Abstract
Oral surgery in patients with bleeding disorders is associated with a high risk of bleeding during and after surgery. This article is aimed to present the case of an eight-year-old girl suffering from severe Fanconi anemia with pancytopenia who underwent a dental extraction. The hemostatic effect of local administration of tranexamic acid in combination with a primary suture seems to be extremely helpful in order to reduce the necessity of blood products and the risk of postoperative bleeding.
Keywords: Surgery, Fanconi Anemia, Pancytopenia, Tranexamic Acid
INTRODUCTION
Fanconi anemia (FA) is a rare autosomal recessive disorder characterized by congenital malformations, progressive bone marrow hypoplasia, and a high risk of malignancies. The existing literature describes occurrence frequency of FA with approximately 1:360,000 [1] with a female-to-male ratio of 1:2 [2]. Fifteen genetic subtypes can be distinguished: FA-A, -B, -C, -D1, -D2, -E, -F, -G, -I, -J, -L, -M, -N, -O, and –P. The majority of FA patients (∼85%) belong to subtypes A (∼60%), C (∼10–15%), or G (∼10%), while a minority (∼15%) is distributed over the remaining 12 subtypes [3]. Cells of FA patients are characteristically sensitive to chromosome breakage by DNA cross-linking agents [4].
The diagnosis is usually made between the age of 5 and 10 years [5]. Whereas, one third of the affected patients do not have any obvious abnormalities, in the majority of cases, physical signs, such as abnormal skin pigmentation, short stature, thumb and radial anomalies, structural renal defects, microcephaly, and delayed development are recognizable at birth and during early childhood.
The most frequent oral manifestations are gingivitis, periodontitis, rotated teeth, and dental agenesis [6]. The high prevalence of periodontal diseases and gingivitis may be related to frequent immune system deficiencies, anemia and leukopenia. However, patients affected by FA often display deficits in their oral hygiene as well [7].
Furthermore, they are predisposed to the development of squamous cell carcinoma (SCC), especially in the oral cavity [8].
Many FA patients experience bone marrow failure, which is generally characterized by an increasing pancytopenia, often initially with thrombocytopenia or leukopenia. In general, bone marrow transplantation is currently the most effective treatment to cure severe aplastic anemia or acute myeloid leukemia and the myelodysplastic syndrome [9]. All patients designated for transplantation should be examined for presence of any active infection. Dentists should carefully exam the oral situation within the pre-transplant evaluation. Decayed teeth have to be restored and destroyed teeth must be removed before transplantation.
It is difficult to find the optimal management for patients with FA when they need oral surgery, because of their susceptibility to infections and pancytopenia. The following report discusses the management of such a patient, who underwent a dental extraction.
CASE REPORT
The patient was an eight-year-old Syrian girl (body weight, 28 kilograms; body height, 127 centimeters).
Diagnosed with FA, she was referred to our clinic to evaluate her dental health before planning bone marrow transplantation. Her parents and her siblings (9- and 11-year-old brothers) were not affected by the disease.
The physical examination showed a young girl with clinical signs of FA including skin hypopigmented areas, hypertrichosis, scoliosis, abnormal ribs and multiple hematomas in the region of the thighs (Fig 1).
Fig 1.
Physical examination: skin hypopigmented areas and hypertrichosis. Photograph of the entire body of the patient shows scoliosis.
The hexadactyly of her left hand was surgically corrected three months after birth. The oral examination revealed several decayed teeth (Fig 2 and 3).
Fig 2.
Panoramic radiograph before dental treatment
Fig 3.
Preoperative photograph. Teeth number 74 and 75 are completely destroyed.
In the lower jaw 74, 75, 84 and 85 were totally destroyed by caries and therefore, indicated for extraction.
The results of the laboratory tests are mentioned in Table 1.
Table 1.
Laboratory Findings
| Reference range | Findings | |
|---|---|---|
| White blood cells | 4,3–11,4×103/μL | 2,0×103/μL |
| Red blood cells | 3,9–5,0×106/μL | 3,5*106/μL |
| Hemoglobin | 6,6–8,2 mmol/L | 6,3 mmol/L |
| Hematocrit | 0,32–0,40 | 0,28 |
| Platelets | 150.000–400.000/μL | 5.000/μL |
| INR | 0,8–1,2 | 0,9 |
| Partial thromboplastin time | 30–50 s | 35 s |
The most noticeable aspect was the severe thrombocytopenia (5,000/μL). A mild hypersensitivity reaction after platelet transfusion in the past was recorded in her file.
Preparation of dental treatment was carried out in close cooperation with the Hemotology Oncology Unit of our hospital. Due to the FA related severe thrombocytopenia, a platelet concentrate was necessary to raise the platelet count above 50,000/μL. After the intravenous (IV) premedication with 50 mg glucocorticoid (prednisolone) and 3 mg antihistaminic agent (dimetinden), which was required to avoid another transfusion reaction, a single unit of leuko-depleted and irradiated apheresis platelets, was given intravenously. A platelet count of 75,000/μL was achieved. The treatment was performed under general anesthesia to avoid age-related noncompliance. Prophylactic antibiotic treatment using 1.5 g ampicillin/sulbactam IV (ampicillin HEXAL® comp, Hexal, Holzkirchen, Germany) was given to reduce the risk of postoperative infection.
A buccal infiltration using a total of 2.0 mL of 4.0% articain with adrenaline 1:200,000 (Ultracain D-S, Sanofi-Aventis, Paris, France) was conducted for local anaesthesia.
We started with the restorative treatment of teeth 54, 65, 16, 26, 36 and 46.
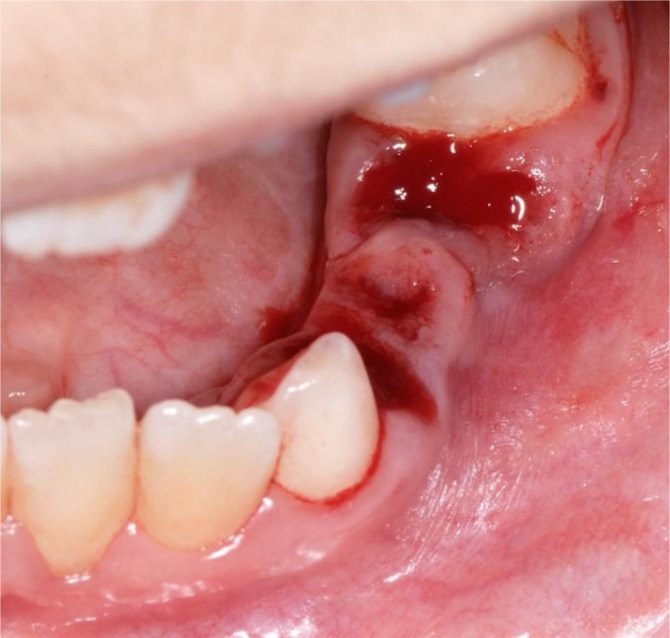
Following that, 74, 75, 84 and 85 were extracted. The sockets were immediately covered with tranexamic acid (TA) (Cyklokapron, Meda Pharma, Bad Homburg, Germany) and closed with a 4x0 resorbable suture (Vicryl, Ethicon Johnson & Johnson, New Brunswick, USA) (Fig 4 and 5).
Fig 4.
Immediate postoperative photograph. The sockets are closed with resorbable suture
Fig 5.
Perioperative photograph. The teeth sockets 74 and 75 after elevation and flooded with TA.
Gauzes saturated with TA were placed onto the teeth sockets for five minutes. Following this protocol, an excellent hemostasis was achieved. The patient was kept in hospital for observation. Gauzes saturated with TA were applied for local hemostasis during the following three days to avoid any late postoperative bleeding caused by early fibrinolysis and hyperfibrinolysis, Postoperative pain was controlled by paracetamol.
The diet (consisted of cool liquids followed by semi-solid food for 3 days after operation. No postoperative bleeding appeared during the observational period.
The platelet count was observed after surgery. No further blood products were necessary despite a decrease to 6,000/μL on the sixth day after operation.
DISCUSSION
The predominant symptom of FA is the progressive bone marrow failure usually during early childhood with increasing pancytopenia, often initial with thrombocytopenia or leukopenia. Because of the important role of platelets in hemostasis in forming the initial plug in vessel injuries, severe thrombocytopenia presents a high risk for a postoperative hemorrhage after dental extraction in these patients.
The threshold platelet count for thrombocytopenic patients who have to undergo surgical procedures remains unclarified in the current literature. It is however, common practice to increase the platelet count to at least 50,000/μL. For procedures in critical regions, such as the brain or eyes and for major surgery, the platelet count should be raised to at least 100,000/μL [10,11].
Obtaining a post transfusion platelet count is required before starting surgery. Depending on the expected level of hemorrhage, additional platelet concentrates can be transfused intraoperatively and postoperatively [11]. In addition, the use of local hemostatic measures is necessary to reduce the need of blood products and the risk of postoperative bleeding. We used TA, a synthetic derivative of the amino acid lysine that causes an antifibrinolytic activity in humans. It reversibly attaches to the lysine-binding site of plasminogen and blocks the binding to fibrin as well as its activation and transformation to plasmin. Thereby, the fibrinolysis and blood clot degradation is retarded [12]. In some countries, TA is not available. Epsilon aminocaproic acid (EA) has a similar effect. However, EA exhibits a slightly shorter plasma half-life and shows less antifibrinolytic activity than TA [13]. Various reports referred to the successful local use of TA in order to reduce oral bleeding and the need of blood products after oral surgery in patients with bleeding disorders [14,15]. Other local measures such as collagen fleece [16], oxidized cellulose [17], gelatin sponge [11], fibrin glue [18] and cyanoacrylate [19] are also reported to be helpful for local hemostasis. Furthermore, these measures may be combined with antifibrinolytic drugs to potentiate the hemostatic effect. In addition, systemic EA or TA may be used if local hemostasis and platelet transfusion appear inadequate. A loading dose of 10 g intravenous followed by 1 g/h is recommended to achieve an effective plasma concentration [11]. In order to prevent secondary bleeding, resorbable suture material was used to avoid suture removal. Non-steroidal, anti-inflammatory drugs and aspirin, which inhibit the platelet function, must be avoided for pain control. Paracetamol is a safe alternative to prevent postoperative pain.
There are successful treatment protocols in the current literature for patients affected by thrombocytopenia. Engel et al. [16] describe the successful management of a female FA patient undergoing bone grafting and dental implant placement. The platelet count in this case was 25,000/μL. The patient received 1 U of single donor platelets preoperatively. EA was given via continuous infusion during the procedure at the rate of 500 mg/h. Additional pooled platelets and packed red blood cells were given intraoperatively. After taking the graft from the anterior iliac crest they used collagen fleece for local hemostasis. Two units of fresh frozen plasma were given postoperatively and the EA infusion was continued at 500 mg/h for 8 hours. A total blood loss of 1,000 mL was registered. Because of a platelet count of 64,000/μL, no blood products were necessary for the abutment placement in the mandible after 4 months and the maxilla after 8 months. The patient received only 500 mg EA orally, every 6 hours starting the day before the procedure and continuing 2 days postoperatively. All implants were noted to have solid osseointegration without difficulty and with adequate hemostasis.
Henderson et al. [11] reported three treatment protocols for patients with thrombocytopenia undergoing dental extraction. Platelet transfusions were combined in one case with gelatin sponge for local hemostasis. In all cases, the surgical sites were closed primarily.
One patient presented postoperative hemorrhage, which was managed systemically with multiple transfusions of packed red blood cells and platelets and topically with thrombin-soaked gauze over the surgical sites as an adjunctive hemostatic measure. This is an indication that postoperative bleeding after oral surgery in patients exhibiting thrombocytopenia is difficult to predict, even though defined treatment protocols are used. Postoperative monitoring of these patients is required to prevent excessive hemorrhage.
While the risk for hematologic diseases is greater during early childhood, solid tumors, particularly SCC, are most frequently observed in adults [4,20].
SCC in FA patients develops earlier than in the general population and shows a more aggressive behavior [4]. An average age of 27 years in FA patients (21) and an average time between the age of FA diagnosis and cancer development of 10.5 years [22] have been reported in the literature. The commonest localizations of SCC in FA patients in descending order are: the tongue, anogenital region, pharynx, larynx, oral mucosa, mandible and skin [23].
There are indications that the incidence of malignancy development is greater after FA patients have endured bone marrow transplantation, maybe by adding risk factors, such as pre-transplantation conditioning with chemotherapy and radiotherapy, immunosuppressive agents given for prophylaxis of graft versus host disease (GVHD) and GVHD itself [23]. Concerning the high risk of development malignancies, a biannual screening of the oral cavity and oropharynx starting between the ages of 15 and 20 is recommended. FA patients with a history of leukoplakia or recurrent oral lesions should be followed every six or eight weeks [4]. If malignancies are diagnosed, operative procedures in FA patients with head and neck cancer are similar to the general population with similar pathologies [24,25].
The associated bone marrow failure in FA patients requires preoperative consultation in a Hematology Oncology Treatment Centre. The need of blood and platelet transfusion before surgery must be considered [24]. Besides the risk of development of wound infections and hematoma, FA patients can have serious problems in adjuvant therapy [4,26]. Because of the increased susceptibility to mutagenic stimuli [27], standard doses of radiotherapy and chemotherapy are generally reduced [24].
CONCLUSION
The topical administration of TA in combination with primary suture and perioperative transfusion of platelets seems to be effective in the prevention of postoperative hemorrhage in patients with severe thrombocytopenia undergoing dental extraction. It is essential to follow defined surgical protocols in the management of patients with bleeding disorders. A specialized dental service should perform these procedures in close cooperation with a hematology oncology treatment centre. The high risk of developing an oral squamous cell carcinoma for patients affected by FA, requires a frequent oral examination by dental practitioners.
REFERENCES
- 1.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003 Feb;101(4):1249–56. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 2.Schofield IDF, Worth AT. Malignant mucosal change in Fanconi’s anemia. J Oral Surg. 1980 Aug;38(8):619–22. [PubMed] [Google Scholar]
- 3.Stoepker C, Hain K, Schuster B. SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat Genet. 2011 Feb;43(2):138–41. doi: 10.1038/ng.751. [DOI] [PubMed] [Google Scholar]
- 4.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003 Jan;129(1):106–12. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JE, Tolar J, Levran O. Germline mutations in BRCA2: shared genetic susceptibility to breast cancer, early onset leukemia, and Fanconi anemia. Blood. 2004 Apr;103(8):3226–9. doi: 10.1182/blood-2003-09-3138. [DOI] [PubMed] [Google Scholar]
- 6.Falci SGM, Corrêa-Faria P, Tataounoff J, Rocha dos Santos CR, Marques LS. Fanconi’s anemia in dentistry: a case report and brief literature review. Rev Odonto Cienc. 2011 Aug;26(3):272–6. [Google Scholar]
- 7.Araujo MR, Oliveira Ribas M, Koubik AC, Mattioli T, de Lima AA, França BH. Fanconi’s anemia: clinical and radiographic oral manifestations. Oral Dis. 2007 May;13(3):291–5. doi: 10.1111/j.1601-0825.2006.01282.x. [DOI] [PubMed] [Google Scholar]
- 8.Salum FG, Martins GB, de Figueiredo MA, Cherubini K, Yurgel LS, Torres-Pereira C. Squamous Cell Carcinoma of the Tongue After Bone Marrow Transplantation in a Patient with Fanconi Anemia. Braz Dent J. 2006 Mar;17(2):161–5. doi: 10.1590/s0103-64402006000200015. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman E, Wagner JE. Hematopoietic stem cell transplantation in childhood inherited bone marrow failure syndrome. Bone Marrow Transplant. 2008 Jan;41(2):127–32. doi: 10.1038/sj.bmt.1705960. [DOI] [PubMed] [Google Scholar]
- 10.British Committee for Standards in Haematology. Blood Transfusion Task Force Guidelines for the use of platelet transfusions. Br J Haematol. 2003 Jul;122(1):10–23. [Google Scholar]
- 11.Henderson JM, Bergman S, Salama A, Koterwas G. Management of the oral and maxillofacial surgery patient with thrombocytopenia. J Oral Maxillofac Surg. 2001 Apr;59(4):421–7. doi: 10.1053/joms.2001.21881. [DOI] [PubMed] [Google Scholar]
- 12.Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981 Feb;673(1):75–85. [PubMed] [Google Scholar]
- 13.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998 Jul;339(4):245–53. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 14.Franchini M, Rossetti G, Tagliaferri A, Pattacini C, Pozzoli D, Lorenz C, et al. Dental procedures in adult patients with hereditary bleeding disorders: 10 years experience in three Italian Hemophilia Centers. Haemophilia. 2005 Sep;11(5):504–9. doi: 10.1111/j.1365-2516.2005.01132.x. [DOI] [PubMed] [Google Scholar]
- 15.Frachon X, Pommereuil M, Berthier AM, Lejeune S, Hourdin-Eude S, Quéro J, et al. Management options for dental extraction in hemophiliacs: a study of 55 extractions (2000–2002) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005 Mar;99(3):270–5. doi: 10.1016/j.tripleo.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 16.Engel JD, Ruskin JD, Tu HK. Hematologic management of a patient with Fanconi’s anemia undergoing bone grafting and implant surgery. J Oral Maxillofac Surg. 1992 Mar;50(3):288–92. doi: 10.1016/0278-2391(92)90329-x. [DOI] [PubMed] [Google Scholar]
- 17.Rayen R, Hariharan VS, Elavazhagan N, Kamalendran N, Varadarajan R. Dental management of hemophiliac child under general anesthesia. J Indian Soc Pedod Prev Dent. 2011 Jan-Mar;29(1):74–9. doi: 10.4103/0970-4388.79954. [DOI] [PubMed] [Google Scholar]
- 18.Jackson MR, MacPhee MJ, Drohan WN, Alving BM. Fibrin sealant: current and potential clinical applications. Blood Coagul Fibrinolysis. 1996 Nov;7(8):737–46. [PubMed] [Google Scholar]
- 19.Al-Belasy FA, Amer MZ. Hemostatic effect of n-butyl-2-cyanoacrylate (histoacryl) glue in warfarin-treated patients undergoing oral surgery. J Oral Maxillofac Surg. 2003 Dec;61(12):1405–9. doi: 10.1016/j.joms.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003 Feb;101(3):822–6. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AW, Hart WR. Multiple squamous-cell carcinomas in Fanconi’s anemia. Cancer. 1982 Aug;50(4):811–4. doi: 10.1002/1097-0142(19820815)50:4<811::aid-cncr2820500432>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Lustig JP, Lugassy G, Neder A, Sigler E. Head and neck carcinoma in Fanconi’s anaemia-report of a case and review of the literature. Eur J Cancer B Oral Oncol. 1995 Jan;31(1):68–72. doi: 10.1016/0964-1955(94)00044-5. [DOI] [PubMed] [Google Scholar]
- 23.Jansisyanont P, Pazoki A, Ord RA. Squamous cell carcinoma of the tongue after bone marrow transplantation in a patient with Fanconi’s anemia. J Oral Maxillofac Surg. 2000 Dec;58(12):1454–7. doi: 10.1053/joms.2000.19212. [DOI] [PubMed] [Google Scholar]
- 24.Gasparini G, Longobardi G, Boniello R, Di Petrillo A, Pelo S. Fanconi anemia manifesting as a squamous cell carcinoma of the hard palate: a case report. Head Face Med. 2006 Jan;2(1):1. doi: 10.1186/1746-160X-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao JW, Cohen BD, Rohde CH, Kutler DI, Spector JA. Free fibular flap reconstruction of the mandible in a patient with Fanconi anemia. Plast Reconstr Surg. 2010 Feb;125(2):61–3. doi: 10.1097/PRS.0b013e3181c72625. [DOI] [PubMed] [Google Scholar]
- 26.Alter BP. Radiosensitivity in Fanconi’s anemia patients. Radiother Oncol. 2002 Mar;62(3):345–7. doi: 10.1016/s0167-8140(01)00474-1. [DOI] [PubMed] [Google Scholar]
- 27.Tischkowitz MD, Hodgson SV. Fanconi anaemia. J Med Genet. 2003 Jan;40(1):1–10. doi: 10.1136/jmg.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]