Abstract
Background and Purpose
Spinal astrocytes have emerged as important mechanistic contributors to the genesis of mechanical allodynia (MA) in neuropathic pain. We recently demonstrated that the spinal sigma non-opioid intracellular receptor 1 (σ1 receptor) modulates p38 MAPK phosphorylation (p-p38), which plays a critical role in the induction of MA in neuropathic rats. However, the histological and physiological relationships among σ1, p-p38 and astrocyte activation is unclear.
Experimental Approach
We investigated: (i) the precise location of σ1 receptors and p-p38 in spinal dorsal horn; (ii) whether the inhibition of σ1 receptors or p38 modulates chronic constriction injury (CCI)-induced astrocyte activation; and (iii) whether this modulation of astrocyte activity is associated with MA development in CCI mice.
Key Results
The expression of σ1 receptors was significantly increased in astrocytes on day 3 following CCI surgery. Sustained intrathecal treatment with the σ1 antagonist, BD-1047, attenuated CCI-induced increase in GFAP-immunoreactive astrocytes, and the treatment combined with fluorocitrate, an astrocyte metabolic inhibitor, synergistically reduced the development of MA, but not thermal hyperalgesia. The number of p-p38-ir astrocytes and neurons, but not microglia was significantly increased. Interestingly, intrathecal BD-1047 attenuated the expression of p-p38 selectively in astrocytes but not in neurons. Moreover, intrathecal treatment with a p38 inhibitor attenuated the GFAP expression, and this treatment combined with fluorocitrate synergistically blocked the induction of MA.
Conclusions and Implications
Spinal σ1 receptors are localized in astrocytes and blockade of σ1 receptors inhibits the pathological activation of astrocytes via modulation of p-p38, which ultimately prevents the development of MA in neuropathic mice.
Tables of Links
| LIGANDS |
|---|
| BD-1047 |
| SB203580 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c,,).
Introduction
Chronic pain, such as peripheral neuropathic pain, can be characterized by sensory disorders that include mechanical allodynia (MA, lowering of response threshold to light tactile stimuli) and thermal hyperalgesia (TH, an increased response to a noxious thermal stimulus). The development of neuropathic pain is associated with a variety of pathophysiological changes (Ueda, 2006; Latremoliere and Woolf, 2009) including peripheral sensitization (the increased sensitivity of nociceptive primary afferent neurons) and central sensitization (hyperexcitability of nociceptive neurons in the dorsal horn of the spinal cord). The precise spinal cord mechanisms underlying the development of MA and TH remain to be clearly defined, despite the fact that a number of studies have reported different signalling pathways involved with the development of MA versus TH (Ossipov et al., 1999; Roh et al., 2008a).
Sigma non-opioid intracellular receptors 1 (σ1 receptors) are involved in a variety of cellular mechanisms but this involvement appears to occur via a common mechanism of regulating intracellular Ca2+ concentrations (Guitart et al., 2004; Su et al., 2010). The σ1 receptor is characterized by a unique mode of action via the regulation of both Ca2+ entry at the plasma membrane level and Ca2+ mobilization from endoplasmic stores (Monnet, 2005). The role of σ1 in modulating central sensitization associated with the development of neuropathic pain has recently been investigated. De la Puente et al. reported that σ1 receptor knockout mice did not show cold and MA and also did not exhibit increased phosphorylation of ERK in the spinal cord after sciatic nerve injury (de la Puente et al., 2009). These results are in agreement with the observation that intrathecal (i.t.) injection of the σ1 antagonist, BD-1047, attenuated MA, but not TH, when administered during the induction phase (days 0–5 following sciatic nerve chronic constriction injury, CCI), but did not attenuate MA when administered during the maintenance phase (days 15–20 after CCI) in CCI rats (Roh et al., 2008c). Recently, a study from our laboratory showed that the activation of spinal σ1 receptors is associated with the phosphorylation of p38 MAPK (p-p38) in the spinal cord dorsal horn (Moon et al., 2013). Based on these findings, we hypothesized that σ1 receptor modulation of p38 activation plays an important role in the induction of MA in neuropathic pain.
Although it is well recognized that σ1 receptors are widely distributed in mammalian peripheral organs and throughout the CNS (Hellewell et al., 1994; Alonso et al., 2000; Palacios et al., 2003), the identity of specific cell types expressing σ1 receptors in the spinal cord dorsal horn is unknown. Identification of the cell type expressing the σ1 receptors would provide an important clue to understanding the role of σ1 receptors and p-p38 in relation to development of chronic MA. It is increasingly recognized that glial cells (astrocytes and microglia) play an important role in chronic pain processing (Gosselin et al., 2010). In particular, astrocytes represent the most abundant cells in the CNS and dynamically modulate neuronal function under both physiological and pathological conditions (Halassa et al., 2007; Gao and Ji, 2010b; Wang et al., 2012). Accumulating evidence suggests that astrocyte activation contributes to the development and maintenance of chronic pain induced by nerve injury, inflammation, paclitaxel and paw incision. (Xu et al., 2007; Gao et al., 2009; Ikeda et al., 2012; Zhang et al., 2012). Furthermore, Ji et al. recently reported that MA was dose dependently attenuated by i.t. administration of L-α-aminoadipate (a specific astrocyte inhibitor) in a rat chemotherapy-induced neuropathic pain model (Ji et al., 2013). Moreover, i.t. administration of L-α-aminoadipate reversed complete Freund's adjuvant (CFA)-induced MA, while it produced no effect on the CFA-induced heat hyperalgesia (Gao and Ji, 2010a). These data imply that spinal astrocytes can play an important role in regulating MA but not TH under chronic pain conditions.
Therefore, the present study was designed to examine: (i) the precise cellular location of σ1 receptors and p-p38 in spinal cord dorsal horn; (ii) whether the inhibition of σ1 receptors or p38 modulates CCI-induced astrocyte activation; and finally (iii) whether this modulation of astrocyte activity is associated with MA development in CCI mice.
Methods
Animal preparation
Male ICR mice (25–30 g) were purchased from the Laboratory Animal Center of Seoul National University (Seoul, Korea). They had free access to food and water and were maintained in temperature- and light-controlled rooms (23 ± 2°C, 12/12h light/dark cycle with lights on at 08:00) for at least 1 week before beginning an experiment. The experimental protocols for animal usage were reviewed and approved by the SNU Animal Care and Use Committee and conform to NIH guidelines (NIH publication No. 86-23, revised 1985). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A CCI of the common sciatic nerve was performed by setting three loose ligatures of 6-0 silk according to the method described by Bennett and Xie (1988). Mice were anaesthetized by i.p. injection with 50 μL of a combination of Zoletil 50® (Virbac, Carros, France), Rompun® (Bayer AG, Leverkusen, Germany) and saline (a ratio of 2:1:2 respectively). The total number of mice used in the study was 242.
Intrathecal (i.t.) drug injection
For i.t. injection, we used the modified method of direct transcutaneous i.t. injection in mice (Hylden and Wilcox, 1980). The flick of the tail was considered indicative of a successful i.t. administration. All drugs administered to experimental and control mice were dissolved in 5 μL of vehicle. I.t. treatments with the drugs were performed twice a day on post-operative days 0–3 (induction period).
The following drugs were used: N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino) ethylamine dihydro-bromide [BD-1047, a σ1 receptor antagonist; 100 nmol, Tocris Cookson Ltd (Bristol, UK)]; 4-(4-fluorophenyl)-2-(4-methylsulfonylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580, a p38 inhibitor; 0.1, 0.3, 1, 3, 10 nmol); fluorocitrate (an astroglial metabolic inhibitor, 0.03, 0.001, 0.003 nmol). SB203580 and fluorocitrate were supplied by Sigma-Aldrich (St. Louis, MO, USA). The doses of drugs used in the present study were based on those used in previous studies from our laboratories showing maximal effects with no detectable side effects (Roh et al., 2008b,c,; Kang et al., 2011; Moon et al., 2013). BD-1047 and fluorocitrate were dissolved in physiological saline, while SB203580 was dissolved in 1% DMSO in saline. The nomenclature regarding receptors conforms to the British Journal of Pharmacology's Concise Guide to PHARMACOLOGY (Alexander et al., 2013a,b,).
Behaviour assessments
Behavioural tests were performed 1 day before CCI or sham surgery in all mice to obtain normal baseline values of withdrawal response to mechanical and thermal stimuli. Animals were tested again following CCI or sham surgery for a period of 21 days as described above. Animals were randomly assigned to experimental groups and all behavioural analyses were performed blindly.
Responses to mechanical and heat stimuli were evaluated as described in previous studies from our laboratories (Roh et al., 2008b; 2011,). Sensitization to innocuous mechanical stimulation (MA) was examined using a 0.16 g von Frey filaments (North Coast Medical, Morgan Hill, CA, USA). The results of the mechanical response testing in each experimental animal were expressed as % withdrawal response frequency (PWF, %) to 10 applications of the filament. To assess thermal nociceptive responses (TH), paw withdrawal response latency (s) was measured by using the plantar paw-flick latency test as previously described by Hargreaves et al. (1988). The test was repeated in the ipsilateral hind paw of each animal, and the mean withdrawal latency was calculated. Cut-off time in the absence of a response was set at 20 s.
Western blotting analysis
After CCI or sham surgery, the mouse spinal cords were removed and collected to examine possible increases in σ1 receptor and GFAP expression. The ipsilateral dorsal quadrant (which included the entire ipsilateral dorsal horn) from each CCI or sham mouse was subsequently processed for Western blot analysis according to the method detailed in our previous reports (Roh et al., 2011; Moon et al., 2013). The membranes were blocked with 5% skim milk for 1 h at room temperature (RT) and incubated at 4°C overnight with a primary antibody specific for β-actin (1:5000, loading control, Santa Cruz Biotechnology Inc., CA, USA), GFAP (1:1000, Chemicon International Inc., CA, USA) or for σ1 receptor (1:1000, anti-OPRS1 antibody, ab53852, Abcam Inc., Cambridge, MA, USA). The membranes were washed and primary antibodies were detected using goat anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase. The bands were visualized with enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK). The positive pixel area of specific bands was measured with a computer-assisted image analysis system and normalized against the corresponding β-actin loading control bands. The mean value of the ratio in sham injury animals was set at 100%. Thus, the % change relative to the sham surgery condition was then calculated for each time point in each group.
Immunohistochemistry
Mice were administered an overdose of anaesthetic and perfused with fixative, and σ1 receptor immunohistochemical staining was subsequently performed on spinal cord sections according to the method detailed in our previous reports (Roh et al., 2011; Moon et al., 2013). To confirm the specificity of the σ1 receptor antibody immunoreactivity, we performed a pre-absorption test in which the antibody was mixed with the OPRS1 recombinant protein (25 μg of peptide·mL−1 of diluted primary antibody, Novus Biologicals, Littleton, CO, USA) overnight at 4°C prior to staining.
In CCI mice, on day 3 post-surgery, transverse spinal cord sections were incubated in blocking solution for 1 h at RT and then incubated for 48 h at 4°C with one of the following two primary antibodies: rabbit anti-σ1 receptor antibody (1:1000) or mouse anti-GFAP (1:1000). Following incubation, tissue sections were washed and incubated for 1 h at RT in secondary antibodies. Cyanine (Cy3) anti-rabbit IgG (1:200, Jackson ImmunoResearch, West Grove, PA, USA) and Alexa fluor 488 anti-mouse IgG (1:200, 1 h at RT, Invitrogen, Carlsbad, CA, USA) antibodies were used as the secondary antibodies respectively. Double-immunofluorescence labelling was used to study the distribution of σ1 receptors and p-p38 in spinal cord dorsal horn cells. For double immunofluorescence staining, floating sections were first incubated overnight at RT with a rabbit anti-σ1 receptor or a rabbit anti-p-p38 (1:1000, #4511, Cell Signaling technology, Beverly, MA, USA). After being washed with TPBS, the sections were then incubated for 2 h at RT with a Cy3-conjugated anti-rabbit IgG antibody (1:200). Then the slices were washed again and incubated overnight at RT with GFAP, neuronal-specific nuclear protein (NeuN) (mouse, 1:1000; Millipore, Billerica, MA, USA) or Iba-1 (goat, 1:500, Abcam) followed by Alexa fluor 488 anti-mouse IgG secondary antibody (1:200) for 2 h at RT. The slides were viewed under a confocal microscope (Fluoview4.3; Olympus, Shinjuku, Tokyo, Japan).
To analyse images, three to five spinal cord sections from the L4-5 lumbar spinal cord segments were randomly selected from each animal, and were analysed using a computer-assisted image analysis system (Metamorph version 7.7.2; Molecular Devices Corporation, Downingtown, PA, USA). The average number of GFAP-ir cells from each animal was obtained and these values were averaged across each group and presented as group data. To maintain a constant threshold for each image and to compensate for subtle variabilities in the immunostaining, we only counted cells that were at least 45% brighter than the average level of each image after background subtraction and shading correction. To analyse colocalization images, pairs of fluorescent images were acquired on the confocal microscope as green and red channels. A qualitative analysis of σ1 receptor and p-p38 antibody colocalization was performed using Metamorph. The background for each image was subtracted by an automatic algorithm without user intervention before analysis. Overlap of the red/green images was visualized in merged images as yellow pixels, and areas of overlap were considered colocalized. The extent of colocalization of σ1 receptors with GFAP, NeuN or Iba-1 was analysed using Pearson's correlation coefficient for Metamorph. The range of values of the correlation coefficient is −1.0 to +1.0. A value of 1.0 shows that the data are perfectly matched with one another and a value of −1.0 is observed when there is an inverse relationship between intensities in the two images (Dunn et al., 2011). As negative control, the same images were used, but one of the images was rotated by 90° and then the same analysis was repeated. The extent of colocalization of p-p38 with NeuN was measured as the number of areas of overlap between the two fluorescent probes in each spinal cord region using Metamorph. To analyse the extent of colocalization of p-p38 in GFAP-labelled cells, we directly quantified the number of cells showing astrocytic nuclei that contained p-p38 immunolabelling. We quantified immunostaining in the following three dorsal horn regions: (i) the superficial dorsal horn (SDH, laminae I and II); (ii) the nucleus proprius (NP, laminae III and IV); and (iii) the neck region (NECK, laminae V and VI) as previously reported (Moon et al., 2013). All analytical procedures described above were performed blindly without knowledge of the experimental conditions.
Statistical analysis
All values are expressed as the mean ± SEM. Statistical analysis was performed using Prism 5.0 (Graph Pad Software, San Diego, CA, USA). Repeated measures two-way anova was used to determine overall differences in the time-course of all nociceptive behavioural tests. One-way anova was used to determine differences across all experimental groups (immunohistochemistry and Western blot assay). Post-hoc analysis was performed using the Bonferroni's multiple comparison test in order to determine the P-value among experimental groups. P < 0.05 was considered statistically significant.
Results
CCI-induced changes in σ1 receptor expression in the dorsal horn of neuropathic mice
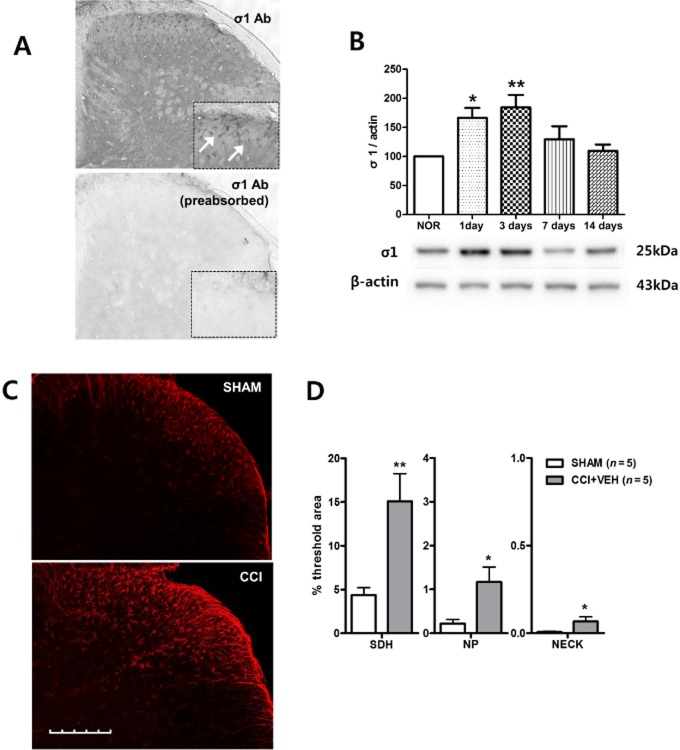
In this study, we utilized a σ1 receptor antibody (anti-OPRS1 antibody) to stain mouse lumbar spinal cord sections 3 days after CCI surgery. The specificity of the antibody was first tested using a pre-absorption test with a σ1 receptor recombinant protein. σ1 receptor-immunoreactivity was not detected in any of the spinal sections processed with pre-absorbed σ1 receptor antibody (Figure 1A). The lack of immunostaining in the specificity controls validates the specificity of the antibody. Next, we performed Western blot analysis because spinal σ1 receptor expression has not been previously reported in CCI mice. There was a significant CCI-induced increase in σ1 receptor expression on Western blots that peaked at 3 days post-surgery and this increased expression was restored to normal pre-CCI values by 7 days post-surgery (Figure 1B). The increase of σ1 receptor expression induced by CCI in mice was similar to that of CCI rats as previously reported (Roh et al., 2008c). To observe the distribution of σ1 receptor expression in the spinal cord, we performed immunohistochemistry using the anti-σ1 receptor antibody on day 3 after sham or CCI surgery (Figure 1C). In the sham group, σ1 receptor-ir cells were observed only in the SDH. Much fewer σ1 receptor-ir cells were detected in the deeper laminae (Figure 1C and D). However, on day 3 post-CCI surgery, σ1 receptor expression was significantly increased in the SDH, but also in the NP and NECK as compared with that of the sham group.
Figure 1.
Sigma non-opioid intracellular receptor 1 (σ1) expression is significantly increased on day 3 post-CCI in the mouse spinal cord dorsal horn. (A) σ1 receptor-immunoreactivity was not detected in any of the spinal sections processed with σ1-antisera pre-absorbed with a σ1 peptide overnight. (B) Western blot analysis indicated that σ1 receptor expression was significantly increased by postoperative day 1 and reached a peak level by postoperative day 3 when compared with σ1 receptor expression in sham surgery (NOR) animals (n = 9 at each time point in the CCI or sham groups). A graph depicting the change in σ1 receptor expression is shown in the upper portion, and the representative bands of σ1 receptor and β-actin expression are presented in the lower portion. (C, D) Representative photomicrographs of σ1 receptor-immunoreactive (ir) cells in the superficial layers of the ipsilateral spinal cord dorsal horn and a graph quantifying the changes in σ1 receptor expression in sham and CCI animals. On post-operative day 3, σ1 receptor expression was significantly increased in the ipsilateral superficial layers of the dorsal horn, but was also increased in the deeper laminae of CCI mice compared with the SHAM-operated controls. *P < 0.05 and **P < 0.01 as compared with those of the SHAM group. Scale bar, 200 μm.
Cellular distribution of σ1 receptors in the spinal cord dorsal horn in CCI mice
To determine which specific cell types express σ1 receptors on the ipsilateral dorsal horn in CCI mice, double staining was performed at day 3 post-CCI using an anti-σ1 receptor antibody in combination with antibodies specific for astrocytes (GFAP), neurons (NeuN), or microglial cells (Iba-1). Double immunostaining with GFAP showed that the increased expression of σ1 receptors was located to astrocytes (Figure 2A and D). No co-expression of σ1 receptor was observed with the NeuN, a neuronal marker (Figure 2B), or Iba-1, a microglial marker (Figure 2C). Pearson's coefficient (r) was used to quantify the degree of colocalization of σ1 receptor with GFAP, NeuN or Iba-1 (Figure 2E). There was a high correlation between σ1 receptor and GFAP-ir cells in the spinal cord of CCI mice (r = 0.801). The average correlation coefficient dropped when the same region was analysed again after one of the two images of the image pair had been rotated 90 degrees (r = 0.011). In contrast, the average correlation coefficient between σ1 receptor and NeuN was −0.0893. While the correlation between the σ1 receptor and Iba-1 was a little higher (r = 0.129), there was no significant difference in the average when it was compared with the average of the same images, when one member of the pair was rotated 90 degrees (Figure 2E). These results indicate that the co-expression values that we obtained provided a meaningful measure of the relative colocalization σ1 receptor and GFAP expression in spinal cord sections (Dunn et al., 2011) and suggest that σ1 receptor expression occurs primarily in astrocytes. 2–12.
Figure 2.
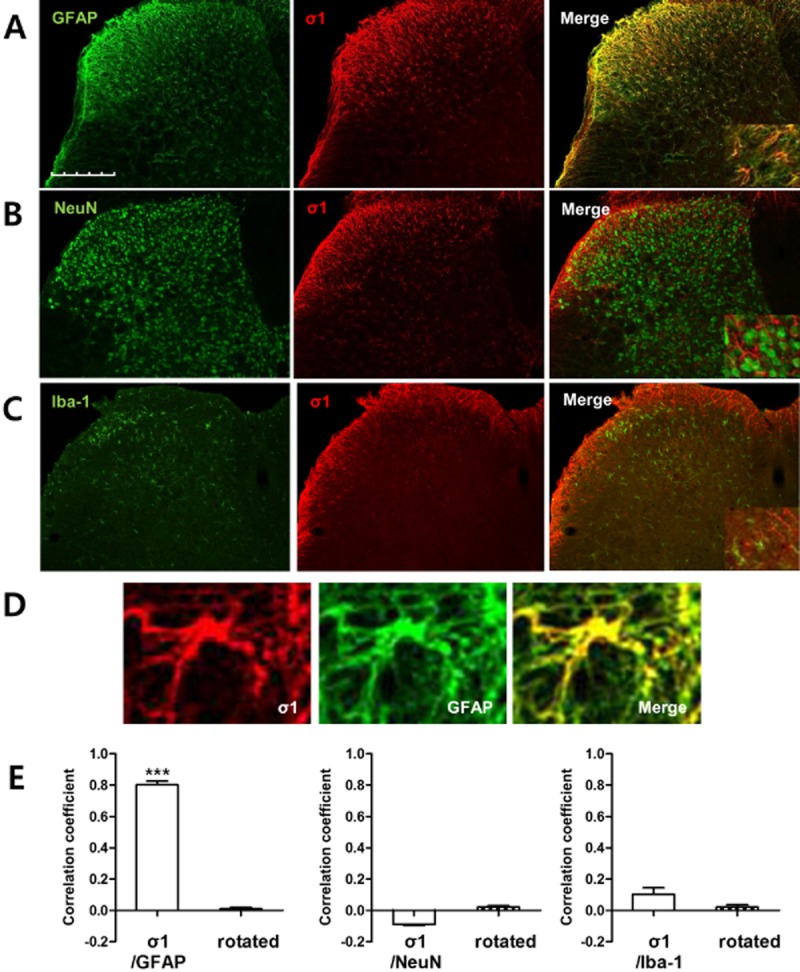
Sigma non-opioid intracellular receptor 1 (σ1) expression was selectively increased in astrocytes after CCI. Transverse sections through the lumbar spinal cord obtained from mice at day 3 post-CCI were processed for immunocytochemistry. (A–C) Immunohistochemical labelling was performed with an antibody against σ1 receptor (σ1, red) and double labelled with GFAP, a marker for astrocytes, NeuN, a marker for neurons or Iba-1, a marker for microglia, antibodies (green). σ1 receptor-ir cells were colocalized with GFAP-ir cells, but not with NeuN or Iba-1 immunostained cells at postoperative day 3 in the ipsilateral dorsal horn spinal cord of CCI mice. (D) Representative fluorescent photomicrographs depicting immunolabelling for σ1 receptor (red) and the astrocyte marker, GFAP (green). Double immunolabelling for σ1 receptor and GFAP (yellow). (E) The average correlation coefficient between σ1 receptor and GFAP was 0.801, but this correlation coefficient was significantly reduced when a region within the spinal cord dorsal horn in one of the images was rotated 90° with respect to the other image. In contrast, the average correlation coefficient of σ1 receptor with either NeuN or Iba-1 was very low (n = 6 for each of the CCI groups). ***P < 0.001 as compared with those of the rotated group. Scale bar, 200 μm.
Effects of i.t. BD-1047 administration on the expression of GFAP in CCI mice
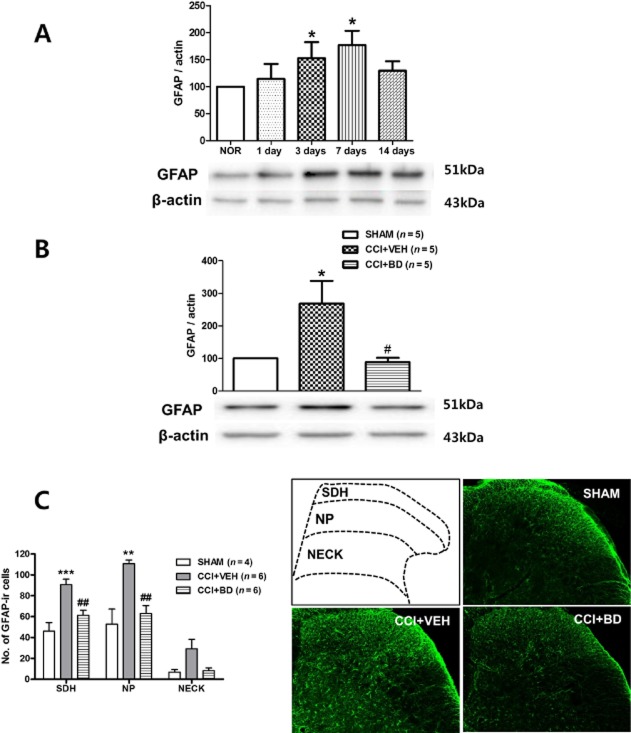
We performed a Western blot analysis and immunohistochemistry to examine whether the CCI-induced increase in GFAP expression was regulated by σ1 receptor activation during the induction phase. In Western blot analysis, the expression of GFAP on immunoblots was significantly increased from days 3 to 7 after CCI (Figure 3A). I.t. administration of the σ1 receptor antagonist, BD-1047 (100 nmol, CCI + BD) on post-operative days 0–3 significantly attenuated the CCI-induced increase in GFAP expression on day 3 post-CCI surgery as compared with the vehicle-treated group (Figure 3B). Immunohistochemistry analysis also confirmed that i.t. administration of BD-1047 effectively attenuated the CCI-induced increase in the number of GFAP-ir cells in the SDH and NP region (Figure 3C).
Figure 3.
The CCI-induced increase in GFAP expression is blocked by spinal injection of the σ1 receptor antagonist, BD-1047. (A) Western blot analysis indicated that GFAP expression was significantly increased by post-operative day 3 and reached a peak level by post-operative day 7 when compared with that of sham surgery (NOR) animals (n = 5 at each time point in the CCI or sham groups). (B) Western blot analysis showed that i.t. injection of BD-1047 (CCI + BD, 100 nmol, administered from days 0 to 3 after surgery) significantly decreased the level of CCI-induced GFAP expression as compared with the vehicle-treated group. A graph depicting the change in the GFAP is shown in the upper portion, and the representative bands of GFAP and β-actin expression are presented in the lower portion of (B). (C) Immunohistochemistry also demonstrated that i.t. treatment with BD-1047 robustly suppressed the number of GFAP-ir cells in ipsilateral spinal cord dorsal horn as compared with the vehicle-treated group. Photomicrographs of representative L4-5 spinal cord sections illustrating GFAP-ir cells in the sham group (SHAM), in the saline-treated CCI group (CCI + VEH) and in the BD-1047-treated CCI group (CCI + BD). An illustration depicting the location of the different spinal cord regions analysed in this study is shown in the upper left panel of this figure. *P < 0.05, **P < 0.01 and ***P < 0.001 as compared with those of the SHAM group, and #P < 0.05 and ##P < 0.01 as compared with those of the CCI + VEH group.
Effects of BD-1047, fluorocitrate or concomitant fluorocitrate and BD-1047 treatment on the development of CCI-induced MA and TH
We first confirmed the antinociceptive effect of the σ1 receptor antagonist, BD-1047 (BD) during the induction phase (Figure 4A and B). Sustained i.t. treatment with BD-1047 reduced the CCI-induced increase in PWF (%) to innocuous mechanical stimuli in a dose-dependent manner (Figure 4A). On the other hand, repeated i.t. administration of BD-1047 did not affect the CCI-induced TH (Figure 4B). These effects of BD-1047 treatment are similar to those reported previously by our laboratories in rats (Roh et al., 2008c; Moon et al., 2013). To confirm the contribution of astrocyte activation to the CCI-induced pain behaviour, the astroglial metabolic inhibitor, fluorocitrate was injected intrathecally on postoperative days 0–3. Similar to the antinociceptive effect of BD-1047, i.t. treatment with fluorocitrate (0.003, 0.01, 0.03 nmol) significantly attenuated the CCI-induced MA in a dose-dependent manner (Figure 4C). On the other hand, CCI-induced TH was not influenced by repeated i.t. treatment of fluorocitrate (Figure 4D). While i.t. treatment of either a low dose of BD-1047 (10 nmol) alone or a low dose of fluorocitrate (0.003 nmol) alone did not alter the MA, the combination of the two treatments (Fc + BD) significantly suppressed MA development (Figure 4E). These results suggest a significant interaction between σ1 receptors and astrocyte activation. However, CCI-induced TH was not affected by concomitant BD-1047 and fluorocitrate treatment (Figure 4F).
Figure 4.
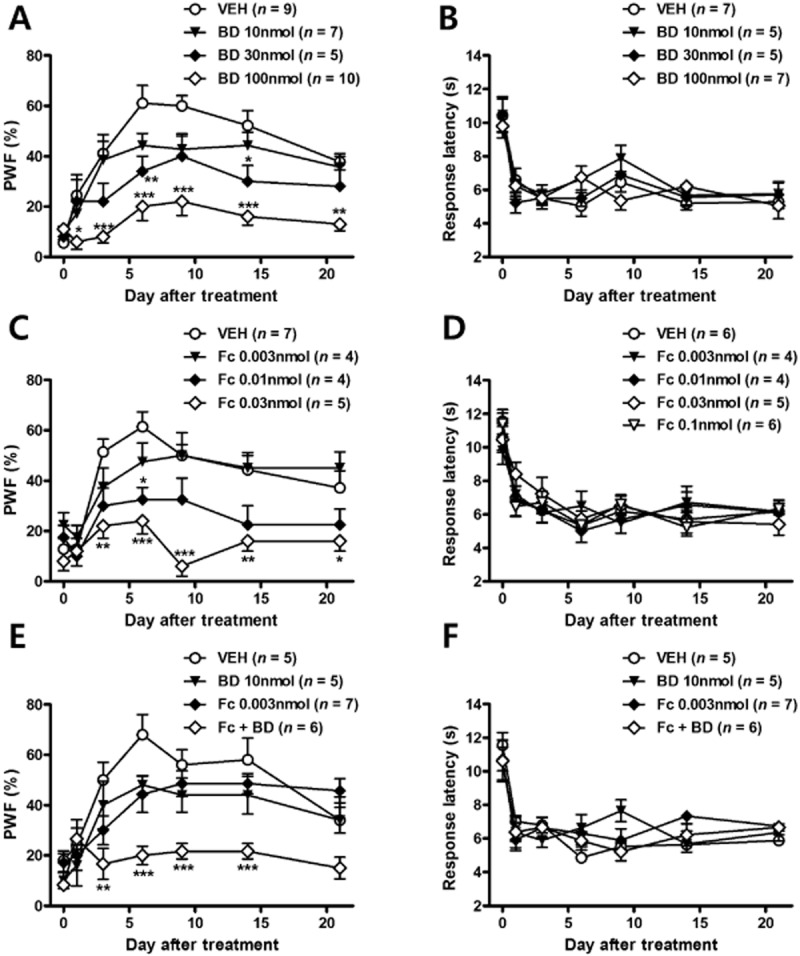
The concomitant treatment with low doses of BD-1047 and fluorocitrate also reduced the development of MA, but had no effect on CCI-induced TH. (A) I.t. injection of BD-1047 in CCI mice blocked the increase in response frequency (%) that occurring in vehicle-treated CCI mice in a dose-dependent manner. (B) However, the decrease in response latency (s) to heat stimuli was unaffected by repeated i.t. treatment with even the highest dose of BD-1047 tested (100 nmol). (C, D) Repeated daily treatment with fluorocitrate significantly attenuated MA, but not TH as compared with the vehicle-treated group (E, F). When a combination of BD-1047 and fluorocitrate was given, MA was reduced, compared with the effects shown in either the BD-1047 or fluorocitrate alone treatment groups. TH was not affected by concomitant BD-1047 and fluorocitrate treatment. *P < 0.05, **P < 0.01 and ***P < 0.001 as compared with those of CCI + VEH group.
The localization of p-p38 in spinal cord dorsal horn after CCI
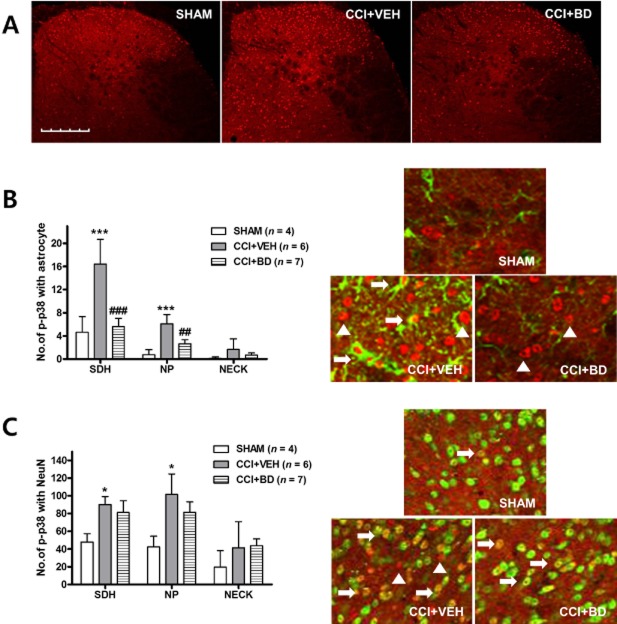
We recently reported that the activation of p38 in the spinal cord contributes to the generation of MA and that p-p38, the active form of p38, is regulated by σ1 receptor activation in CCI rats (Moon et al., 2013). We used an anti-phospho-p38 antibody to confirm the cellular distribution of p-p38 in mice lumbar spinal cord sections on day 3 following CCI surgery. To determine which cell types express p-p38 in the spinal cord dorsal horn in CCI mice, double staining was performed using NeuN, GFAP or an Iba-1 antibody. We found that the majority of the p38 staining was in the nucleus of astrocytes (Figure 5A) or neurons (Figure 5B). There was no evidence of p-p38 staining in Iba-1-positive microglia (Figure 5C).
Figure 5.
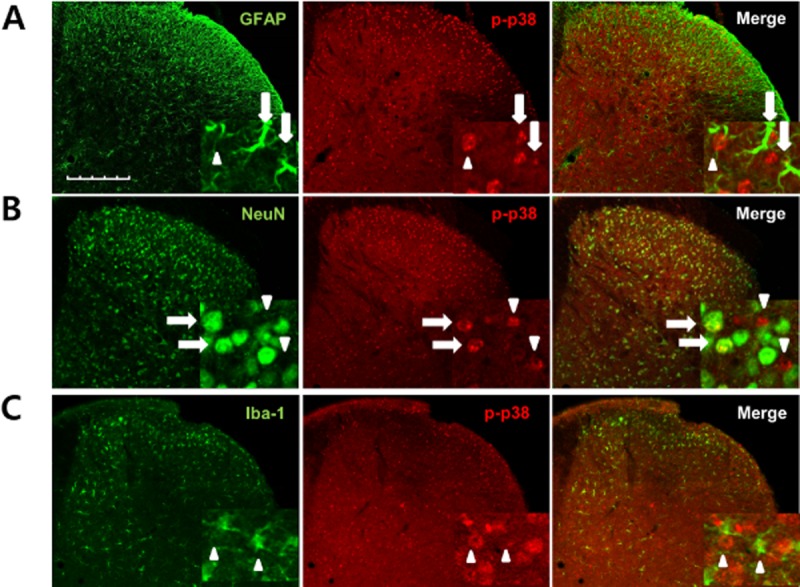
Phosphorylated (p)-p38 was increased in spinal astrocytes and neurons following CCI in mouse ipsilateral spinal cord dorsal horn. (A–C) Transverse sections through the lumbar spinal cord segment 3 days after CCI were labelled with an antibody against p-p38 (red) and double labelled with GFAP, Iba-1 or NeuN antibodies (green). The p-p38-ir staining was preferentially located in the nuclei of GFAP-positive and NeuN-positive cells. (A) Some of the p-p38-labelled cells were GFAP-positive astrocytes (arrows), while others were not GFAP positive (arrowheads). (B) p-p38-labelled cells were clearly double labelled with NeuN antisera, a neuronal marker (arrows), although some phospho-p38-labelled cells were not NeuN-positive neurons (arrowheads). (C) No colocalization was detected for p-p38 and Iba-1, a microglia marker (arrowheads). Scale bar, 200 μm.
Effects of i.t. BD-1047 administration on the expression of p-p38 in astrocytes or neurons in CCI mice
The CCI-induced increased in p-p38 expression was decreased by BD-1047 treatment during the induction phase on day 3 after CCI surgery (Figure 6A). We next performed double staining to examine whether the p-p38 located in astrocytes or neurons or both is regulated by σ1 receptor activation. There was a CCI-induced increase in p-p38 immunostaining in both GFAP- and NeuN-positive cells in the SDH and NP regions of the dorsal horn as compared with that of the sham group (Figure 6B and C). Repeated daily, i.t. administration of BD-1047 significantly decreased the level of CCI-induced p-p38 expression in GFAP-labelled cells, but not in NeuN-labelled cells, as compared with the vehicle-treated group (Figure 6B and C).
Figure 6.
I.t. treatment with the σ1 receptor antagonist, BD-1047 decreased the level of CCI-induced p-p38 expression located in astrocytes, but not in neurons. (A) The CCI-induced increase in p-p38 expression was suppressed by BD-1047 treatment during the induction phase on day 3 after CCI surgery. (B) I.t. administration of BD-1047 decreased the level of CCI-induced p-p38 expression colocalized with GFAP as compared with the vehicle group. (C) However, increased p-p38 expression colocalized with NeuN was not altered by i.t. treatment with BD-1047. *P < 0.05 and ***P < 0.001 and as compared with those of SHAM group, and ##P < 0.01 and ###P < 0.001 as compared with those of the CCI + VEH group. Scale bar, 200 μm.
Effects of i.t. SB203580 administration on the expression of GFAP in CCI mice
We performed a Western blot analysis and immunohistochemistry to examine whether the CCI-induced increase in GFAP expression was regulated by p38 activation. Sustained i.t. administration of the p38 inhibitor, SB203580, (3 nmol, CCI + SB) on post-operative days 0–3 significantly reduced the CCI-induced increase in GFAP expression, as compared with vehicle-treated CCI mice (Figure 7A). Immunohistochemistry analysis also confirmed that the i.t. administration of SB203580 during the induction phase effectively attenuated the CCI-induced increase in the number of GFAP-ir cells in all dorsal horn laminae (Figure 7B).
Figure 7.
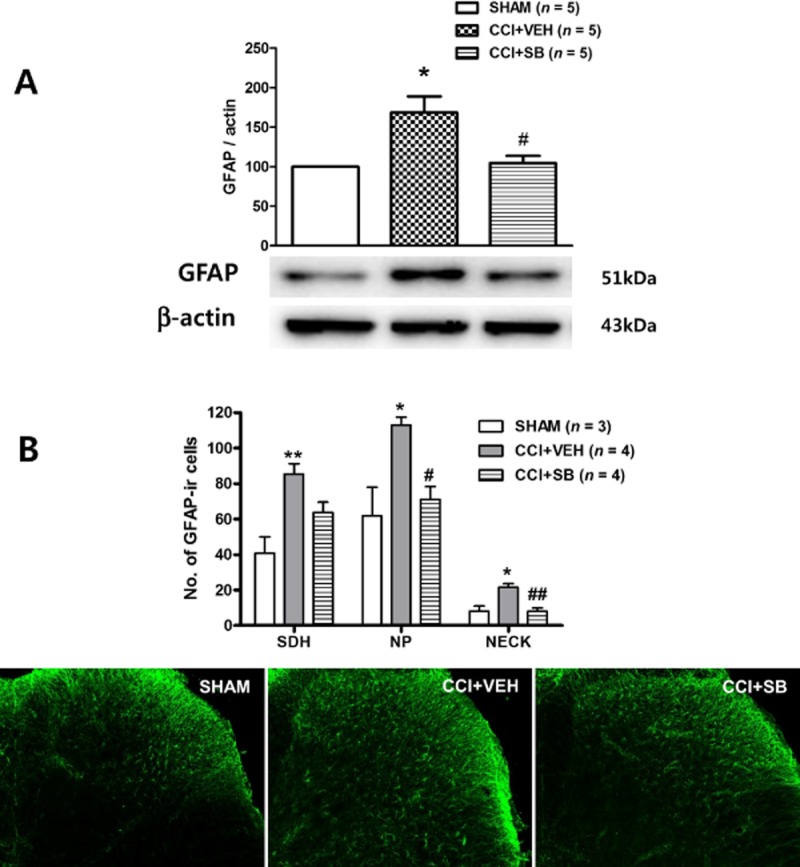
The CCI-induced GFAP increase is blocked by spinal injection of the p38 inhibitor, SB203580. (A) Western blot analysis showed that i.t. injection of SB203580 (CCI + SB, 3 nmol, administered from days 0 to 3 after surgery) significantly decreased the level of CCI-induced GFAP expression as compared with the vehicle-treated group. A graph depicting the change in GFAP is shown in the upper portion of (A), and the representative bands of GFAP and β-actin expression are presented in the lower portion. (B) Immunohistochemistry also demonstrated that i.t. treatment with SB203580 robustly suppressed the number of GFAP-ir cells in the ipsilateral dorsal horn as compared with the vehicle-treated group. Photomicrographs of representative L4-5 spinal cord sections illustrating GFAP-immunoreactive cells in the sham group (SHAM), in the saline-treated CCI group (CCI + VEH) and in the SB203580-treated CCI group (CCI + SB). *P < 0.05 and **P < 0.01 as compared with those of SHAM group, and #P < 0.05 and ##P < 0.01 as compared with those of the CCI + VEH group.
Effects of SB203580 or concomitant fluorocitrate and SB203580 treatment on the development of CCI-induced MA and TH
To confirm the relation between p-p38 expression and astrocyte activation to CCI-induced pain behaviours, we intrathecally administered SB203580 or fluorocitrate on post-operative days 0–3. Sustained i.t. treatment with SB203580 (0.3, 1, 3 nmol) significantly attenuated the CCI-induced increase in PWF (%) to innocuous mechanical stimuli in a dose-dependent manner (Figure 8A). However, SB203580 administration did not affect the CCI-induced TH (Figure 8B). This effect of SB203580 treatment is similar to that previously reported in rats from our laboratory (Moon et al., 2013). While i.t. treatment of either a low dose of SB203580 (0.3 nmol) or a low dose of fluorocitrate (0.003 nmol) alone did not alter CCI-induced MA, the combination of the two treatments (Fc + SB) significantly suppressed MA development (Figure 8C). These results suggest that there is a significant interaction between p-p38 and astrocyte activation. Conversely, CCI-induced TH was not affected by concomitant SB203580 and fluorocitrate treatment (Figure 8D).
Figure 8.
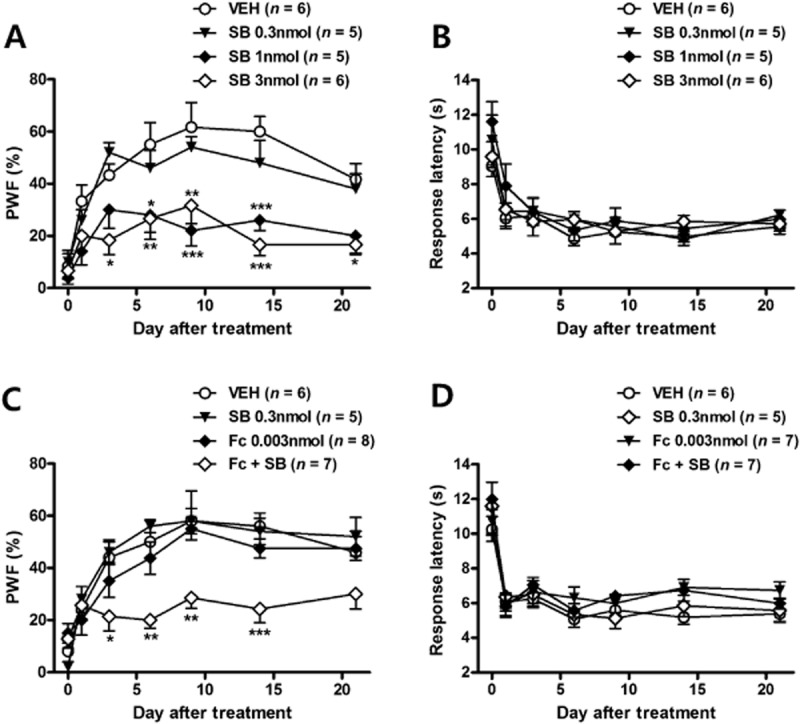
The concomitant treatment with a low dose of SB203580 and a low dose of fluorocitrate also reduced the development of MA, but had no effect on TH. (A) I.t. injection of SB203580 blocked the increase in response frequency (%) that was observed in vehicle-treated CCI mice in dose-dependent manner. (B) However, the decrease in response latency (s) to heat stimuli was unaffected by repeated i.t. treatment with SB203580. (C, D) The lowest dose of SB203580 had no effect on CCI-induced MA and TH. When a combination of SB203580 and fluorocitrate was given, MA was reduced, compared with the effects shown in either the SB203580 or fluorocitrate alone treated group. TH was not affected by concomitant SB203580 and fluorocitrate treatment. *P < 0.05, **P < 0.01 and ***P < 0.001 as compared with those of CCI + VEH group.
Discussion
Although the role of σ1 receptors in central sensitization and pain hypersensitivity has been reported in several pain models (de la Puente et al., 2009; Carlsson et al., 2010; Nieto et al., 2012), the cellular distribution of σ1 receptors in the spinal cord dorsal horn has not been reported previously, particularly as it relates to a chronic pain condition. There are two possibilities with respect to the mechanism underlying the action of spinal σ1 receptors in the induction of chronic pain. The first hypothesis is that σ1 receptors are up-regulated in spinal neurons and can directly modulate these neurons (central terminals of primary afferent neurons or second-order neuron in dorsal horn) under conditions of chronic pain; and the second hypothesis is that spinal cord σ1 receptors can indirectly modulate neuronal activity via a signalling mechanism associated with glial cells (astrocytes and/or microglia). Interestingly, the first finding of the present study demonstrated that σ1 receptor expression is significantly and selectively increased only in astrocytes and not in spinal cord neurons on day 3 post-CCI surgery. This is consistent with the work of Ruscher et al. (2011), which demonstrated a significant increase in σ1 receptor expression in reactive astrocytes and not neurons or microglia after experimental stroke (Ruscher et al., 2011).
A growing number of studies have used Ca2+ as an indicator of astrocytic activity and demonstrated that an increase in cytoplasmic Ca2+ concentration ([Ca2+]i) in astrocytes is correlated with gliotransmitter release and modulation of neuronal activity (Agulhon et al., 2008; Ben Achour et al., 2010). Astrocytes exhibit a large number of G protein-coupled metabotropic receptors (GPCRs) linked to Ca2+ mobilization from internal stores or ionotropic glutamate channels or receptors linked to extracellular Ca2+ entry (Agulhon et al., 2008; Miyano et al., 2010). The stimulation of the GPCRs coupled to PLC hydrolyses the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) to generate DAG and inositol triphosphate (IP3), leading to IP3 receptor (IP3 R) activation and Ca2+ release from the endoplasmic reticulum (ER). It has been reported that σ1 receptors normally reside at a mitochondrion-associated ER membrane where σ1 receptors regulate ER-mitochondrion Ca2+ signalling and ER-nucleus crosstalk (Su et al., 2010). When cells are stimulated by σ1 receptor ligands or undergo prolonged stress, σ1 receptors have been shown to activate IP3-induced Ca2+ efflux from the ER (Hayashi and Su, 2001). In addition, activated σ1 receptors translocate to plasma membrane, thus regulating functional proteins, including ion channels, receptors and kinases (Su et al., 2009; 2010,). Recent studies from our laboratories have also demonstrated that spinal σ1 receptor-mediated nociceptive action is associated with Ca2+-dependent second-messenger cascades including PLC and PKC (Roh et al., 2008b; 2010,), which are also known to be closely linked to an increase in [Ca2+]i in astrocytes. These findings suggest that the increased expression or up-regulation of σ1 receptors in CCI animals occurs primarily in spinal cord astrocytes and thus they can regulate a variety of cellular functions via [Ca2+]i modulation in these glial cells. Conversely, there are several reports that σ1 receptors are also located in motor neurons of spinal cord ventral horn (Mavlyutov et al., 2010; Mancuso et al., 2012) and that σ1 receptor mRNA is found in cultured microglial cells (Gekker et al., 2006) and that σ1 receptors are present in retinal microglia (Zhao et al., 2014) as well as brain astrocytes (Francardo et al., 2014). Clearly, there are some discrepancies among the various studies with regard to which cell types express σ1 receptors and this could be due to the use of different animal species (mouse versus rat versus human) and to the use of different σ1 receptor antibodies. It is also likely that the pattern of cellular distribution of σ1 receptors may different under different physiological or pathological conditions and among different animal models of disease or pain. However, in the mouse CCI model used here, the only significant change in σ1 receptor expression occurred in astrocytes in the spinal cord dorsal horn.
The present study verified that sustained i.t. injection of a σ1 receptor antagonist reduced the CCI-induced increase in GFAP expression as well as the induction of MA. We have previously shown that i.t. injection of the BD-1047 blocked both MA and increases in spinal NMDA receptor GluN subunit1 (GluN1) expression and phosphorylation during the induction phase in CCI rats (Roh et al., 2008c). We also reported that spinal σ1 receptor-induced sensitization is mediated by an increase in neuronal NOS, which is associated with an NO-induced increase in PKC-dependent phosphorylated GluN1 (pGluN1) expression (Roh et al., 2011). However, it is currently unclear if and how σ1 receptors in astrocytes modulate pGluN1 expression in neurons. One possibility is that σ1 receptor activation in astrocytes may release proinflammatory cytokines (such as IL-1β) or chemokines [monocyte chemoattractant protein-1 (MCP-1)], and that these indirectly mediate NMDA receptor activation in neurons. It is known that activated astrocytes can release IL-1β and MCP-1 after nerve injury (Guo et al., 2007; Gao et al., 2009; Zhang et al., 2011a). Several recent studies show that IL-1 receptors colocalize with the GluN1 subunits in neurons of the spinal cord, and IL-1β may bind to its IL-1 receptor to enhance the pGluN1, which ultimately facilitates pain behaviours in inflammatory or bone cancer pain (Guo et al., 2007; Zhang et al., 2008a,b,). In addition, MCP-1 also rapidly enhances NMDA-induced inward currents, which has been strongly implicated in central sensitization and hyperalgesia (Gao et al., 2009; Gao and Ji, 2010b). It is possible that the effect of the σ1 receptor antagonist is mediated by the inhibition of cytokines or chemokines via the modulation of astrocyte activation; however, the precise mechanism of σ1 receptor's action in spinal cord astrocytes must be determined in future studies.
The present study shows that i.t. treatment with the astroglial inhibitor, fluorocitrate, as well as the σ1 receptor antagonist, BD-1047, given during the induction phase of neuropathic pain, significantly reduced the development of MA, but not TH, in CCI mice. Interestingly, low doses of fluorocitrate produced synergic suppressive effects on MA development when combined with low doses of BD-1047 or the p38 inhibitor, SB203580. In addition, the CCI-induced increase in GFAP expression was significantly reduced when BD-1047 or SB203580 were injected intrathecally during the induction phase. Recently, several studies have also reported that spinal astrocytes can directly contribute to the development of MA, but not TH in various pain conditions. GAO et al. reported that i.t. administration of the L-α-aminoadipate on post-CFA day 2 reversed CFA-induced bilateral MA, but not TH (Gao and Ji, 2010a). They also reported that spinal i.t. injection of TNF-α-activated astrocytes produce MA by releasing MCP-1 in naïve mice (Gao et al., 2010c). In addition, Zhang et al. reported that MA can be induced by the i.t. administration of exogenous brain-derived neurotrophic factor-stimulated astrocytes to naïve rats (Zhang et al., 2011b). Furthermore, a single i.t. injection of spinal astrocytes activated by a PKC activator failed to produce TH in naïve mice, while i.t. injection of a microglia cell line activated by ATP significantly decreased paw withdrawal latency to a thermal stimulus (Narita et al., 2006). These results imply that the spinal σ1 receptors can modulate astrocyte activation via phosphorylation of p38 and contribute to the development of MA, but not TH in neuropathic mice.
Finally, we found that the activation of p38 occurs predominantly in both spinal dorsal horn neurons and astrocytes and that i.t. treatment with a σ1 receptor antagonist during the induction phase significantly reduced p-p38 expression in astrocytes, but not in neurons. Although many studies using chronic pain models in rats have reported that p38 is activated exclusively in microglia (Tsuda et al., 2004; Terayama et al., 2008; Wen et al., 2009), we found that p-p38-ir staining was preferentially localized to the nucleus of GFAP-positive astrocytes and NeuN-positive neurons. These results are in line with several studies using chronic pain models in mice. Xu et al. reported p38 was activated in the nucleus of astrocytes or neurons, but that there was no evidence of p-p38 staining in microglia, after partial sciatic nerve ligation (pSNL) in lumbar spinal sections in mice (Xu et al., 2007). They reported that multiple i.t. injections of a p38 inhibitor reduced spinal astrocyte proliferation after pSNL. Zhang et al. also reported recently that the inhibitory effect of TNFα on GABAergic neurons is mediated by p-p38, which is expressed in neurons and astrocytes (Zhang et al., 2010). The expression of TNF receptor 1 and p-38 in spinal astrocytes suggests that astrocytes are involved in the TNF-α-induced spinal disinhibition. Collectively, these results together with our data suggest that the p38 pathway may play an important role in astrocyte modulation and the subsequent induction of MA under chronic pain conditions. Moreover, the fact that i.t. BD-1047 injection specifically inhibited p-p38 expression in astrocytes, but not neurons, provides evidence that furthers our understanding of the possible relationship between p-p38 modulation and the cellular distribution of σ1 receptors in the spinal cord.
In addition, it was previously reported that p38 has at least four different isoforms, α, β, γ and δ, which differ in their substrate preference, activation modes and response to inhibitors (Kumar et al., 2003). The conventional p38 inhibitor, SB203580 non-selectively inhibits both p38α and p38β2 (Barone et al., 2001). In addition, among the four isoforms, p38α and p38β are constitutively expressed in the spinal cord (Svensson et al., 2005). Svensson et al. also demonstrated that intraplantar formalin and i.t. substance P in rats produced nocifensive flinching and p-p38 expression, and this was prevented when spinal p38β, but not p38α, was down-regulated. Meanwhile, in mouse brain, both p38α and p38β are present in neurons, while p38β is also expressed in glial cells (Lee et al., 2000). Thus, this diversity in the pattern of p-p38 subtype expression might reflect the fact that p38 subtypes can be differentially activated under a variety of pain conditions. Thus, it is possible that the different p-p38 subtypes, especially p38α and p38β, are differentially distributed in the spinal cord dorsal horn in CCI mice and affected differentially by σ1 receptor activation.
In conclusion, the current study has demonstrated that i.t. treatment with a σ1 receptor antagonist during the induction phase of CCI-induced neuropathic pain significantly reduces the CCI-induced pathological activation of astrocytes in the spinal cord dorsal horn. Moreover, this effect of a σ1 receptor antagonist on spinal astrocyte activation is mediated in part by the inhibition of p-p38, which can dramatically suppress the induction of MA, but not TH, in neuropathic mice. Collectively, these findings suggest that the pharmacological inhibition of spinal σ1 receptors may be a useful approach for the management of astrocyte-mediated MA development in neuropathic pain patients.
Acknowledgments
This research was supported by the National Research Foundation (NRF) Grant (2014R1A2A2A01007695 and 2012R1A3A2048834) funded by the Korean Government (MSIP), Korea.
Glossary
- [Ca2+]i
cytoplasmic Ca2+ concentration
- BD-1047
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino) ethylamine dihydro-bromide
- CCI
chronic constriction injury
- CFA
complete Freund's adjuvant
- ER
endoplamic reticulum
- GluN1
NMDA receptor GluN1 subunit
- i.t
intrathecal
- IP3
inositol triphosphate
- MA
mechanical allodynia
- MCP-1
monocyte chemoattractant protein-1
- NECK
neck region
- NP
nucleus proprius
- pGluN1
phosphorylated NMDA receptor GluN1 subunit
- PIP2
phosphatidylinositol 4,5-bisphosphate
- p-p38
phosphorylation of p38 MAPK
- PWF
withdrawal response frequency
- SB203580
4-(4-fluorophenyl)-2- (4-methylsulfonylphenyl)-5-(4-pyridyl)-1H-imidazole
- SDH
superficial dorsal horn
- TH
thermal hyperalgesia
- σ1 receptor
sigma non-opioid intracellular receptor 1
Author contributions
J. Y. M. contributed to the writing of the article, analysed the data and carried out the experiments. D. H. R. and S. Y. Y. contributed to the construction of the animal models and the revised article. S. R. C., S. G. K., H. S. C. and S. Y. K. performed the behavioural experiments and collected the samples. H. J. H. performed the histological examinations and molecular biological techniques. A. J. B. helped to revise the article. S.B. O. and J.H. L. designed the experimental programme and revised the article.
Conflict of interest
None.
References
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, et al. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013a;170:449–458. doi: 10.1111/bph.12444. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, et al. Immunocytochemical localization of the sigma-1 receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, et al. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med Res Rev. 2001;21:129–145. doi: 10.1002/1098-1128(200103)21:2<129::aid-med1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ben Achour S, Pont-Lezica L, Bechade C, Pascual O. Is astrocyte calcium signaling relevant for synaptic plasticity? Neuron Glia Biol. 2010;6:147–155. doi: 10.1017/S1740925X10000207. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Ohsawa M, Hallberg M, Nyberg F, Kamei J. Substance P(1–7) induces antihyperalgesia in diabetic mice through a mechanism involving the naloxone-sensitive sigma receptors. Eur J Pharmacol. 2010;626:250–255. doi: 10.1016/j.ejphar.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300:C723–C742. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francardo V, Bez F, Wieloch T, Nissbrandt H, Ruscher K, Cenci MA. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain. 2014;137:1998–2014. doi: 10.1093/brain/awu107. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem. 2010a;115:505–514. doi: 10.1111/j.1471-4159.2010.06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurother. 2010b;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 2010c;58:1871–1880. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. Int Immunopharmacol. 2006;6:1029–1033. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology (Berl) 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Kiritoshi T, Murase K. Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat. Mol Pain. 2012;8:43. doi: 10.1186/1744-8069-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XT, Qian NS, Zhang T, Li JM, Li XK, Wang P, et al. Spinal astrocytic activation contributes to mechanical allodynia in a rat chemotherapy-induced neuropathic pain model. PLoS ONE. 2013;8:e60733. doi: 10.1371/journal.pone.0060733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Kim CY, Roh DH, Yoon SY, Park JH, Lee HJ, et al. Chemical stimulation of the ST36 acupoint reduces both formalin-induced nociceptive behaviors and spinal astrocyte activation via spinal alpha-2 adrenoceptors. Brain Res Bull. 2011;86:412–421. doi: 10.1016/j.brainresbull.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Park J, Che Y, Han PL, Lee JK. Constitutive activity and differential localization of p38alpha and p38beta MAPKs in adult mouse brain. J Neurosci Res. 2000;60:623–631. doi: 10.1002/(SICI)1097-4547(20000601)60:5<623::AID-JNR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Mancuso R, Olivan S, Rando A, Casas C, Osta R, Navarro X. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurother. 2012;9:814–826. doi: 10.1007/s13311-012-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience. 2010;167:247–255. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyano K, Morioka N, Sugimoto T, Shiraishi S, Uezono Y, Nakata Y. Activation of the neurokinin-1 receptor in rat spinal astrocytes induces Ca2+ release from IP3-sensitive Ca2+ stores and extracellular Ca2+ influx through TRPC3. Neurochem Int. 2010;57:923–934. doi: 10.1016/j.neuint.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Monnet FP. Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol Cell. 2005;97:873–883. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- Moon JY, Roh DH, Yoon SY, Kang SY, Choi SR, Kwon SG, et al. Sigma-1 receptor-mediated increase in spinal p38 MAPK phosphorylation leads to the induction of mechanical allodynia in mice and neuropathic rats. Exp Neurol. 2013;247:383–391. doi: 10.1016/j.expneurol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Narita M, Yoshida T, Nakajima M, Narita M, Miyatake M, Takagi T, et al. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochem. 2006;97:1337–1348. doi: 10.1111/j.1471-4159.2006.03808.x. [DOI] [PubMed] [Google Scholar]
- Nieto FR, Cendan CM, Sanchez-Fernandez C, Cobos EJ, Entrena JM, Tejada MA, et al. Role of sigma-1 receptors in paclitaxel-induced neuropathic pain in mice. J Pain. 2012;13:1107–1121. doi: 10.1016/j.jpain.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Bian D, Malan TP, Jr, Lai J, Porreca F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain. 1999;79:127–133. doi: 10.1016/s0304-3959(98)00187-0. [DOI] [PubMed] [Google Scholar]
- Palacios G, Muro A, Vela JM, Molina-Holgado E, Guitart X, Ovalle S, et al. Immunohistochemical localization of the sigma1-receptor in oligodendrocytes in the rat central nervous system. Brain Res. 2003;961:92–99. doi: 10.1016/s0006-8993(02)03892-1. [DOI] [PubMed] [Google Scholar]
- de la Puente B, Nadal X, Portillo-Salido E, Sanchez-Arroyos R, Ovalle S, Palacios G, et al. Sigma-1 receptors regulate activity-induced spinal sensitization and neuropathic pain after peripheral nerve injury. Pain. 2009;145:294–303. doi: 10.1016/j.pain.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, et al. Depletion of capsaicin-sensitive afferents prevents lamina-dependent increases in spinal N-methyl-D-aspartate receptor subunit 1 expression and phosphorylation associated with thermal hyperalgesia in neuropathic rats. Eur J Pain. 2008a;12:552–563. doi: 10.1016/j.ejpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, et al. Intrathecal administration of sigma-1 receptor agonists facilitates nociception: involvement of a protein kinase C-dependent pathway. J Neurosci Res. 2008b;86:3644–3654. doi: 10.1002/jnr.21802. [DOI] [PubMed] [Google Scholar]
- Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, et al. Intrathecal injection of the sigma-1 receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology. 2008c;109:879–889. doi: 10.1097/ALN.0b013e3181895a83. [DOI] [PubMed] [Google Scholar]
- Roh DH, Yoon SY, Seo HS, Kang SY, Moon JY, Song S, et al. Sigma-1 receptor-induced increase in murine spinal NR1 phosphorylation is mediated by the PKCalpha and epsilon, but not the PKCzeta, isoforms. Neurosci Lett. 2010;477:95–99. doi: 10.1016/j.neulet.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Roh DH, Choi SR, Yoon SY, Kang SY, Moon JY, Kwon SG, et al. Spinal neuronal NOS activation mediates sigma-1 receptor-induced mechanical and thermal hypersensitivity in mice: involvement of PKC-dependent GluN1 phosphorylation. Br J Pharmacol. 2011;163:1707–1720. doi: 10.1111/j.1476-5381.2011.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, Gisselsson L, et al. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134:732–746. doi: 10.1093/brain/awq367. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Vaupel DB. When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci Signal. 2009;2:pe12. doi: 10.1126/scisignal.261pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- Terayama R, Omura S, Fujisawa N, Yamaai T, Ichikawa H, Sugimoto T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience. 2008;153:1245–1255. doi: 10.1016/j.neuroscience.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Ueda H. Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther. 2006;109:57–77. doi: 10.1016/j.pharmthera.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Wang S, Song L, Tan Y, Ma Y, Tian Y, Jin X, et al. A functional relationship between trigeminal astroglial activation and NR1 expression in a rat model of temporomandibular joint inflammation. Pain Med. 2012;13:1590–1600. doi: 10.1111/j.1526-4637.2012.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110:155–165. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- Xu M, Bruchas MR, Ippolito DL, Gendron L, Chavkin C. Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase. J Neurosci. 2007;27:2570–2581. doi: 10.1523/JNEUROSCI.3728-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Lv MM, Wang S, Chen L, Qian NS, Tang Y, et al. Spinal astrocytic activation is involved in a virally-induced rat model of neuropathic pain. PLoS ONE. 2011a;6:e23059. doi: 10.1371/journal.pone.0023059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J Neurosci. 2010;30:12844–12855. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yoon SY, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain. 2012;13:293–303. doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008a;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, et al. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. 2008b;154:1533–1538. doi: 10.1016/j.neuroscience.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang J, Zhou Q, Xu Y, Pu S, Wu J, et al. Brain-derived neurotrophic factor-activated astrocytes produce mechanical allodynia in neuropathic pain. Neuroscience. 2011b;199:452–460. doi: 10.1016/j.neuroscience.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ha Y, Liou GI, Gonsalvez GB, Smith SB, Bollinger KE. Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest Ophthalmol Vis Sci. 2014;55:3375–3384. doi: 10.1167/iovs.13-12823. [DOI] [PMC free article] [PubMed] [Google Scholar]