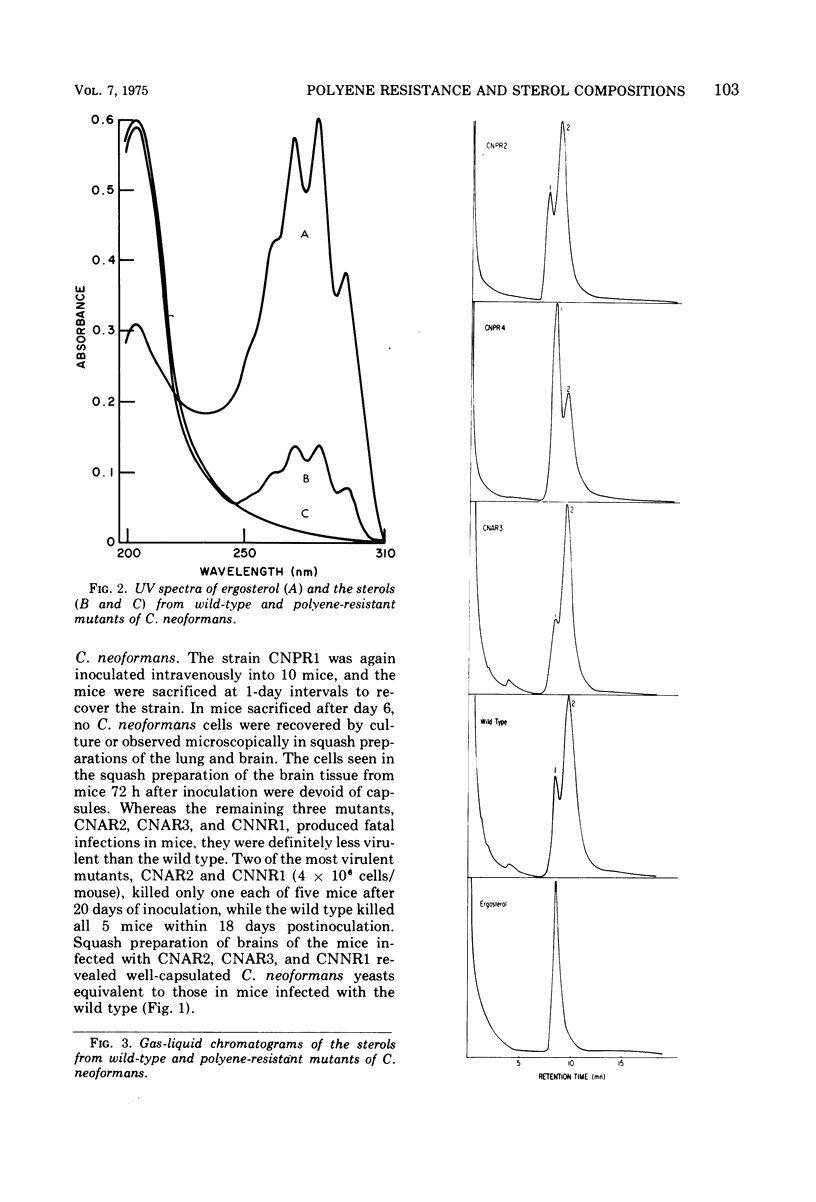
Abstract
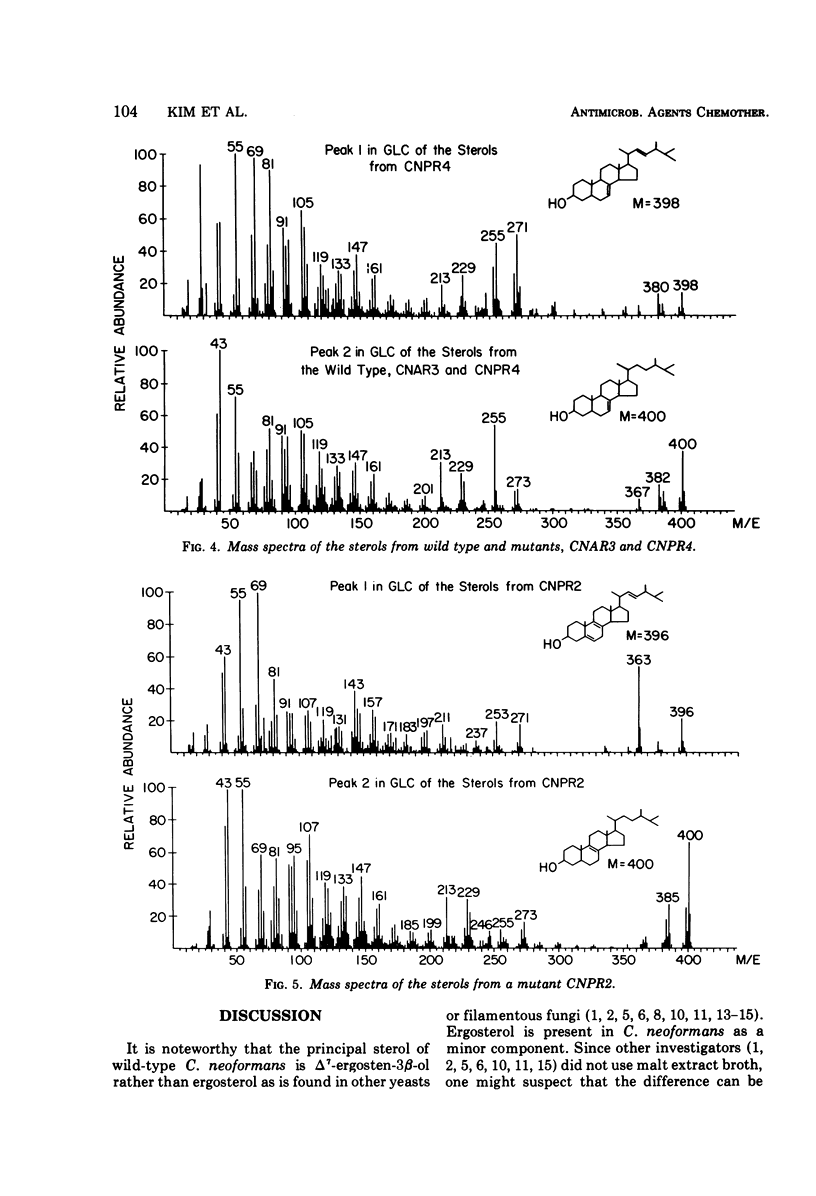
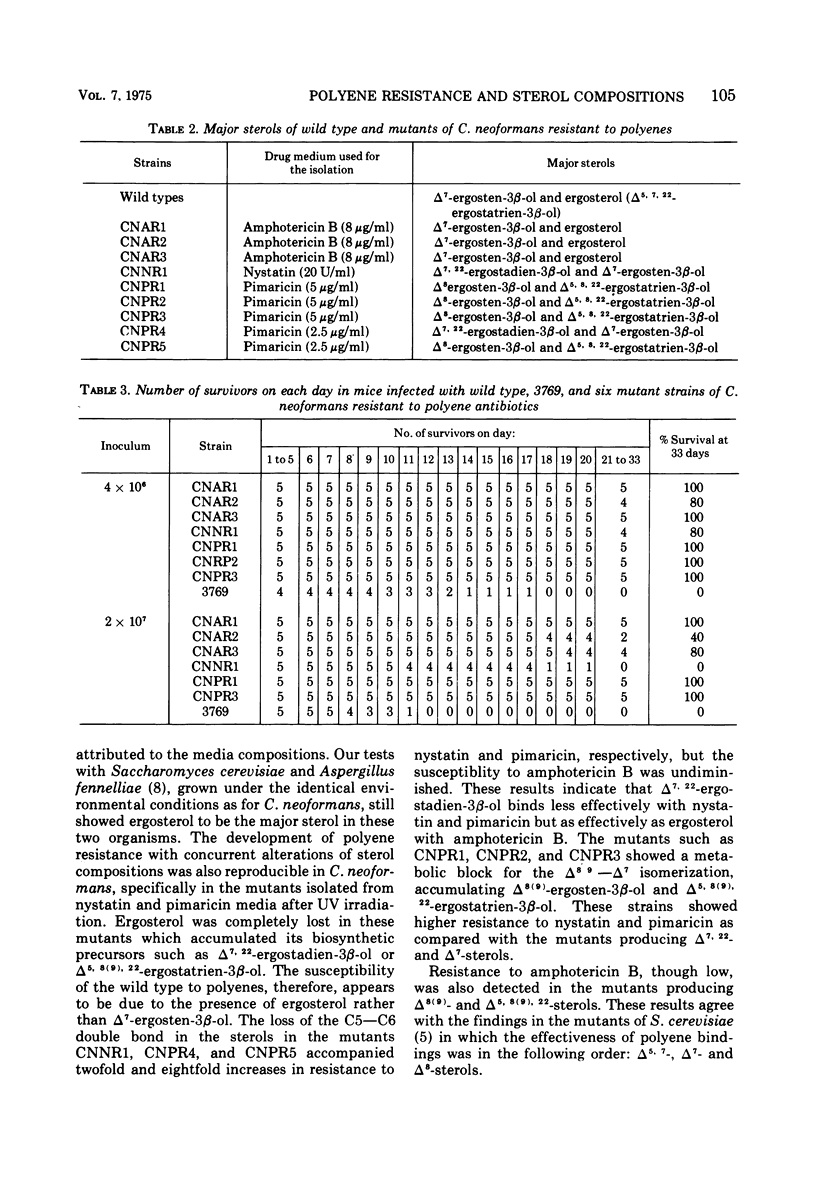
Six mutants of Cryptococcus neoformans resistant to nystatin and pimaricin and three mutants resistant to amphotericin B were isolated by ultraviolet irradiation techniques from two wild-type strains. The major sterols of the wild-type strains were Δ7-ergosten-3β-ol and ergosterol. All six mutants resistant to nystatin and pimaricin showed either loss of ergosterol and concurrent production of Δ7, 22-ergostadien-3β-ol and Δ7-ergosten-3β-ol, or loss of both the wild-type sterols, with production of Δ8(9)-ergosten-3β-ol and Δ5, 8(9), 22-ergostatrien-3β-ol. The mutants producing Δ7, 22-ergostadien-3β-ol and Δ7-ergosten-3β-ol showed relatively low levels of resistance to nystatin and pimaricin, whereas the mutants producing Δ8(9)-ergosten-3β-ol and Δ5, 8(0), 22-ergostatrien-3β-ol showed a high level of resistance to either drug. Although highly resistant to amphotericin B, however, the three mutants produced sterol compositions identical to those of the wild types, indicating that the strains acquired resistance other than by alteration of the membrane sterols. The mutants producing Δ8(9) and Δ5, 8(9), 22 sterols were not virulent for mice, showed reduced growth rates at 25 C, and failed to grow at 37 C. The other mutants showed a slightly reduced rate of growth both at 25 and 37 C, and the virulence in mice was slightly reduced in comparison with that of the wild types. These comparisons were on gross observations and were not statistically analyzed.
Full text
PDF

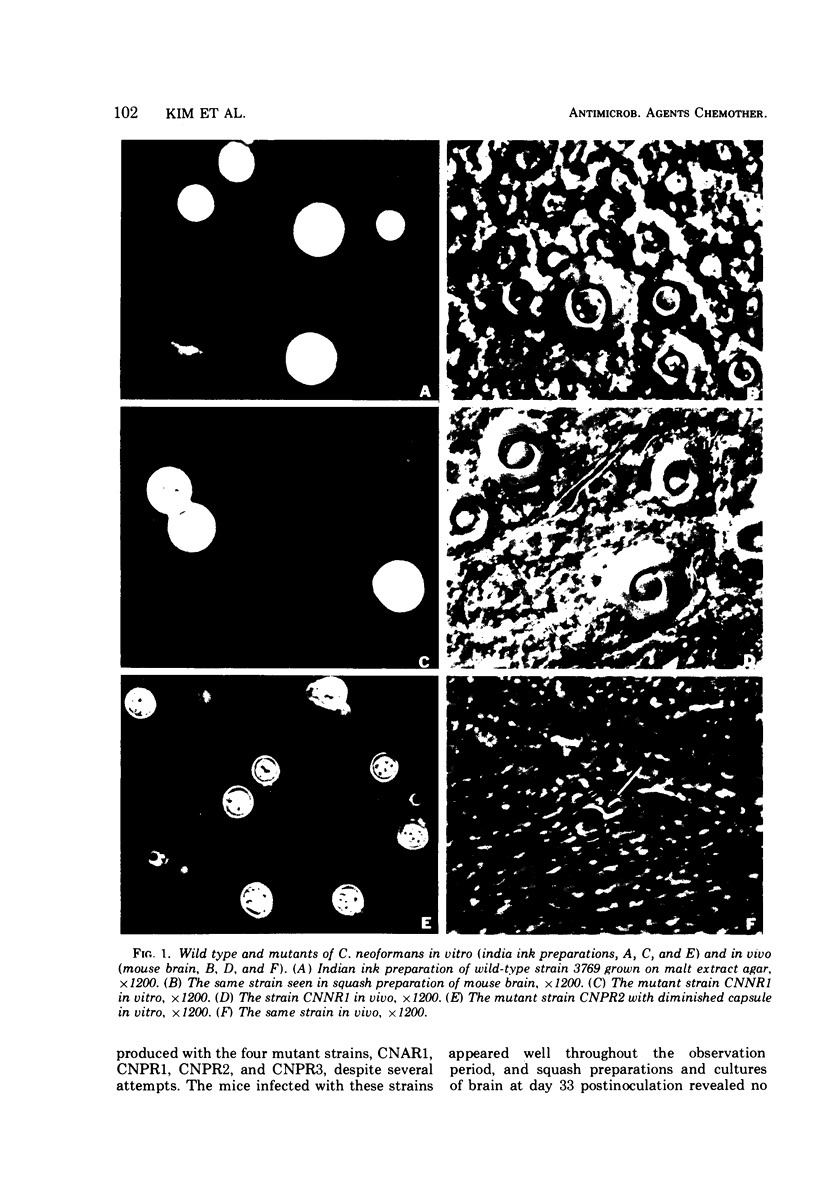

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bard M. Biochemical and genetic aspects of nystatin resistance in saccharomyces cerevisiae. J Bacteriol. 1972 Sep;111(3):649–657. doi: 10.1128/jb.111.3.649-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenhoff J. Development of strains of Cryptococcus neoformans resistant to nystatin, amphotericin B, trichomycin and polymyxin B. Acta Pathol Microbiol Scand. 1968;73(4):572–582. doi: 10.1111/j.1699-0463.1968.tb03215.x. [DOI] [PubMed] [Google Scholar]
- Fryberg M., Oehlschlager A. C., Unrau A. M. Sterol biosynthesis in antibiotic-resistant yeast: nystatin. Arch Biochem Biophys. 1974 Jan;160(1):83–89. doi: 10.1016/s0003-9861(74)80011-1. [DOI] [PubMed] [Google Scholar]
- Grindle M. Sterol mutants of Neurospora crassa: their isolation, growth characteristics and resistance to polyene antibiotics. Mol Gen Genet. 1973 Feb 2;120(3):283–290. doi: 10.1007/BF00267158. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Sterols from polyene-resistant mutants of Candida albicans. J Gen Microbiol. 1972 Nov;73(1):201–203. doi: 10.1099/00221287-73-1-201. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kwon-Chung K. J. Polyene-resistant mutants of Aspergillus fennelliae: sterol content and genetics. Antimicrob Agents Chemother. 1974 Jul;6(1):102–113. doi: 10.1128/aac.6.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molzahn S. W., Woods R. A. Polyene resistance and the isolation of sterol mutants in Saccharomyces cerevisiae. J Gen Microbiol. 1972 Sep;72(2):339–348. doi: 10.1099/00221287-72-2-339. [DOI] [PubMed] [Google Scholar]
- Parks L. W., Bond F. T., Thompson E. D., Starr P. R. 8(9),22 -Ergostadiene-3 -ol, an ergosterol precursor accumulated in wild-type and mutants of yeast. J Lipid Res. 1972 May;13(3):311–316. [PubMed] [Google Scholar]
- Woods R. A., Bard M., Jackson I. E., Drutz D. J. Resistance to polyene antibiotics and correlated sterol changes in two isolates of Candida tropicalis from a patient with an amphotericin B-resistant funguria. J Infect Dis. 1974 Jan;129(1):53–58. doi: 10.1093/infdis/129.1.53. [DOI] [PubMed] [Google Scholar]
- Woods R. A. Nystatin-resistant mutants of yeast: alterations in sterol content. J Bacteriol. 1971 Oct;108(1):69–73. doi: 10.1128/jb.108.1.69-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]