Abstract
Functional connectivity in the resting state based on BOLD functional MRI (fMRI) has been used mainly to observe brain networks while subjects ‘do nothing’. The same principle, however, can be used for any other steady-state brain condition. In this study, we compared the connectivity of the motor area during simple finger tapping with and without real time neuro-feedback of the activation in the hand motor area. The presence of the neuro-feedback not only induces correlations between the visual and motor areas, but also increases basal ganglia involvement and bilateral motor cortex connectivity.
I. Introduction
Neuro-feedback uses neurophysiological data to update the subject about his or her own performance with the goal to improve behavior. While EEG-based techniques have been used for many years [1] – [4], real-time fMRI (rt-fMRI) based neuro-feedback is a more recent technology that allows brain activity, as extracted from BOLD fMRI, to be used as the signal to feed back to the subject. This technique is implemented by reading, in real time, the BOLD images and using the intensity of activity from a region of interest as a parameter for the display the subject sees while in the MRI scanner. One main advantage of fMRI neuro-feedback is that the signal to be fed back to the subject can simply be the average of the BOLD activity in a brain area of interest, contrary to the complex pattern recognition and machine training needed in EEG based techniques. Rt-fMRI has been successfully used in motor and motor imagery tasks [5] – [7] using areas of interest in the motor cortex or supplementary motor area (SMA). This technique has also been used to modulate activity in higher cognitive areas such as the amygdala [8] and insular cortex [9].
While these studies explored the ability of the subject to modulate a particular brain area, to our knowledge, no study has yet looked at the effects the feedback has on functional connectivity. In this work, we explored the effect of the neuro-feedback signal on the functional connectivity of the motor cortex.
II. Methodology
Subjects performed a right hand finger-tapping task in the scanner with and without feedback of activity from the contralateral motor cortex. Electromyography (EMG) was used to monitor task performance. Post-hoc, we investigated changes in connectivity due to the presence of the neuro-feedback.
A. Paradigm
Task conditions were presented in a block design fashion. During the run without feedback (called GO), subjects saw the word “GO” on the screen, instructing them to tap their fingers, and a fixation cross to indicate rest. During the feedback runs (FBK), finger tapping was indicated by a red column, the height of which was modulated by the amplitude of their brain activity during finger tapping, and rest was indicated by a fixation cross. Five blocks of 20 seconds task and 20 seconds rest were collected for each condition.
B. Subjects
Five healthy subjects (3 males, 2 females; ages: 25 to 34) participated in the study after giving informed consent. All subjects were right handed and had normal neurological examination.
C. Imaging
Images were collected on a 3T GE scanner using an 8 channel head coil. After a scout image, 14 slices were selected in the axial plane, covering the motor cortex. EPI was collected using the following parameters: FOV: 22.4cm; resolution 64×64, 14 slices, slice thickness 5.0 mm, gap: 0.5mm. TR was set to 0.8 seconds, and flip angle at 50 degrees. 294 images were acquired at each run.
Full head MPRAGE was collected from all subjects and used for data normalization for group analysis.
D. Real time fMRI
BOLD signal from real-time fMRI was used to generate neuro-feedback. It was implemented using the real time plug-in in AFNI [10] and an in house program written in python [11].
The region of interest (ROI) for the neuro-feedback run was selected in the motor cortex, based on the maximum regional activations during the GO run. A second ROI was drawn in a slice distant from the motor area to correct for global effects. Each run started with a resting period for MRI signal stabilization (5 seconds). The following 20 seconds subjects were at rest, and data from this period was averaged to create reference signal ‘Signal_ref’.
The mean value from each ROI was computed in AFNI and read by the python program. The neuro-feedback signal was computed as the relative change between the signal in both ROIs at time ‘t’ and the reference signal:
During the feedback blocks, subjects saw a red column on the screen with a continuously updated height that was proportional to Signal_t. Signal_t was updated after each TR and has a delay of 1 TR (0.8s) plus about 1 second. The main delay in the rt-fMRI neurofeedback comes from the hemodynamic response.
E. Electromyography
MRI-compatible electrodes were placed on the right first dorsal interosseous muscle and data measured using Brain-products system.
After removing the gradient artifact, the EMG signal was baseline corrected and rectified; finally, we computed the mean activity for the finger tapping blocks for each subject.
A two-tail paired t-test was used to compare the means of the runs with and without feedback.
F. MRI Data Analysis
Data processing was performed in AFNI. Five images from each run were discarded to account for MRI equilibration effects. Pre-processing included: slice timing correction, volume registration, motion correction and scale to percentage signal change. MPRAGE datasets were normalized to the Talairach standard template [12].
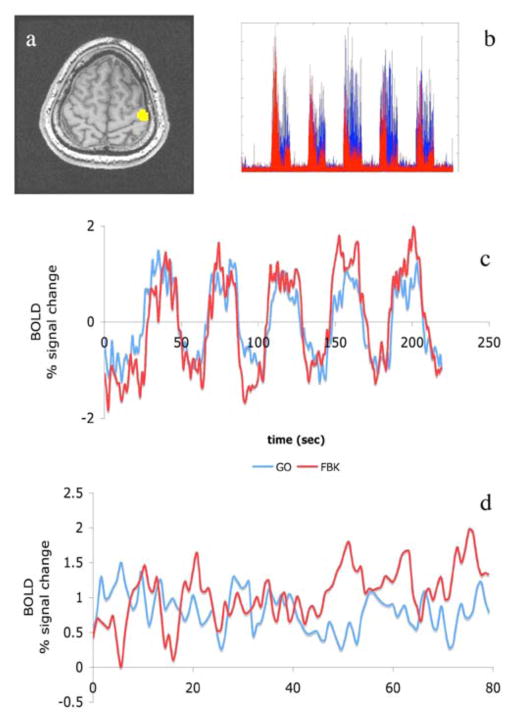
A seed area was selected in the motor cortex (MC), based on activation in the GO task (see figure 1a,c). This is the same area used for the neuro-feedback. We performed connectivity analysis by correlating the time-course of the MC seed during the blocks of activity with all voxels of the brain (see figure 1d).
Figure 1.
Single subject data. a) Motor Cortex seed; b) EMG activity measured over 5 blocks; c) BOLD signal time courses during the GO (blue) and FBK (red) runs; d) BOLD signal time course for the blocks of motor activity.
Correlation coefficients were converted to z-scores using Fisher’s transformation (z=0.5*((1−r)/(1+r)). A map of the MC connectivity during finger tapping was constructed for the GO and the FBK runs by averaging the z-scores across subjects.
In order to test the differences due to the presence of the feedback signal, the GO and FBK connectivity maps were compared using a paired t-test.
For each subject, we computed connectivity during finger-tapping for the GO and FBK runs.
We defined regions of interest in the right and left hand knob, by drawing an ROI on the structural data; and in the putamen by conjunction of the t-test results and the anatomical templates provided by AFNI. For each of these ROIs, we computed the average correlation coefficient per subject and per run.
III. Results
EMG
All subjects performed the task as requested, showing EMG activity during the GO/FBK times and a quiet EMG during the resting period (see figure 1b).
There was no significant difference in EMG amplitude between the GO and neuro-feedback runs (p= 0.19)
fMRI connectivity
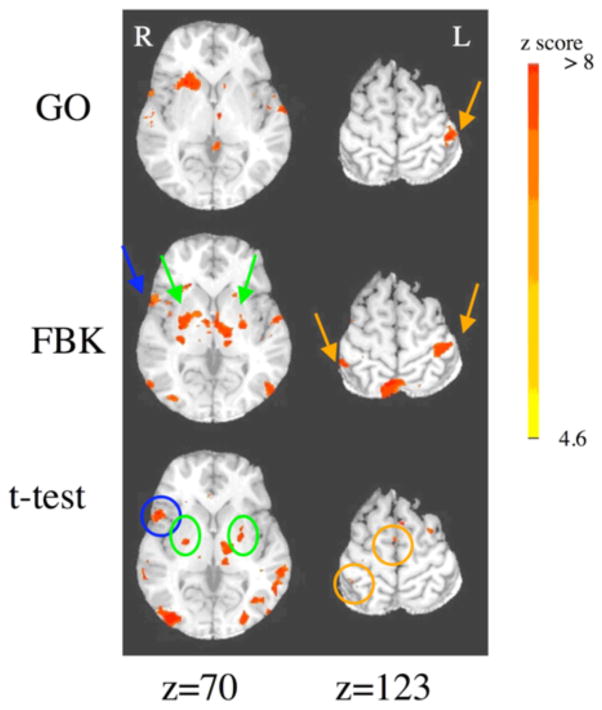
The MC seed, during the GO paradigm, showed less bilateral connections than what typically is seen during the resting state [13]. Notably, the right motor cortex did not demonstrate any significant correlation to the left motor cortex seed region (see figure 2).
Figure 2.
Connectivity maps with the MC seed, data from five subjects. Orange arrows signal motor cortices, green putamen and blue insula cortex. Circles indicate the same areas in the t-test map.
The t-test of the connectivity maps between GO and FBK paradigms shows the strongest differences in connectivity in the basal ganglia, supramarginal gyri and insula cortex (see figure 2). The contralateral motor area and pre-motor areas showed increased connectivity. A difference is also seen in visual areas, more likely due to the increased visual stimulation during the FBK condition.
ROI analysis
The mean correlation coefficient between the seed region and the left hand motor area was 0.54 (p<0.003) for the GO and 0.74 (p<0.00013) for the FBK condition. The right hand motor area was significantly correlated to the seed (r= 0.22, p<0.0012) for the FBK condition, but did not reach significance for the GO condition (r=0.03, p>0.1). The putamen was correlated to the MC seed in both conditions, but the strength of the correlation was significantly higher in the FBK condition (Left GO: r=0.25 (p<0.015), FBK: r=0.45 (p<0.0001).; Right GO: r=0.16 (p<0.015), FBK: r=0.38 (p<0.0001). See figure 3.
Figure 3.
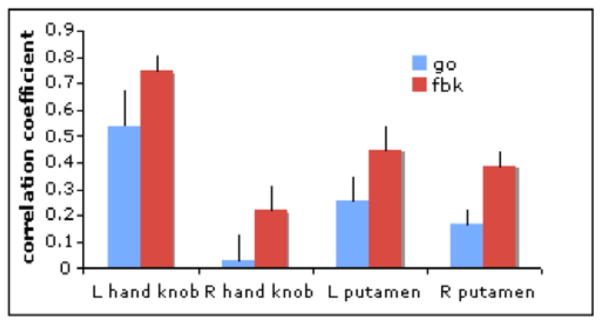
Mean correlation for selected ROIs
IV. Discussion
Functional connectivity of the motor area has extensively been studied at rest. Less often, it has been studied as subjects perform a sustained amount of activity. Here, we showed that the connectivity is affected by the presence of neuro-feedback. Even though we studied a small sample so far, the patterns of connectivity were affected by the presence of neuro-feedback.
It has been reported that the connectivity of the motor cortex becomes more unilateral during task performance [14]. However, when feedback was present, the connectivity became more bilateral, as seen in figures 2 and 3.
The EMG level was not sufficient to account for the significant increase in connectivity between MC and putamen during the neuro-feedback runs. Ventral striatum has been related to feedback activity in monitoring performance and rewards [15]. Thus, it is possible that the motor and executive circuits of the basal ganglia are engaged during neuro-feedback, and that basal ganglia increases its connectivity with the motor cortex [16].
V. Conclusion
We showed that introduction of neuro-feedback during a simple motor task has an effect on the connectivity of the motor circuits. These results are independent of the amount of activity. Therefore, the use of rt-fMRI as a neuro-feedback tool may enable subjects to modify functional connectivity within motor circuits and could create new possibilities for clinical interventions.
Acknowledgments
The authors thank Dr. Jerzy Bodurka for his help in setting up the real-time fMRI at NIH.
This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, NIH.
Contributor Information
Silvina G. Horovitz, Email: horovits@mail.nih.gov, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892 USA (phone: 301-435-2163; fax: 301-480-2286)
Brian D. Berman, Email: bermanb2@mail.nih.gov, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892 USA
Mark Hallett, Email: hallettm@mail.nih.gov, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892 USA.
References
- 1.Mulholland TB. Feedback method: a new look at functional EEG, Electroencephalogr. Clin Neurophysiol. 1969;27:688. doi: 10.1016/0013-4694(69)91310-8. [DOI] [PubMed] [Google Scholar]
- 2.Peper E. Reduction of efferent motor commands during alpha feedback as a facilitator of EEG alpha and a precondition for changes in consciousness. Kybernetik. 1971;9:226–31. doi: 10.1007/BF00289584. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer AE, Usselman LB. A versatile amplitude analyzer for EEG signals to provide feedback stimuli to the subject. Med Biol Eng. 1970;8:209–11. doi: 10.1007/BF02509333. [DOI] [PubMed] [Google Scholar]
- 4.Tan G, Thornby J, Hammond DC, Strehl U, Canady B, Arnemann K, Kaiser DA. Meta-analysis of EEG biofeedback in treating epilepsy. Clin EEG Neurosci. 2009;40:173–9. doi: 10.1177/155005940904000310. [DOI] [PubMed] [Google Scholar]
- 5.Yoo SS, Jolesz FA. Functional MRI for neurofeedback: feasibility study on a hand motor task. Neuroreport. 2002;13:1377–81. doi: 10.1097/00001756-200208070-00005. [DOI] [PubMed] [Google Scholar]
- 6.deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JD. Learned regulation of spatially localized brain activation using real-time fMRI. Neuroimage. 2004;21:436–43. doi: 10.1016/j.neuroimage.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Papageorgiou TD, Curtis WA, McHenry M, LaConte SM. Neurofeedback of two motor functions using supervised learning-based real-time functional magnetic resonance imaging. Conf Proc IEEE Eng Med Biol Soc. 2009:5377–80. doi: 10.1109/IEMBS.2009.5333703. [DOI] [PubMed] [Google Scholar]
- 8.Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage. 2003;18:760–8. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 9.Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage. 2007;35:1238–46. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 Jun;29(3):162–173. doi: 10.1006/cbmr.1996.0014. http://afni.nimh.nih.gov. [DOI] [PubMed] [Google Scholar]
- 11.Python programming language. www.python.org.
- 12.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: an approach to cerebral imaging. Thieme; 1988. [Google Scholar]
- 13.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 14.Amann M, Hirsch JG, Gass A. A serial functional connectivity MRI study in healthy individuals assessing the variability of connectivity measures: reduced interhemispheric connectivity in the motor network during continuous performance. Magn Reson Imaging. 2009;27:1347–59. doi: 10.1016/j.mri.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 16.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 2986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]