Abstract
Background
Effective interventions to improve medication adherence are usually complex and expensive.
Objective
To assess the impact of a low-cost intervention designed to improve medication adherence and clinical outcomes in post-discharge patients with CVD.
Method
A pilot RCT was conducted at a teaching hospital. Intervention was based on the four-item Morisky Medication Adherence Scale (MMAS-4). The primary outcome measure was medication adherence assessed using the eight-item MMAS at baseline, at 1 month post hospital discharge and re-assessed 1 year after hospital discharge. Other outcomes included readmission and mortality rates.
Results
61 patients were randomized to intervention (n = 30) and control (n = 31) groups. The mean age of the patients was 61 years (SD 12.73), 52.5% were males, and 57.4% were married or living with a partner. Mean number of prescribed medications per patient was 4.5 (SD 3.3). Medication adherence was correlated to intervention (p = 0.04) and after 1 month, 48.4% of patients in the control group and 83.3% in the intervention group were considered adherent. However, this difference decreased after 1 year, when adherence was 34.8% and 60.9%, respectively. Readmission and mortality rates were related to low adherence in both groups.
Conclusion
The intervention based on a validated patient self-report instrument for assessing adherence is a potentially effective method to improve adherent behavior and can be successfully used as a tool to guide adherence counseling in the clinical visit. However, a larger study is required to assess the real impact of intervention on these outcomes.
Keywords: Cardiovascular Diseases, Medication Adherence, Patient Discharge, Patient Discharge Summaries, Randomized Controlled Trial
Trial registration: Registro Brasileiro de Ensaios Clínicos RBR-26ydc3.
Introduction
Medication adherence can be defined as the extent to which patients follow the instructions they are given for prescribed treatments1. Adherence is a determinant of high-quality outcomes, yet studies indicate that 20% to 50% of patients - across gender, age, and ethnic cohorts and with various medical disorders - do not take their medications as prescribed2,3. This is a growing concern to clinicians and healthcare systems because of mounting evidence that non-adherence is prevalent and associated with adverse outcomes and higher costs of care4,5. In the setting of chronic medical conditions, such as hypertension and diabetes, medication non-adherence leads to worse medical treatment outcomes, higher hospitalization rates and increased health care costs6-8. Even after hospitalization, adherence problems may persist due to inadequate knowledge about the treatment, lack of understanding or excessive complexity9,10.
A variety of interventions have been tested with the goal of improving patient adherence. However, despite several types of interventions demonstrating effectiveness in improving medication adherence in chronic medical conditions, few significantly affected clinical outcomes11. A Cochrane Database review concluded that almost all of the interventions that were effective for long-term care were complex, including combinations of more convenient care, information, reminders, self-monitoring, reinforcement, counseling, family therapy, psychological therapy, crisis intervention, manual telephone follow-up and supportive care. Even the most effective interventions did not lead to large improvements in adherence and treatment outcomes1.
We have developed our intervention based on patient responses to the questions of a self-reporting instrument for assessing adherence to medications12,13. Based on a previous study14, we hypothesized that this low-cost intervention would improve measured adherence and reduce exacerbations requiring emergency department visits or hospitalization. The present study assessed an intervention in post-discharge patients with CVD taking prescribed drugs with the aim of improving medication adherence and clinical outcomes.
Methods
Design
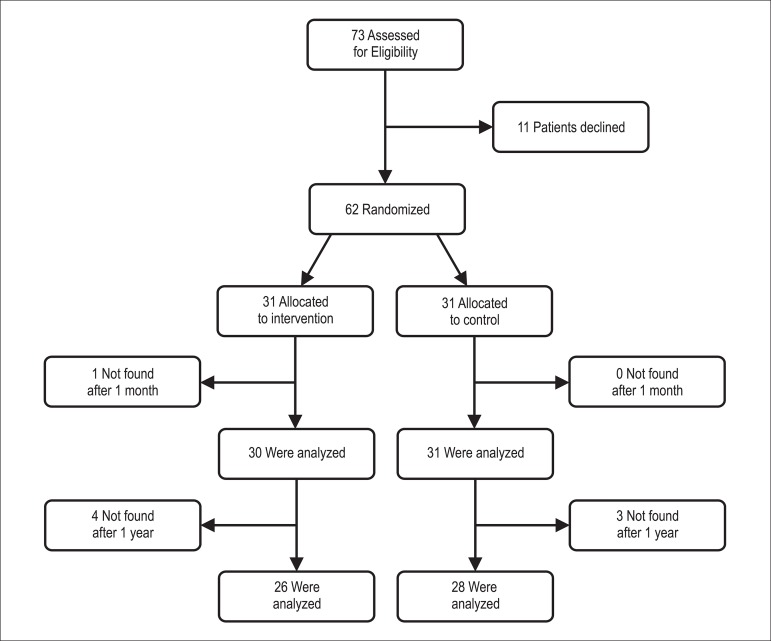
We performed a randomized controlled, single-center trial with patients allocated to intervention versus usual care between October 2010 and April 2011. A computer program randomized patients using minimization with a random element to ensure that the 2 trial arms are not significantly different on 5 key variables: age, gender, number of drugs per patient, surgical procedure during hospitalization and diagnosis. Figure 1 shows the flow of patients throughout the study.
Figure 1.
Flow diagram.
Setting
A 300-bed cardiovascular clinic of a private urban teaching hospital, in the city of Maceió, state of Alagoas, Northeast Brazil. The state of Alagoas has one of the worst human development indices (HDI) in Brazil.
Recruitment
We recruited and obtained consent from consecutive patients who were discharged from cardiovascular clinics and who were prescribed antihypertensive medication.
Patients were randomly allocated to brief intervention or usual care and we used an intent-to-treat approach and thus ignored subsequent changes to regimens, treatment interruptions, and treatment termination.
Data Collection
We had to strike a careful balance between early intervention and enough time for participants to demonstrate variance in medication adherence, thus our decision was to assess adherence at baseline and 1 month post-hospital discharge. Later, we re-assessed adherence 1 year after hospital discharge, as well as readmission and mortality rates; both clinical outcome measures were included to correlate adherence and effectiveness of intervention. Therefore, consenting participants were contacted by phone call by a trained lay interviewer at 2 moments: a) 1 month after their event, when they were asked to answer the Morisky Medication Adherence Scale (MMAS-8)15; b) 1 year after their event, when they were once again asked to complete MMAS-8 and to report the number of hospitalizations related to CVD during the study year. Patients that were randomized to the intervention group were contacted again by phone on the same day (+ 5 days) of the first application of MMAS-8, during this telephone contact. Questions about the medications were discussed with these patients by the research pharmacists. Deaths were confirmed via death certificate. The study was closed 12 months after the last patient had been discharged. Patients who had been readmitted to the study site hospital during the 12-month follow-up period did not receive the intervention again.
Inclusion criteria
Aiming maximum inclusiveness, we recruited all patients who had a discharge diagnosis of CVD and who were on antihypertensive medication.
Exclusion criteria
Patients were excluded if they reported they were already using any tool to improve adherence.
Ethical Approval
The Federal University of Alagoas institutional review board approved the study protocol and consent form.
Measuring Adherence
Medication adherence - the primary outcome measure in the evaluation of this trial was assessed by phone call through the MMAS-8, an 8-item self-report scale for measuring medication-taking behavior developed from a previously validated 4-item scale15 and supplemented with additional items to better assess barriers to adherence behavior (Chart 1 and 2). Each of the 8 items measures a specific medication-taking behavior and not a determinant of adherence behavior. The new scale has been determined to have higher reliability compared with the 4-item scale (a = .83 vs a = .61)15. The MMAS scores can range from 0 to 8 and have been previously trichotomized into the following 3 levels of adherence to facilitate use in clinical practice: high adherence (score = 8), medium adherence (score, 6 to < 8), and low adherence (score, < 6)15. Prior research revealed that the new scale is significantly associated with blood pressure control in patients with hypertension15,16. In our study, we used a validated version in Portuguese of the MMAS-817. Patients were considered adherent if they had a score equal to 8 (high adherence)16.
Chart 1.
The 4-Item Morisky Medication Adherence Scale (MMAS-4)
| 1. Do you ever forget to take your (name of health condition) medicine? | Yes / No |
| 2. Do you ever have problems remembering to take your (name of health condition) medicine? | Yes / No |
| 3. When you feel better, do you sometimes stop taking your (name of health condition) medicine? | Yes / No |
| 4. Sometimes if you feel worse when you take your (name of health condition) medicine, do you stop taking it? | Yes / No |
Chart 2.
The 8-Item Morisky Medication Adherence Scale (MMAS-8)
| 1. Do you sometimes forget to take your high blood pressure pills? | Yes / No |
| 2. Over the past two weeks, were there any days when you did not take your high blood pressure medicine? | Yes / No |
| 3. Have you ever cut back or stopped taking your medication without telling your doctor because you felt worse when you took it? | Yes / No |
| 4. When you travel or leave home, do you sometimes forget to bring along your medications? | Yes / No |
| 5. Did you take your high blood pressure medicine yesterday? | No / Yes |
| 6. When you feel that your blood pressure is under control, do you sometimes stop taking your medicine? | Yes / No |
| 7. Do you ever feel hassled about sticking to your blood pressure treatment plan? | Yes / No |
| 8. How often do you have difficulty remembering to take all your blood pressure medication? | Never-Rarely/Once in a while/ Sometimes/Usually/All the time |
Intervention
As the MMAS-4 measures non-adherence using 4 items and identifies the 2 main types of non-adherence - unintentional non-adherence (which occurs when patients wish to adhere to medications, but are prevented from taking medications due to some reason; it is based on forgetfulness and clarification regarding the medical regimen) and intentional non-adherence (which occurs when patients deliberately do not take their medications; it is based on withdrawing medications when feeling better or worse)18,19, we have developed our intervention based on MMAS-4 questions, which has been shown to be predictive of adherence to cardiovascular medications and blood pressure control, aiming to prevent both intentional and unintentional non-adherence behavior, INAB and UNAB, respectively.
This protocol consisted of 2 distinct parts: patient-centered verbal instructions (to prevent INAB and UNAB) and written material about prescribed medications (to prevent UNAB). Interventions to enhance medication adherence in chronic medical conditions may be grouped by type10,20,21, as follows: (I) informational interventions describe cognitive strategies designed primarily to educate and motivate patients by instructional means, based on the concept that patients who understand their condition and its treatment will be more informed, empowered, and likely to comply (e.g.: face-to-face oral, telephone, written, or audiovisual education; didactic group class; and mailed instructional material - not including reminders or prompts to comply); (II) behavioral interventions are strategies designed to influence behavior through shaping, reminding (cues), or rewarding desired behavior (reinforcement), examples include skill building by a health care professional, pillboxes, calendars, a change in packaging, or other steps intended to remind the patient; changes in dosage schedule to simplify the regimen or tailor the regimen to the patient's daily routine (i.e., reduce its behavioral demands); and rewards and reinforcement (e.g., assessment of adherence with feedback to the patient); (III) family and social interventions involve social support strategies, whether provided by family or another group (e.g. support groups and family counseling)10. Our combined intervention included features of I and II of the preceding categories.
The main elements of the discharge counseling were as follows: a) information about disease process/prognosis; b) information about discharge medications (e.g., therapeutic goals; how to monitor drug treatment, especially the consequences of abrupt cessation of antihypertensive treatments and adverse drug reactions that may be causes of withdrawal; and how to handle inaccurate dosing systems or unusual dosage forms). Subjects considered critical to success of treatment were discussed with the patient (Chart 3 - please see additional File 2). Additional information (e.g., dosage schedule) was written on a drug treatment card adapted as a refrigerator magnet.
Chart 3.
Points to discuss with patients and suggested recommendations
| MMAS-4 question | Do you ever forget to take your medicine? | Do you ever have problems remembering to take your medicine? | When you feel better do you sometimes stop taking your medicine? | When you sometimes feel worse when you take the medicine, do you stop taking it? |
|---|---|---|---|---|
| Specific goal | To improve memory for medication taking | To adapt the regimen to the patient's daily schedule to address carelessness | To avoid stopping the treatment when feeling better | To avoid stopping the treatment when feeling worse |
| Procedure 1 | Procedure 2 | Procedure 3 | ||
| Provide an easy reminder and identify a daily activity or cue that the patient does regularly at about the time he or she should take medications and explain to the patient to take medications at this time | Explain fundamentals of hypertension and its management to patient in terms he or she can understand. Explain how the medication works in a simple way and what are the specific consequences or effects if the patient stops taking it. | Teach patient how to monitor the most common side effects of his or her treatment (withdrawal rates due to side effects must be investigated). Support and encourage patient to report the problem to the physician. | ||
| Therapeutic class information | ||||
| Agents acting on the renin- angiotensin system, Beta blocking agents and Calcium channel blockers | This medicines act by dilating the blood vessels (and, in some cases*, reducing heart muscles contractility). Therefore amlodipine and losartan may take a few weeks to show noticeable effects. | |||
| Linking medication taking to patient's daily schedule31 | Withdrawal symptoms | Potential causes of withdrawal | ||
| Amlodipine | Abrupt withdrawal of this agent can precipitate coronary vasospasm, which may result in myocardial infarction. | Peripheral edema, fatigue, palpitation, headache, dyspepsia, nausea. | ||
| May be taken with food. | ||||
| Atenolol | Tachycardia, palpitation, excessive sweating, chest pain, heart attack, death. | Hypotension, bradycardia, bronchospasm, cold extremities. | ||
| May be taken with food (although the presence of food may reduce the bioavailability of atenolol by 20%). | ||||
| Captopril | Abrupt withdrawal of these agents can precipitate hypertensive rebound in diabetic patients with chronic renal failure | Dry cough, hyperkalemia (especially if used with spironolactone; main symptoms: palpitations and muscle weakness), postural hypotension. | ||
| May be taken without food. | ||||
| Carvedilol | Tachycardia, palpitation, excessive sweating, chest pain, heart attack, death. | Fatigue, hypotension, diarrhea, asthenia, bradycardia, dizziness, edema. | ||
| May be taken with food. | ||||
| Enalapril | Abrupt withdrawal of these agents can precipitate hypertensive rebound in diabetic patients with chronic renal failure | Hypotension, dry cough. | ||
| May be taken with food. | ||||
| Losartan | There is little evidence of rebound effect after abrupt withdrawal of losartan therapy | Dizziness, diarrhea, tiredness. | ||
| May be taken with food (concurrent use of losartan and grapefruit juice may result in increased half-life and decreased area under the concentration time curve of losartan's active metabolite). | ||||
| Therapeutic class information | ||||
| Diuretics | These medications increase water loss by inhibiting sodium and chloride resorption in the kidneys. When taking these antihypertensives, one will urinate a lot because of the increased water loss. | |||
| Linking medication taking to patient's daily schedule | Withdrawal symptoms | Potential causes of withdrawal | ||
| Hydrochlorothiazide | If diuretics are withdrawn suddenly in patients with a normal sodium intake, there will be rebound retention of sodium and water (with consequent edema), because compensatory mechanisms that maintain sodium balance in the face of diuretics continue to act for several days after diuresis has worn off. | Hypokalemia (symptoms include muscular weakness, myalgia, and muscle cramps), weakness. | ||
| May be taken with food. | ||||
| Furosemide | Hypokalemia (symptoms include muscular weakness, myalgia, and muscle cramps), hyperglycemia, hyponatremia (symptoms include nausea and vomiting, headache, fatigue, appetite loss). | |||
| May be taken with food. | ||||
| Spironolactone | There is no apparent rebound effect after abrupt withdrawal of spironolactone therapy | Hyperkalemia (main symptoms: palpitations and muscle weakness). | ||
| May be taken with food. | ||||
| Therapeutic class information | ||||
| Platelet aggregation inhibitors | Treatment with blood thinners prevents blood clots from forming in blood vessels. It may be used to prevent or treat heart attacks and stroke. | |||
| Linking medication taking to patient's daily schedule | Withdrawal symptoms | Potential causes of withdrawal | ||
| Acetylsalicylic acid | Optimal dosing time: in the morning and/or in the evening with food (bedtime administration of aspirin decreases morning surge of platelet aggregation while kept same antiplatelet efficacy during other time of the day compared to taking these drugs at day-time). | The withdrawal of acetylsalicylic acid may be associated with traditional cardiovascular risk factors and thrombosis. | Gastrointestinal side effects (pain, heartburn, indigestion), bleeding. | |
| Clopidogrel | Optimal dosing time: in the morning and/or in the evening with food (bedtime administration of clopidogrel decreases morning surge of platelet aggregation while kept same antiplatelet efficacy during other time of the day compared to taking these drugs at day-time). | Clopidogrel withdrawal is associated with a rebound prothrombotic and/ or proinflammatory response. Premature cessation of clopidogrel in patients receiving drug-eluting stents is a clear risk factor for stent thrombosis. | Bleeding, Gastrointestinal side effects (pain, heartburn, indigestion, diarrhea), rash. | |
| Therapeutic class information | ||||
| Lipid modifying agent | These agents are used along with a proper diet to help lower 'bad' cholesterol and fats and raise 'good' cholesterol in the blood | |||
| Linking medication taking to patient's daily schedule | Withdrawal symptoms | Potential causes of withdrawal | ||
| Rosuvastatin | Statin withdrawal abrogates this beneficial effect in patients initially responsive to this therapy and may cause rebound inflammatory effect. | Muscle pain, fatigue and weakness, myalgia, cognitive loss. | ||
| May be taken without food. | ||||
The research pharmacists were previously trained in discharge counseling approach for 6 months at the study setting. The enhanced medication review provided by pharmacists to patients in the intervention group on the day of discharge consisted of 9 steps with mean total duration of 32 minutes:
The doctor confirms the patient's hospital discharge and sends the outpatient prescription for analysis by the pharmacist.
The pharmacist transcribes data from medical records and prescription to a form specifically designed for this study.
The pharmacist reviews the following data for each drug: indication, dosage and schedule, treatment duration, method of use, adverse reactions, main drug-drug and drug-food interactions. Current drug-drug interactions or other drug-related problems are communicated to the prescriber before discharge. The communicated problems did lead to an adaptation/correction of the prescription.
The pharmacist reviews the following data for each patient: diagnosis, age, sex and drugs used before hospitalization.
After reviewing patient data, the pharmacist highlights critical points to the success of treatment after hospital discharge. (Chart 3)
Main advice and schedules are written on a drug treatment card adapted as a refrigerator magnet.
Subjects considered critical to successful treatment and schedules are discussed with the patient. In this step, the health-disease process is also discussed, as well as measures to be taken in case of a forgotten dose. (Chart 3)
The instructions about drug treatment are checked with the patient.
The drug treatment card is given to the patient as well as a phone number for contact.
The research pharmacists were also encouraged to minimize regimen complexity for their patients by identifying potential simplifications during discharge prescription reviews and discussing these changes with hospital doctors and patients.
The intervention pertained to all discharge medications. However, medication adherence was ascertained just for the anti-hypertensive medications.
Control condition
Participants in the control group received hospital's usual care and answered the same questions (from the MMAS-8 and about readmission events) by phone at the same moments as the intervention group. Moreover, they were visited before discharge by a research-team member in order to disguise the lack of testing intervention.
Sample Size
We estimated that a sample size of at least 19 patients in each group would provide 80% power to detect a 40% difference, based on baseline adherence and results from a previously performed pilot investigation in medication adherence by using a t-test with a 2-sided α level of 0.0522. Unlike this, difference in adherence between groups is generally small in randomized clinical trials1,10,21. However, we did not find any study that included concurrent post-discharge patients with CVD and MMAS as the adherence measure, or even studies with an intervention based on its own assessment tool. We believe that this shared mechanism leads to higher adherence scores in intervention group and consequently increases effect size.
Statistical Analysis
Bivariate comparisons between groups were performed using chi-square test for equal proportions (or Fisher's exact tests where numbers are small) and reported as numbers and percentages. Continuous normally distributed variables (determined by Kolmogorov-Smirnov test) were compared using ANOVA and reported as means (standard error). Backward stepwise logistic regression analysis was conducted to determine independent correlates of medication adherence. These statistical analyses were performed using the statistical program package SPSS version 16.0 (SPSS Inc., Chicago, Illinois). The researcher (S.F.N.) responsible for analyzing readmission and adherence data was blinded regarding the group to which the patients had been randomized.
The magnitude of the intervention effect (Cohen's d) was calculated by subtracting the MMAS-8 mean score of the control group from the MMAS-8 mean score of the intervention group and dividing the result by the pooled standard deviation23. A Cohen's d of 0.5 thus indicates that the mean of the intervention group is half a standard deviation larger than the mean of the control group. Values of d from 0.56 to 1.2 can be assumed to be large, 0.33 to 0.55 are moderate, and 0 to 0.32 are small23. This measure is independent from the method of adherence measurement used, thus permitting comparison of different interventions across studies. In this way, it provides more information than a simple test of significance comparing outcomes in intervention and control groups10.
Results
Of 73 patients invited to participate in the study, 11 declined. Of 62 randomized patients (31 intervention and 31 control), 1 patient was not found after 1 month and was excluded from further analyses. This left 61 eligible patients. Figure 1 shows the flow of patients throughout the study. The groups were well balanced with respect to baseline characteristics (Table 1). The mean age of the patients was 61 years (SD 12.73), 52.5% were males, and 57.4% were married or living with a partner. Mean number of prescribed medications per patient was 4.5 (SD 3.3). Most prescribed drugs were: aspirin (63.9%), atenolol (57.4), losartan (55.7), rosuvastatin (39.3), clopidogrel (32.8), amlodipine (21.3), carvedilol (19.7), spironolactone (18.0), furosemide (14.8), hydrochlorothiazide (11.5), enalapril (8.2), captopril (6.6), digoxin (5.2), atorvastatin (3.3), and others (29.5). Among the most commonly prescribed drugs (used by more than 10% of patients), only carvedilol and hydrochlorothiazide were not statistically significantly associated with adherence (p = 0.489 and p = 0.179 respectively).
Table 1.
Baseline Characteristics of Patients by Group
| Characteristic | Intervention Group (n = 30) | Control Group (n = 31) | p values |
|---|---|---|---|
| Sex, N°. (%) | |||
| Male | 16 (53.3) | 16 (51.6) | 0.90a |
| Female | 14 (46.7) | 15 (48.4) | |
| Age, mean (SD) | 60.93 (12.69) | 61.07 (12.99) | 0.96b |
| Married or living with partner, N°. (%) | 18 (60.0) | 17 (54.8) | 0.36a |
| N°. of prescribed medications at discharge, mean (SD) | 4.46 (1.72) | 4.5 (1.93) | 0.94b |
| Underwent surgery during hospitalization, N° (%) | 10 (33.3) | 9(29.0) | 0.93a |
| Main diagnosis, N°. (%) | |||
| Hypertension | 16 (53.3) | 15 (48.4) | 0.70a |
| Heart failure | 6(20.0) | 8(25.8) | |
| Coronary artery disease | 5 (16.7) | 7(22.6) | |
| Diabetes mellitus | 7(23.3) | 5 (16.1) | |
| Other | 2(6.7) | 0 |
Chi-square test
ANOVA
Medication Adherence at baseline
Medication adherence was 41.9% and 58.1% in the usual care and intervention groups at baseline (p = 0.710).
Medication Adherence after 1 Month
Medication adherence improved between the baseline and follow-up surveys in both groups, 48.4% and 83.3% in the usual care and intervention groups, respectively, but was significantly greater (p = 0.004) in the intervention group (Cohen's d = 0.741). Potential factors that may affect medication adherence (e.g. gender, age, marital status, number of prescribed medications, undergoing surgery during hospitalization and main diagnosis) were not related to medication adherence after 1 month. In the logistic regression, having received the intervention was the only significant risk factor for medication adherence.
Medication Adherence after 1 Year
The effects of the intervention decreased (p = 0.203) during the 12-month post intervention follow-up period, in which medication adherence was 34.8% and 60.9% for the control group and intervention group, respectively (Cohen's d = 0.643).
Readmission and Mortality rate
The numbers of hospitalizations and deaths during the study are summarized in Table 2. Of 61 patients analyzed after 1 month, 7 were lost to follow-up after 1 year (4 intervention and 3 control). Of the 54 patients, 9 ([3 intervention and 6 control]; p = 0.27, Fisher exact test) died before the end of the 12-month follow-up period; 8 patients died of their underlying disease and 1 of septicemia (intervention group). 20/54 patients (7/26 intervention and 13/28 control]; p = 0.20, Fisher exact test) were re-hospitalized during the course of the study. Main cause of re-hospitalization was CVD (25 events), followed by respiratory problems (2) and cancer (1). As for the intervention group, there was a 19.5% reduction in re-hospitalization, although this was not significant at the .05 level. Adherent patients in both groups had significantly lower rates of readmission and mortality (p = 0.03 and p = 0.02 respectively). The mortality rate was also influenced by diagnosis and presence of comorbidities.
Table 2.
Summary of Outcomes at 12-Months Follow Up
| Value (%) | |||
|---|---|---|---|
| Variable | Intervention Group (n = 30) | Control Group (n = 31) | p Values |
| Readmissions | 6 (20%) | 15 (48%) | 0.20 |
| Deaths | 3 (10%) | 6 (19%) | 0.43 |
Discussion
In 2001, Cooper et al. showed in a review of 12 meta-analyses that educational interventions were generally poorly described, and failed to adhere to theoretical models24. Subsequent reviews have found few consistent associations between the characteristics and effectiveness of interventions to improve adherence1,10,20,21,25. Kripalani et al11 observed that behavioral interventions that reduced dosing demands of individual therapies consistently improved adherence10; other successful interventions usually contained multiple elements delivered over time. However, none of these reviews focused on medication adherence after hospital discharge. In a Cochrane Database review1, no evidence of difference in adherence to medication was observed after two pharmacy discharge plans.
In contrast to these findings, our intervention based on MMAS-4 improved medication adherence score after 1 month. In addition, control of potential confounding factors (e.g. age, gender, marital status, number of prescribed medications, undergoing surgery during hospitalization and main diagnosis) did not influence the results. This effect may be due to several causes. First, we used a measure of medication-taking behavior based on the same pattern of intervention; although the eight-item scale has shown better psychometric properties than the original 4-item scale15, some questions are identical.
Second, although 12 different classes of drugs were used by the patients' physicians, a narrow variety of drugs inside theses classes were prescribed (mostly aspirin, enalapril, losartan, clopidogrel and rosuvastatin). Such rigid adherence to medical guidelines, although leading to excessive costs, permitted us to focus our efforts on a restricted number of therapies.
Third, our intervention was centered on subjects considered critical to treatment success and it may be related to a MMAS-8 question commonly answered incorrectly among non-adherent patients: "Have you ever cut back or stopped taking your medication without telling your doctor because you felt worse when you took it?" Burnier26 states that it appears that an effective, convenient drug regimen that is relatively free of side effects, combined with a positive and supportive approach to treatment, will therefore yield the best results in terms of facilitating adherence and persistence with antihypertensive therapy26. In our study, physicians reported that some patients came back or contacted them by phone after an adverse drug event for which they were warned during a counseling session, instead of stop taking their medication.
Fourth, the presence of a Hawthorne effect was considered and must not be ignored; however, patients in the control group were visited by a research-team member in order to disguise the lack of testing intervention. A systematic review performed by Schlenk et al21 observed that, because of the nature of the interventions, the investigators were not able to blind the participants and interventionists to group assignment, which could produce Hawthorne, novelty, and experimenter effects in the intervention group subjects. For all practical purposes, our patients were blinded about the treatment that was used.
Fifth, in the pilot study we observed that longer counseling sessions (e.g. 20 to 40 minutes) led to a few patients' refusal of continuation of intervention. Thus, we believe that a shorter intervention tends to be more comprehensive and effective. It is also noted that acceptability of intervention among patients was high.
However, the positive effect of the intervention decreased significantly (p = 0.203) after 1 year, which may be due to the fact that this study did not have sufficient power to detect a 26% reduction in adherence (Cohen's d = 0.643) as a meaningful difference. Kripalani et al11 state that many similar studies were relatively small and not sufficiently powered to detect differences in clinical outcomes. On the other side, a high effect size - as foreseen in this pilot study - does not require a large sample and both probability of detecting a difference and the power are both high as well. A sample size of at least 30 patients in each group would provide 80% power to detect a 33.5% increase in medication adherence; and we observed a 35% difference after 1 month. However, as a pilot study, our study was in fact underpowered for more nuanced differences in long-term adherence and other outcomes.
Our study had other limitations. For example, the intervention was delivered by 2 pharmacists, which limits the generalizability of our findings. Because of multiple components of intervention (e.g. verbal counseling based on INAB and UNAB and written materials) we could not attribute intervention effects to any single component. Although patients, prescribers and the researcher responsible for analyzing study data were blinded to the group, the pharmacists who performed data collection were not. We also had limited information about other medications used during the follow-up year. Intervention itself was limited by lack of ongoing contact. Carter et al27 suggest that continued interventions may be necessary to maintain high rates of BP control, especially in those patients who lose BP control. And this may be why the intervention did not led to better clinical outcomes after 1 year. At this time point, adherent patients in both groups had significantly lower rates of readmission and mortality, and once the effect of intervention on adherence dissipated, we believe that the same intervention involving ongoing contact may positively influence these clinical outcomes. Murray et al28, for instance, provided a 9-month multilevel intervention to support medication management in outpatients with heart failure who had low health literacy and limited resources, with a 3-month post-study phase, improving adherence to cardiovascular medications and decreasing health care use and costs28. Finally, 8 (11%) patients declined to participate. While one patient refused to participate because of the possibility of being assigned to the placebo condition, five patients claimed not having time to participate and two declared themselves anxious to go home. Although this occurred at pre-randomization and does not affect internal validity, it may affect the generalizability of our study.
While non-adherence to medications is common for patients with CVD, who might be less likely to take their medication as prescribed because hypertension is asymptomatic, many studies have described the association between medication adherence and clinical outcomes7. A meta-analysis revealed that the odds of good blood pressure control among patients adherent to anti-hypertensive medications, compared to those who were non-adherent was 3.44 [95% confidence interval 1.6-7.37]25 . Although we did not measure blood pressure as part of the study and cannot determine the association between self-reported medication adherence and blood pressure control, previous studies demonstrated a significant association between the MMAS-8 score and blood pressure control15-17.
Nair et al29 state that medication adherence appears to be a patterned behavior established through the creation of a routine and a reminder system for taking the medication. In addition, control of hypertension - as one of the most important interventions to prevent coronary heart disease, stroke, heart failure and end stage renal disease - may be achieved by continued efforts on development of simple and feasible interventions to improve medication adherence. Gehi et al30 findings suggest that self-reported adherence may be a simple and straightforward method to identify patients at risk for adverse cardiovascular outcomes in patients with Coronary Heart Disease. Similarly, it seems plausible that interventions designed to improve self-reported adherence can positively affect clinical outcomes. Our findings suggest that self-report methods of measuring medication-taking behavior, such as MMAS-4, may be integrated into the clinical visit in which the health care provider interacts with the patients in assessing adherence behavior and provides immediate feedback and counseling as to how barriers - such as forgetting, misunderstandings - can be addressed, as well as provide reinforcement for good adherence behavior31.
Despite the encouraging results, we need to examine other behavioral determinants that may influence medication adherence and clinical outcomes. These results need to be replicated using a multicenter, randomized strategy, and such a study is already being carried out in patients with chronic CVD.
Conclusion
Based on these findings, we conclude that a brief intervention based on a patient self-report instrument for assessing medication adherence is a potentially effective method to improve adherent behavior - which was correlated, in our investigation, to readmissions and mortality rate - in post-discharge patients with CVD taking prescribed drugs. However, a larger study is required to assess the real impact of intervention on these outcomes. Although updated by MMAS-8 as a research method for assessing adherence to medications, MMAS-4 can still be used as a guide for an educational intervention in the clinical setting, whereby the health care provider asks the intentional and unintentional adherence questions to the patient and provides guidance and counseling to the patient based on the response.
Acknowledgments
We acknowledge the assistance provided by Hospital do Açúcar staff in the implementation of this intervention study.
This research was fully sponsored by the Research Foundation for the State of Alagoas (FAPEAP), with grant number EFP00000889.
The content is solely the responsibility of the authors and does not necessarily represent the official views of FAPEAL or Hospital do Açúcar.
We also thank all patients who participated in this study.
Footnotes
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception and design of the research: Oliveira-Filho AD, Morisky DE, Lyra-Jr DP; Acquisition of data: Pacheco ST; Analysis and interpretation of the data: Oliveira-Filho AD, Costa FA, Neves SF; Statistical analysis: Neves SF; Obtaining financing: Oliveira-Filho AD; Writing of the manuscript: Oliveira-Filho AD, Morisky DE, Pacheco ST, Neves SF; Critical revision of the manuscript for intellectual content: Oliveira-Filho AD, Morisky DE, Costa FA, Lyra-Jr DP.
Sources of Funding
This study was funded by FAPEAL.
Role of the Sponsor
The funders had no role in the design and conduction of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Study Association
This article is part of the thesis of Doctoral submitted by Alfredo D. Oliveira-Filho, from Universidade Federal de Sergipe.
References
- 1.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008 Apr 16;(2): doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 2.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 3.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 4.Cole JA, Norman H, Weatherby LB, Walker AM. Drug copayment and adherence in chronic heart failure: effect on cost and outcomes. Pharmacotherapy. 2006;26(8):1157–1164. doi: 10.1592/phco.26.8.1157. [DOI] [PubMed] [Google Scholar]
- 5.Murray MD, Tu W, Wu J, Morrow D, Smith F, Brater DC. Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills. Clin Pharmacol Ther. 2009;85(6):651–658. doi: 10.1038/clpt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 8.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Caballos M, Ramos-Diaz F, Jimenez-Moleon JJ, Bueno-Cavanillas A. Drug-related problems in older people after hospital discharge and interventions to reduce them. Age Ageing. 2010;39(4):430–438. doi: 10.1093/ageing/afq045. [DOI] [PubMed] [Google Scholar]
- 10.Harris CM, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9(3):150–153. doi: 10.1097/PTS.0b013e318286f87d. [DOI] [PubMed] [Google Scholar]
- 11.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 12.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Morisky DE, Malotte CK, Choi P, Davidson P, Rigler S, Sugland B, et al. A patient education program to improve adherence rates with antituberculosis drug regimens. Health Educ Q. 1990;17(3):253–267. doi: 10.1177/109019819001700303. [DOI] [PubMed] [Google Scholar]
- 14.Andrade TN, Jabbur-Lopes MO, Oliveira-Filho AD, Chen TF, Lyra-Jr DP. Evaluation of pharmaceutical counseling after hospital discharge of post-surgery patients. Lat Am J Pharm. 2011;20(1):107–111. [Google Scholar]
- 15.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Oliveira-Filho AD, Barreto-Filho JA, Neves SJ, Lyra DP., Junior Association between the 8-item Morisky Medication Adherence Scale (MMAS-8) and blood pressure control. Arq Bras Cardiol. 2012;99(1):649–658. doi: 10.1590/s0066-782x2012005000053. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira-Filho AD, Morisky DE, Neves SJ, Costa FA, de DP., Lyra Junior The 8-item Morisky Medication Adherence Scale: validation of a Brazilian-Portuguese version in hypertensive adults. Res Social Adm Pharm. 2014;10(3):554–561. doi: 10.1016/j.sapharm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Lowry KP, Dudley TK, Oddone EZ, Bosworth HB. Intentional and unintentional nonadherence to antihypertensive medication. Ann Pharmacother. 2005;39(7-8):1198–1203. doi: 10.1345/aph.1E594. [DOI] [PubMed] [Google Scholar]
- 19.Unni EJ, Farris KB. Unintentional non-adherence and belief in medicines in older adults. Patient Educ Couns. 2011;83(2):265–268. doi: 10.1016/j.pec.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Laba TL, Bleasel J, Brien JA, Cass A, Howard K, Peiris D, et al. Strategies to improve adherence to medications for cardiovascular diseases in socioeconomically disadvantaged populations: a systematic review. Int J Cardiol. 2013;167(6):2430–2440. doi: 10.1016/j.ijcard.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 21.Schlenk EA, Bernardo LM, Organist LA, Klem ML, Engberg S. Optimizing medication adherence in older patients: a systematic review. J Clin Outcomes Manag. 2008;15(12):595–606. [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira-Filho AD, Pacheco ST, Neves SJ, Gama DP, Costa DP., Lyra Jr Ministério da . Secretaria de Ciência. Tecnologia e Insumos Estratégicos. Departamento de Ciência e Tecnologia . Avaliação de tecnologias em saúde: seleção de estudos apoiados pelo Decit. Brasília: MS; 2011. Otimização da adesão terapêutica pós-alta hospitalar de pacientes com doenças cardiovasculares crônicas: resultados preliminares de um ensaio clínico randomizado; pp. 82–92. [Google Scholar]
- 23.Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment: confirmation from meta-analysis. Am Psychol. 1993;48(12):1181–1209. doi: 10.1037//0003-066x.48.12.1181. [DOI] [PubMed] [Google Scholar]
- 24.Cooper H, Booth K, Fear S, Gill G. Chronic disease patient education: lessons from meta-analyses. Patient Educ Couns. 2001;44(2):107–117. doi: 10.1016/s0738-3991(00)00182-8. [DOI] [PubMed] [Google Scholar]
- 25.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19(11):1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Carter BL, Doucette WR, Franciscus CL, Ardery G, Kluesner KM, Chrischilles EA. Deterioration of blood pressure control after discontinuation of a physician-pharmacist collaborative intervention. Pharmacotherapy. 2010;30(3):228–235. doi: 10.1592/phco.30.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146(10):714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 29.Nair KV, Belletti DA, Doyle JJ, Allen RR, McQueen RB, Saseen JJ, et al. Understanding barriers to medication adherence in the hypertensive population by evaluating responses to a telephone survey. Patient Prefer Adherence. 2011;5:195–206. doi: 10.2147/PPA.S18481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167(16):1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu LL, Zhou Q, Yan XF, Zeng S. Optimal time to take once-daily oral medications in clinical practice. Int J Clin Pract. 2008;62(10):1560–1571. doi: 10.1111/j.1742-1241.2008.01871.x. [DOI] [PubMed] [Google Scholar]