Abstract
Objective:
This study compared the marginal adaptation of mineral trioxide aggregate (MTA) and MTA-like materials as root-end fillings after incubation in phosphate buffer saline (PBS), a synthetic tissue fluid, for either 1 week or 2 months.
Materials and Methods:
In this experimental study, seventy-two extracted human single-rooted teeth were prepared and obturated with gutta-percha and AH26 sealer. The apical 3 mm of the roots were resected. Root-end cavities were prepared with an ultrasonic retrotip. The specimens were randomly divided into three groups (n=24) and filled with either ProRoot MTA, OrthoMTA, or RetroMTA. Half of the specimens in each group were stored in PBS for 1 week the other half for 2 months. Epoxy resin replicas from the resected root-end surfaces and longitudinally sectioned roots were fabricated. The gaps at the material/dentin interface were measured using a scanning electron microscope (SEM). Transversal, longitudinal, and overall gap sizes were measured. The data were analyzed using Kruskal-Wallis and Mann-Whitney tests. The significance level was set at p < 0.05.
Results:
There were no significant differences between the marginal adaptation of ProRoot MTA, RetroMTA, and OrthoMTA in both transverse and longitudinal sections after incubation for either 1 week or 2 months (p > 0.05). In addition, the test groups were not significantly different regarding the overall mean gap values (p > 0.05).
Conclusion:
Under the conditions of this study, there was no difference between the marginal adaptation of ProRoot MTA, OrthoMTA, and RetroMTA as root-end filling materials after exposure to PBS for either 1 week or 2 months.
Keywords: Marginal Adaptation, Dental; Mineral Trioxide Aggregate; Scanning Electron Microscopy
INTRODUCTION
The main purpose of placing root-end filling materials during periradicular surgery is to seal the communications between the root canal system and periradicular tissues [1]. Several materials have been used for root-end filling in endodontic surgery [2–6]. Since the introduction of mineral trioxide aggregate (MTA), it has been widely used for root-end filling, perforation repair, pulpotomy, pulp capping, and formation of an apical barrier in necrotic open apices of the teeth because of the good biocompatibility, sealing ability, and its ability to promote hard-tissue formation [7–12]. MTA is composed of tricalcium silicate, dicalcium silicate, tricalcium aluminate and tetracalcium aluminoferrite, bismuth oxide, and other mineral oxides [13, 14]. The powder consists of fine hydrophilic particles that form a colloidal gel in the presence of water or moisture, which solidifies to hard cement [13, 15]. Recently, a new type of MTA, BioMTA (Meta Biomed Co., LTD, Seoul, Korea) has been introduced to the market. It is manufactured in both forms of OrthoMTA and RetroMTA for clinical applications similar to those described for ProRoot MTA. OrthoMTA consists of tricalcium silicate, dicalcium silicate, tricalcium aluminate, tetracalcium aluminoferrite, free calcium oxide, and bismuth oxide [16]. However, according to the manufacturer, RetroMTA is composed of calcium carbonate, silicon dioxide, aluminum oxide and zirconium oxide. Limited information is available regarding BioMTA in the literature. Chang et al. [16] showed that the components of OrthoMTA and ProRoot MTA are similar except for the absence of arsenic (As) and the less amount of chromium (Cr) in OrthoMTA. However, it has been reported that the biocompatibility of OrthoMTA was lower compared with that of ProRoot MTA [17].
The interaction of MTA and MTA-like materials with different types of storage media should be considered in studies on the physicochemical properties of biomaterials. Several investigations showed the formation of apatite crystalline structures as a result of the interaction between MTA and synthetic tissue fluids such as phosphate-buffered saline solution (PBS) [11, 18–21], which might increase the sealing ability of the biomaterials [22]. It has been shown that the aggregation of apatite crystals begins within the first hours of exposure of MTA to PBS [22], which increases substantially over time [21, 23–25].
Marginal adaptation has been considered as an indirect indicator of the sealing capacity of a root-end filling material [26]. Therefore, SEM evaluation of the marginal adaptation of root-end filling materials may provide information on the sealing ability of these materials [26, 27]. Several investigations have shown the superior marginal adaptation of MTA compared with that of amalgam [3, 28], IRM [3, 29], and Super EBA [3, 5, 29]. However, Oliveira et al. [30] showed that the marginal adaptation of ProRoot MTA, IRM, amalgam, and SuperEBA are comparable.
To the best of our knowledge, there is limited information regarding the effect of synthetic tissue fluid on the marginal adaptation of MTA as a root-end filling material. Therefore, this study was aimed to compare the marginal adaptation of a new type of MTA (BioMTA) to that of ProRoot MTA exposed to PBS for 1 week and 2 months using resin replicas.
MATERIALS AND METHODS
Preparation of the specimens
In this ex-vivo study, 72 extracted single-rooted human teeth were selected. Crowns were removed at the cemento-enamel junction with a high-speed diamond fissure bur (Tizkavan, Tehran, Iran) under a continuous water spray. The working length was determined by subtracting 1mm from the lengths recorded when tips of #15 K-files were visible at the apical foramina. The root canals were then prepared using ProTaper rotary system (Dentsply, Maillefer, Ballaigues, Switzerland) to an apical preparation file size F3. The root canals were irrigated with 5.25% sodium hypochlorite during canal preparation. Subsequently, the root canals were obturated using laterally compacted gutta-percha (Meta Biomed Co., LTD, Seoul, Korea) and AH26 sealer (Dentsply, De Trey, Konstanz, Germany). After canal obturation, the teeth were stored in 100% humidity for 1 week.
Then the apical 3 mm of the roots were resected perpendicular to the long axis of the teeth with a diamond fissure bur in high-speed handpiece under water spray. Afterwards, 3-mm deep root-end cavitie was prepared with a diamond-coated retrotip (E32D, NSK, Japan) attached to an ultrasonic unit (Varios 970, NSK, Japan) set at power level 6 according to the manufacturer’s instructions. Specimens were then randomly divided into three groups each of 24 and were filled with a mixture of tooth-colored ProRoot MTA (Dentsply Tulsa Dental, Tulsa, OK, USA), OrthoMTA (Meta Biomed Co., LTD, Seoul, Korea), or RetroMTA (Meta Biomed Co., LTD, Seoul, Korea). The root-end filling materials were prepared according to the manufacturer’s instructions. After the cavities were filled, the root-end materials were gently packed with appropriate pluggers and paper points. Excessive material was removed with a plastic instrument and cotton pellet.
Each group was then randomly divided into two subgroups each of 12.
In one subgroup, the roots were stored in PBS solution (pH = 7.4) at 37ºC and incubated for 1 week and in the other subgroup, for 2 months. The PBS solution was replaced every 5 days to replenish the buffering capacity of PBS [21].
SEM analysis
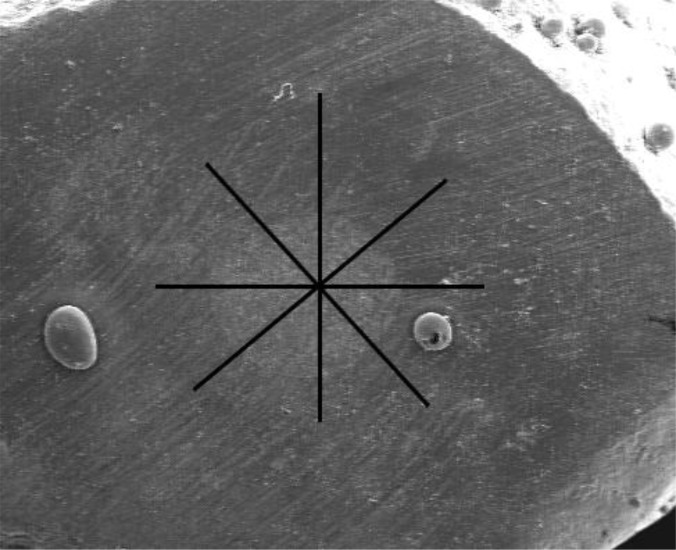
In the present study, the marginal adaptation of tested root-end filling materials was evaluated using resin replicas fabricated from the apical surface of the resected root-ends as well as longitudinal root sections defined as transverse and longitudinal replicas, respectively [31]. For obtaining transverse resin replicas, impressions were taken using a polyvinylsiloxane material (Panasil, Kettenbach GmbH & Co. KG, Germany). A low viscosity epoxy resin (Epoxiran, Tehran, Iran) was mixed according to the manufacturer’s instructions and then delivered into the impressions. In order to evaluate the longitudinal interface between root-end filling materials and the walls of the root-end cavity, the roots were ground longitudinally by a diamond bur until the gutta-percha and root-end filling materials were completely exposed. After that, the resin replicas were prepared from longitudinal sections in the manner described for transverse replicas. Then resin replicas were mounted on metallic stubs, sputter-coated with gold, and examined under SEM (Vega II XMU, Tescan, Czech Republic). Measurement of marginal adaptation was performed similar to that used in a previous study [31]. The perimeter of root-end cavities was divided into eight sections (Fig 1A).
Fig 1A.
The perimeter of root-end cavities was divided into eight sections to measure the gap between dentin and root-end filling material in the transverse section.
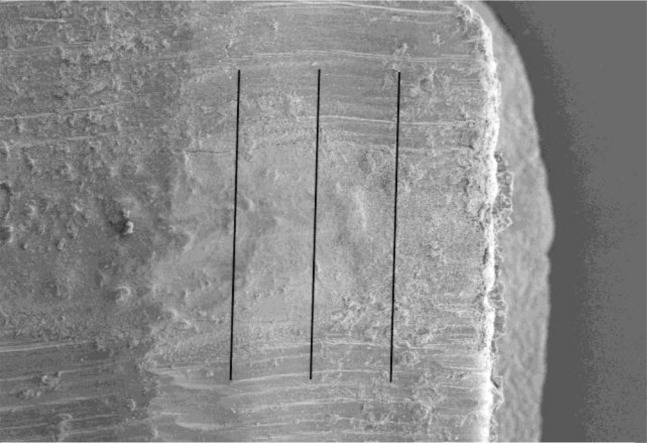
In each section, the maximum distance between the root-end filling materials and cavity walls was recorded. For longitudinal replicas, the 3-mm root-end filling material was divided into four sections (Fig 1B).
Fig 1B.
3-mm root-end filling material was divided into four sections in the longitudinal plane.
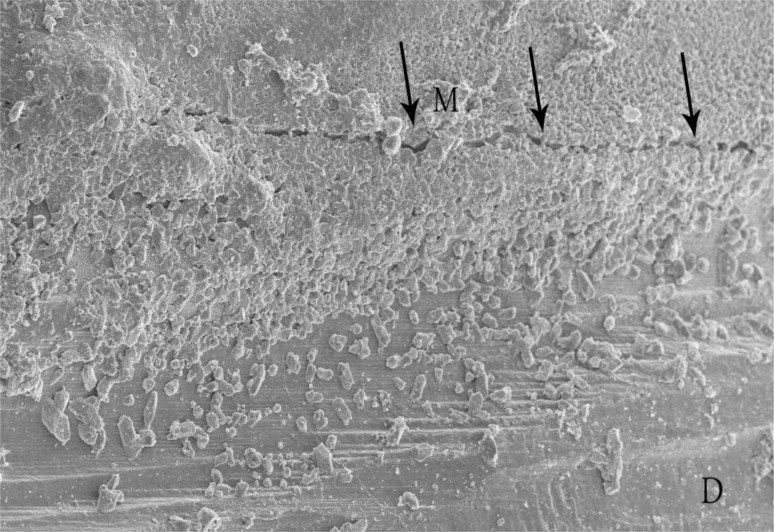
In each section, the maximum gap observed between the root-end filling material and both cavity walls were measured (Figs 2 and 3).
Fig 2.
SEM photomicrograph shows no gap between root-end filling material (M) and dentin (D).
Fig 3.
A gap is evident at the root-end filling material (M) and dentin (D) interface.
The average of eight recorded numbers in each transverse and longitudinal section was calculated.
The overall maximum gap value was measured by calculating the average of all 16 numbers recorded for both transverse and longitudinal replicas.
The data were analyzed using the Kruskal-Wallis test to compare the tested materials in each section and each interval. Mann-Whitney test was used to compare the marginal adaptation of each material for each section at two intervals. The significance level was set at p < 0.05.
RESULTS
The mean ± standard deviations (in μm) of gaps seen in different groups are shown in the Table 1. The results showed that there were no significant differences among the marginal adaptation of ProRoot MTA, RetroMTA, and OrthoMTA in both transverse (p = 0.82) and longitudinal (p = 0.87) sections at the 1-week interval. Furthermore, no significant differences were found between the values of the tested material in both transverse (p = 0.43) and longitudinal (p = 0.45) sections at 2 months. The results showed that the root-end materials were not significantly different regarding the overall mean gap values (p > 0.05).
Table 1.
Mean Gap Values (μm) for Three Tested Root-End Filling Materials
| Material | Interval | Section | Mean (± SD) | Minimum | Maximum |
|---|---|---|---|---|---|
| ProRoot MTA | 1-week | Transverse | 1.14 (±1.35) | 0.00 | 16.81 |
| Longitudinal | 0.83 (±0.97) | 0.00 | 12.00 | ||
| Overall | 0.99 (±1.16) | 0.00 | 14.40 | ||
| 2-month | Transverse | 0.20 (±0.40) | 0.00 | 10.25 | |
| Longitudinal | 0.63 (±1.11) | 0.00 | 16.60 | ||
| Overall | 0.42 (±0.76) | 0.00 | 13.43 | ||
|
| |||||
| OrthoMTA | 1-week | Transverse | 1.60 (±1.59) | 0.00 | 22.7 |
| Longitudinal | 1.35 (±2.13) | 0.00 | 31.64 | ||
| Overall | 1.48 (±1.86) | 0.00 | 27.17 | ||
| 2-month | Transverse | 0.30 (±0.70) | 0.00 | 12.51 | |
| Longitudinal | 0.99 (±1.13) | 0.00 | 12.60 | ||
| Overall | 0.65 (±0.92) | 0.00 | 12.55 | ||
|
| |||||
| RetroMTA | 1-week | Transverse | 1.1 (±1.17) | 0.00 | 26.8 |
| Longitudinal | 0.50 (±0.72) | 0.00 | 16.52 | ||
| Overall | 0.80 (±0.95) | 0.00 | 21.66 | ||
| 2-month | Transverse | 0.74 (±1.13) | 0.00 | 15.96 | |
| Longitudinal | 0.76 (±1.00) | 0.00 | 25.80 | ||
| Overall | 0.75 (±1.07) | 0.00 | 20.88 | ||
DISCUSSION
In order to evaluate the quality of the apical seal obtained by root-end filling material, several methods have been used such as penetration of the dye, radioisotope, and bacteria as well as fluid filtration means, electrochemical technique, confocal microscopy, and scanning electron microscopy (SEM) [3]. In this study, adaptation of ProRoot MTA, RetroMTA, and OrthoMTA was evaluated by the resin replica technique using SEM. SEM examination of original tooth specimens has several shortcomings. Preparation of the root before SEM observation may cause separation of the material from root-end cavity walls as well as dehydration of the material and tooth structure resulting crack formation and artifacts [3, 32].
Therefore, resin replica technique has been suggested for assessment of marginal adaptation of dental material [3, 30, 32]. It has been shown that fabricating replicas using epoxy resin accurately reproduced details of 1 to 2 microns [33]. Accordingly, Gondim et al. [34] showed that the details in hard tissue were not different in images taken from both original teeth and resin replicas. In the present study, marginal adaptation of the tested material was assessed by resin replicas taken from both the surface of the resected root-ends and longitudinally-sectioned roots for more accurate investigation of the material/dentin interface. The results of this study showed that there was no significant difference between the marginal adaptation of ProRoot MTA, RetroMTA, and OrthoMTA in both transverse and longitudinal sections. It has been stated that the plane of the root section might affect the results of marginal adaptation studies [3].
A material may show good adaptation to cavity walls in one plane, but not in the entire specimen. However, in this study, the plane of the root section influenced the marginal adaptation of none of the three tested materials. This finding is in accordance with the results of the previous study [31] that showed similar marginal adaptation in transverse and longitudinal sections of root-end cavities filled with ProRoot MTA.
On the other hand, Torabinejad et al. [3] and Badr [28] showed smaller gap sizes in transverse resin replicas of root-end cavities filled with gray ProRoot MTA compared with longitudinal sections. These different results might be because of the type of used material, which was white ProRoot MTA in the present study and gray ProRoot MTA in the other mentioned studies.
In this study, specimens were exposed to PBS for 1 week and 2 months to partially simulate the in vivo conditions [11].
The findings of the current study showed no difference among marginal adaptation of specimens exposed to PBS for 1 week and 2 months.
Several studies have shown that the interaction between MTA and phosphate containing solutions such as PBS and synthetic tissue fluids resulted in the aggregation of apatite crystals over MTA, which increased over time [18, 20, 25]. Although the results of the present study showed lower mean gap values in 2-month specimens of the most experimental groups, there was no difference between the mean gap sizes at both time periods. Previously, Reyes-Carmona et al. [19] showed that the exposure of 5-mm thick MTA plugs to PBS from both apical and coronal sides for 2 months resulted in the formation of an interfacial layer at MTA/dentin interface with intratubular mineralization at all levels of the material. However, no interfacial layer and/or intratubular mineralization formation was observed at the cervical third of MTA plug exposed to PBS only from the apical side. In addition, formation of the interfacial layer was shown after immersion of 2-mm thick root sections filled with MTA in PBS for 2 months [18, 20, 22].
In the above-mentioned studies, the surface of the material within the root slices was larger than the root-end fillings in the present study. In this study, similar mean gap values in 1-week and 2-month specimens might be attributed to the small surface of the root-end filling materials exposed to PBS.
It is worth mentioning that it is not possible to compare data from the literature, because the methodologies of marginal adaptation assessment vary widely from one study to another. The design of the studies, plane of root sectioning, and gap measurement methods are different in studies on marginal adaptation of the root-end filling materials. Furthermore, in some studies, the specimens were stored in 100% humidity and the type of storage media have not been stated [28, 35], but in the others, the roots were stored in water [29] or wrapped in moist gauze and kept in 100% humidity [36]. Exposure of root-end fillings to different conditions before evaluation of marginal adaptation could influence the findings of the studies. It has been stated that exposure to blood during the setting had a negative effect on the marginal adaptation of MTA compared with the material exposed to synthetic tissue fluid [37]. However, no published studies have examined the marginal adaptation of MTA at different time periods, thus making it impossible to compare the results of this study to those of other studies.
CONCLUSION
Under the conditions of this ex vivo study, it could be concluded that there was no significant difference between the marginal adaptation of ProRoot MTA, OrthoMTA, and RetroMTA as root-end fillings after exposure to PBS for either 1 week or 2 months.
Acknowledgments
This study was part of a M.S. thesis supported by Tehran University of Medical Sciences (grant no: 22939). The authors wish to thank Dr. Mohammad J. Kharrazifard for his assistance in data analysis and Azad Tejarat Pars Ltd. for providing some of the root-end filling material in this study.
REFERENCES
- 1.Torabinejad M, Pitt Ford TR. Root end filling materials: a review. Endod Dent Traumatol. 1996 Aug;12(4):161–78. doi: 10.1111/j.1600-9657.1996.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernabe PF, Holland R, Morandi R, de Souza V, Nery MJ, Otoboni Filho JA, et al. Comparative study of MTA and other materials in retrofilling of pulpless dogs' teeth. Braz Dent J. 2005;16(2):149–55. doi: 10.1590/s0103-64402005000200012. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Smith PW, Kettering JD, Pitt Ford TR. Comparative investigation of marginal adaptation of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995 Jun;21(6):295–9. doi: 10.1016/S0099-2399(06)81004-6. [DOI] [PubMed] [Google Scholar]
- 4.Peters CI, Peters OA. Occlusal loading of EBA and MTA root-end fillings in a computer-controlled masticator: a scanning electron microscopic study. Int Endod J. 2002 Jan;35(1):22–9. doi: 10.1046/j.1365-2591.2002.00449.x. [DOI] [PubMed] [Google Scholar]
- 5.Xavier CB, Weismann R, de Oliveira MG, Demarco FF, Pozza DH. Root-End Filling Materials: Apical Microleakage and Marginal Adaptation. J Endod. 2005 Jul;31(7):539–42. doi: 10.1097/01.don.0000152297.10249.5a. [DOI] [PubMed] [Google Scholar]
- 6.Maltezos C, Glickman GN, Ezzo P, He J. Comparison of the sealing of Resilon, Pro Root MTA, and Super-EBA as root-end filling materials: a bacterial leakage study. J Endod. 2006 Apr;32(4):324–7. doi: 10.1016/j.joen.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod. 1995 Dec;21(12):603–8. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 8.Faraco IM, Jr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent traumatol. 2001 Aug;17(4):163–6. doi: 10.1034/j.1600-9657.2001.170405.x. [DOI] [PubMed] [Google Scholar]
- 9.Holland R, de Souza V, Murata SS, Nery MJ, Bernabe PF, Otoboni Filho JA, et al. Healing process of dog dental pulp after pulpotomy and pulp covering with mineral trioxide aggregate or Portland cement. Braz Dent J. 2001;12(2):109–13. [PubMed] [Google Scholar]
- 10.Camp JH. Diagnosis dilemmas in vital pulp therapy: treatment for the toothache is changing, especially in young, immature teeth. J Endod. 2008 Jul;34(7 Suppl):S6–12. doi: 10.1016/j.joen.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005 Feb;31(2):97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Nakano T, Tomokiyo A, Fujii S, Wada N, Monnouchi S, et al. Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. J Endod. 2010 Apr;36(4):647–52. doi: 10.1016/j.joen.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005 Nov;38(11):834–42. doi: 10.1111/j.1365-2591.2005.01028.x. [DOI] [PubMed] [Google Scholar]
- 14.Asgary S, Parirokh M, Eghbal MJ, Stowe S, Brink F. A qualitative X-ray analysis of white and grey mineral trioxide aggregate using compositional imaging. J Mater Sci Mater Med. 2006 Feb;17(2):187–91. doi: 10.1007/s10856-006-6823-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004 Feb;25(5):787–93. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 16.Chang SW, Baek SH, Yang HC, Seo DG, Hong ST, Han SH, et al. Heavy metal analysis of ortho MTA and ProRoot MTA. J Endod. 2011 Dec;37(12):1673–6. doi: 10.1016/j.joen.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Lee BN, Son HJ, Noh HJ, Koh JT, Chang HS, Hwang IN, et al. Cytotoxicity of newly developed ortho MTA root-end filling materials. J Endod. 2012 Dec;38(12):1627–30. doi: 10.1016/j.joen.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Reyes-Carmona JF, Felippe MS, Felippe WT. The biomineralization ability of mineral trioxide aggregate and Portland cement on dentin enhances the push-out strength. J Endod. 2010 Feb;36(2):286–91. doi: 10.1016/j.joen.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Carmona JF, Felippe MS, Felippe WT. A phosphate-buffered saline intracanal dressing improves the biomineralization ability of mineral trioxide aggregate apical plugs. J Endod. 2010 Oct;36(10):1648–52. doi: 10.1016/j.joen.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Shokouhinejad N, Nekoofar MH, Razmi H, Sajadi S, Davies TE, Saghiri MA, et al. Bioactivity of EndoSequence root repair material and bioaggregate. Int Endod J. 2012 Dec;45(12):1127–34. doi: 10.1111/j.1365-2591.2012.02083.x. [DOI] [PubMed] [Google Scholar]
- 21.Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. J Endod. 2006 May;32(5):425–8. doi: 10.1016/j.joen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white portland cement with dentin in a phosphate-containing fluid. J Endod. 2009 May;35(5):731–6. doi: 10.1016/j.joen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Gandolfi MG, Ciapetti G, Taddei P, Perut F, Tinti A, Cardoso MV, et al. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent Mater. 2010 Oct;26(10):974–92. doi: 10.1016/j.dental.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Gandolfi MG, Taddei P, Tinti A, De Stefano Dorigo E, Rossi PL, Prati C. Kinetics of apatite formation on a calcium-silicate cement for root-end filling during ageing in physiological-like phosphate solutions. Clin Oral Investig. 2010 Dec;14(6):659–68. doi: 10.1007/s00784-009-0356-3. [DOI] [PubMed] [Google Scholar]
- 25.Gandolfi MG, Taddei P, Tinti A, Prati C. Apatite-forming ability (bioactivity) of ProRoot MTA. Int Endod J. 2010 Oct;43(10):917–29. doi: 10.1111/j.1365-2591.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- 26.Stabholz A, Friedman S, Abed J. Marginal adaptation of retrograde fillings and its correlation with sealability. J Endod. 1985 May;11(5):218–23. doi: 10.1016/s0099-2399(85)80063-7. [DOI] [PubMed] [Google Scholar]
- 27.Tanzilli JP, Raphael D, Moodnik RM. A comparison of the marginal adaptation of retrograde techniques: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1980 Jul;50(1):74–80. doi: 10.1016/0030-4220(80)90335-7. [DOI] [PubMed] [Google Scholar]
- 28.Badr AE. Marginal adaptation and cytotoxicity of bone cement compared with amalgam and mineral trioxide aggregate as root-end filling materials. J Endod. 2010 Jun;36(6):1056–60. doi: 10.1016/j.joen.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Gondim E, Zaia AA, Gomes BP, Ferraz CC, Teixeira FB, Souza-Filho FJ. Investigation of the marginal adaptation of root-end filling materials in root-end cavities prepared with ultrasonic tips. Int Endod J. 2003 Jul;36(7):491–9. doi: 10.1046/j.1365-2591.2003.00679.x. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira HF, Alencar AHG, Figueiredo JAP, Guedes OA, de Almeida Decurcio D, Estrela C. Evaluation of Marginal Adaptation of Root-End Filling Materials Using Scanning Electron Microscopy. Iran Endod J. 2013 Fall;8(4):182–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Shokouhinejad N, Nekoofar MH, Ashoftehyazdi K, Zahraee S, Khoshkhounejad M. Marginal Adaptation of New Bioceramic Materials and Mineral Trioxide Aggregate: A Scanning Electron Microscopy Study. Iran Endod J. 2014 Spring;9(2):144–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Gandolfi MG, Sauro S, Mannocci F, Watson TF, Zanna S, Capoferri M, et al. New tetrasilicate cements as retrograde filling material: an in vitro study on fluid penetration. J Endod. 2007 Jun;33(6):742–5. doi: 10.1016/j.joen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Teaford MF, Oyen OJ. Live primates and dental replication: new problems and new techniques. Am J Phys Anthropol. 1989 Sep;80(1):73–81. doi: 10.1002/ajpa.1330800109. [DOI] [PubMed] [Google Scholar]
- 34.Gondim E, Jr, Gomes BP, Ferraz CC, Teixeira FB, Souza-Filho FJ. Effect of sonic and ultrasonic retrograde cavity preparation on the integrity of root apices of freshly extracted human teeth: scanning electron microscopy analysis. J Endod. 2002 Sep;28(9):646–50. doi: 10.1097/00004770-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Bidar M, Moradi S, Jafarzadeh H, Bidad S. Comparative SEM study of the marginal adaptation of white and grey MTA and Portland cement. Aust Endod J. 2007 Apr;33(1):2–6. doi: 10.1111/j.1747-4477.2007.00053.x. [DOI] [PubMed] [Google Scholar]
- 36.Shipper G, Grossman ES, Botha AJ, Cleaton-Jones PE. Marginal adaptation of mineral trioxide aggregate (MTA) compared with amalgam as a root-end filling material: a low-vacuum (LV) versus high-vacuum (HV) SEM study. Int Endod J. 2004 May;37(5):325–36. doi: 10.1111/j.0143-2885.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- 37.Salem Milani A, Rahimi S, Froughreyhani M, Vahid Pakdel M. Effect of Blood Contamination on Marginal Adaptation and Surface Microstructure of Mineral Trioxide Aggregate: A SEM Study. J Dent Res Dent Clin Dent Prospects. 2013;7(3):157–63. doi: 10.5681/joddd.2013.025. [DOI] [PMC free article] [PubMed] [Google Scholar]