or two-way anova with a Tukey's test or Student's t-test for post hoc analysis, and non-parametric analysis was performed using Kruskal–Wallis test/Friedman test with a Dunn's test or Mann–Whitney test for post hoc analysis.
Materials
All reagents, including caffeine, IBMX, MDL-12,330A hydrochloride (MDL), brefeldin A (BFA), 8-bromoadenosine cAMP (8-Br-cAMP), 8-(4-chlorophenylthio)-cGMP sodium salt (8-Br-cGMP), ryanodine and H-89 dihydrochloride hydrate (H-89) were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. N6, 2′-O-dibutyryl-cAMP (DB-cAMP) was from Life Science Institute, Inc. (Saitama, Japan). DMSO was from Wako Pure Chemical Industries, Ltd. (Saitama, Japan).
Results
Caffeine and IBMX lengthen circadian period and increase circadian amplitude in MEFs
To confirm a lengthening effect of caffeine in vitro, effects of caffeine on circadian period were examined using MEFs derived from PER2::LUC mice treated with vehicle or caffeine (0.5–10 mM). Treatment with caffeine caused a dose-dependent increase in the period (Figure 1A and B). The amplitude of LUC expression was enhanced by caffeine at concentrations between 0.5 and 5 mM, but was decreased at the highest concentration (10 mM) (Figure 1C). As caffeine also inhibits PDE activity, we confirmed the effect of PDE inhibition on circadian period by treating MEFs with another PDE inhibitor, IBMX (250 μM), or the corresponding vehicle (DMSO). The period was prolonged by application of IBMX, and when combined with caffeine the increase in the period was greater than in cells treated with caffeine only (Figure 1D). The amplitude of LUC expression was enhanced by IBMX treatment (Figure 1E).
Ryanodine facilitated caffeine-induced period length and amplitude increase in MEFs
Because caffeine is a known stimulant for the ryanodine receptor, the effect of ryanodine alone or caffeine plus ryanodine on period and amplitude of rhythm was examined. Low or high doses of ryanodine given in the absence of caffeine did not affect the period or the amplitude (Supporting Information Fig. S1). The caffeine-induced lengthened period was potentiated by the high dose of ryanodine, whereas the caffeine-induced increase of amplitude was reduced by this treatment. The data suggest that ryanodine receptor-associated Ca2+ may be important for period and amplitude.
Circadian period and amplitude in MEFs are modified by treatment with cAMP analogues, AC inhibitor, exchange protein directly activated by cAMP (Epac) inhibitor and PKA inhibitor
As inhibition of PDE activity leads to increased intracellular levels of cAMP and/or cGMP, increased cAMP activates the PKA signalling pathway as well as downstream Epac. To test whether cAMP was involved, cells were treated with the cell-permeant cAMP analogues DB-cAMP and 8-Br-cAMP, which lengthened the period without affecting the amplitude (Supporting Information Fig. S2). The presence of both cAMP analogues potentiated the period-lengthening effect of caffeine (Supporting Information Fig. S2A and C), although the amplitude of LUC expression was reduced (Supporting Information Fig. S2B and D). Neither the period length nor the amplitude was affected by 8-Br-cGMP (a cGMP analogue) application (Supporting Information Fig. S2E and F).
To further examine the role of cAMP in determining circadian period, cells were treated with the AC inhibitor MDL. A shortened period and reduced amplitude were observed after MDL treatment alone in a dose-dependent manner (Supporting Information Fig. S3A). Caffeine-induced lengthening of the period and augmentation of amplitude were blocked by MDL (Supporting Information Fig. S3). These data suggest that the increased cellular cAMP, but not cGMP, was involved in period-lengthening effects of caffeine.
In the next experiment, we examined the effect of a PKA inhibitor on caffeine-induced modification of circadian rhythm. Treatment of cells with the PKA inhibitor H-89 alone or in conjunction with caffeine had no effect on period (Supporting Information Fig. S4A and B). The amplitude was slightly reduced by H-89, and caffeine-induced augmentation of amplitude was significantly and dose-dependently blocked by H-89. These data suggest that PKA activation is not involved in determination of period, but is associated with the maintenance of amplitude. As Epac is known to be a downstream target of cAMP, we examined the effect of the Epac inhibitor BFA on caffeine-induced modification of circadian rhythm (Supporting Information Fig. S4). According to a previous report (O'Neill et al., 2008), we used 5 and 10 μM BFA, but these doses irreversibly inhibited the oscillation in the present experiment. BFA at 0.5 μM did not affect period and amplitude in the presence or absence of caffeine (Supporting Information Fig. S4). BFA alone at 1 μM did not affect period but reduced amplitude. BFA at 1 μM potentiated caffeine-induced lengthening of period, but it attenuated the caffeine-induced increase in amplitude (Supporting Information Fig. S4). These data suggest that activation of Epac is necessary for maintenance of amplitude and period.
Effect of caffeine and caffeine plus MDL on cAMP contents in MEFs
Caffeine (1 mM) treatment for 1 h significantly increased the cellular cAMP content (1.8 ± 0.4 pM per 106 cells, n = 4 for vehicle, 5.2 ± 0.8 pM per 106 cells, n = 6 for caffeine, P < 0.05 by Student's t-test). Co-administration of 1 mM caffeine plus 5 μM MDL did not reverse the caffeine-induced increase, but did augment the caffeine effect (5.8 ± 0.6 pM per 106 cells, n = 3 for caffeine, 7.4 ± 0.3 pM per 106 cells, n = 5 for caffeine plus MDL, P > 0.05 by Student's t-test).
Transient caffeine treatment at specific times causes a circadian phase shift in MEFs
To test whether transient exposure to caffeine was sufficient to alter circadian rhythmicity, MEFs were treated with caffeine for 30 min. No effects were observed when caffeine was applied just after the times corresponding to the peak or trough of LUC expression (Figure 2A, B, D and E). However, the peak in LUC expression was advanced when cells were treated with caffeine immediately prior to the time corresponding to the trough in expression (Figure 2G and H). There were no significant effects on the amplitude of LUC expression whether caffeine was applied just after the peak (Figure 2C) or trough (Figure 2F); however, the amplitude was enhanced when caffeine was applied just prior to the trough (Figure 2I). These data suggest that transient caffeine treatment near the bottom of the decreasing phase of rhythm causes a phase advance.
Figure 2.
Transient treatment with caffeine at specific times during the 24 h cycle causes a phase shift in the rhythmic expression of PER2::LUC in mouse embryonic fibroblasts. (A, D, G) Representative detrended data for cells treated with water as vehicle (VEH) or 1 mM caffeine (CAF). Arrows pointing up or down indicate the time point of caffeine application and peak phase, respectively, and were used to analyse the phase shift. (B, E, H) Treatment with 1 mM caffeine at a time point immediately preceding the trough in oscillatory PER2::LUC expression caused a phase delay in the first peak, as well as an increase in amplitude (C, F, I). VEH-treated cells were designated as time 0. #P < 0.05, ##P < 0.01 versus VEH (Student's t-test). All values are expressed as means ± SEM (n = 4 per group).
Chronic caffeine intake causes a phase delay in peripheral circadian clocks of mice
To determine whether the effects of caffeine on MEF rhythm extend to mouse peripheral clocks, LUC activity in the liver, kidney and submandibular gland of mice that were chronically treated with caffeine was examined ex vivo. Mice were provided with water containing 0.1% caffeine and maintained under a normal light–dark cycle or constant darkness. There were no differences in locomotor activity between caffeine-treated and control mice in terms of onset or duration of activity under light–dark cycle (Figure 3A), and onset and free-running period under constant darkness (Supporting Information Fig. S5A).
Figure 3.
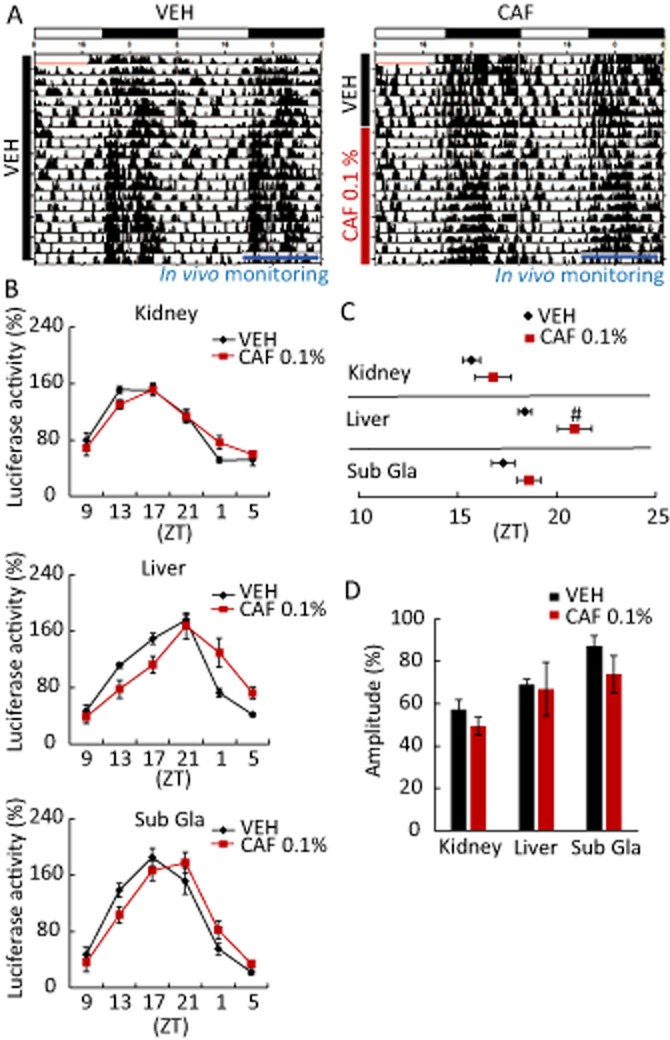
Chronic caffeine intake causes a phase delay in peripheral circadian clocks. Mice maintained under a 12:12 h light–dark cycle were given water with 0.1% caffeine (CAF) for 14 days after pretreatment with untreated water for 7 days. In vivo monitoring of PER2::LUC bioluminescence was initiated at ZT 9. (A) Representative double-plotted actograms for mice administered water (VEH) or CAF. Horizontal white and black bars indicate light and dark periods, respectively, in the 24 h cycle; vertical black bars indicate recorded activity; blue bars indicate the period of in vivo monitoring. (B) Oscillatory PER2::LUC bioluminescence in peripheral organs of mice treated with VEH or CAF. Phase advance was observed in the liver. (C) Average peak phase of PER2::LUC bioluminescence in each tissue from (B). #P < 0.05 versus VEH (Student's t-test). (D) Daily average bioluminescence in each tissue. All values are expressed as mean ± SEM (n = 4 per group).
After 2 weeks of chronic caffeine intake under light–dark cycle, a phase delay was observed in the liver (Figure 3B and C) compared with vehicle-treated control mice; however, the amplitude of peripheral clock rhythms was unaffected by caffeine consumption (Figure 3D). Phase delays were also observed in the liver and submandibular gland of caffeine-treated animals under constant darkness (Supporting Information Fig. S5B); amplitude was unaffected in these tissues, but was reduced in the kidney (Supporting Information Fig. S5C). The ex vivo data suggested that the chronic treatment caused phase delay in peripheral organ clocks without affecting locomotor activity rhythms.
Transient caffeine treatment at specific times causes a circadian phase shift in peripheral clocks of mice
Because the effect of caffeine on circadian phase of MEFs was found to depend on the time point in the 24 h circadian cycle, peripheral organs of mice transiently exposed to caffeine were examined in vivo to determine whether a similar phase shift occurred. Mice were injected with vehicle (saline) or caffeine (20 mg·kg−1) at ZT 1, 5, 12 or 21 for three consecutive days (Figure 4A). Compared with vehicle-treated or un-injected controls, bioluminescence rhythms showed significant phase advances in all peripheral organs of caffeine-treated mice at ZT 5 (Figure 4B and C). Moreover, the peak of LUC expression was advanced, delayed or unaffected depending on caffeine treatment time (Figure 4C; Supporting Information Fig. S6B). The amplitude and direction of the phase shift was expressed as a function of caffeine treatment time to generate phase–response curves for each peripheral organ (Figure 4E). Caffeine treatment at a time point corresponding to early in the day caused a phase advance, whereas a treatment late at night caused a delay. The amplitude was unaffected when caffeine was injected at ZT 5, 12 and 21, but was reduced upon injection at ZT 1 (Figure 4D; Supporting Information Fig. S6C). The sleep/wake cycle of female mice fluctuates with the oestrous cycle, and these changes possibly influence the CAF-induced phase reset seen in female mice. To confirm that caffeine causes the circadian phase reset, the experiment was conducted separately on male mice. These mice that were given 20 mg·kg−1 caffeine over 3 days exhibited clear and significant phase advances of all peripheral clocks (Supporting Information Fig. S7). These data suggest that caffeine causes a phase advance when administered during the daytime, and phase delay during late night-time in vivo.
Figure 4.
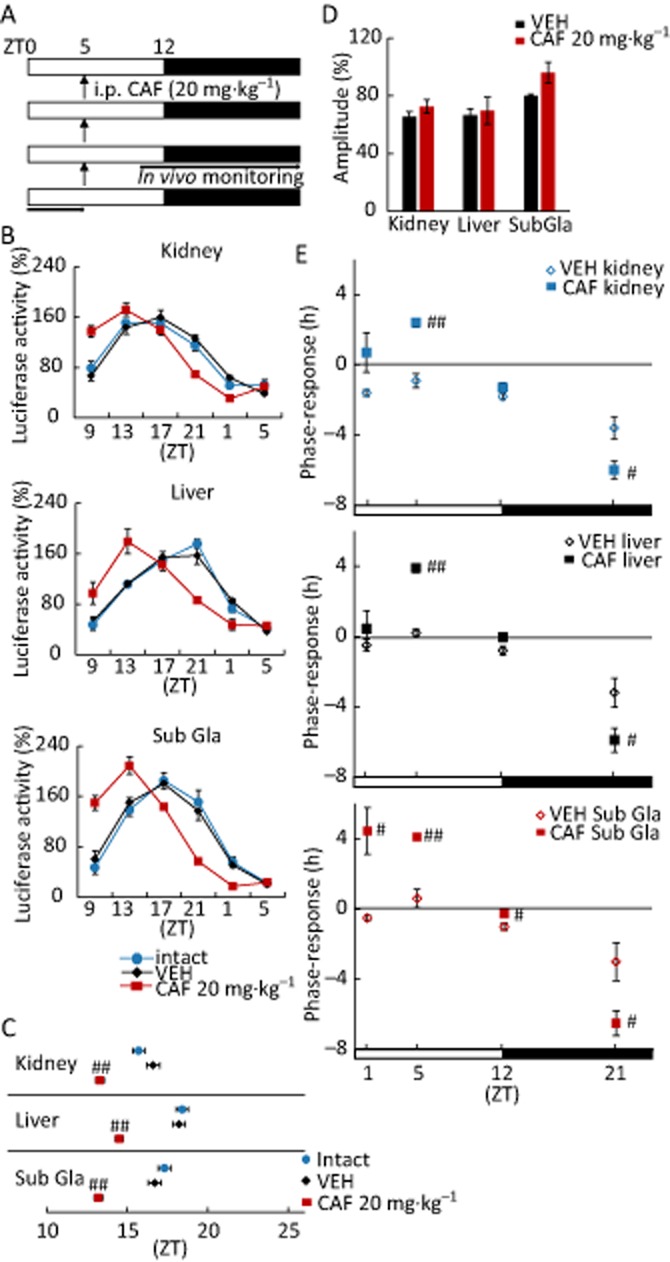
Transient treatment with caffeine at specific times during the 24 h cycle induces a phase shift in peripheral clocks in female mice. Mice maintained under 12:12 h light–dark conditions were given i.p. injections of vehicle (VEH)or 20 mg·kg−1 caffeine (CAF) for three consecutive days at ZT 1, 5, 12 or 21; in vivo monitoring was initiated at ZT 9. (A) Representative protocol for injection at ZT 5. (B) Oscillatory PER2::LUC bioluminescence in various tissues following the protocol from (A). Phase advances were observed in the kidney, liver and submandibular gland (Sub Gla) upon treatment with CAF. (C) Peak phase of PER2::LUC oscillations in each tissue indicated in (B). ##P < 0.01 versus VEH (Student's t-test). (D) Daily average bioluminescence amplitude in each tissue. (E) The amplitude and direction of the phase shift (y-axis) was plotted against caffeine treatment time (ZT; x-axis), to obtain phase–response curves for each tissue. Positive and negative values indicated phase advance and delay respectively. #P < 0.05, ##P < 0.01 versus VEH (Student's t-test, except for Sub Gla at ZT 1 for Mann–Whitney test). All values are expressed as mean ± SEM (n = 4 per group).
Phase shifts induced by transient exposure to caffeine do not involve the SCN
To determine whether the phase shift of peripheral clocks induced by caffeine occurs via the central clock, the SCN and liver of mice injected with vehicle (saline) or with caffeine (20 mg·kg−1) at ZT 5 for three consecutive days were examined in an ex vivo tissue culture system. Caffeine treatment had no effect on circadian phase in the SCN (Figure 5A and B), but caused phase advance in the liver (Figure 5D and E); no effect on the circadian period was observed in either tissue (Figure 5C and F). These data suggest that the effects of caffeine on peripheral circadian rhythm are mediated not through the central clock, but directly in peripheral tissues.
Figure 5.
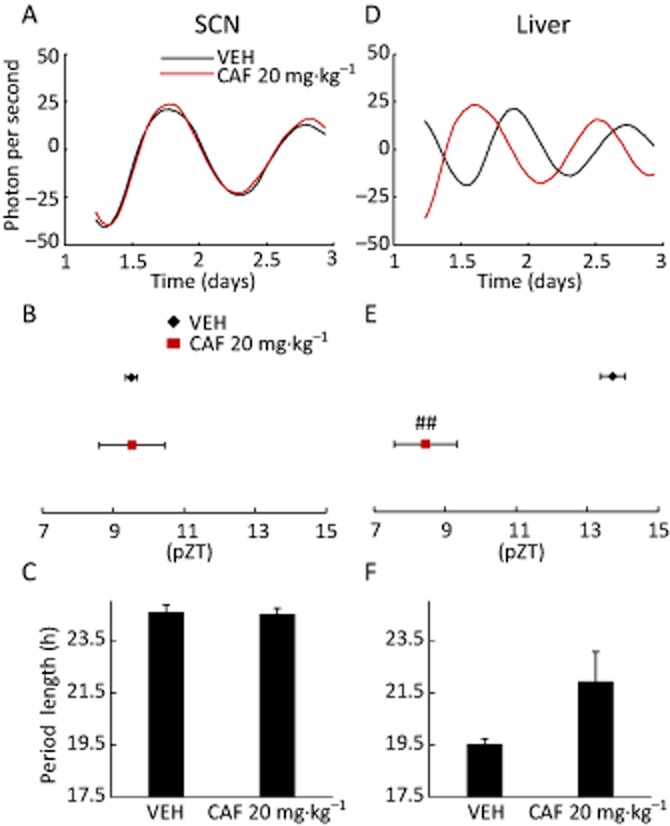
Circadian phase shifts induced by transient treatment with caffeine do not depend on the SCN. Mice maintained under 12:12 h light–dark conditions were injected with vehicle (VEH) or 20 mg·kg−1 caffeine (CAF) at ZT 5 for three consecutive days, then killed at ZT 7 to assess oscillatory PER2::LUC bioluminescence in the liver or SCN. (A, D) Representative detrended data from the SCN (A) or liver (D) of vehicle (VEH)- or caffeine-treated animals. Caffeine caused a phase advance in the liver but not the SCN. (B, E) Average peak phase of PER2::LUC bioluminescence in each tissue indicated in (A) and (D). ##P < 0.01 versus VEH (Student's t-test). (C, F) Period of rhythmic PER2::LUC expression in each tissue indicated in (A) and (D). The distance between peaks 1 and 2 represents period length. All values are expressed as mean ± SEM (n = 4 per group).
Discussion
In the present study, the effects of caffeine on the amplitude, period and phase of circadian rhythm were examined in vivo and ex vivo by monitoring the bioluminescence of PER2::LUC knock-in mice (Tahara et al., 2012) and in vitro using MEFs derived from these mice. In Table 1 we summarize the present data. Caffeine lengthened the period of oscillation in a dose-dependent manner. It was not possible to monitor the effect of caffeine on period in peripheral organ clocks in vivo due to technical limitations. However, the phase of the circadian rhythm was delayed by 2–3 h in peripheral organs of mice maintained under a normal light–dark cycle or in constant darkness after free caffeine consumption, although the periodicity of the free-running activity rhythm of mice under constant darkness was unaffected. These data suggest that the period of circadian rhythm in mouse peripheral organs can be lengthened by chronic caffeine intake.
Table 1.
Summary of caffeine action
| MEF | Ex vivo | In vivo | In vivo | ||
|---|---|---|---|---|---|
| SCN | Liver | IVIS | Locomotor | ||
| Free running | ↑ | − | ↑ | ↑ | − |
| Amplitude | ↓ | − | − | −↓ | − |
| Phase shift | + | − | + | + | Not yet |
↑, increase; ↓, decrease; +, effective; −, no effect.
Chronic caffeine treatment in vitro increased the amplitude of circadian oscillation in a dose-dependent manner, except at the highest dose of 10 mM, which reduced the amplitude (Figure 1). In ex vivo experiments, intake of 0.1% caffeine did not affect the amplitude of all peripheral clocks in mice under light–dark cycle or in constant darkness, except for kidney. In vitro, low doses of caffeine may facilitate the synchronization of circadian clocks and high doses may reduce synchronization. As SCN-lesioned mice showed low amplitudes of peripheral clocks resulting from a decrease in synchronization (Tahara et al., 2012), caffeine may reduce the synchronization of kidney clocks. Caffeine injection at ZT 1 reduced the amplitude of peripheral clocks (Supporting Information Fig. S6), but not at other ZTs. The external stimuli at ZT 1 caused a singularity effect of caffeine on oscillation, because the oscillation is unstable at ZT 1. Indeed, singularity effects on oscillation have previously been reported (Ukai et al., 2007); thus, amplitude might be reduced in the present experiment.
Caffeine treatment induced phase shifts in the circadian rhythm of peripheral clocks, as determined by in vivo monitoring experiments, and the effect was dependent on the time of caffeine application during the 24 h cycle. Phase advance was observed when caffeine was administered at ZT 1 and 5, but delayed at ZT 21, while treatment at ZT 12 produced no effect in female mice (Figure 4). Similar to female mice, caffeine administration at ZT 5 produced a large phase advance in male mice. A similar result was obtained from in vitro experiments, in which caffeine caused a phase advance in MEFs when applied during the decreasing phase of PER2::LUC bioluminescence, corresponding to the ZT 5 time point. To our knowledge, this is the first observation that caffeine causes a phase shift in peripheral clocks. Therefore, we confirmed whether a phase shift in the SCN may result in a phase shift in peripheral clocks. Surprisingly, transient caffeine injection at ZT 5 caused phase advance in the liver but not the SCN in ex vivo experiments (Figure 5). The current experiment was conducted under light–dark entrained conditions, and therefore the entraining effect of caffeine may be weak for the SCN clock, but may be sufficiently robust for peripheral clocks. Some previous reports have suggested that the SCN is a target tissue for caffeine (Ding et al., 1998; Oike et al., 2011; Vivanco et al., 2013). It is worth noting that the mouse strains, environmental lighting conditions, and the injected and consumed amounts of caffeine were different between the published studies and the present experiments. Therefore, additional studies are required to clarify the factors that account for the differences in the findings. Because the SCN is not affected by CAF, we wonder about the pharmacological relevance of altering the oscillator present in peripheral tissues by consuming caffeine. As feeding-induced phase shifts of peripheral clocks are independent of SCN oscillation (Hara et al., 2001; Shibata et al., 2010), food or drink containing caffeine may be useful for feeding-induced entrainment of peripheral clocks.
In the current experiment, we should consider the possibility that the influence of caffeine on circadian rhythm may be related to the influence of caffeine on sleep. Sleep deprivation by gentle handling during inactive periods led to phase-advanced circadian rhythms in hamsters (Mistlberger et al., 2002). Caffeine injection during inactive periods such as ZT 0 and ZT 5 resulted in awake behaviour similar to gentle handling and induced phase advance in the IVIS experiments. Additional experiments using psychomotor stimulants such as amphetamine and modafinil are required to confirm this hypothesis.
In another set of experiments, we tried to elucidate the role of cAMP and/or Ca2+ signalling in cellular rhythms in vitro, that is, the amplitude and period. Wefound that increased cytosolic levels of cAMP can cause lengthening of circadian period in cultured cells. This was demonstrated by administration of the PDE inhibitor IBMX, the AC inhibitor MDL and cAMP analogues. In addition, the simultaneous administration of cAMP analogues and caffeine had a greater effect on period length than either compound alone. However, the augmentation observed after caffeine plus cAMP analogues is likely to reflect an additive effect of both drugs, because cAMP analogues used here are resistant to PDE. As caffeine caused an increase in cellular cAMP content whereas MDL did not reverse this effect, the caffeine-induced lengthening of the period could not be fully explained by cAMP levels alone. In the Drosophila brain, caffeine caused an increase in circadian period resulting from higher levels of cAMP and corresponding activation of PKA (Wu et al., 2009). Non-competitive (p-site) inhibitors of AC have shown a dose-dependent suppression of cAMP signalling in the SCN (An et al., 2011), as well as reversibly lengthening in vitro circadian period and behavioural periodicity in mice (O'Neill et al., 2008). Although the reasons for the discrepancy between this and other studies are unclear, they might be attributed to the differences in the cell types examined and the various mechanisms of action of the inhibitors.
The period of rat liver explants was reversibly lengthened by inhibiting the release and import of Ca2+ in the endoplasmic reticulum and by treatment with membrane-permeant Ca2+ chelators (Báez-Ruiz and Díaz-Muñoz, 2011). It is possible that transient caffeine exposure increases Ca2+ levels, but that chronic caffeine administration reduces intracellular Ca2+ via the ryanodine receptor, because high doses of ryanodine facilitated caffeine-induced lengthening of period (Supporting Information Fig. S1).
DB-cAMP attenuated the caffeine-induced increase in amplitude, suggesting that a high concentration of cAMP reversely reduced the rhythm amplitude. In the absence of caffeine, H89 slightly and BFA strongly reduced the rhythm amplitude. The caffeine-induced increase in amplitude was reversed by treatment with BFA and H89. BFA was shown to reduce the amplitude of mPer1-Luc expression in the SCN, while a reduction in amplitude induced by AC inhibition was rescued by the Epac agonist Sp-8-CPT-2′-O-Me-cAMP (O'Neill et al., 2008). In the current experiment, the PDE inhibitors caffeine and IBMX increased, and the AC inhibitor MDL decreased the rhythm amplitude. Co-administration of caffeine plus IBMX or DB-cAMP reversely inhibited the caffeine-induced amplitude increase, perhaps due to excess activation of cAMP, because a high dose of caffeine (10 mM) reduced the amplitude (Figure 1). Taken together, these findings suggest that circadian rhythm amplitude is increased via cAMP–Epac and cAMP–PKA signalling. Alternatively, cAMP/Ca2+ signalling may be involved, as treatments that reduce intracellular Ca2+ levels have been shown to reduce amplitude, presumably resulting in desynchronization, in a dose-dependent manner (Shibata et al., 1984; Ikeda et al., 2003; Lundkvist et al., 2005; Maywood et al., 2006). In the present experiment, a high dose of ryanodine strongly attenuated the caffeine-induced increase of amplitude, suggesting a critical role of intracellular Ca2+ levels in rhythm amplitude.
The role of cAMP/Ca2+ signalling in phase shifts has been well investigated. Glutamate-induced, Ca2+-mediated phase resetting in the SCN has been reported (Kim et al., 2005), which may be achieved through the effectors Ca2+/calmodulin-dependent PK, MAPK and PKC (Lee et al., 2010; Welsh et al., 2010; O'Neill et al., 2013). Thus, a plausible mechanism for the effects of acute caffeine administration would be increased cytosolic Ca2+ concentration through activation of the ryanodine receptor, with consequent activation of cAMP/Ca2+ signalling, culminating in rhythmic CRE activation at Per gene promoters, leading to a shift in circadian phase (Obrietan et al., 1999; Tischkau et al., 2003; O'Neill et al., 2013).
In summary, caffeine caused phase advance or delay of peripheral clocks in vivo when administered early in the day or night, respectively, and caused a lengthening of circadian period, increased amplitude in vitro but not in vivo. These results suggest that caffeine intake with food/drink may help in food-induced resetting of peripheral circadian clocks.
Acknowledgments
This study was partially supported by a Grant-in-Aid for Scientific Research (B) (23300278) and (S) (26220201) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (S.S.).
Glossary
- 8-Br-cAMP
8-bromoadenosine cAMP
- 8-Br-cGMP
8-(4-chlorophenylthio)-cGMP
- BFA
brefeldin A
- BMAL1
brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1
- CLOCK
circadian locomotor output cycles kaput
- CRE
cAMP response element
- CRY
cryptochrome
- DB-cAMP
N6, 2′-O-dibutyryl-cAMP
- Epac
exchange protein directly activated by cAMP
- IVIS
in vivo imaging system
- LUC
luciferase
- MDL
MDL-12,330A hydrochloride
- MEF
mouse embryonic fibroblasts
- PER
period
- SCN
suprachiasmatic nucleus
- ZT
Zeitgeber time
Author contributions
S. N. and Y. T. designed and performed the experiments and wrote the paper. S. O., M. K., A. S., Y. I. and M. K. performed the experiments and analysed data. S. S. conceived, supervised and designed experiments, and wrote the paper.
Conflict of interest
The authors have no conflicts of interest to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site: http://dx.doi.org/10.1111/bph.12890
Effect of treatment with ryanodine on circadian rhythmicity in mouse embryonic fibroblasts. (A) The increase in the period of rhythmic PER2::LUC expression induced by caffeine (CAF) was potentiated by 100 μM ryanodine compared with cells treated with caffeine. (B) Ryanodine 100 μM reversed the caffeine-induced increase in amplitude of oscillatory PER2::LUC expression. #P < 0.05, ##P < 0.01 versus vehicle (VEH); *P < 0.05, **P < 0.01 versus 1 mM caffeine, Tukey's test. All values are expressed as means ± SEM (n = 4 per group).
Treatment with cAMP analogues DB-cAMP and 8-Br-cAMP lengthens circadian period in mouse embryonic fibroblasts. (A, C, E) The increase in the period of rhythmic PER2::LUC expression induced by caffeine (CAF) was potentiated by DB-cAMP and 8-Br-cAMP, but unaltered by the cGMP analogue 8-Br-cGMP compared with cells treated with the vehicle (VEH) water. (B, D, F) DB-cAMP, but not 8-Br-cAMP or 8-Br-cGMP, reversed the CAF-induced increase in amplitude of oscillatory PER2::LUC bioluminescence. #P < 0.05, ##P < 0.01 versus vehicle; *P < 0.05, **P < 0.01 versus 1 mM caffeine, Tukey's test. All values are expressed as means ± SEM (n = 4 per group).
Treatment with the AC inhibitor MDL shortens circadian period in a concentration-dependent manner in mouse embryonic fibroblasts. (A) The period of rhythmic PER2::LUC expression was decreased by treatment with MDL, caffeine (CAF) or both compared with cells treated with the vehicle (VEH) water. (B) MDL treatment blocked the caffeine-induced increase in amplitude of oscillatory PER2::LUC expression. #P < 0.05, ##P < 0.01 versus vehicle; *P < 0.05, **P < 0.01 versus 1 mM caffeine; Tukey's test. All values are expressed as means ± SEM (n = 4 per group, except of 1 mM caffeine group; n = 6).
Effect of treatment with the PKA inhibitor H-89 and exchange protein directly activated by cAMP 1 (Epac1) antagonist BFA on circadian rhythmicity in mouse embryonic fibroblasts. (A, B) The period of oscillatory PER2::LUC bioluminescence was unchanged by application of H-89, but the caffeine (CAF)-induced increase in amplitude was attenuated by H-89. #P < 0.05, ##P < 0.01 versus vehicle (VEH); *P < 0.05, **P < 0.01 versus 1 mM caffeine; Tukey's test. (C, D) BFA enhanced caffeine (CAF)-induced lengthen of period, but suppressed caffeine-induced increase of amplitude. (C) ##P < 0.01 versus vehicle (VEH); **P < 0.01 versus 1 mM caffeine. $$ P < 0.01 versus 1 μM BFA; Tukey's test. (D) *P < 0.05 versus 1 mM caffeine; Dunn's test. All values are expressed as means ± SEM (n = 4 per group).
Chronic consumption of caffeine causes a phase delay in peripheral clocks under constant darkness. Mice maintained in constant darkness were given water with 0.1% caffeine (CAF) for 14 days after pretreatment with regular water for 7 days. On the last day, in vivo monitoring of PER2::LUC expression was initiated at ZT 9. (A, upper panels) Double-plotted actograms for mice treated with water (vehicle) or CAF. Horizontal black bars indicate the period of constant darkness; vertical black bars indicate recorded activity; blue bars indicate the period of in vivo monitoring. (Lower panels) The free-running activity period was assessed by χ2 periodogram analysis for 7 days before (PRE) and after (POST) caffeine application. (B) Average peak phase of PER2::LUC oscillations in each peripheral tissue. Caffeine treatment caused phase shifts in the liver and submandibular gland (Sub Gla). #P < 0.05 versus vehicle (VEH) (Mann–Whitney test) (n = 4 for VEH; n = 5 for CAF). (C) Daily average bioluminescence amplitude in each tissue. ##P < 0.01 versus VEH (Student's t-test). All values are expressed as mean ± SEM.
Transient treatment with caffeine at specific times during the 24 h cycle induces phase shifts in peripheral clock oscillations. Mice maintained under 12:12 h light–dark conditions were injected with vehicle (VEH) or 20 mg·kg−1 caffeine (CAF) for three consecutive days at ZT 1, 5, 12 or 21; in vivo monitoring of oscillatory PER2::LUC bioluminescence was initiated at ZT 9. (A) Rhythmic PER2::LUC in peripheral organs of mice injected with caffeine at ZT 12, 21 or 1. Phase shifts were observed in the kidney and submandibular gland (Sub Gla) when injections were performed at ZT 21 and 1. (B) Average peak phases of PER2::LUC oscillations in each tissue from (A). ZT 12: n = 4 (VEH), n = 5 (CAF); ZT 21: n = 4 for each group; ZT 1: n = 4 (VEH), n = 5 (CAF). #P < 0.05, ##P < 0.01 versus VEH (Student's t-test, except of Sub Gla at ZT 1 for Mann–Whitney test). (C) Daily average bioluminescence amplitude in each tissue. #P < 0.05, ##P < 0.01 versus VEH (Student's t-test). All values are expressed as mean ± SEM.
Transient treatment with caffeine at ZT 5 induces a phase advance in peripheral clocks in male mice. Male mice maintained under 12:12 h light–dark conditions were given i.p. injections of vehicle (VEH) or 20 mg·kg−1 caffeine (CAF) for three consecutive days at ZT 5; in vivo monitoring was initiated at ZT 9. (A) Oscillatory PER2::LUC bioluminescence in various tissues. Phase advances were observed in the kidney, liver and submandibular gland (Sub Gla) upon treatment with caffeine. (B) Daily average bioluminescence amplitude in each tissue. (C) Peak phase of PER2::LUC oscillations in each tissue indicated in (A). ##P < 0.01 versus VEH (Student's t-test). All values are expressed as mean ± SEM (n = 4 per group).
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Irwin RP, Allen CN, Tsai C, Herzog ED. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011;105:2289–2296. doi: 10.1152/jn.00966.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Ruiz A, Díaz-Muñoz M. Chronic inhibition of endoplasmic reticulum calcium-release channels and calcium-ATPase lengthens the period of hepatic clock gene Per1. J Circadian Rhythms. 2011;9:6. doi: 10.1186/1740-3391-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Takahashi JS. Molecular components of the mammalian circadian clock. Handb Exp Pharmacol. 2013;217:3–27. doi: 10.1007/978-3-642-25950-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2011;51:363–373. doi: 10.1080/10408390903586412. [DOI] [PubMed] [Google Scholar]
- Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, et al. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- Feldman JF. Circadian periodicity a neurospora: alteration by inhibitors of cyclic AMP phosphodiesterase. Science. 1975;190:789–790. doi: 10.1126/science.173018. [DOI] [PubMed] [Google Scholar]
- Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SH, Sekhar KR, Ke H, Corbin JD. Inhibition of cyclic nucleotide phosphodiesterases by methylxanthines and related compounds. Handb Exp Pharmacol. 2011;200:93–133. doi: 10.1007/978-3-642-13443-2_4. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Goodenough JE, Bruce VG. The effects of caffeine and theophylline on the phototactic rhythm of Chlamydomonas reinhardtii. Biol Bull. 1980;159:649–655. [Google Scholar]
- Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- Guerreiro S, Marien M, Michel PP. Methylxanthines and ryanodine receptor channels. Handb Exp Pharmacol. 2011;200:135–150. doi: 10.1007/978-3-642-13443-2_5. [DOI] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, et al. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, O'Neill JS. Cellular circadian pacemaking and the role of cytosolic rhythms. Curr Biol. 2008;18:R805–R815. doi: 10.1016/j.cub.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Hayasaka N, Yaita T, Kuwaki T, Honma S, Honma K, Kudo T, et al. Optimization of dosing schedule of daily inhalant dexamethasone to minimize phase shifting of clock gene expression rhythm in the lungs of the asthma mouse model. Endocrinology. 2007;148:3316–3326. doi: 10.1210/en.2007-0010. [DOI] [PubMed] [Google Scholar]
- Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, et al. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Choi HJ, Kim JS, Kim YS, Jeong DU, Shin HC, et al. Voltage-gated calcium channels play crucial roles in the glutamate-induced phase shifts of the rat suprachiasmatic circadian clock. Eur J Neurosci. 2005;21:1215–1222. doi: 10.1111/j.1460-9568.2005.03950.x. [DOI] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee J, Kwon I, Nakajima Y, Ohmiya Y, Son GH, et al. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J Cell Sci. 2010;123:3547–3557. doi: 10.1242/jcs.070300. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Genetics of circadian rhythms in mammalian model organisms. Adv Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Belcourt J, Antle MC. Circadian clock resetting by sleep deprivation without exercise in Syrian hamsters: dark pulses revisited. J Biol Rhythms. 2002;17:227–237. doi: 10.1177/07430402017003006. [DOI] [PubMed] [Google Scholar]
- Müller CE, Jacobson KA. Xanthines as adenosine receptor antagonists. Handb Exp Pharmacol. 2011;200:151–199. doi: 10.1007/978-3-642-13443-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- Ohta H, Xu S, Moriya T, Iigo M, Watanabe T, Nakahata N, et al. Maternal feeding controls fetal biological clock. PLoS ONE. 2008;3:e2601. doi: 10.1371/journal.pone.0002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike H, Kobori M, Suzuki T, Ishida N. Caffeine lengthens circadian rhythms in mice. Biochem Biophys Res Commun. 2011;410:654–658. doi: 10.1016/j.bbrc.2011.06.049. [DOI] [PubMed] [Google Scholar]
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill JS, Maywood ES, Hastings MH. Cellular mechanisms of circadian pacemaking: beyond transcriptional loops. Handb Exp Pharmacol. 2013;217:67–103. doi: 10.1007/978-3-642-25950-0_4. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, et al. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, et al. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38:312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Shiratsuchi A, Liou SY, Ueki S. The role of calcium ions in circadian rhythm of suprachiasmatic nucleus neuron activity in rat hypothalamic slices. Neurosci Lett. 1984;52:181–184. doi: 10.1016/0304-3940(84)90371-9. [DOI] [PubMed] [Google Scholar]
- Shibata S, Tahara Y, Hirao A. The adjustment and manipulation of biological rhythms by light, nutrition, and abused drugs. Adv Drug Deliv Rev. 2010;62:918–927. doi: 10.1016/j.addr.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Otsuka M, Fuse Y, Hirao A, Shibata S. Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbα with shifts in the liver clock. J Biol Rhythms. 2011;26:230–240. doi: 10.1177/0748730411405958. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Kuroda H, Saito K, Nakajima Y, Kubo Y, Ohnishi N, et al. In vivo monitoring of peripheral circadian clocks in the mouse. Curr Biol. 2012;22:1029–1034. doi: 10.1016/j.cub.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J Biol Chem. 2003;278:718–723. doi: 10.1074/jbc.M209241200. [DOI] [PubMed] [Google Scholar]
- Ukai H, Kobayashi TJ, Nagano M, Masumoto KH, Sujino M, Kondo T, et al. Melanopsin-dependent photo-perturbation reveals desynchronization underlying the singularity of mammalian circadian clocks. Nat Cell Biol. 2007;9:1327–1334. doi: 10.1038/ncb1653. [DOI] [PubMed] [Google Scholar]
- Vivanco P, Studholme KM, Morin LP. Drugs that prevent mouse sleep also block light-induced locomotor suppression, circadian rhythm phase shifts and the drop in core temperature. Neuroscience. 2013;254:98–109. doi: 10.1016/j.neuroscience.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J Neurosci. 2009;29:11029–11037. doi: 10.1523/JNEUROSCI.1653-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, et al. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci U S A. 2005;102:2608–2613. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of treatment with ryanodine on circadian rhythmicity in mouse embryonic fibroblasts. (A) The increase in the period of rhythmic PER2::LUC expression induced by caffeine (CAF) was potentiated by 100 μM ryanodine compared with cells treated with caffeine. (B) Ryanodine 100 μM reversed the caffeine-induced increase in amplitude of oscillatory PER2::LUC expression. #P < 0.05, ##P < 0.01 versus vehicle (VEH); *P < 0.05, **P < 0.01 versus 1 mM caffeine, Tukey's test. All values are expressed as means ± SEM (n = 4 per group).
Treatment with cAMP analogues DB-cAMP and 8-Br-cAMP lengthens circadian period in mouse embryonic fibroblasts. (A, C, E) The increase in the period of rhythmic PER2::LUC expression induced by caffeine (CAF) was potentiated by DB-cAMP and 8-Br-cAMP, but unaltered by the cGMP analogue 8-Br-cGMP compared with cells treated with the vehicle (VEH) water. (B, D, F) DB-cAMP, but not 8-Br-cAMP or 8-Br-cGMP, reversed the CAF-induced increase in amplitude of oscillatory PER2::LUC bioluminescence. #P < 0.05, ##P < 0.01 versus vehicle; *P < 0.05, **P < 0.01 versus 1 mM caffeine, Tukey's test. All values are expressed as means ± SEM (n = 4 per group).
Treatment with the AC inhibitor MDL shortens circadian period in a concentration-dependent manner in mouse embryonic fibroblasts. (A) The period of rhythmic PER2::LUC expression was decreased by treatment with MDL, caffeine (CAF) or both compared with cells treated with the vehicle (VEH) water. (B) MDL treatment blocked the caffeine-induced increase in amplitude of oscillatory PER2::LUC expression. #P < 0.05, ##P < 0.01 versus vehicle; *P < 0.05, **P < 0.01 versus 1 mM caffeine; Tukey's test. All values are expressed as means ± SEM (n = 4 per group, except of 1 mM caffeine group; n = 6).
Effect of treatment with the PKA inhibitor H-89 and exchange protein directly activated by cAMP 1 (Epac1) antagonist BFA on circadian rhythmicity in mouse embryonic fibroblasts. (A, B) The period of oscillatory PER2::LUC bioluminescence was unchanged by application of H-89, but the caffeine (CAF)-induced increase in amplitude was attenuated by H-89. #P < 0.05, ##P < 0.01 versus vehicle (VEH); *P < 0.05, **P < 0.01 versus 1 mM caffeine; Tukey's test. (C, D) BFA enhanced caffeine (CAF)-induced lengthen of period, but suppressed caffeine-induced increase of amplitude. (C) ##P < 0.01 versus vehicle (VEH); **P < 0.01 versus 1 mM caffeine. $$ P < 0.01 versus 1 μM BFA; Tukey's test. (D) *P < 0.05 versus 1 mM caffeine; Dunn's test. All values are expressed as means ± SEM (n = 4 per group).
Chronic consumption of caffeine causes a phase delay in peripheral clocks under constant darkness. Mice maintained in constant darkness were given water with 0.1% caffeine (CAF) for 14 days after pretreatment with regular water for 7 days. On the last day, in vivo monitoring of PER2::LUC expression was initiated at ZT 9. (A, upper panels) Double-plotted actograms for mice treated with water (vehicle) or CAF. Horizontal black bars indicate the period of constant darkness; vertical black bars indicate recorded activity; blue bars indicate the period of in vivo monitoring. (Lower panels) The free-running activity period was assessed by χ2 periodogram analysis for 7 days before (PRE) and after (POST) caffeine application. (B) Average peak phase of PER2::LUC oscillations in each peripheral tissue. Caffeine treatment caused phase shifts in the liver and submandibular gland (Sub Gla). #P < 0.05 versus vehicle (VEH) (Mann–Whitney test) (n = 4 for VEH; n = 5 for CAF). (C) Daily average bioluminescence amplitude in each tissue. ##P < 0.01 versus VEH (Student's t-test). All values are expressed as mean ± SEM.
Transient treatment with caffeine at specific times during the 24 h cycle induces phase shifts in peripheral clock oscillations. Mice maintained under 12:12 h light–dark conditions were injected with vehicle (VEH) or 20 mg·kg−1 caffeine (CAF) for three consecutive days at ZT 1, 5, 12 or 21; in vivo monitoring of oscillatory PER2::LUC bioluminescence was initiated at ZT 9. (A) Rhythmic PER2::LUC in peripheral organs of mice injected with caffeine at ZT 12, 21 or 1. Phase shifts were observed in the kidney and submandibular gland (Sub Gla) when injections were performed at ZT 21 and 1. (B) Average peak phases of PER2::LUC oscillations in each tissue from (A). ZT 12: n = 4 (VEH), n = 5 (CAF); ZT 21: n = 4 for each group; ZT 1: n = 4 (VEH), n = 5 (CAF). #P < 0.05, ##P < 0.01 versus VEH (Student's t-test, except of Sub Gla at ZT 1 for Mann–Whitney test). (C) Daily average bioluminescence amplitude in each tissue. #P < 0.05, ##P < 0.01 versus VEH (Student's t-test). All values are expressed as mean ± SEM.
Transient treatment with caffeine at ZT 5 induces a phase advance in peripheral clocks in male mice. Male mice maintained under 12:12 h light–dark conditions were given i.p. injections of vehicle (VEH) or 20 mg·kg−1 caffeine (CAF) for three consecutive days at ZT 5; in vivo monitoring was initiated at ZT 9. (A) Oscillatory PER2::LUC bioluminescence in various tissues. Phase advances were observed in the kidney, liver and submandibular gland (Sub Gla) upon treatment with caffeine. (B) Daily average bioluminescence amplitude in each tissue. (C) Peak phase of PER2::LUC oscillations in each tissue indicated in (A). ##P < 0.01 versus VEH (Student's t-test). All values are expressed as mean ± SEM (n = 4 per group).