Abstract
Objective:
This study aimed to assess the anticariogenic and hemolytic activity of crude plant seed protein extracts against tooth decaying bacteria.
Materials and Methods:
The proteins from seeds of 12 different plants were extracted and used for antimicrobial assay against six different organisms. The extraction was carried out in 10mM of sodium phosphate buffer (pH 7.0). Protein concentrations were determined as described by Bradford method. Anticariogenic activity was studied by agar well diffusion method and Minimum Inhibitory Concentration (MIC) was evaluated by the two-fold serial broth dilution method. Hemolytic activity, treatment of proteinase K and Kinetic study in Mimusops elengi crude seed protein extract.
Results:
The anticariogenic assay demonstrated the activity of Mimusops elengi against Staphylococcus aureus and Streptococcus pyogenes. A minor activity of Glycine wightii against Streptococcus mutans was also found. The protein content of Mimusops elengi seed protein extract was 5.84mg/ml. The MIC values for Staphylococcus aureus and Streptococcus pyogenes against Mimusops elengi seed protein extract were 364.36μg/ml and 182.19μg/ml, respectively. Kinetic study further elucidated the mode of inhibition in the presence of the Mimusops elengi plant seed protein with respect to time. The concentration of crude extract which gave 50% hemolysis compared to Triton X-100 treatment (HC50) value was 1.58 mg/ml; which is more than five times larger than that of the MIC. Treatment with proteinase K of the Mimusops elengi seed protein resulted in absence of the inhibition zone; which clearly indicates that the activity was only due to protein.
Conclusion:
Our results showed the prominence of Mimusops elengi plant seed protein extract as an effective herbal medication against tooth decaying bacteria.
Keywords: Mimusops elengi, Seeds, Anticariogenic activity, Hemolysis
INTRODUCTION
Dental caries are among the most common diseases in the world. The link between oral diseases and the activities of microbial species that form part of the microbial flora of the oral cavity has been well established [1]. More than 750 bacterial species are present in the oral cavity; out of which, more than 50% are identified to be responsible for oral diseases.
Anaerobic bacterial organisms, such as Porphyromonas gingivalis, Actinobacillus sp, Prevotella sp. and Fusobacterium sp. that reside in subgingival level, are responsible for periodontal diseases [2].
In periodontal diseases, the areas at or below the gingival crevice become infected and cause a cellular inflammatory response in the gingiva and the surrounding connective tissues. Many plant extracts are reported to be efficient against microbial and parasitic infections [4].
It is necessary to remove such types of inflammation from the teeth and bone and therefore, it is imperative to investigate plant extracts for elimination of periodontal infection [4]. Seeds have different types of proteins. Some of these proteins have antimicrobial properties, which play an important role in plant defense mechanisms. One of these proteins, Chitinase is present in high concentrations in seeds [7].
Many people believe that herbal products cause less or no side effect compare to synthetic drugs [8]. Some plants are a potential source of phytochemicals that have antimicrobial properties present in new antibiotic prototypes [9].
The major objective is to isolate active compounds, such as phytochemical and proteins responsible for this antibacterial activity [10, 11].
Many researchers are attracted to antimicrobial proteins (AMPs) because of their use in antibiotics and antifungals. Antimicrobial proteins are present in simple and complex organisms, including single cell microorganisms [12]. AMPs are responsible for the growth, multiplication and spread of bacteria and allow plants and animals to resist infection by environmental bacterial species. The objective of this study is to investigate the inhibitory effect of the seed protein extracts from some medicinal plants on cariogenic organisms, under in vitro conditions, and in human red blood cells in order to provide a scientific validity for their use for controlling oral organisms. There are few data regarding bioactivity of seed protein extracts of medicinal plants against cariogenic microorganisms. The results may be useful in finding new cost-effective antibacterial drugs of natural origin with different mechanisms of action from those in current use.
MATERIALS AND METHODS
Collection of plant materials
Different plant seeds were collected between June and July 2011 form different parts of Gujarat and the surrounding Vallabh Vidyanagar region (Table 1). Disease free plant seeds were used to examine their anticariogenic and haemolytic activities.
Table 1.
Details of selected plant seeds
| S.N | BOTANICAL NAME | FAMILY | LOCAL NAME | COLLECTION SITE |
|---|---|---|---|---|
| 1 | Butea monosperma (Lam.) Taub. | Papilionaceae | Khakhro | Anand |
| 2 | Delonix regia (Boj.) Raf. | Caesalpiniaceae | Gulmahor | V.V. Nagar |
| 3 | Derris indica (Lam.) Bennet | Papilionaceae | Karanj | V.V.Nagar |
| 4 | Emblica officinalis Gaertn. | Euphorbiaceae | Amla | Gana |
| 5 | Eucalyptus globules Labill. | Myrtaceae | Nilgiri | Mogari |
| 6 | Glycine wightii (Grah. ex W. & A.) Verdcourt | Papilionaceae | Soyabean | Anand |
| 7 | Mimusops elengi L. | Sapotaceae | Borsali | New V.V. Nagar |
| 8 | Piper nigrum L. | Piperaceae | Mari | Anand |
| 9 | Sesamum indicum L. | Pedaliaceae | Tal | Napa |
| 10 | Saraca ashoka L. | Comberetaceae | Ashoka | V.V. Nagar |
| 11 | Vigna radiate (L.) Wilezek var. Sublobata (Roxb.) Verdcourt | Papilionaceae | Mag | New V. V. Nagar |
| 12 | Zea mays L. | Poaceae | Makai | Bakrol |
The seeds were identified by Dr. Kalpesh Ishnava (Plant Taxonomist) at Ashok and Rita Patel Institute of Integrated Study & Research in Biotechnology and Allied Sciences (ARIBAS), New VallabhVidyanagar, Gujarat, India.
Extraction of seed proteins
Antimicrobial proteins were extracted from plant seeds using 10mM of sodium phosphate buffer (pH 7.0). The extraction was performed as described by Salahudin et al. in 2011 [13] with minor modifications. The buffer was prepared and seeds of all plants were grinded in pre-cooled mortar and pestle with extraction buffer in 1:3 ratios i.e. 1 gm of seed in 3 ml of buffer; and the extract was harvested in 50 ml centrifuge tube. This extract was centrifuged at 10,000 rpm at 4°C for 20 minutes.
The crude extract isolated (supernatant) was saturated with 80% ammonium sulfate. Subsequently, this saturated crude extract was centrifuged at 13,000 rpm at 4°C for 30 minutes to pellet down the precipitated proteins. Pellet was further resuspended in the buffer and underwent purification of protein concentrate using dialysis bag.
Protein Purification
Bradford methodology was used to determine protein concentrations [14]. Different aliquots of appropriately diluted samples (10–50 μL) were mixed with 200μl crude extract to a total of 1 mL with distilled water at room temperature for 5 minutes. The absorbance was read at 595 nm, and the protein content was calculated with a bovine serum albumin (BSA) as standard.
Cariogenic Bacterial Strains and Preparation of Inoculums
Bacteria were selected based on their role in tooth decay and purchased from the Microbial Type Culture Collection (MTCC) bank, Chandigarh. The cultivation of the bacterial culture was revived using specific bacterial growth media and glycerol stocks for long-term storage. The bacteria responsible for dental caries used for this study included Lactobacillus acidophilus: MTCC No. *447; Lactobacillus casei: MTCC No. 1423; Staphylococcus aureus: MTCC No. 96; Streptococcus mutans: MTCC No.890; Candida albicans: MTCCNo.183 and Streptococcus pyogenes: MTCC No.442 and their growth conditions followed standards protocols. We took fresh bacterial suspension cultures and streaked them into specific selective media and incubated them at optimal temperature to maintain uniform growth rate of each organism. The fresh bacterial culture media were compared with 0.5 McFarland standard turbidity, which is equivalent to approximately 1×108 bacterial cell count per mL [15].
Bioassay for Antimicrobial Activity: Agar Well Diffusion Method
In the present study, we used 12 different plant seed extracts to test antibacterial activity. The antibacterial activity was studied by agar well diffusion method. From the stock, 100 mg of each plant extract was suspended in one milliliter of dimethyl sulfoxide (DMSO). In order to make agar plates, the Petri dishes were thoroughly washed using detergent, then dried and autoclave sterilized at 121 °C and 15 lbs pressure for 15 minutes. We subsequently poured 25 ml of sterilized selective medium into each sterile Petri dish and allowed it to solidify at room temperature. The plates were incubated at 37º C for sterility checking overnight. Agar plates were marked and divided into four equal parts, labeled for specific organism and extract number. One hundred microliter of fresh bacterial culture including 108 CFU/ml was spread on each agar plate with sterile glass spreader. A well 7 mm in diameter was punched off in the agar medium with sterile cup borer and subsequently was filled with 100 μl of respective plant seed protein extract. Plates were placed in the refrigerator for 30 minutes to allow the diffusion of the extracts and then incubated at 37°C (or pre-specified temperature) for 24 hours or more, depending on the organisms, until the zone of inhibition appeared. The zone of inhibition (excluding well diameter) was measured as a property of antibacterial activity. Ampicillin, amoxicillin and tetracycline were used as standard at a concentration of 100 μg/mL. Bioassay was performed in duplicate and was repeated twice.
Minimum Inhibitory Concentration (MIC) Assay:
The MIC of the crude seed protein extract of Mimusops elengi against Staphylococcus aureus and Streptococcus pyogenes was evaluated by the two-fold serial broth dilution method previously described by Chattoapadhyay et al (1998) [16]. The MIC was tested in the concentration range between 2.92 mg/mL and 0.05 mg/mL. The MIC was defined as the lowest concentration of the crude extract, which gave no visible growth on the plate.
Kinetic Study of the Crude Seed Protein Extracts of Mimusops Elengi Against Staphylococcus Aureus and Streptococcus Pyogenes:
An overnight broth culture of Staphylococcus aureus and Streptococcus pyogenes was taken for the study and different assemblies were prepared for the kinetic study. The kinetic study used the standard protocol of Kareem et al (2008) [17]. The optical density (427nm) was determined at 60-minute intervals for six hours, using spectrophotometer to obtain both the curves of normal growth in culture flask and inhibitory effect in the test flask for Staphylococcus aureus and Streptococcus pyogenes.
Hemolytic Activity of Mimusops Elengi Crude Protein Extract:
Hemolytic activity was evaluated as described previously by Andra et al. (2008) with a slight modification [18].
A suspension of human erythrocyte was washed with phosphate buffered saline (PBS: 1.5 mM KH2PO4, 2.7 mM KCl, 8.1 mM Na2HPO4, 135 mM NaCl, pH-7.4) and then centrifuged at 6,000 g for 10 min and washed with PBS until the supernatant was colorless. The human erythrocytes were then resuspended and diluted to 10 times of the original volume with PBS. This was referred to as the stock erythrocyte suspension. Subsequently, 150 μL of Mimusops elengi crude extract (577.0μg/mL) in PBS was incubated with 150 μL of stock human erythrocyte suspension (final human erythrocytes concentration, 4% v/v) for 60 minutes at 37° C.
After the incubation period, it was centrifuged at 1,000 x g for 10 minutes to remove the intact erythrocytes. We then collected the released hemoglobin from 10 fold-dilution of the supernatant and measured at 540 nm. We calculated the percentage of hemolysis and expressed it as hemolytic activity. The following formula was used to find out the hemolytic activity.
Where ‘A sample’ is A540 of red blood cells with peptide solution in PBS, ‘A buffer’ is A540 of red blood cells in PBS, and ‘A max’ is A540 of red blood cells with 1% (v/v) Triton X-100 in PBS. No hemolysis (0%) and full hemolysis (100%) were observed in the presence of PBS and 1% (v/v) Triton X-100, respectively.
Treatment of Mimusops Elengi Crude Seed Protein Extract by Proteinase K:
The crude extract was treated with proteolytic enzyme proteinase K. Proteinase K was added to 200 μL of crude extract (5.84 mg/mL protein), in which the ratio of protein substrate to proteolytic enzymes was 1:8. The treatment reaction was performed at 37°C for 20 hours [21].
After 10,000 rpm centrifugation for 5 minutes, the supernatant was collected and further used for antibacterial activity studies.
RESULTS
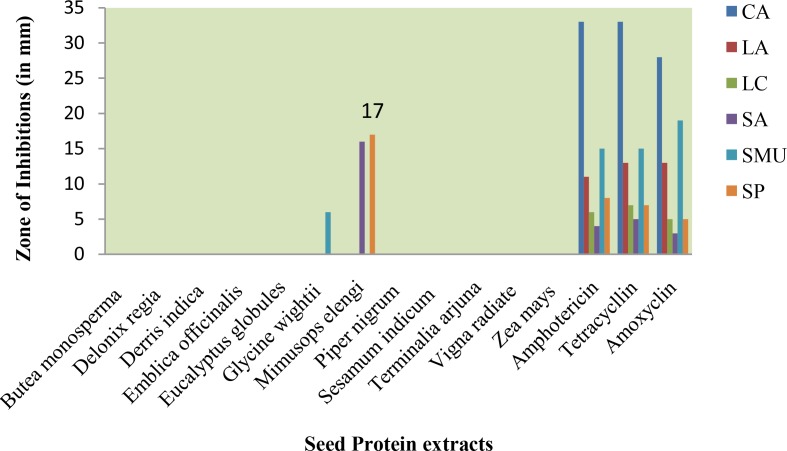
The sensitivity of crude plant seed protein extracts against the tooth decaying organisms (CA, LA, LC, SA, SMU and SP) was assessed by visualizing the presence or absence of inhibition zone and measuring the diameter of the zone of inhibition.
Antimicrobial Activity
The sensitivity of crude plant seed protein extracts against the tooth decaying organisms is shown in Figure 1. All the selected plant seed protein extracts were found to be inactive against Candida albicans. Similarly, the plant seed protein extracts did not show activity against Lactobacillus acidophilus and Lactobacillus casei.
Fig 1.
Antibacterial activity of seed protein extracts against cariogenic microorganisms (Zone in mm).
Amphotericin, tetracycline and amoxicillin demonstrated 4, 5 and 3mm zone of inhibition against Staphylococcus aureus, respectively. Mimusops elengi seed protein extract had 16mm zone of inhibition against Staphylococcus aureus.
The zone of inhibition of the plant seed extract of Glycine wightii against this organism was 6 mm; which is a moderate level of activity in comparison with three antibiotics. Babu Shankar Ponnusha et al. (2011) reported the Glycine max plant extracts for antibacterial activity against different organisms [18].
Mimusops elengi seed protein extract showed the activity against Staphylococcus pyogenes. Amphotericin, tetracycline and amoxicillin demonstrated 8, 7 and 5mm zones of inhibition, respectively. Mimusops elengi seed protein extract showed 17mm zone of inhibition. Mimusops elengi seed protein extract was found to be much more effective in our study compared to the study by Disha (2010) using different organic extracts of seeds [19].
Protein Content of Crude Extract from Mimusops Elengi Seeds:
One gram of dry seed of Mimusops elengi was extracted in 3 ml of 10mM Sodium phosphate buffer. The resulting protein concentration was 5.84 mg/ml. This concentration was reconfirmed by Nanodrop; showing same reading for the amount of protein present in the sample.
Minimum Inhibitory Concentration (MIC):
Minimum inhibitory concentration values of the crude extract against these two strains were evaluated.
In the case of Mimusops elengi seed protein extract, MIC values were 364.36μg/ml against Staphylococcus aureus and 182.19μg/ml for Streptococcus pyogenes. Mimusops elengi seed crude protein extract showed higher sensitivity towards Streptococcus pyogenes compared to Staphylococcus aureus.
Mimusops elengi crude extract showed interesting antibacterial activities which could inhibit all tested gram positive microorganisms.
A significant MIC value against the tested bacteria indicates that this extract has a high potential for widespread application.
Kinetics of Antibacterial Activity:
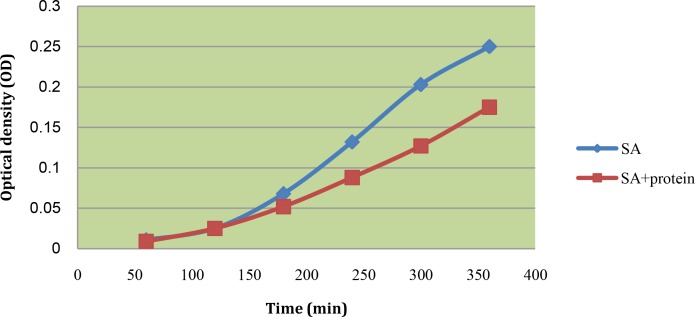
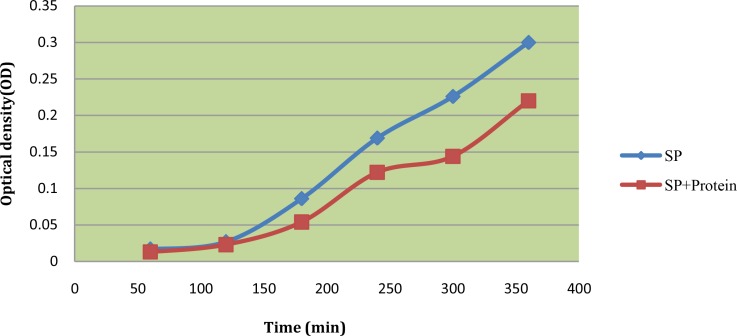
The study of the effect of plant seed protein extract of Mimusops elengi on the growth dynamics of Staphylococcus aureus and Streptococcus pyogenes was done in comparison with the normal growth (Figures 1 and 2).
Fig 2.
Kinetics of antimicrobial activities of seed protein extract of Mimusops elengi against Staphylococcus aureus (SA)
We took the plant seed extract at doses equivalent to MIC. The population of both bacteria declined after the 2 hours (Figures 2 and 3).
Fig 3.
Kinetics of antimicrobial activities of seed protein extracts of Mimusops elengi against Streptococcus pyogenes (SP)
The microorganisms survived and the mechanism of action of the antimicrobial agents are poorly understood [20].
It was also noted that antimicrobial growth dynamics were both dose and time dependent and this is a more rational basis for determining optimal dosage for antimicrobial treatment regimens [21].
Hemolytic Activity
Many antimicrobial peptides have been reported to show cytotoxic activity in eukaryotic cells. Mimusops elengi seed protein extract is tested for hemolytic activity against human erythrocytes.
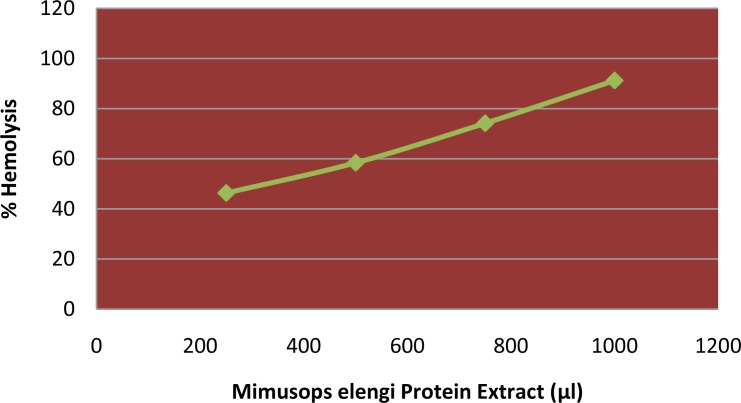
Mimusops elengi seed protein extract showed hemolytic activity against human erythrocytes in a dose-dependent manner.
The crude protein extract concentration causing 50% hemolytic activity compared to Triton –X-100 treatment equaled to 1.58mg/ml (Figure 3).
Where ‘Asample’ is A540 of red blood cells with protein extract in stock human erythrocyte suspension, ‘Abuffer’ is A540 of red blood cells in PBS, and ‘Amax’ is A540 of red blood cells with 1% (v/v) Triton X-100 in PBS. No hemolysis (0%) and full hemolysis (100%) were observed in the presence of PBS and 1% (v/v) Triton X-100, respectively. Thus, according to graph, HC50 value for the sample was1.58 mg compared to Triton X-100. HC50 value was about 10 times higher than the MIC value against Streptococcus pyogenes and four times that of Staphylococcus aureus (Figure 4). The Mimusops elengi crude seed protein extract after treatment with proteinase K revealed no further activity of the extract against both Staphylococcus aureus and Streptococcus pyogenes which confirms that the antimicrobial activity was only in the presence of fully structured protein and after its destruction or hydrolysis by enzyme the activity vanish (Table 2).
Fig 4.
The susceptibility of freshly collected erythrocytes to hemolytic (%) with seed protein extract of Mimusops elengi
Table 2.
Inhibition zone of Mimusops elengi crude extract after treatment with proteinase K
| Samples | Loaded protein (μl) | Inhibition zone (mm) | |
|---|---|---|---|
|
| |||
| Staphylococcus aureus (SA) | Streptococcus pyogenes (SP) | ||
| Crude extract | 100 | 16 | 17 |
| Crude extract + Proteinase K | 100 | 0 | 0 |
DISCUSSION
This study concludes that use of 10mM of sodium phosphate buffer (pH 7.0) for the extraction is proficient for retrieving good amount of the protein from the plant sample and is a reliable extraction buffer. Mimusops elengi plant seed protein extract resulted in three times larger zones of inhibition compared to antibiotics for both Staphylococcus aureus and Streptococcus pyogenes. This seed protein extract clearly shows the prominence of the inhibitory protein present. Moreover, MIC study indicates that Streptococcus pyogenes is slightly more susceptible to Mimusops elengi plant seed protein compared to Staphylococcus aureus by showing lower MIC value compared to Staphylococcus aureus. Kinetic study further elucidated the inhibition and confirmed the inhibitory activity in presence of Mimusops elengi plant seed protein with respect to time. Treatment with proteinase K confirmed that the inhibitory activity was only due to the protein present in the sample, as after its treatment with proteinase K, the sample lost its inhibitory activity against both organisms as protein was hydrolyzed by this enzyme.
Finally, hemolytic activities of the Mimusopselengi plant seed protein give HC50 value at a much higher range than that of the MIC values against both organisms and thus suggests its application as a successful herbal remedy in practice. Different plant seed protein extracts have antibacterial activity. Some of the peptides can inhibit different types of microorganisms including the fungi and viruses. Plants synthesize large number of different proteins; they may serve as future reservoir of novel drugs and therapeutic agents. Many of these proteins have shown activity against oral pathogens [3]. The protein extracts are at least three times more active in comparison to the three antibiotics tested against the selected cariogenic microorganisms.
Based on our results, one can conclude that seed protein extract of Mimusops elengiis is slightly more effective against Streptococcus pyogenes than Staphylococcs aureus [22]. Disha (2010) reported that seed extracts have antibacterial activity against oral organisms [20].
The seed extraction procedure used different organic solvents like hexane, ethyl acetate, methanol and distilled water extracts. All the seed extracts showed no activity against these organisms. The plant seed protein extraction method using sodium phosphate buffer is more suitable compared to other extraction methods and these proteins have a potential to be developed as drugs against tooth decaying organisms.
In the case of Mimusops elengi seed protein extracts, MIC values were 364.36 μg/ml against Staphylococcus aureus and 182.19 μg/ml against Streptococcus pyogenes. The extract of Mimusops elengi seeds was active against Gram positive bacteria. It has been reported that crude protein extracts of cruciferous vegetables had greater inhibitory effects against Gram negative bacteria than Gram positive bacteria [23].
It is possible that the responsible molecule for antibacterial activity in Mimusops elengi crude extract might be a protein or a peptide. Crude protein extract of Moringa oleifera seeds has been reported to possess antibacterial activities against S. aureus (MIC 24.0 mg/mL).
These MIC values are much higher than our results, showing that crude extract of Mimusops elengi seeds are more potent for bacterial inhibition [24].
We took the plant seed extract at doses equivalent to MIC. The population of both bacteria declined after 2 hours. The kinetic activity study provided clear idea for the vehicle system used to deliver antibacterial agents to the oral cavity [25]. An agent capable of killing oral bacteria within minutes of exposure can be used in a product like toothpaste or mouthwash since brushing or rinsing usually takes only a few minutes [26]. These formulations are added to a delivery vehicle such as chewing gum, gel or varnish that can sustain its release over a period of time. Further studies are required to determine the side effects and the antibacterial activity of extracts when used over a long period of time in the oral cavity. Mimusops elengi crude extract was tested for hemolytic activity against human erythrocytes.
Hemolytic activity against human erythrocytes in a dose-dependent manner with concentration of protein at which 50% hemolytic compared to Triton X-100 treatment (HC50) value equaled to 1.58 mg/ml [27]. The HC50 value for Mimusops elengi crude extract is far larger than that of Melittin and MIC values against the tested bacteria. This implies that the crude extract may be safely used in humans. After treatment with proteinase K, Mimusops elengi crude seed protein extract showed no further activity against Staphylococcus aureus or Streptococcus pyogenes. This confirms that the antimicrobial activity was only present in the structurally intact protein, and that its activity vanishes after destruction or hydrolysis by proteinase K.
CONCLUSION
This study concludes that 10mM sodium phosphate buffer (pH- 7.0) is competent for retrieving an adequate amount of the protein from the plant sample and is proven as a reliable buffer for extraction. Mimusops elengi plant seed protein extract showed inhibition zones, for Staphylococcus aures and Streptococcus pyogenes that were more than three times that of antibiotics.
This clearly shows the prominence of the inhibitory protein. Moreover, MIC study indicates that Streptococcus pyogenes is slightly more susceptible to Mimusops elengi plant seed protein compared to Staphylococcus aureus by showing lower MIC value compared to Staphylococcus aureus. Kinetic study further elucidates the kinetics of inhibition and confirms the inhibitory activity in the presence of the Mimusops elengi plant seed protein with respect to time. Treatment with Proteinase K confirms that the inhibitory activity was only due to the protein present in the sample because after its treatment with proteinase K sample lost its inhibitory activity against both organisms. Finally, hemolytic activity of the Mimusops elengi plant seed protein gives HC50 value at a much higher range than the MIC values against both organisms and thus suggests its application as an herbal product in practice.
Acknowledgments
Authors are thankful to Charutar Vidya Mandal (CVM), Vallabh Vidyanagar, Gujarat, India and Dr. Pradip S Patel, Director of Ashok and Rita Patel Institute of Integrated Studies and Research in Biotechnology and Allied Sciences (ARIBAS), New Vallabh Vidyanagar-388121, Gujarat, INDIA for providing necessary support for research and laboratory facility.
REFERENCES
- 1.Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005 Dec;13(12):589–95. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Tichy J, Novak J. Extraction, assay and analysis of antimicrobials from plants with activity from plants with activity against dental pathogenes (Streptococcus spp.) Journal of Altern. Complement Med. 1998 Jan;4(1):39–45. doi: 10.1089/acm.1998.4.1-39. [DOI] [PubMed] [Google Scholar]
- 3.Ellof JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol. 1998 Feb;60(1):1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 4.Palombo EA, Semple SJ. Antibacterial activity of traditional medicinal plants. J Ethnopharmacol. 2001 Oct;77(2–3):151–7. doi: 10.1016/s0378-8741(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 5.Cowan MM. Plant products as antimicrobial agents. Clinical Microbiol Rev. 1999 Oct;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina A, Gorlach J, Volrath S, Ryals J. Wheat genes encoding two types of PR-1 proteins are pathogen inducible, but do not respond to activators of systemic acquired resistance. Mol Plant Microbe Interact. 1999 Jan;12(1):53–8. doi: 10.1094/MPMI.1999.12.1.53. [DOI] [PubMed] [Google Scholar]
- 7.Shariff N, Sudarshana MS, Umesha S, Hariprasad P. Antimicrobial activity of Rauvof iatetraphylla and Physalis minima leaf and callus extracts. Afr. J. Biotechnol. 2006;5(10):946–950. [Google Scholar]
- 8.Afolayan AJ. Extracts from the shoots of Arctotis arctotoides inhibit the growth of bacteria and fungi. Pharm. Biol. 2003;41(1):22–25. [Google Scholar]
- 9.Gottlieb OR, Borin MR, Brito NR. Integration of ethnobotany and phytochemistry: dream or reality? Phytochemistry. 2002 Feb;60:145–152. doi: 10.1016/s0031-9422(02)00088-2. [DOI] [PubMed] [Google Scholar]
- 10.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000 Jun;17(3):215–34. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 11.Jenssen H. Anti-herpes simplex virus activity of lactoferrin/lactoferricin an example of antiviral activity of antimicrobial protein/peptide. Cell Mol Life Sci. 2005 Dec;:3062–3013. doi: 10.1007/s00018-005-5228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defense. Curr Opin Immunol. 1999 Feb;11(1):23–7. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 13.Salahudin MA, Allah B, Rauf Ahmad M, Irfan M, Sadia L. Comparison of the antimicrobial activity of seed protein extracts from six medicinal plants against Staphylococcus aureus. Emir. Journal. Food Agric. 2011;23(1):103–109. [Google Scholar]
- 14.Thimmaiah SR. Standard Methods of Biochemical Analysis. 2006:96–97. [Google Scholar]
- 15.Perilla MJ. Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in this developing world. WHO. 2003:209–214. [Google Scholar]
- 16.Chattopadhyay D, Maiti K, Kundu AP, Chakraborty MS, Bhadra R, Mandal SC, et al. Antimicrobial activity of Alstonia macrophylla - folklore of Bay Island. J Ethnopharmacol. 2001 Sep;77(1):49–55. doi: 10.1016/s0378-8741(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 17.Andra J, Jakovkin I, Grotzinger J, Hecht O, Krasnosdembskaya AO, Goldmann T, et al. Structure and mode of action of the antimicrobial peptide arenicin. Biochem J. 2008 Feb 15;410(1):113–22. doi: 10.1042/BJ20071051. [DOI] [PubMed] [Google Scholar]
- 18.Babu Shankar P, Sathiyamoorthy S, Palanisamy P, Boopathi S, Rajaram V. Antioxidant and antimicrobial properties of Glycine Max-A review. Int J Cur Bio Med Sci. 2011;1(2):49–62. [Google Scholar]
- 19.Wang W-X, Dgany O, Wolf SG, Levy I, Algom R, Pouny Y, Wolf A, Altman A, Shoseyov O, Aspen SP. An exceptional thermal, protease and detergent-resistant self-assembled nano-particle. Biotechnol Bioeng. 2006 Sep 5;95(1):161–8. doi: 10.1002/bit.21010. [DOI] [PubMed] [Google Scholar]
- 20.Disha M. 2010. Studies on the anticariogenic potential of medicinal plant extracts; pp. 1–65. Dissertation Thesis Report. Sardar Patel University, Vallabh Vidyanagar, Gujarat, India. [Google Scholar]
- 21.Hud SH, Wanga JC, Kunga HF, Wanga JT, Leeb WL, Yangc YH. Antimicrobial effect of extracts of cruciferous vegetables. Kaohsiung J Med Sci. 2004 Dec;20(12):591–9. doi: 10.1016/S1607-551X(09)70264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabeen R, Shahid M, Jamil A, Ashraf M. Microscopic evaluation of the antimicrobial activity of seed extracts of Moringa oleifera, Pakistan J. Botany. 2008;40(4):1349–1358. [Google Scholar]
- 23.Woolfrey BF, Enright M. Ampicillin killing curve patterns for Ampicillin susceptible non typeable Haemophilus influenza strains by the agar dilution plate count method. Antimicrobial Agents and Chemotherapy. 1990 Jun;39:1074–1087. doi: 10.1128/aac.34.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalkley LJ, Koornholf HJ. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa, Escharichia coli and Staphylococcus aureus determined by the kill curve method: Antibiotics comparison and synergistic interactions. Antimicrobial Agents and Chemotherapy. 1985;28(2):331–342. doi: 10.1128/aac.28.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emilson CG. Potential efficacy of chlorhexidine against mutans streptococci and human dental caries. J Dent Res. 1994 Mar;73(3):682–91. doi: 10.1177/00220345940730031401. [DOI] [PubMed] [Google Scholar]
- 26.Chung JY, Choo MH, Lee JK, Hwangj K. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against. Streptococcus mutans Phytomedicine. 2006 Mar;13(4):261–6. doi: 10.1016/j.phymed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima Y, Ishibashi J, Yukuhiro F, Asaoka A, Taylor D, Yamakawa M. Antibacterial activity and mechanism of action of tick defense in against Gram positive bacteria. Biochim Biophys Acta. 2003 Dec 5;1624(1–3):125–30. doi: 10.1016/j.bbagen.2003.10.004. [DOI] [PubMed] [Google Scholar]