Abstract
Background: Transmembrane protease serine 4 (TMPRSS4), one of the type II transmembrane serine proteases (TTSPs), is elevated in various cancers and is associated with multiple malignant phenotypes. However, the expression pattern and biologic significance of TMPRSS4 in thyroid cancer are largely unknown. In this study, we investigated the expression of TMPRSS4 in thyroid cancer and assessed the pro-proliferative role of TMPRSS4 in thyroid cancer.
Methods: Immunohistochemistry and real-time reverse transcription-polymerase chain reaction (RT-PCR) assays were performed to assess the expression of TMPRSS4 in thyroid cancer. We evaluated in vitro cell proliferation using MTT, colony formation, anchorage-independent growth, flow cytometry analysis, and 5-ethynyl-2'-deoxyuridine (EdU) incorporation assays. Western blot, real-time RT-PCR, and luciferase assays were conducted to reveal the underlying mechanisms.
Results: TMPRSS4 is overexpressed in thyroid cancer and is associated with the grade of malignancy. Depletion of TMPRSS4 in thyroid cancer cells significantly suppressed proliferation. Moreover, the proliferation of thyroid cancer cells with TMPRSS4 overexpression was significantly enhanced. We also show that cyclic adenosine monophosphate response element-binding protein (CREB)-cyclin D1 signaling mediates, at least partially, the role of TMPRSS4 in thyroid cancer cell proliferation.
Conclusions: TMPRSS4 is overexpressed in thyroid cancer and TMPRSS4-CREB signaling is needed to sustain thyroid cancer cell proliferation.
Introduction
Thyroid cancer is the most frequent malignant tumor in endocrine systems and its incidence is showing an increasing prevalence in many regions worldwide (1). Thyroid neoplasia includes differentiated papillary and follicular carcinomas (PTC and FTC) and undifferentiated anaplastic carcinoma (ATC) (2). Molecular genetic studies have demonstrated that thyroid cancer, like other malignant tumors, is associated with gradual accumulation of a series of genetic and epigenetic events, resulting in increased function of oncogenes and loss of function of tumor suppressor genes (3,4). In order to expand our understanding of tumorigenesis of thyroid cancer and obtain new insights into the management of thyroid neoplasia, more studies still need to be carried out to explore novel molecular events critical for thyroid cancer development and progression.
Transmembrane protease serine 4 (TMPRSS4), a novel type II transmembrane serine protease (TTSP), was first identified in pancreatic cancer where its expression is strongly elevated (5). Subsequent studies demonstrated that TMPRSS4 is highly expressed in several types of cancers, such as gastric cancer (6), cervical squamous cell carcinoma (7), breast cancer (8), colon cancer (9), and lung cancer (9,10), and that it promotes cancer cell proliferation, epithelial-mesenchymal transition (EMT), invasion, and metastasis. It has also been reported that TMPRSS4 is overexpressed in thyroid cancer (11,12). In a study aimed at assessing differential gene expression between PTC and normal thyroid tissue, Jarzab et al. (11) demonstrated that TMPRSS4 is significantly overexpressed in PTC compared with normal thyroid tissue. Moreover, TMPRSS4 expression was significantly higher in malignant (PTC, FTC, and ATC) than in benign (follicular adenoma) thyroid neoplasms, and was an independent predictor of thyroid cancer (12). However, the oncogenic significance and underlying molecular mechanisms of TMPRSS4 in malignant thyroid tumors are largely unknown.
Cyclic adenosine monophosphate response element-binding protein (CREB), a ubiquitous transcription factor, has been implicated in tumorigenesis of various types of cancers, including thyroid cancer (13). The transcriptional activity of CREB is stimulated when it is phosphorylated on serine 133 and this subsequently promotes the expression of target genes that are associated with cell growth (14). Although the implication of CREB has been investigated extensively in several kinds of cancers, the involvement of CREB in thyroid tumorigenesis remains to be elucidated. Here, we investigate TMPRSS4 expression in human thyroid cancer and attempt to elucidate the molecular mechanisms mediating its oncogenic role.
Materials and Methods
Cell lines
The K1 (papillary cancer), WRO (follicular cancer), and 8505C (anaplastic cancer) thyroid cancer cell lines were from the European Collection of Cell Cultures (ECACC, Salisbury, United Kingdom). Cell lines were maintained in 10% fetal bovine serum.
Patients and tissue specimens
This study was conducted on a total of 113 cases of paraffin-embedded thyroid carcinomas (73 cases of PTC, 28 cases of FTC, and 12 cases of ATC), 15 cases of goiters, and 18 cases of adenoma samples, which had been clinically and histologically diagnosed at the First Affiliated Hospital of Sun Yat-sen University between 2008 to 2013. Twelve freshly collected thyroid cancer specimens and the matched adjacent noncancerous thyroid tissues were collected, frozen, and stored in liquid nitrogen until assayed. Informed consent from patients and ethics approval from the Institutional Research Ethics Committee was obtained.
Immunohistochemistry
Immunohistochemistry (IHC) analysis was performed to study altered protein expression in formalin-fixed and paraffin-embedded human thyroid lesions. The degree of immunostaining was examined and scored independently by two observers by combining both the proportion of positively staining tumor cells and the staining intensity as previously described (15,16). In brief, the proportion of tumor cells was scored as follows: 0 (no positive tumor cells), 1 (<10% positive tumor cells), 2 (10–50% positive tumor cells), and 3 (>50% positive tumor cells). The intensity of staining was graded according to the following criteria: 0 (no staining); 1 (weak staining=light yellow), 2 (moderate staining=yellow brown), and 3 (strong staining=brown). The staining index (SI) was calculated as staining intensity score×proportion of positive tumor cells. Using this method of assessment, we evaluated the expression of the studied proteins by determining the SI, which scored as 0, 1, 2, 3, 4, 6 and 9. An SI score of ≥4 was used to define tumors with high expression, and ≤3 as tumors with low expression.
Vectors and retroviral infection
A TMPRSS4 construct was generated by subcloning polymerase chain reaction (PCR)-amplified full-length human TMPRSS4 cDNA into pMSCV. For silencing of TMPRSS4, two human siRNA sequences were cloned into pSuper-retro-puro to generate pSuper-retro-TMPRSS4-RNAi#1 and pSuper-retro-TMPRSS4-RNAi#2, respectively, and the sequences were RNAi#1, CCAAGCCTACTAGAGCAAGAA and RNAi#2, CCAAGCCTACTAGAGCAAGAA. Retroviral production and infection were performed as described previously (17). Stable cell lines were selected by treatment with 0.5 μg/mL puromycin for 10 days, beginning 48 hours after infection. The reporter plasmids containing wild-type (Plasmid #32727) and mutated CREB binding sites in the cyclin D1 promoter (Plasmid #32732) were purchased from Addgene (Cambridge, MA). The CREB reporter plasmid was created by inserting annealed oligonucleotides (18) 5′-CTGACGTCAGAGTGACGTCAGAGTGACGTCAGAGTGACGTCAA-3′ and 5′-GATCTTGACGTCACTCTGACGTCACTCTGACGTCACTCTGACGTCAGGTAC-3′ (the response element in the forward strand is underlined) into the KpnI and BglII sites of the pTA-Luc vector (Clontech, Palo Alto, CA).
Western blotting
Western blotting was performed according to a standard method as described previously (17). The following primary antibodies were used: anti-TMPRSS4 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-CREB and anti-phospho-CREB (Ser133) (Cell Signaling, Beverly, MA), and anti-α-tubulin (Sigma-Aldrich, St. Louis, MO).
RNA extraction and real-time reverse transcription-polymerase chain reaction
RNA extraction, reverse transcription (RT), and real-time polymerase chain reaction (PCR) were performed as described previously (15). The primers were as follows: TMPRSS4 forward, 5′-TCTCAGCTCCAGGCTACAGG-3′ and reverse, 5′-GGTTTGACATCGAGGCTGTT-3′; cyclin D1 forward, 5′-AACTACCTGGACCGCTTCCT-3′ and reverse, 5′-CCACTTGAGCTTGTTCACCA-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse, 5′-AGAGGCAGGGATGATGTTCTG-3′.
Cell growth assay
In vitro cell growth was measured using the MTT method; 5×103 cells were plated per well in 96-well plates and cultured overnight. Cell proliferation was evaluated with a MTT assay every 24 hours over a period of 6 days. Each assay was repeated three independent times in triplicates.
5-ethynyl-2'-deoxyuridine incorporation assay
5-ethynyl-2'-deoxyuridine (EdU) incorporation assay was performed according to the manufacturer's protocol (RiboBio, Guangzhou, China). Briefly, the cells were cultured in triplicate in 24-well plates for 24 hours, and then treated with 50 μM of EdU for 2 hours at 37°C. After fixation with 4% formaldehyde for 10 minutes and premeabilization with 0.5% Triton X-100 for 10 minutes at room temperature, the cells were treated with 1×Apollo reaction cocktail for 30 minutes. Subsequently, the nuclei of the cells were stained with Hoechst 33342 and visualized under a fluorescent microscope.
Colony-formation assay
Cells were plated at 500 per well into 6-well plates and cultured for 10 days. The colonies were stained with crystal violet.
Anchorage-independent growth
Two milliliters of 0.66% agar medium was added to each well of 6-well plates to form the bottom agar. Fifteen thousand cells were mixed with 2 mL of 0.33% agar medium and were then layered onto the bottom agar and incubated at 37°C in 5% CO2, and 0.5 mL of culture medium was added every week to keep the soft agar from drying and to supply nutrition.
Flow cytometry analysis
Cells kept in a culture dish were harvested and washed in ice-cold phosphate-buffered saline (PBS), followed by fixation in 80% ice-cold ethanol in PBS. Before staining, the cells were spun down in a cooled centrifuge and resuspended in cold PBS. Bovine pancreatic RNAase (Sigma-Aldrich, St. Louis, MO) was added at a final concentration of 2 μg/mL, and cells were incubated at 37°C for 30 minutes, followed by incubation in 20 μg/mL of propidium iodide (Sigma-Aldrich) for 20 minutes at room temperature. Analysis was performed with a flow cytometer (Beckman-Coulter, Hialeah, FL).
Reporter assay
Dual-Luciferase® Reporter Assays (Promega, Madison, WI) were performed according to the manufacturer's instructions and as described previously (15). Briefly, the cells were seeded in triplicate in 24-well plates and allowed to settle for 24 hours. Cotransfection of plasmids and 1 ng pRL-TK Renilla was performed using Lipofectamine 2000 Reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer's instruction. Luciferase activities were assessed at 24 hours after transfection and firefly luciferase activities were normalized to Renilla luciferase activities. Experiments were repeated three times.
Statistical analysis
All statistical analyses were carried out using the SPSS 13.0 statistical software package (SPSS Inc., Chicago, IL). All values represent at least three independent experiments and are expressed as the means±standard deviation (SD). The differences between two experimental conditions were compared on a one-to-one basis using the Student's t tests. p<0.05 was considered statistically significant.
Results
TMPRSS4 is overexpressed in thyroid cancer tissue
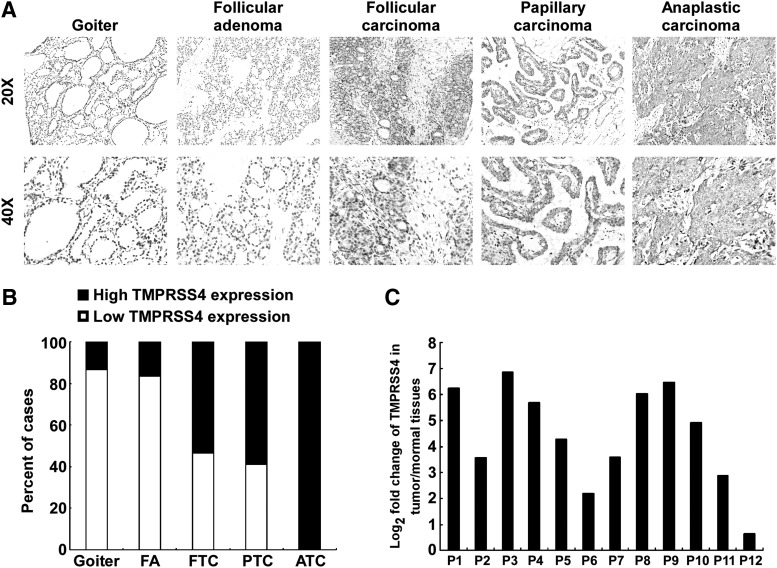
To investigate the clinical relevance of TMPRSS4 in thyroid cancer, we analyzed the expression of TMPRSS4 in 15 cases of goiters, 18 cases of adenomas, and 113 cases of thyroid cancers by performing IHC. As shown in Figure 1A and 1B, the protein level of TMPRSS4 in thyroid malignant lesions was significantly increased in comparison with that in benign lesions (goiters and adenomas). Of note, low expression of TMPRSS4 was observed in most goiters (13/15) and adenoma specimens (15/18), whereas TMPRSS4 is highly expressed in 61.9% of thyroid cancers (70/113). Intriguingly, high expression of TMPRSS4 was detected in all ATC specimens tested (12/12). In contrast, high expression of TMPRSS4 was present in 53.6% (15/28) or 58.9% (43/73) of FTC or PTC samples, respectively. Moreover, we performed real-time RT-PCR assay to assess the expression of TMPRSS4 in 12 pairs of thyroid cancer tissues and their adjacent noncancerous thyroid tissues. As shown in Figure 1C, the expression levels of TMPRSS4 in cancer tissues were markedly elevated as compared with that in the adjacent normal tissues. Moreover, TMPRSS4 expression was significantly correlated with tumor-node-metastasis staging (p<0.001): T classification (p=0.009), and N classification (p=0.017) (Table 1). The TMPRSS4 expression levels did not differ significantly by age and sex. Taken together, these results indicate that TMPRSS4 is overexpressed in thyroid cancer and they suggest that the observed elevated expression of TMPRSS4 in thyroid cancer may play a biologic role in the disease.
FIG. 1.
Expression of transmembrane protease serine 4 (TMPRSS4) is elevated in thyroid cancer. (A) Representative images of immunohistochemistry (IHC) assays on TMPRSS4 expression in thyroid tissue specimens from the studied cohort. (B) Quantification of TMPRSS4 expression levels in thyroid tissue specimens. (C) Log2-transformed fold changes in TMPRSS4 expression of 12 paired tumors and adjacent nonmalignant tissues.
Table 1.
Association Between Clinicopathologic Parameters and Level of Transmembrane Protease Serine 4 Protein Expression in Patients with Thyroid Cancer
| TMPRSS4 | |||
|---|---|---|---|
| Clinicopathologic variables | Low | High | p value |
| Age | |||
| <45 yr | 27 | 34 | 0.141 |
| ≥45 yr | 16 | 36 | |
| Sex | |||
| Male | 14 | 15 | 0.188 |
| Female | 29 | 55 | |
| TNM stage | |||
| I and II | 41 | 38 | <0.001 |
| III and IV | 2 | 32 | |
| T classification | |||
| T1 and T2 | 40 | 51 | 0.009 |
| T3 and T4 | 3 | 19 | |
| N classification | |||
| N0 | 29 | 31 | 0.017 |
| N1 | 14 | 39 | |
TMPRSS4, transmembrane protease serine 4.
Depletion of endogenous TMPRSS4 inhibits the proliferation of thyroid cancer cells
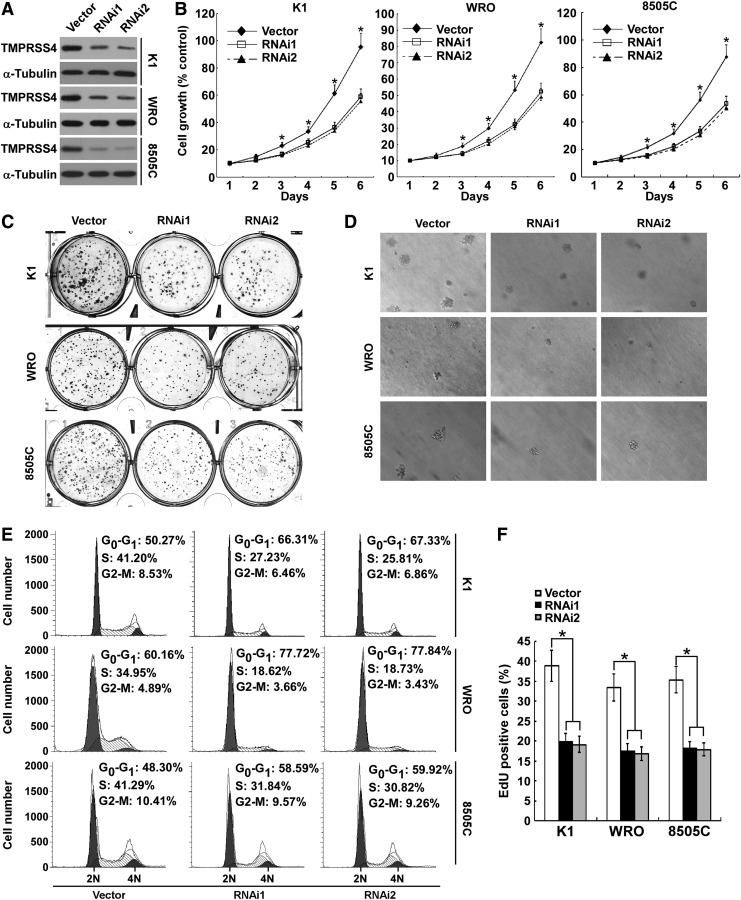
To determine the function of TMPRSS4 in thyroid cancer cell proliferation, we stably knocked down TMPRSS4 expression in thyroid cancer cell lines K1, WRO, and 8505C using retroviral short hairpin RNA (shRNAs) (Fig. 2A). Initially, we investigated the effect of TMPRSS4 on thyroid cancer cell growth by performing MTT assays. As shown in Figure 2B, silencing of TMPRSS4 resulted in a significantly decreased rate of cell growth in comparison with control cells. Furthermore, the capabilities of colony formation of cells with knocked down TMPRSS4 were markedly suppressed as compared with control cells (Fig. 2C and Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/thy). Next, we evaluated the effect of TMPRSS4 depletion on anchorage-independent growth of thyroid cancer cells. As presented in Figure 2D and Supplementary Figure S1B and S2A, fewer and smaller colonies were formed in TMPRSS4 knocked down cells as compared with control cells. To investigate how TMPRSS4 regulates cell cycle progression, fluorescence-activated cell sorting (FACS) analyses of the cell cycle distribution were performed. As shown in Figure 2E, depletion of TMPRSS4 increased the proportion of cells in G1 phase. Moreover, the TMPRSS4 knockdown-induced cell proliferation inhibition was also demonstrated using an EdU incorporation assay, and the results showed that the number of EdU-positive cells was significantly decreased in TMPRSS4-silenced cells (Fig. 2F). Taken together, these data suggest that depletion of TMPRSS4 inhibits thyroid cancer cell proliferation.
FIG. 2.
Depletion of transmembrane protease serine 4 (TMPRSS4) inhibits the proliferation of thyroid cancer cells. (A) Protein expression of TMPRSS4 was analyzed by Western blotting assay. α-Tubulin was used as a loading control. (B) MTT assay was conducted to investigate the effect of TMPRSS4 on the proliferation of thyroid cancer cells at the indicated time points. (C) Representative images of colony formation assays of K1, WRO, and 8505C cells with TMPRSS4 knockdown. (D) Representative images of anchorage-independent colonies formed by the indicated cells. (E) Flow-cytometric determination of proportion of the studied cells in distinct cell-cycle phases. (F) Quantification of 5-ethynyl-2'-deoxyuridine (EdU) incorporation assays. For (B) and (F), the data are reported as mean±standard deviation of three independent experiments. *p<0.05.
Overexpression of TMPRSS4 enhances the proliferation of thyroid cancer cells
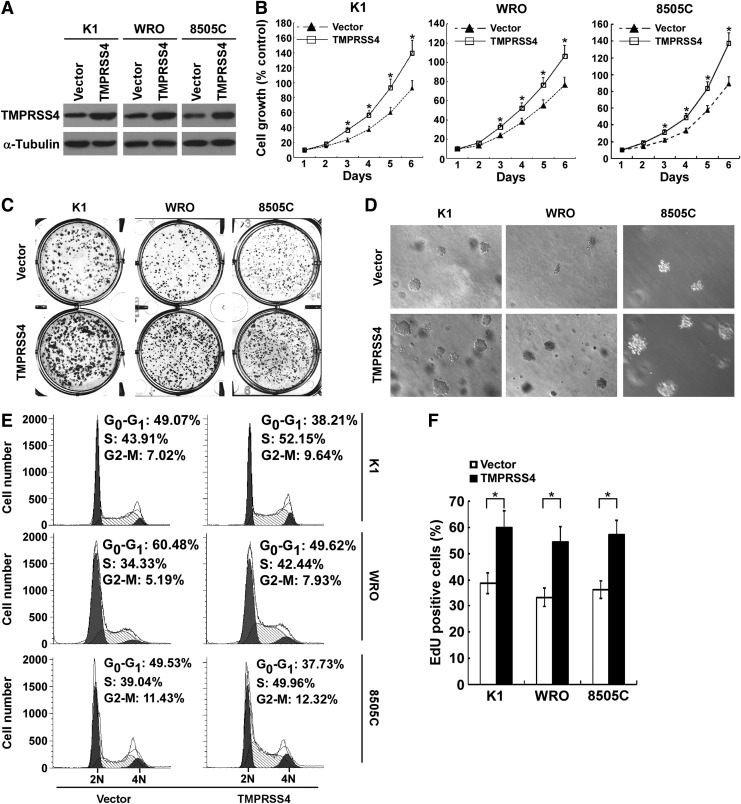
To further investigate the role of TMPRSS4 in thyroid cancer cell proliferation, TMPRSS4 was stably transduced into K1, WRO and 8505C cells, respectively (Fig. 3A). As shown in Figure 3B, ectopic expression of TMPRSS4 significantly increased the rate of proliferation as compared with vector-control cells. Overexpression of TMPRSS4 enhanced the capabilities of colony formation of thyroid cancer cells (Fig. 3C and Supplementary Fig. S1C). Furthermore, soft agar assays indicated that TMPRSS4-overexpressing thyroid cancer cells formed more and larger colonies (Fig. 3D and Supplementary Figs S1D and S2B).
FIG. 3.
Overexpression of transmembrane protease serine 4 (TMPRSS4) enhances the proliferation of thyroid cancer cells. (A) Overexpression of TMPRSS4 in thyroid cancer cell lines was analyzed by Western blotting. α-Tubulin was used as a loading control. (B) MTT assays were performed to evaluate the effect of TMPRSS4 overexpression on the proliferation of the studied thyroid cancer cells at the indicated time points. (C) Representative micrographs of colony formation assays of the studied cells. (D) Representative images of anchorage-independent colonies formed. (E) Flow-cytometric determination of proportion of studied cells in distinct cell-cycle phases. (F) Quantification of 5-ethynyl-2'-deoxyuridine (EdU) incorporation assays. For (B) and (F), the data are reported as mean±standard deviation of three independent experiments. *p<0.05.
TMPRSS4-overexpressing thyroid cancer cells showed a substantial decrease in G1 phase cells compared with that in vector-control cells (Fig. 3E). Moreover, the number of EdU-positive cells was significantly increased in TMPRSS4-overexpressing thyroid cancer cells (Fig. 3F). Taken together, TMPRSS4 is involved in proliferation of thyroid cancer cells.
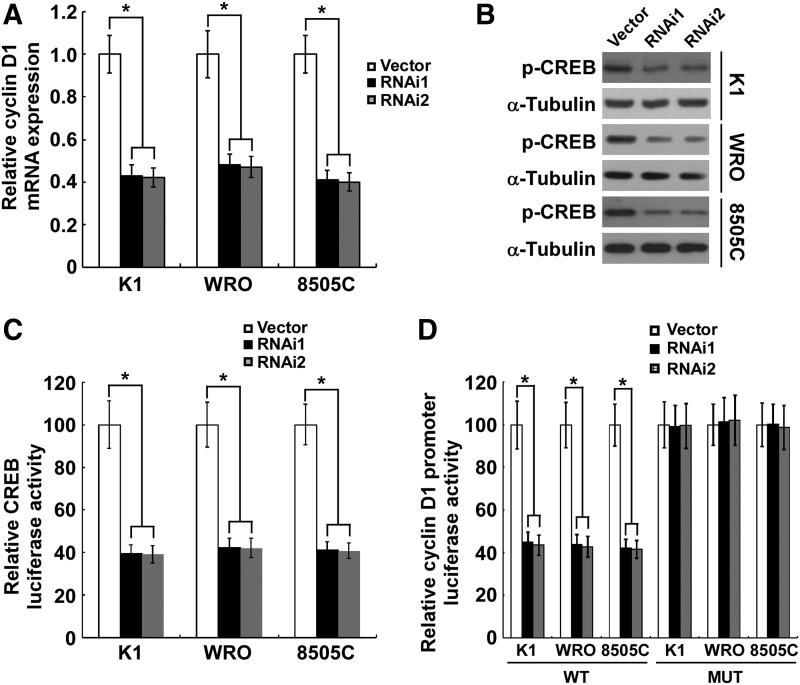
TMPRSS4 knockdown inhibits CREB-induced cyclin D1 transcription
In exploring the mechanism underlying TMPRSS4 depletion-induced proliferation inhibition, we found that the mRNA level of cyclin D1 was significantly attenuated subsequent to the knockdown of TMPRSS4 in thyroid cancer cells (Fig. 4A). To investigate how TMPRSS4 regulates cyclin D1 transcription, we found that the Ser133 phosphorylation level of CREB, a well-known transcription factor involved in cyclin D1 modulation, was significantly inhibited in thyroid cancer cells with knocked down TMPRSS4 (Fig. 4B), indicating that CREB might mediate the effect of TMPRSS4 on cyclin D1 transcription. Furthermore, depletion of TMPRSS4 resulted in a strongly diminished CRE reporter activity in thyroid cancer cells (Fig. 4C). As a further confirmation that CREB mediates the TMPRSS4-induced decrease in cyclin D1, we found that knockdown of TMPRSS4 inhibited the luciferase activity of the cyclin D1 promoter, while mutation of the CRE site decreased the TMPRSS4-depletion–induced inhibition of cyclin D1 promoter luciferase activity (Fig. 4D), suggesting a role of TMPRSS4 in CREB-cyclin D1 signaling.
FIG. 4.
Silencing of transmembrane protease serine 4 (TMPRSS4) suppresses the cyclic adenosine monophosphate response element-binding protein (CREB)-induced cyclin D1 transcription. (A) Cellular mRNA levels of cyclin D1 in studied cells were analyzed by real-time reverse transcription-polymerase chain reaction (RT-PCR) using GAPDH as a loading control. (B) Dephosphorylation of CREB (Ser133) in TMPRSS4 knocked down K1, WRO and 8505C cells. α-tubulin was used as a loading control. (C) Indicated cells were transduced with the CREB firefly luciferase reporter plasmid, using cotransfected pRL-TK to normalize the luciferase values. Data are presented as fold induction relative to vector-control cells. (D) Depletion of TMPRSS4 suppressed the luciferase activity of cyclin D1 promoter and the effect was diminished when the CRE site in cyclin D1 promoter was mutated. For (A), (C), and (D), results derived from three independent experiments are expressed as mean±standard deviation. *p<0.05.
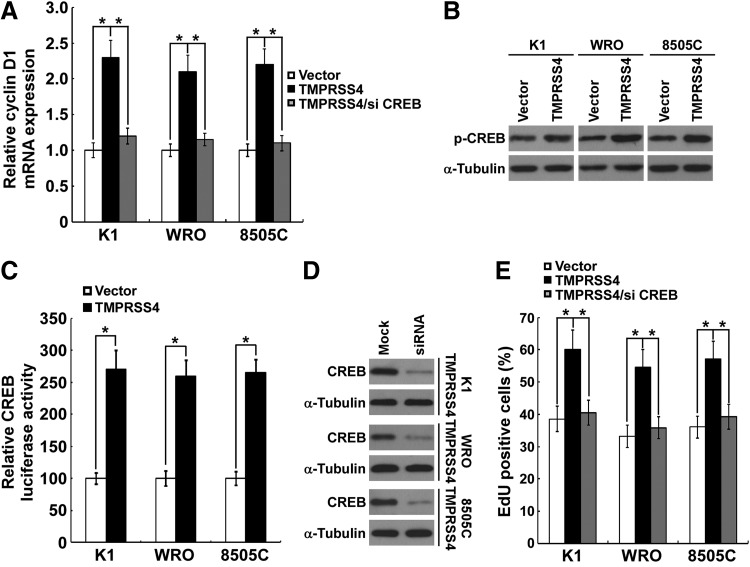
Ectopic overexpression of TMPRSS4 promotes CREB-induced cyclin D1 transcription
We next investigated the role of TMPRSS4 overexpression on CREB-cyclin D1 signaling. As shown in Figure 5A, overexpression of TMPRSS4 in K1, WRO, and 8505C thyroid cancer cells significantly enhanced the mRNA level of cyclin D1. As expected, the Ser133 phosphorylation level of CREB was significantly increased in TMPRSS4-overexpressing cells (Fig. 5B). Meanwhile, TMPRSS4 overexpression significantly activated the CRE reporter in thyroid cancer cells (Fig. 5C). To further confirm the involvement of CREB in TMPRSS4-induced cyclin D1 expression and cell proliferation, we knocked down CREB by siRNA (Santa Cruz Biotechnology, sc-29281) in TMPRSS4-overexpressing thyroid cancer cells (Fig. 5D). As shown in Figure 5A and 5E, TMPRSS4-induced CREB downstream cyclin D1 expression and cell growth could be reversed by CREB knockdown. Taken together, the results support a pro-proliferative role of TMPRSS4 in thyroid cancer cells that is mediated, at least partially, through CREB-cyclin D1 signaling.
FIG. 5.
Ectopic overexpression of transmembrane protease serine 4 (TMPRSS4) promotes the cyclic adenosine monophosphate response element-binding protein (CREB)-induced cyclin D1 transcription. (A) Cellular mRNA levels of cyclin D1 were elevated in TMPRSS4-overexpressing thyroid cancer cells. TMPRSS4-induced cyclin D1 expression could be reversed by CREB knockdown. (B) Ectopic expression of TMPRSS4 in the studied cells promoted the phosphorylation of CREB (Ser133). α-tubulin served as the sample loading control. (C) Indicated cells were transduced with the CREB firefly luciferase reporter plasmid, using cotransfected pRL-TK to normalize the luciferase values. Data are presented as fold induction relative to vector-control cells. (D) Knockdown of CREB in TMPRSS4-overexpressing thyroid cancer cell lines was analyzed by Western blotting. α-Tubulin was used as a loading control. (E) TMPRSS4-induced cell growth could be reversed by CREB knockdown. For (A), (C), and (E), results derived from three independent experiments are expressed as mean±standard deviation. *p<0.05.
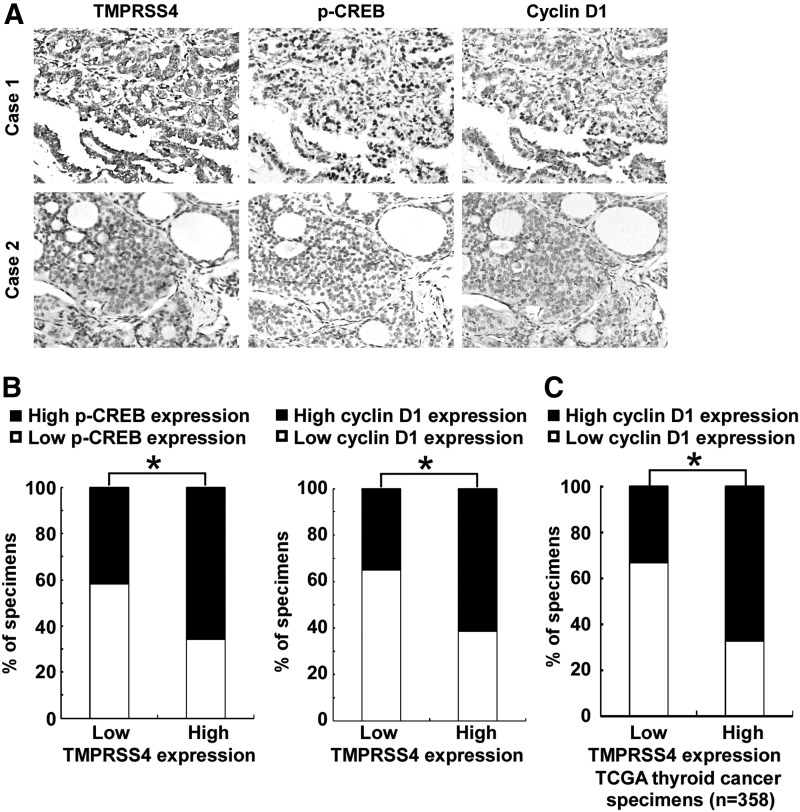
TMPRSS4 expression correlates with CREB-cyclin D1 signaling in thyroid primary cancer
We next asked whether there is an association between the expression levels of TMPRSS4 and p-CREB, as well as cyclin D1 in the clinical specimens. As shown in Figure 6A and 6B, 58.1% (25 cases) and 65.1% (28 cases) of samples with low TMPRSS4 expression (43 cases), respectively, exhibited low levels of p-CREB and cyclin D1, whereas 34.3% (24 cases) and 38.6% (27 cases) of samples with high TMPRSS4 expression (70 cases) showed low levels of p-CREB and cyclin D1, respectively (p<0.05). Moreover, we analyzed the expression of TMPRSS4 and cyclin D1 in 358 cases of thyroid cancer specimens using RNAseqV2 data sets deposited on The Cancer Genome Atlas (TCGA) website (https://tcga-data.nci.nih.gov/tcga). The patients were categorized into 2 groups: low TMPRSS4 expression (less than the median; n=179) and high TMPRSS4 expression (greater than the median; n=179). As shown in Figure 6C, 32.9% (59 cases) of samples with low TMPRSS4 expression exhibited high levels of cyclin D1, whereas 67.1% (120 cases) of samples with high TMPRSS4 expression showed high levels of cyclin D1, suggesting that TMPRSS4 expression significantly correlated (p<0.05) with the level of cyclin D1 in thyroid cancers. Taken together, our data suggest that TMPRSS4 overexpression in thyroid cancer lesions is associated with activation of CREB-cyclin D1 signaling, which further contributes to the proliferation of thyroid cancer.
FIG. 6.
Transmembrane protease serine 4 (TMPRSS4) expression correlates with cyclic adenosine monophosphate response element-binding protein (CREB)-cyclin D1 signaling in thyroid primary cancer. (A) Expression of TMPRSS4 is associated with p-CREB and cyclin D1 expression levels in clinical thyroid cancer specimens. Two representative cases are shown. (B) Percentage of specimens showing low or high TMPRSS4 expression in relation to the expression levels of p-CREB and cyclin D1. (C) The association of TMPRSS4 and cyclin D1 in 358 cases of thyroid cancer specimens using RNAseqV2 data sets deposited on TCGA website. For (B) and (C), *p<0.05.
Discussion
In the current study, we found a novel functional link between TMPRSS4 and the CREB transcription factor. Our data show that TMPRSS4 is overexpressed in thyroid cancers and associated with the grade of malignancy. We also demonstrate that CREB-cyclin D1 signaling mediates, at least partially, the role of TMPRSS4 in thyroid cancer cell proliferation.
Proteases play important roles in various biological processes and their importance in tumor development and progression have gained an increasing interest. TTSPs, recently discovered proteases, have been implicated in many important cellular processes and in tumorigenesis. TMPRSS4, one of the TTSPs, is upregulated in several types of cancers and associated with many malignant phenotypes. For example, Sheng et al. (6) showed that the expression level of TMPRSS4 was elevated in gastric cancer and was associated with invasion and lymph node metastasis. TMPRSS4 can facilitate EMT and thus promote invasion and metastasis of human cancers (9). Moreover, TMPRSS4 was found to be overexpressed in non-small cell lung cancer and is associated with poor clinical outcomes of the patients with the disease (10). Although it has been demonstrated that TMPRSS4 might be a diagnostic marker of malignant thyroid nodules (12), the expression pattern and biologic significance of TMPRSS4 in thyroid cancer remain unclear. Here, we demonstrate that the expression of TMPRSS4 is elevated in thyroid cancers and that it correlates with the grade of malignancy.
Nevertheless, the mechanisms underlying the upregulation of TMPRSS4 in cancers are largely unknown. Further studies are needed to investigate the mechanism that upregulate TMPRSS4 in thyroid cancers, which might reveal new insights into the understanding of their pathogenesis.
Previous studies mostly focused on the pro-EMT and pro-metastasic roles of TMPRSS4 in tumorigenesis (19). However, few studies have examined the pro-proliferative effect of TMPRSS4 in human cancers. For instance, TMPRSS4 was involved in the development and progression of colorectal cancer via regulating the proliferation and self-renewal ability of colorectal cancer cells (20). Consistent with these studies, our data demonstrate that depletion of TMPRSS4 significantly inhibits the growth of thyroid cancer cells, indicating a possible requirement of TMPRSS4 in uncontrolled growth of thyroid cancer cells. Notably, our findings show that the activation of CREB signaling was TMPRSS4-dependent in the tested thyroid cancer models. It is well known that CREB is associated with proliferation in many cancers, including thyroid cancer (21). CREB plays a role in the growth of normal thyroid follicular cells (22). De Falco et al. (23) demonstrated that CD44 enhances the proliferation of thyroid cancer cells via phosphorylation of CREB. The phosphorylation of CREB at a key regulatory residue, Ser133, results in CREB activation (24,25). Cyclin D1 plays a pivotal role in modulating the G1/S phase transition, and is involved in cell proliferation and tumorigenesis (26). Indeed, the data shown in the current study indicate that TMPRSS4 promotes Ser133 phosphorylation of CREB, which leads to transcriptional activation of cyclin D1 and cell growth.
In conclusion, we reveal that TMPRSS4 is overexpressed in thyroid cancer and promotes the proliferation of thyroid cancer cells via CREB-cyclin D1 signaling, potentially offering new molecular targets for treatment of thyroid cancer.
Supplementary Material
Acknowledgments
This research was supported by grants from the Natural Science Foundation of China (No. 81370076); Doctoral Fund of Ministry of Education, China (No. 20130171110067); Guangzhou Municipal Science and Technology special fund (No. 1346000270); Industrial Technology Research and Development funding projects, Guangdong Province (No. 2012A030400006); Young Teachers Cultivate Projects of Sun Yat-sen University (No. 13ykpy15); Key Medical Laboratory of Guangdong Province; Guangdong Department of Science & Technology Translational Medicine Center grant (No. 2011A080300002).
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A.2013Cancer statistics, 2013. CA Cancer J Clin 63:11–30 [DOI] [PubMed] [Google Scholar]
- 2.Bukhari MH, Niazi S, Anwar M, Nasir A.2009An audit of local experience, histological classification of primary tumours of the thyroid according to WHO revised criteria with a critical account. Histopathology 55:120–124 [DOI] [PubMed] [Google Scholar]
- 3.Kouniavsky G, Zeiger MA.2010Thyroid tumorigenesis and molecular markers in thyroid cancer. Curr Opin Oncol 22:23–29 [DOI] [PubMed] [Google Scholar]
- 4.Nikiforov YE, Nikiforova MN.2011Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 7:569–580 [DOI] [PubMed] [Google Scholar]
- 5.Wallrapp C, Hahnel S, Muller-Pillasch F, Burghardt B, Iwamura T, Ruthenburger M, Lerch MM, Adler G, Gress TM.2000 A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res 60:2602–2606 [PubMed] [Google Scholar]
- 6.Sheng H, Shen W, Zeng J, Xi L, Deng L.2014Prognostic significance of TMPRSS4 in gastric cancer. Neoplasma 61:213–217 [DOI] [PubMed] [Google Scholar]
- 7.Cheng D, Liang B, Li Y.2013High TMPRSS4 expression is a predictor of poor prognosis in cervical squamous cell carcinoma. Cancer Epidemiol 37:993–997 [DOI] [PubMed] [Google Scholar]
- 8.Liang B, Wu M, Bu Y, Zhao A, Xie F.2013Prognostic value of TMPRSS4 expression in patients with breast cancer. Med Oncol 30:497. [DOI] [PubMed] [Google Scholar]
- 9.Jung H, Lee KP, Park SJ, Park JH, Jang YS, Choi SY, Jung JG, Jo K, Park DY, Yoon JH, Park JH, Lim DS, Hong GR, Choi C, Park YK, Lee JW, Hong HJ, Kim S, Park YW.2008TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene 27:2635–2647 [DOI] [PubMed] [Google Scholar]
- 10.Larzabal L, Nguewa PA, Pio R, Blanco D, Sanchez B, Rodriguez MJ, Pajares MJ, Catena R, Montuenga LM, Calvo A.2011Overexpression of TMPRSS4 in non-small cell lung cancer is associated with poor prognosis in patients with squamous histology. Br J Cancer 105:1608–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarzab B, Wiench M, Fujarewicz K, Simek K, Jarzab M, Oczko-Wojciechowska M, Wloch J, Czarniecka A, Chmielik E, Lange D, Pawlaczek A, Szpak S, Gubala E, Swierniak A.2005Gene expression profile of papillary thyroid cancer: sources of variability and diagnostic implications. Cancer Res 65:1587–1597 [DOI] [PubMed] [Google Scholar]
- 12.Kebebew E, Peng M, Reiff E, Duh QY, Clark OH, McMillan A.2005ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann Surg 242:353–361; discussion 361–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar DB, Cheng JC, Sakamoto KM.2005Role of cyclic AMP response element binding protein in human leukemias. Cancer 104:1819–1824 [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg D, Groussin L, Jullian E, Perlemoine K, Bertagna X, Bertherat J.2002Role of the PKA-regulated transcription factor CREB in development and tumorigenesis of endocrine tissues. Ann NY Acad Sci 968:65–74 [DOI] [PubMed] [Google Scholar]
- 15.Guan H, Wei G, Wu J, Fang D, Liao Z, Xiao H, Li M, Li Y.2013Down-regulation of miR-218-2 and its host gene SLIT3 cooperate to promote invasion and progression of thyroid cancer. J Clin Endocrinol Metab 98:E1334–1344 [DOI] [PubMed] [Google Scholar]
- 16.Guan H, Liu L, Cai J, Liu J, Ye C, Li M, Li Y.2011Sphingosine kinase 1 is overexpressed and promotes proliferation in human thyroid cancer. Mol Endocrinol 25:1858–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan H, Cai J, Zhang N, Wu J, Yuan J, Li J, Li M.2012Sp1 is upregulated in human glioma, promotes MMP-2-mediated cell invasion and predicts poor clinical outcome. Int J Cancer 130:593–601 [DOI] [PubMed] [Google Scholar]
- 18.Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB.2010Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. J Biol Chem 285:142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Kang HY, Nam EH, Choi MS, Zhao XF, Hong CS, Lee JW, Lee JH, Park YK.2010TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis 31:597–606 [DOI] [PubMed] [Google Scholar]
- 20.Huang A, Zhou H, Zhao H, Quan Y, Feng B, Zheng M.2013TMPRSS4 correlates with colorectal cancer pathological stage and regulates cell proliferation and self-renewal ability. Cancer Biol Ther 15:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu YT, Jin DY.2007CREB—a real culprit in oncogenesis. FEBS J 274:3224–3232 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen LQ, Kopp P, Martinson F, Stanfield K, Roth SI, Jameson JL.2000 A dominant negative CREB (cAMP response element-binding protein) isoform inhibits thyrocyte growth, thyroid-specific gene expression, differentiation, and function. Mol Endocrinol 14:1448–1461 [DOI] [PubMed] [Google Scholar]
- 23.De Falco V, Tamburrino A, Ventre S, Castellone MD, Malek M, Manie SN, Santoro M.2012CD44 proteolysis increases CREB phosphorylation and sustains proliferation of thyroid cancer cells. Cancer Res 72:1449–1458 [DOI] [PubMed] [Google Scholar]
- 24.Ginty DD, Bonni A, Greenberg ME.1994Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell 77:713–725 [DOI] [PubMed] [Google Scholar]
- 25.De Cesare D, Jacquot S, Hanauer A, Sassone-Corsi P.1998Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene. Proc Natl Acad Sci USA 95:12202–12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seybt TP, Ramalingam P, Huang J, Looney SW, Reid MD.2012Cyclin D1 expression in benign and differentiated malignant tumors of the thyroid gland: diagnostic and biologic implications. Appl Immunohistochem Mol Morphol 20:124–130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.