Abstract
Background
Ventricular and supraventricular premature complexes (PC) are frequent and usually symptomatic. According to a previous study, magnesium pidolate (MgP) administration to symptomatic patients can improve the PC density and symptoms.
Objective
To assess the late follow-up of that clinical intervention in patients treated with MgP or placebo.
Methods
In the first phase of the study, 90 symptomatic and consecutive patients with PC were randomized (double-blind) to receive either MgP or placebo for 30 days. Monthly follow-up visits were conducted for 15 months to assess symptoms and control electrolytes. 24-hour Holter was performed twice, regardless of symptoms, or whenever symptoms were present. In the second phase of the study, relapsing patients, who had received MgP or placebo (crossing-over) in the first phase, were treated with MgP according to the same protocol.
Results
Of the 45 patients initially treated with MgP, 17 (37.8%) relapsed during the 15-month follow-up, and the relapse time varied. Relapsing patients treated again had a statistically significant reduction in the PC density of 138.25/hour (p < 0.001). The crossing-over patients reduced it by 247/hour (p < 0.001). Patients who did not relapse, had a low PC frequency (3 PC/hour). Retreated patients had a 76.5% improvement in symptom, and crossing-over patients, 71.4%.
Conclusion
Some patients on MgP had relapse of symptoms and PC, indicating that MgP is neither a definitive nor a curative treatment for late follow-up. However, improvement in the PC frequency and symptoms was observed in the second phase of treatment, similar to the response in the first phase of treatment.
Keywords: Ventricular Premature Complexes; Arrhythmias, Cardiac; Magnesium
Introduction
The incidence of premature ventricular complexe (PVC) and premature supraventricular complexe (PsVC) increases with age, their prevalence being estimated at as much as 50% of the general population1-5. The long-term prognosis is benign, but, when symptomatic, such premature complexe (PC) can be uncomfortable or even disabling. Several pharmacological and non-pharmacological measures for their clinical control have been suggested. However, under certain conditions, the results are limited or the risk-benefit relationship is arguable.
Magnesium (Mg) is an alkaline earth metal that participates in several metabolic processes, such as reactions in ATP generation. It is the second intracellular cation and the fourth cation of the human body6-9. That metal plays a significant role in maintaining proper cardiac rhythm, because of its action on the Na/K- ATPase pump6,7 and its interaction with calcium10. Approximately half of the body Mg is within soft tissue cells, while the other half is within bone tissue8,11. Less than 1% of Mg is found in the blood, and approximately 0.3%, in the serum12,13. Thus, total body Mg has a weak correlation with its serum levels, which can be normal in the presence of low intracellular values14,15.
The Mg intake has decreased in the past century8, probably due to the increased consumption of processed foods8,14,16, which can be a risk factor for Mg deficiency. That deficiency can be associated with cardiovascular disease, such as cardiac arrhythmias8. In selected patients, based on a simple treatment strategy, antiarrhythmics drugs, which can be harmful or poorly tolerated under certain circumstances, can be avoided. Recently, we have shown that Mg pidolate (MgP), after a 30-day continuous supplementation, can decrease ectopic beats and improve related symptoms17. Based on those initial findings, we assessed the outcome of this cohort of patients in the late follow-up.
This study was aimed at: 1) assessing the late clinical outcome of patients initially treated with placebo and/or MgP; 2) assessing whether patients with arrhythmia relapse in a 15-month follow-up, when treated for the second time, have the same response of the first treatment; and 3) assessing the response of patients to MgP after initially receiving placebo.
Methods
This study's methodology has been previously described when assessing the 30-day results17. In the first state of the treatment, ninety consecutive symptomatic patients were randomized (double-blind) to receive either 3.0 g/day of MgP, the equivalent of 260 mg of the Mg element, or placebo, for 30 days. All patients underwent transthoracic echocardiography, and had normal kidney function (Cockcroft-Gault formula) and structurally normal heart18.
The serum levels of Mg, sodium, calcium and potassium were measured before and on the 15th and 30th day after randomization. The follow-up visits were monthly conducted to assess symptoms by using a specific questionnaire. As previously described17, the questionnaire was elaborated with the following questions: 1- failures or "leaps", like a 'somersault' in the chest; 2- cough with palpitations; 3- dizziness; 4- dyspnea; 5- sweating and/or chest pain. According to the frequency of symptoms, points were attributed, which resulted in a score, and a two-point drop was considered a criterion of improvement. In addition, a categorical classification was conducted by asking the patients whether the symptoms improved, the answers being merely "yes" or "no" (Figure 1). In the long-term follow-up, all patients underwent 24-hour Holter monitoring twice, independently of symptoms, to assess the PC frequency. In addition, Holter monitoring (3 channels) was performed whenever symptoms appeared (before and 30 days of use of MgP).
Figure 1.
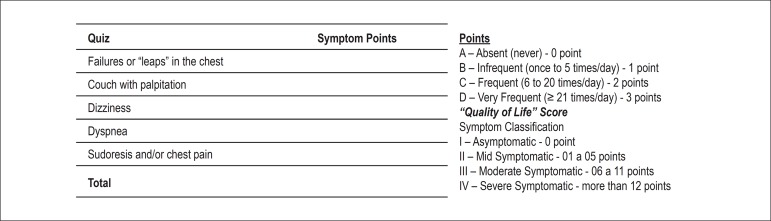
This figure shows the score system to assess improvement of symptoms before and after the drugs in both groups (placebo and magnesium pidolate).
In the second phase of the study, the patients with symptom relapse, of both the treatment and placebo groups ("crossing over"), received MgP for 30 more days, according to the same protocol.
Statistical analysis
The densities of PC in both phases of treatment (pre- and post-intervention) were measured by using mean, standard deviation, median, minimum and maximum values19.
The PC densities were compared between times of treatment and between treatments by using repeated-measures two-way analysis of variance (ANOVA)20. A correlation matrix of symmetrical components was calculated between asssessments20. Multiple comparisons by using Bonferroni adjustment21 were performed between the treatment times, and contrast was created to assess whether the improvement in the first treatment (pre-post 1st treatment) differed from that in the second treatment (pre-post 2nd treatment).
The improvement in symptoms was described at each treatment phase in both groups of patients, and McNemar tests19 were performed to assess whether there was difference in the improvement percentages in each treatment. The results were illustrated by using graphs of mean profiles with the respective standard errors. The 5% significance level was adopted for all tests. The SPSS software, version 20.0, was used for statistical analysis.
Results
The 90 patients participating in the first phase of treatment were followed up for 15 months. Table 1 shows the outcome of the first intervention. Of the 45 patients initially randomized to receive MgP, 41 (91.1%) were asymptomatic after the first month assessment, and four relapsed. In subsequent months, more 13 patients relapsed, adding to a total of 17 relapses (37.8%). All patients were treated again. The relapse time varied during the 15-month follow-up, and no statistically significant relapse concentration in a certain time was observed.
Table 1.
Outcome of the first intervention with Magnesium pidolate (MgP) versus placebo (shown as median)
| Variable | Group | 1st treatment (N = 90) | Improvement (%) | p | |
|---|---|---|---|---|---|
| pre | post 30 days | ||||
| PVC/day | < 0.001 | ||||
| Placebo (N = 45) | 2634 (194 - 24534) | 4211 (42 - 22526) | 1 (-831.7 - 78.4) | ||
| MgP (N = 45) | 1883 (2 - 38804) | 165 (0 - 6677) | 96 (-1250 - 100) | ||
| PsVC/day | < 0.001 | ||||
| Placebo (N = 45) | 176 (0 - 15965) | 785 (1 - 19861) | -5 (-2840 - 75) | ||
| MgP (N = 45) | 84 (0 - 20019) | 51 (0 - 15548) | 62 (-3025 - 100) | ||
| PC-Density/hour | < 0.001 | ||||
| Placebo (N = 45) | 158 (12.1 - 1022.3) | 199 (24.8 - 938.6) | -3 (-794.7 - 19.1) | ||
| MgP (N = 45) | 121 (21.1 - 1616) | 12 (0 - 647.8) | 87 (-7.7 - 100) | ||
Mann-Whitney test results, PC: premature complexe; PVC: premature ventricular complexes; PsVC: premature supraventricular complexes.
Frequency of premature complexes
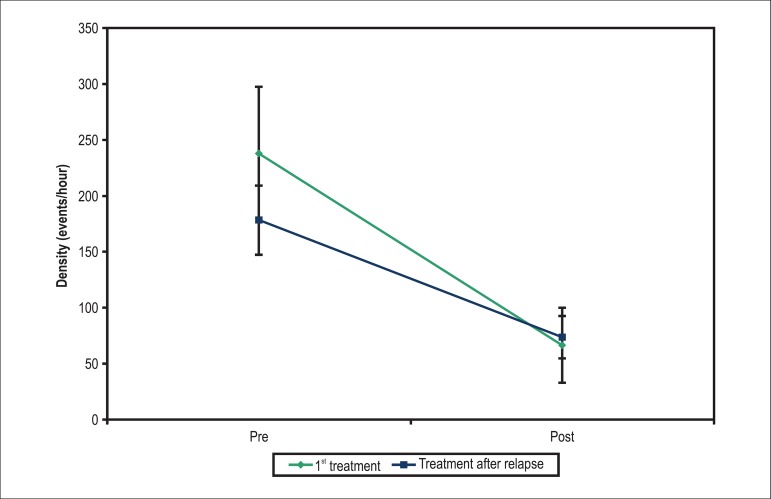
The relapsing patients (17 in the MgP group) who were retreated had a mean reduction in the PC density of 138.25/hour in both treatments (p < 0.001). No mean difference was observed in the PC density improvement between the successful initial treatment and that after relapse (p = 0.159), that is, patients using MgP repeated the response of the first treatment: significant improvement in symptoms and in PC (Table 2 and Figure 2).
Table 2.
Results of the multiple comparisons of extrasystole density of the groups of treatment
| Group | Comparison | Mean difference | Standard error | t value | gl | p |
|---|---|---|---|---|---|---|
| Crossing | Placebo pre vs post | -22.46 | 37.74 | -0.59 | 20 | > 0.999 |
| MgP pre vs post | 224.55 | 37.74 | 5.95 | 20 | < 0.001 | |
| Placebo vs MgP pre | -0.01 | 37.74 | 0.00 | 20 | > 0.999 | |
| Placebo vs MgP post | 246.99 | 37.74 | 6.54 | 20 | < 0.001 | |
| (pre-post) Placebo vs MgP | 247.00 | 53.38 | 4.63 | 60 | < 0.001 | |
| Relapse | Pre vs Post | 138.25 | 24.11 | 5.73 | 16 | < 0.001 |
| (pre-post) 1st Treat vs 2nd Treat | -66.86 | 46.72 | -1.43 | 48 | 0.159 |
vs: versus; result of Bonferroni multiple comparisons.
Figure 2.
Mean profiles of extrasystole density in each treatment of relapsing patients.
The median value of PVC/day after the first treatment was 3, and was 1872 on Holter monitoring after relapse. After the new intervention, this frequency decreased to 280 (p < 0.001). Regarding PsVC, the median after the first treatment was 14 PsVC/day, 15 on Holter monitoring after relapse, increasing to 38 after treatment (p < 0.102) (Table 3). The density of PC/hour after the first treatment was 10.3, increasing to 126.1 on relapse, and decreasing to 50.2 after the new treatment (p < 0.001) (Tables 3 and 4).
Table 3.
Description of ventricular and supraventricular extrasystoles in each group
| Variable | 1st treatment | Follow-up | |||
|---|---|---|---|---|---|
| pre | post 30 days | pre (follow-up) | post (follow-up) | ||
| No relapse | PVC/day | 2034 (0 - 38804) | 264 (0 - 6677) | 7.3 (0 - 710) | NA* |
| PsVC/day | 29.5 (0 - 19386) | 18.5 (0 - 15548) | 5.5 (0 - 973) | NA* | |
| PC/hour | 119.9 (34.9 - 1616) | 15.5 (0 - 647.8) | 3 (0 - 40.5) | NA* | |
| Relapse (N = 17) | PVC/day | 1386 (0 - 6497) | 3 (0 - 1616) | 1872 (0 - 8040) | 280 (0 - 3980) |
| PsVC/day | 78 (0 - 20019) | 14 (0 - 13104) | 15 (0 - 12020) | 38 (0 - 7008) | |
| PC/hour | 132 (21.1 - 839.7) | 10.3 (0 - 546) | 126.1 (33.4 - 511.3) | 50.2 (1 - 292) | |
| Crossing (N = 21) | PVC/day | 3030 (0 - 22502) | 3002 (0 - 21429) | 2508 (0 - 22505) | 689 (0 - 18812) |
| PsVC/day | 2 (0 - 15965) | 3 (0 - 19861) | 4 (0 - 15965) | 1 (0 - 10900) | |
| PC/hour | 264.8 (32.5-938.2) | 299.3(34.6-899.6) | 264.8 (32.5 - 938.3) | 48 (0 - 784) | |
Not applicable, PC: premature complexe; PVC: premature ventricular complexes; PsVC: premature supraventricular complexes.
Table 4.
Description of densities of extrasystoles per hour in each group
| Group/Treatment | Time | Mean | SD | Median | Minimum | Maximum | N |
|---|---|---|---|---|---|---|---|
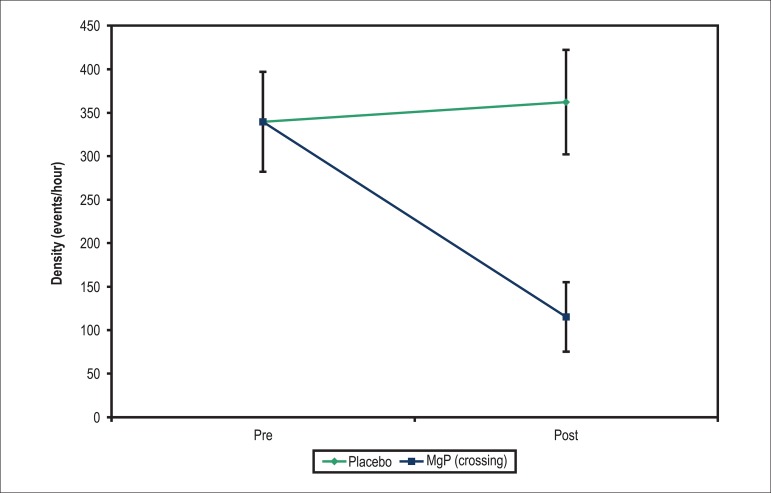
| Placebo | Pre | 339.82 | 263.67 | 264.75 | 32.50 | 938.20 | 21 |
| Post | 362.28 | 275.71 | 299.25 | 34.58 | 899.60 | 21 | |
| MgP (crossing) | Pre | 339.83 | 263.69 | 264.75 | 32.50 | 938.33 | 21 |
| Post | 115.29 | 183.47 | 48.00 | 0.00 | 784.00 | 21 | |
| 1st treatment | Pre | 238.07 | 245.03 | 132 | 21.08 | 839.67 | 17 |
| Post | 66.39 | 137.26 | 10.3 | 0.00 | 546.00 | 17 | |
| Treatment after relapse | Pre | 178.28 | 126.75 | 126.08 | 33.42 | 511.25 | 17 |
| Post | 73.46 | 78.15 | 50.21 | 1.00 | 292.00 | 17 |
SD: standard deviation; MgP: magnesium pidolate
Only three patients relapsed twice, being retreated, and two of them responded similarly, with improvement in symptoms and in PC frequency. Only one patient relapsed three times, and had a good response after the retreatments. The patients who did not relapse had a low PC frequency on Holter monitoring, with a median of 3 PC/hour (Table 3).
Of the 45 patients treated with placebo in the first phase, 6 (13.3%) improved their symptoms and 39 (86.7%) remained symptomatic. Of those 39, 21 agreed to receive MgP and were selected for crossing-over. Patients who did not agree to receive MgP were excluded from the analysis. The crossing-over patients had a statistically significant mean reduction in the PC density when using MgP (p < 0.001), while, when on placebo, no change in density was observed (p > 0.999). The mean improvement in PC density was 247/hour, greater in the treatment with MgP than in the first treatment with placebo (p < 0.001) (Table 2 and Figure 3).
Figure 3.
Mean profiles of density of extrasystoles in each treatment in patients of the placebo group undergoing crossing-over.
The median of PVC decreased from 2508/day to 689/day (p < 0.001), and that of PsVC, from 4/day to 1/day (p < 0.015) (Table 3). The density of PC/hour was 264.8 in the pre-treatment phase, dropping to 48 after the use of MgP (crossing-over) (Tables 3 and 4).
On Holter monitoring, PVC were more frequent, originating mainly (71%) from the outflow tract of the ventricles.
Symptoms
In relapsing patients, the improvement in symptoms of both treatments was statistically the same (p = 0.125) (Table 5). All 17 relapsing patients improved their symptoms in the first treatment, while 76.5% improved their symptoms in the relapse treatment. Similar response was obtained in the score of symptoms and in the categorical response of symptom improvement ('yes' or 'no').
Table 5.
Improvement in symptoms of each group of patients for each treatment, and results of the marginal association test
| Group | Improvement in symptoms | 1st treatment | 2nd treatment | p | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Crossing | No | 18 | 85.7 | 6 | 28.6 | < 0.001 |
| Yes | 3 | 14.3 | 15 | 71.4 | ||
| Total | 21 | 100 | 21 | 100 | ||
| Relapse | No | 0 | 0 | 4 | 23.5 | 0.125 |
| Yes | 17 | 100 | 13 | 76.5 | ||
| Total | 17 | 100 | 17 | 100 | ||
McNemar test results
The patients using placebo in the initial treatment and MgP in the second one (crossing-over) had a statistically greater improvement in symptoms with the second treatment (14.3% versus 71.4%, respectively; p < 0.001) (Table 5).
No significant changes in the measurements of serum Mg, potassium and calcium were observed during the study. No side effects were observed in the long-term follow-up.
Discussion
The follow-up results show that the improvement in symptoms by using MgP was maintained (76.5%) in the relapsing group as compared with the initial treatment. The group using placebo initially (crossing-over) had a 71.4% improvement in symptoms with MgP versus a 14.3% improvement with placebo.
The use of MgP was effective in reducing PC. However, because that is mainly a symptomatic treatment and because the mechanism of PC is multifactorial, symptoms and PC relapsed in approximately 38% of the cases, indicating that MgP is neither a definitive nor a curative treatment in late follow-up. However, the patients who could use MgP again improved their frequency of PC and mainly their symptoms, the latter being this study's major objective, because the group treated had a structurally normal heart.
It is worth noting that the patients initially treated with placebo also improved significantly their symptoms and frequency of PC after using MgP. This confirms that Mg administration is really better than placebo use.
The Mg reduces irregular heartbeats, and Mg deficiency should always be considered a potential factor for cardiac arrhythmias22. One hypothesis of the pathophysiological mechanisms relates to the fact that low Mg levels lead to an increase in intracellular calcium and sodium, and to a decrease in intracellular potassium7,8. Those changes in ionic loads cause membrane potential flotations, destabilizing atrial and ventricular myocardium, causing arrhythmias.
The Framingham study23 has shown that low Mg levels are associated with the development of atrial fibrillation in individuals with no cardiovascular disease. Patients at intensive care units should undergo intravenous replacement of Mg for arrhythmias not responding to conventional drugs, such as torsades de pointes15, and arrhythmias caused by digitalis intoxication24. Similarly to hypomagnesemia, digitalis inhibits the Na/K ATPase pump, increasing intracellular calcium and the contraction power of cardiomyocytes, PC being one of the most common arrhythmias in digitalis intoxication. The Mg deficiency caused by diuretic treatment in heart failure is associated with a higher incidence of arrhythmias, such as ventricular ectopy6,8,25. High concentrations of catecholamines can lead to the exit of intracellular Mg to the extracellular compartment, resulting in Mg reduction in the tissues, causing arrhythmias, especially in cardiac surgeries and in congestive heart failure patients6.
According to Kleivay and Milne26, the recommended daily Mg intake is 320 mg/day. Higher daily doses might be required by patients on diuretics, more prone to Mg level reduction, and, thus, with a higher potential and more susceptible to the development of ES26. Martynov and Akatova27 have followed up, for 15 years, 31 patients with mitral valve prolapse, who, during that time, regularly used Mg preparations for three months, twice a year. A reduction in the following parameters were observed: mean and maximum heart rate; number of tachycardia episodes; QT interval duration; and incidence of paroxysmal supraventricular tachycardia and PC. In addition, improvements in quality of life and in sympathetic tone were observed.
More recently, Del Gobbo et al28 have assessed obese adults with type 2 diabetes, and have shown that low serum Mg levels were associated with a high prevalence of PVC.
Our previous study, assessing 60 individuals with PVC and PsVC, has shown an improvement in the frequency of arrhythmia and mainly in symptoms17. Our current results indicate a significant improvement in PVC of the outflow tract. Right ventricular outflow tract is the most common origin of PVC in patients without structural heart disease29. That site might have remnant cells of the neural crest and of adrenergic modulation. The Mg reduction in those cells might be related to sympathetic modulation, more evident in the ventricles.
All those studies have shown that Mg intake can be a practical alternative to treat some arrhythmias. Considering its low cost, its efficacy and safety, the increase in daily Mg intake should be considered for symptomatic patients with PC and a structurally normal heart.
Limitations
Intracellular Mg was not measured, but the measurement of serum Mg levels has shown that supplementation to be safe and effective. The specific score for symptoms has not been validated, because it does not exist in the literature. However, a categorical assessment ('yes' or 'no') has also been performed, showing a good correlation between the reduction in PC frequency and improvement in symptoms. In addition, this study was not aimed at preventing life-threatening arrhythmias. These data should not be used to justify the treatment of patients for that purpose, especially those with heart diseases.
Conclusion
Some patients on MgP had relapse of symptoms and PC, indicating that MgP is neither a definitive nor a curative treatment in late follow-up. However, improvement in the PC frequency and symptoms was observed in the second phase of treatment, after relapse, being similar to the response in the first phase of treatment. Treatment with MgP, even after the first phase with placebo, improved the symptoms and PC density of the population studied (structurally normal heart).
The increase in daily Mg intake should be considered for patients with PC and structurally normal heart.
Acknowledgements
We thank Prof. Dr. Dalmo Moreira and Prof. Dr. Ricardo Alkmim Teixeira for their precious suggestions in the first phase of this project, Prof. Dr. Antônio Carlos Pereira Barretto and Prof. Dr. Alfredo J. Mansur for all the encouragement, and Rogério Ruscitto Prado for his support in the statistical analysis.
Footnotes
Author contributions
Conception and design of the research: De Falco CNML, Darrieux FCC, Scanavacca M; Acquisition of data: De Falco CNML, Darrieux FCC, Sacilotto L; Analysis and interpretation of the data: De Falco CNML, Darrieux FCC, Sacilotto L, Pisani C; Statistical analysis: De Falco CNML, Darrieux FCC, Pisani C; Obtaining financing: De Falco CNML; Writing of the manuscript: De Falco CNML, Darrieux FCC, Scanavacca M; Critical revision of the manuscript for intellectual content: De Falco CNML, Darrieux FCC, Sacilotto L, Lara S, Ramires JAF, Sosa E, Wu TC, Hachul D, Scanavacca M.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article results from the continuation of the doctoral dissertation by Cristina Nádja M. Lima De Falco, from Faculdade de Medicina da Universidade de São Paulo.
References
- 1.Barret PA, Peter CT, Swan HJ, Singh BN, Mandel WJ. The frequency and prognostic significance of electrocardiographic abnormalities in clinically normal individuals. Prog Cardiovasc Dis. 1981;23(4):299–319. doi: 10.1016/0033-0620(81)90018-9. [DOI] [PubMed] [Google Scholar]
- 2.De Paula RS, Antelmi I, Vincenzi MA, André CD, Artes R, Grupi CJ, et al. Cardiac arrhythmias and atrioventricular block in a cohort of asymptomatic individuals without heart disease. Cardiology. 2007;108(2):111–116. doi: 10.1159/000095950. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen V, Jensen G, Schnohr P, Hansen JF. Premature ventricular beats in healthy adult subjects 20 to 79 years of age. Eur Heart J. 1985;6(4):335–341. doi: 10.1093/oxfordjournals.eurheartj.a061860. [DOI] [PubMed] [Google Scholar]
- 4.Wajngarten M, Grupi C, Bellotti GM, Da Luz PL, Azul LG, Pileggi F. Frequency and significance of cardiac rhythm disturbances in healthy elderly individuals. J Electrocardiol. 1990;23(2):171–176. doi: 10.1016/0022-0736(90)90138-r. [DOI] [PubMed] [Google Scholar]
- 5.Hiss RG, Averill KH, Lamb LE. Electrocardiographic findings in 67,375 asymptomatic subjects. III. Ventricular rhythms. Am J Cardiol. 1960;6:96–107. doi: 10.1016/0002-9149(60)90040-0. [DOI] [PubMed] [Google Scholar]
- 6.Swaminathan R. Magnesium metabolism and disorders. Clin Biochem Rev. 2003;24(2):47–66. [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Carter A. Magnesium: the forgotten electrolyte. Aust Prescr. 2007;30:102–105. [Google Scholar]
- 8.Gums JG. Magnesium in cardiovascular and other disorders. Am J Health Syst Pharm. 2004;61(15):1569–1576. doi: 10.1093/ajhp/61.15.1569. [DOI] [PubMed] [Google Scholar]
- 9.Gums JG, Stier-Carson D, Hedrix GH, Weart GH, De Oca GM. Effect of magnesium and potassium on cardiac function: the role of spironolactone. J Pharm Technol. 1990;6:10–14. [Google Scholar]
- 10.Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res. 2007;35(3):277–289. doi: 10.1177/147323000703500301. [DOI] [PubMed] [Google Scholar]
- 11.Elin RJ. Magnesium: the fifth but forgotten electrolyte. Am J Clin Pathol. 1994;102(5):616–622. doi: 10.1093/ajcp/102.5.616. [DOI] [PubMed] [Google Scholar]
- 12.Elin RJ. Laboratory tests for the assessment of magnesium status in humans. Magnes Trace Elem. 1991;92(2-4):172–181. [PubMed] [Google Scholar]
- 13.Arnaud MJ. Update on the assessment of magnesium status. Br J Nutr. 2008;99(Suppl 3):S24–S36. doi: 10.1017/S000711450800682X. [DOI] [PubMed] [Google Scholar]
- 14.Arsenian MA. Magnesium and cardiovascular disease. Prog Cardiovasc Dis. 1993;35(4):271–310. doi: 10.1016/0033-0620(93)90008-2. [DOI] [PubMed] [Google Scholar]
- 15.Purvis JR, Movahed A. Magnesium disorders and cardiovascular diseases. Clin Cardiol. 1992;15(8):556–568. doi: 10.1002/clc.4960150804. [DOI] [PubMed] [Google Scholar]
- 16.Kawano Y, Matsuoka H, Takishita S, Omae T. Effects of magnesium supplementation in hypertensive patients: assessment by office, home, and ambulatory blood pressures. Hypertension. 1998;32(2):260–265. doi: 10.1161/01.hyp.32.2.260. [DOI] [PubMed] [Google Scholar]
- 17.Falco CN, Grupi C, Sosa E, Scanavacca M, Hachul D, Lara S, et al. Successful improvement of frequency and symptoms of premature complexes after oral magnesium administration. Arq Bras Cardiol. 2012;98(6):480–487. doi: 10.1590/s0066-782x2012005000043. [DOI] [PubMed] [Google Scholar]
- 18.Helou R. Should we continue to use the Cockcroft-Gault formula? Nephron Clin Pract. 2010;116(3):c172–c185. doi: 10.1159/000317197. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood BR, Sterne JAC. Essential medical statistics. 2nd ed. Massachusetts, USA: Blackwell Science; 2006. [Google Scholar]
- 20.Singer JM, Andrade DF. Handbook of statistics . Analysis of longitudinal data. In: Sen P.K., Rao C.R., editors. bio-environmental and public health statistics. Vol. 18. Amsterdam: North Holland; 2000. pp. 115–160. [Google Scholar]
- 21.Kuntner M, Nachtsheim C, Neter J, Li W. Applied linear statistical models. 4th ed. Illinois, USA: McGraw Hill / Irwin; 1996. [Google Scholar]
- 22.Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med. 2005;20(1):3–17. doi: 10.1177/0885066604271539. [DOI] [PubMed] [Google Scholar]
- 23.Khan AM, Lubitz SA, Sullivan LM, Sun JX, Levy D, Vasan RS, et al. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2013;127(1):33–38. doi: 10.1161/CIRCULATIONAHA.111.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iseri LT, Allen BJ, Brodsky MA. Terapia de magnesio en arritmias cardiacas en medicina de cuidados críticos. Revista de Cuidados Intensivos em Medicina. 2005;20(1):3–17. [Google Scholar]
- 25.Ceremuzynski L, Gebalska J, Wolk R, Makowska E. Hypomagnesemia in heart failure with ventricular arrhythmias. Beneficial effects of magnesium supplementation. J Intern Med. 2000;24(1):78–86. doi: 10.1046/j.1365-2796.2000.00585.x. [DOI] [PubMed] [Google Scholar]
- 26.Klevay LM, Milne DB. Low dietary magnesium increases supraventricular ectopy. Am J Clin Nutr. 2002;75(3):550–554. doi: 10.1093/ajcn/75.3.550. [DOI] [PubMed] [Google Scholar]
- 27.Martynov Al, Akatova EV. Fifteen years experience of the use of magnesium preparations in patients with mitral valve prolapse. Kardiologiia. 2011;51(6):60–65. [PubMed] [Google Scholar]
- 28.Del Gobbo LC, Song Y, Poirier P, Dewailly E, Elin RJ, Egeland GM. Low serum magnesium concentrations are associated with a high prevalence of premature ventricular complexes in obese adults with type 2 diabetes. Cardiovasc Diabetol. 2012;11(1):23–23. doi: 10.1186/1475-2840-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darrieux FC, Scanavacca MI, Hachul DT, Melo SL, D'Ávilla AB, Grupi CJ, et al. Radiofrequency catheter ablation of premature ventricular contractions originating in the right ventricular outflow tract. Arq Bras Cardiol. 2007;88(3):265–272. doi: 10.1590/s0066-782x2007000300003. [DOI] [PubMed] [Google Scholar]