Abstract
Purpose
Monitoring patient-reported symptoms is necessary to adjust and improve supportive care during chemotherapy. Continuing advances in computerized approaches to symptom monitoring can enhance communication about unrelieved symptoms between patients and oncology providers and may facilitate intensified symptom treatment.
Methods
An automated IT-based telephone monitoring system was developed to enable oncology providers to receive and act on alert reports from patients about unrelieved symptoms during chemotherapy treatment. Daily, 250 participants (randomized to treatment or attentional control) were asked to call the automated system to report presence, severity, and distress for common chemotherapy-related symptoms (1–10 scale if present). For the treatment group, symptoms exceeding preset thresholds for moderate-to-severe intensity levels generated emailed alert reports to both the patient’s oncologist and oncology nurse.
Results
Patients reported high satisfaction and ease of use of the automated system. Over 80 % of providers reported usefulness of the symptom alert reports. Ten monitored symptoms resulted in, on average, nine moderate-to-severe intensity alerts per patient over 45 study days. However, providers rarely contacted patients after receiving alerts. There were no significant differences in change of symptom severity between the two groups (mean difference=0.06, p=0.58).
Conclusion
Despite patients’ use of a daily symptom monitoring system and providers’ receipt of information about unrelieved symptoms of moderate-to-severe intensity, oncology physicians and nurses did not contact patients to intensify symptom treatment nor did symptoms improve. Further research is indicated to determine if oncology providers initiated follow-up to intensify symptom treatment, whether symptom outcomes would improve.
Keywords: Automated symptom monitoring, Patient–provider communication, Unrelieved symptom alerts, Patient-reported outcomes, Provider response to unrelieved symptoms
Introduction
Patient-reported symptoms are important outcomes in cancer care as well as relevant endpoints in clinical trials research [1–6]. Research consistently demonstrates that clinicians’ ratings of patients’ subjective symptoms underrate severity or entirely miss symptom presence, underscoring the importance of obtaining these assessments directly from patients [2]. Historically, it has been challenging to develop methods that routinely track and identify patients with poorly controlled symptoms so that modification of their symptom treatment can lead to improved outcomes [7, 8]. However with continuing advances in computerized approaches to symptom measurement, a variety of platforms now exist that efficiently capture patient-reported symptoms electronically [9]. Feasibility and patient acceptability of such approaches are widely studied and well received, yet few studies have evaluated whether using automated patient–provider communication about unrelieved symptoms actually results in improved symptom outcomes [10, 11].
Automated approaches to symptom monitoring are ideal for patients receiving ambulatory cancer care where the vast majority of chemotherapy is provided. Ambulatory care requires patients to manage symptoms at home but often patients are inadequately prepared or forget instructions about how to deal with unrelieved symptoms [11, 12]. Instructions to call oncology providers for unrelieved symptoms are often not followed. This may be due to a lack of expectation that symptoms can be improved, hesitancy to bother busy providers, or a willingness to tolerate high levels of symptoms [7, 8]. As a result, cancer symptom management is often suboptimal [13–15]. This may lead to decreased functioning and quality of life [16] or a patient deciding to discontinue treatment due to intolerable symptom burden [14].
Communication technology utilizing the telephone can aid clinicians in monitoring patients with chronic conditions at home including demonstrated acceptable reliability and validity for symptom monitoring [13, 15, 16]. Other work demonstrates that patients tend to reveal more to automated assessments than to clinician-administered assessments, and that they prefer automated systems when disclosing sensitive symptom information [11, 17].
We developed an automated telephone-based symptom monitoring system to bridge the communication gap between patients and oncology providers about unrelieved symptoms during chemotherapy for patients at home [15]. The technology for the system was modeled after that used by the Medical Information Systems Unit at Boston University led by Robert Friedman, M.D., to help clinicians care for patients with chronic health conditions [7, 18] and to help patients engage in self-care [12, 19, 20].
For this study, the telephone monitoring system was designed to assess daily symptoms during chemotherapy for patients at home and send automated alerts of unrelieved symptoms to the patient’s oncology physician and nurse. We explored whether timely provider notification of poorly controlled symptoms would prompt oncology providers to communicate with patients and intensify treatment of unrelieved symptoms and could lead to improved symptom outcomes.
Methods
Design, participants, and settings
A prospective, randomized parallel group clinical trial design was used; eligible and consenting participants were randomized in a 1:1 allocation within 11 oncologist/nurse provider teams to a symptom alert treatment group or attentional control. Our aims were to test whether (1) the treatment group would experience less symptom severity and distress when compared with attentional control, (2) the providers would initiate more contact with their patients in the treatment group, and (3) providers would initiate more changes to symptom treatment in the treatment group. We also explored patient and provider satisfaction with the system.
Participants were recruited from a convenience sample of four ambulatory oncology clinics in two states in the USA, including a community cancer center in the southeast, two community practices in the west, and a clinical cancer center in the west. Eleven provider teams (oncologists and nurses) were approached to participate in the study and all agreed. Patients were screened after their first cycle of chemotherapy. We excluded patients receiving concurrent radiation therapy as theywould already have daily health care contact to address unrelieved symptoms. Eligible patients were to receive at least three chemotherapy cycles, were 18 years or older, had daily access to a touch tone telephone, understood English or Spanish, were physically and mentally able to participate, and reported at least one symptom of moderate or greater intensity during their first chemotherapy cycle.
Treatment and control
All patients were asked to call the automated monitoring system daily to report on ten symptoms—pain, fatigue, nausea/vomiting, fever, trouble sleeping, anxiety, depressed mood, sore mouth, diarrhea, and constipation. The symptoms were selected from the literature and confirmed in our pilot study as most frequently reported by patients receiving chemotherapy [15, 21, 22]. Patients were queried if symptoms were present in the past 24 h and, if present, they rated severity and distress on a 1–10 scale. The 1–10 numeric scale is commonly used clinically and is an accepted standard in the measurement of symptoms [23]; questions could be stated easily on the phone and answered numerically with the touch-tone keypad. If fever was reported, the highest temperature was entered numerically; in addition, distress but not severity was measured for fever. For the treatment group, at completion of the phone call, the system immediately faxed or emailed (based on provider preference) symptom alert reports to the patient’s oncologist and oncology nurse. Alert thresholds varied by symptom; they were initially established by an expert panel and then revised based on pilot work [15]. Two thresholds were set: a simple alert when severity or distress was ≥5 or 7 (depending on the symptom) on the 10-point scale and trend alerts based on a pattern of moderate severity over several days. For example, pain generated an alert when pain was rated at 5 or greater, whereas fatigue generated an alert at 7 or a trend alert based on a pattern of 3 out of the past 7 days reported at moderate levels (4–6). The report included not only severity and distress but a symptom profile including answers to drill-down questions such as the number of vomiting episodes, oral intake, dizziness, and use of antiemetics for nausea. Reports also included graphs of symptom patterns since the first day of chemotherapy.
The attentional control group received equivalent contact time with the automated system including identical voice and assessment questions. They understood that the data they submitted were for research purposes only and were not available for clinical action. On every call, all patients, regardless of group, were advised to call their oncology providers if they had concerns about their symptoms. In all of the participating provider teams, normal usual care procedure for unrelieved symptoms was to instruct patients to call the clinic office for symptom concerns.
Measures
We collected sociodemographic data from patient interviews and disease- and treatment-related variables from the medical record. The daily automated calls provided the symptom presence, severity, and distress data (if a symptom was present, a 1–10 scale was used to report severity and distress). Rather than a summated rating scale, we used single-item indicators to measure severity and distress—an approach recommended in outcomes research due to increased sensitivity to change [24]. Youngblut and Casper reviewed numerous clinical studies that used single-item indicators including items to measure symptom severity and concluded that single-item indicators had acceptable reliability and validity [25]. Daily calls also asked patients if they had received or initiated a contact (call or visit) with their oncology providers. If they entered yes, research staff called the participant to document specifics and whether the contact involved discussion of symptoms. In addition, patients and providers were interviewed at end of study participation about system usability and acceptability.
Procedures
Recruitment
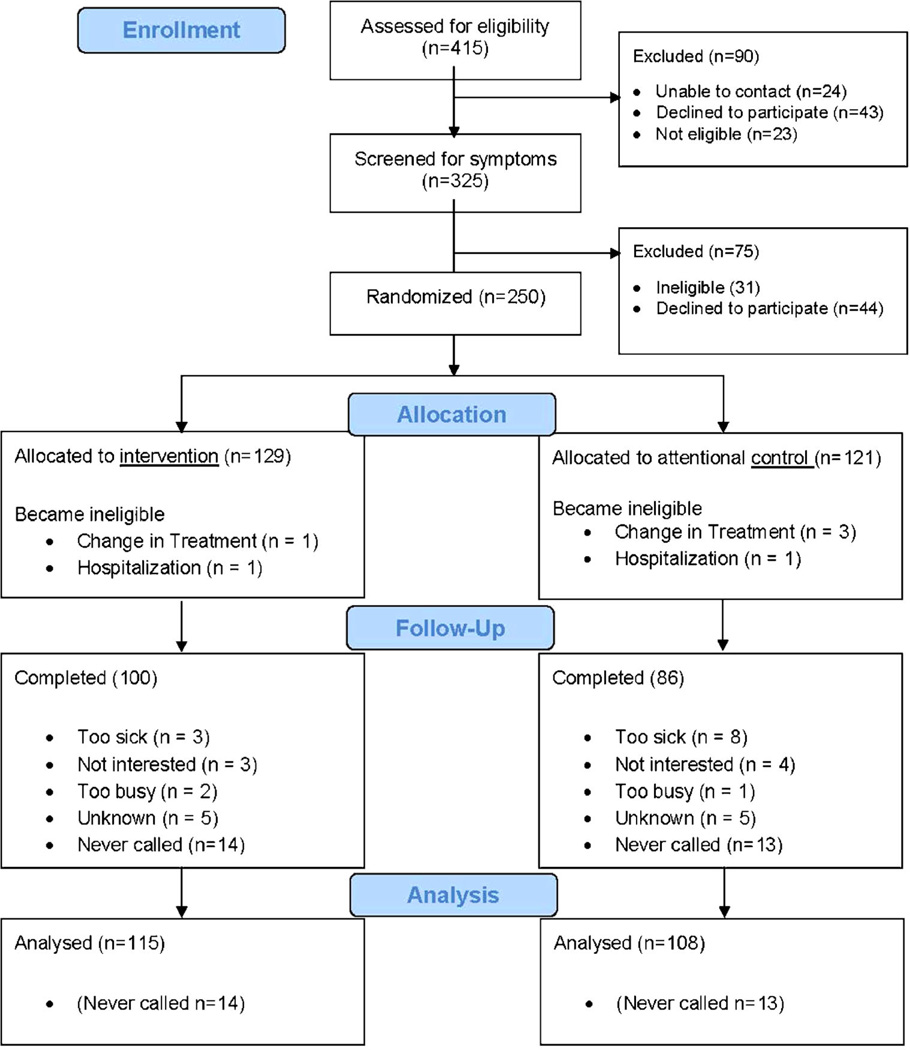
Institutional review board approval was granted at participating organizations. Patients beginning a new chemotherapy protocol were identified, screened for eligibility after the first cycle of chemotherapy, and those eligible were invited to participate for chemotherapy cycles 2 and 3 (Fig. 1). Participating patients and oncology providers signed an informed consent.
Fig. 1.
CONSORT flow diagram
Randomization and masking
Patients were stratified by provider team to ensure equivalency of the treatment and control groups within teams and then randomly assigned to treatment or attentional control. Random assignments in blocks of ten were generated for each provider stratification group. Research staff and patients did not know assignment until after informed consent. Providers were not informed of random assignment but could not be blinded as they would only receive alert reports about treatment group patients.
Research staff trained patient participants in use of the automated system. A toll-free number and personalized password were used to access the system. Calls began 24 h after chemotherapy, beginning with cycle 2, and continued through cycle 3 with an average of 45 observed days per participant.
Analysis
To begin analysis, nonparametric chi-square analysis and independent t tests were used to examine baseline equivalence between study groups, differences between participants and non-participants, and between completing participants and those who withdrew. Then, the three aims were analyzed. To test whether the groups differed on overall symptom levels for aim 1, we conducted an intent-to-treat analysis and applied a random intercepts model using SPSS Version 19. Severity and distress scores across all symptoms were the dependent variables while group was the independent variable. Also, to investigate group differences in the number of reported symptom days, we used generalized linear modeling with a negative binomial distribution and log link function (due to the high dispersion of call-day counts). We ran separate models for the number of severe, moderate, mild, and no-symptom days. For aim 2, we compared two types of contacts—provider initiated versus patient-initiated—using a chi-square analysis. For aim 3, we used chi-square analysis to compare symptom-related contact versus non-symptom-related contact between the groups.
Results
Of the 294 eligible patients, 250 (85 %) consented to participate (Fig. 1). The majority of participants were female (76.0 %), Caucasian (91.4 %), married (72.5 %), with a mean age of 55.5 years (see Table 1). Comparisons of group equivalence at baseline indicated that the treatment group was overrepresented by women (chi-square 4.89; p=0.027) and breast cancer diagnosis (chi-square=9.56; p=0.023).
Table 1.
Comparison of demographic and clinical characteristics at baseline (n=250)
| Demographics | Treatment (n=129) | Control (n=121) | Total (n=250) | Equivalency Tests | ||||
|---|---|---|---|---|---|---|---|---|
| Frequency | Valid % | Frequency | Valid % | Frequency | Valid % | Test value | p value | |
| Gender | ||||||||
| Male | 23 | 17.8 | 37 | 30.6 | 60 | 24.0 | 4.887 | 0.027 |
| Female | 106 | 82.2 | 84 | 69.4 | 190 | 76.0 | ||
| Race | ||||||||
| Caucasian | 112 | 89.6 | 110 | 93.2 | 222 | 91.4 | 0.6013 | 0.438 |
| Other | 13 | 10.4 | 8 | 6.8 | 21 | 8.6 | ||
| Marital status | ||||||||
| Single | 14 | 11.0 | 16 | 13.7 | 30 | 12.3 | 7.43 | 0.190 |
| Separated/divorced | 15 | 11.8 | 7 | 6.0 | 22 | 9.0 | ||
| Married | 91 | 71.7 | 86 | 73.5 | 177 | 72.5 | ||
| Widowed | 7 | 5.5 | 7 | 6.0 | 14 | 5.7 | ||
| Living w/ partner | 0 | 0 | 1 | 0.9 | 1 | 0.4 | ||
| Diagnosis | ||||||||
| Breast | 63 | 48.8 | 36 | 29.8 | 99 | 39.6 | 9.564 | 0.0227 |
| Colon | 9 | 7.0 | 11 | 9.1 | 20 | 8.0 | ||
| Ovarian | 13 | 10.1 | 18 | 14.9 | 31 | 12.4 | ||
| Other | 44 | 34.1 | 56 | 46.3 | 100 | 40.0 | ||
| Diagnosis stage | ||||||||
| I | 7 | 5.6 | 2 | 1.8 | 9 | 3.8 | 4.028 | 0.258 |
| II | 46 | 36.8 | 35 | 30.7 | 81 | 33.9 | ||
| III | 31 | 24.8 | 35 | 30.7 | 66 | 27.6 | ||
| IV | 41 | 32.8 | 42 | 36.8 | 83 | 34.7 | ||
| Age at diagnosis | Mean | Range | Mean | Range | Mean | Range | 0.413 | 0.680 |
| 55.2 | 21–86 | 55.8 | 19–81 | 55.5 | 19–86 | |||
Six participants became ineligible during the study due to a change in treatment (Fig. 1). Twenty-seven participants dropped from the treatment group (21 %) and 31 from the control group (26 %), a non-significant difference (p>0.05). Of those who dropped from the study, 14 of the treatment group (52 % of drops) and 13 of the control group (42 % of drops) never called the system. Of those who tried the system regardless of group assignment, some dropped because they were too sick to call (n=11; 35 %). The mean number of patients participating per practice was 23. A dropped analysis was performed to compare age, gender, and disease stage between the 27 non-callers and the 223 participants that were analyzed. Results indicated that the groups did not differ in age, or disease stage (both p values >0.05), but did differ in gender p=0.031 with males being overrepresented in the non-caller group.
There were a total of 6,509 calls into the system by 223 patients. The overall daily call adherence was 65.0 % of expected days. Results from a negative binomial regression showed no difference in average days called between treatment (M=28.72, SD=15.62) and control (M=29.69, SD=16.78), p=.66. The average call duration was 5:18 min/s (SD=2:49) with no differences between groups (p=0.22).
Symptoms were common in both groups. Table 2 shows the prevalence and mean severity of moderate-to-severe symptoms reported at least once. The frequency at which patients reported symptoms having a moderate or severe level of severity or distress at least once are summarized in Table 3. Fatigue was the most prevalent moderate-to-severe symptom reported by 89.2 % of participants followed by trouble sleeping (74.9 %) and pain (70.4 %). Depressed mood and nausea/vomiting were also prevalent at moderate-to-severe levels in over half the patients.
Table 2.
Mean severity of symptoms in patients who reported symptom severity at moderate or higher intensity (≥4)
| Control | n=108 | Mean | SD |
| Fatigue | 69 | 6.66 | 0.88 |
| Pain | 60 | 6.14 | 0.93 |
| Trouble sleeping | 53 | 7.15 | 1.07 |
| Nausea | 48 | 6.16 | 1.07 |
| Constipation | 36 | 6.13 | 1.22 |
| Depressed mood | 27 | 7.44 | 1.06 |
| Diarrhea | 19 | 6.13 | 1.21 |
| Nervous | 18 | 7.06 | 1.29 |
| Sore mouth | 17 | 5.55 | 0.69 |
| Treatment | n=115 | Mean | SD |
| Pain | 67 | 6.07 | 1.11 |
| Fatigue | 63 | 6.86 | 1.13 |
| Trouble sleeping | 55 | 7.36 | 1.05 |
| Nausea | 50 | 6.12 | 1.17 |
| Depressed mood | 36 | 6.79 | 1.34 |
| Constipation | 33 | 6.29 | 1.39 |
| Diarrhea | 31 | 5.76 | 0.88 |
| Nervous | 27 | 7.16 | 1.18 |
| Sore mouth | 25 | 6.30 | 1.31 |
Table 3.
Number of unique participants reporting symptoms at moderate or severe level at least once. p values reflect chi-square analysis
| Symptom | Control | Treatment | Total | p value | |||
|---|---|---|---|---|---|---|---|
| n=108 | n=115 | N=223 | |||||
| n | % | n | % | N | % | ||
| Fatigue | 96 | 88.9 | 103 | 89.6 | 199 | 89.2 | 0.87 |
| Trouble sleeping | 83 | 76.9 | 84 | 73.0 | 167 | 74.9 | 0.51 |
| Pain | 76 | 70.4 | 83 | 72.2 | 159 | 71.3 | 0.77 |
| Depressed mood | 55 | 50.9 | 64 | 55.7 | 119 | 53.4 | 0.48 |
| Nausea/vomiting | 58 | 53.7 | 66 | 57.4 | 124 | 55.6 | 0.58 |
| Nervous/anxious | 50 | 46.3 | 55 | 47.8 | 105 | 47.1 | 0.82 |
| Constipation | 41 | 38.0 | 44 | 38.3 | 85 | 38.1 | 0.96 |
| Diarrhea | 30 | 27.8 | 37 | 32.2 | 67 | 30.0 | 0.47 |
| Sore mouth | 28 | 25.9 | 36 | 31.3 | 64 | 28.7 | 0.38 |
| Fever distress | 24 | 22.2 | 23 | 20.0 | 47 | 21.1 | 0.68 |
Moderate or severe is any score of ≥4 out of 10
For the primary aim of the study, results indicate no significant difference between symptom severity or distress scores between groups (mean difference=0.06, p=0.58). In addition, there were no differences between groups in average number of no symptom days (0), mild days (1–3), moderate days (4–7), or severe symptom days (8–10), all p values >0.05 (Tables 3 and 4). A post hoc sensitivity analysis was conducted with G*Power to access available statistical power. With a sample size of 223 participants, we had sufficient power (1−B)=0.91 to detect a small effect size Cohen’s d=0.10 and alpha=0.05.
Table 4.
Mean number of days at each severity level
| Mean number of days | ||||
|---|---|---|---|---|
| Severity | Control | Treatment | Total | p value |
| n=108 | n=115 | N=223 | ||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Calls made | 29.69 (16.78) | 28.72 (15.62) | 29.19 (16.17) | 0.66 |
| Asymptomatic | 5.83 (8.86) | 5.76 (10.65) | 5.79 (9.80) | 0.41 |
| Mild | 6.56 (11.21) | 7.24 (9.42) | 6.91 (10.30) | 0.84 |
| Moderate | 11.27 (11.43) | 9.89 (8.69) | 10.56 (10.11) | 0.15 |
| Severe | 6.02 (7.93) | 5.83 (8.84) | 5.92 (8.39) | 0.73 |
p values reflect results of Negative Binomial Regression
Even though alerts were not generated for the control group, for comparison, we calculated the number of times an alert would have been generated and found no differences between groups (p=0.80). The treatment group generated alerts to oncology providers on 1,028 call days (approximately 9 days/patient). Two or more symptoms at moderate-to-severe levels were included on 650 (63.2 %) of these alerts and 457 (44.5 %) of these alerts reported at least one symptom at severe levels (>8). Severe levels were significantly more common in the treatment group (p<0.001).
For the second aim; there were a total of 211 unscheduled contacts between patients and oncology providers (Table 5). There was no significant difference between treatment and control for unscheduled contacts (p=0.73). The frequency of patient-initiated vs. provider-initiated unscheduled contacts was also similar between the two groups (p=0.14).
Table 5.
Unscheduled contacts within each group
| Type of contact | Control group | Treatment group | p value | ||
|---|---|---|---|---|---|
| Cycle 2 | Cycle 3 | Cycle 2 | Cycle 3 | ||
| Patient-initiated | 62 | 34 | 49 | 34 | |
| 84.9 % | 97.1 % | 74.2 % | 91.9 % | ||
| Total by group | 96 (88.8 %) | 83 (80.6 %) | |||
| Provider-initiated | 11 | 1 | 17 | 3 | |
| 15.1 % | 2.9 % | 25.8 % | 8.1 % | ||
| Total by group | 12 (11.2 %) | 20 (19.4 %) | |||
| Total count Percent | 73 | 35 | 66 | 37 | 0.14 |
| 100 % | 100 % | 100 % | 100 % | ||
For the third aim, control participants talked about symptoms at patient-initiated contacts (n=79; 73.0 %) somewhat more often than treatment participants (n=64; 62.0 %). However, this was not statistically different (p=0.19).
Treatment group provider-initiated contacts that resulted in an office visit were infrequent but nearly double compared to control, 18 contacts (17.5 %) versus 10 contacts (9.3 %), respectively. Provider-initiated contacts that were telephone-only were identical for both groups, two calls (1.9 %). Among the 32 provider-initiated unscheduled contacts (those resulting in office visits and/or telephone calls), the treatment group discussed symptoms more often than control, 14 (70.0 %) versus 4 (33.0 %), respectively; likely due to the small numbers, this was not a statistically significant difference, p=0.10.
What is most striking is the very few follow-up contacts by oncology providers in spite of receiving a high number of alerts (1,028) including nearly 45% with at least one symptom at 8 or greater on a 10-point scale. For pain alone, 167 alert reports included pain ratings at 8 or greater. On average, alerts contained reports of three symptoms at moderate or greater intensity levels. In spite of this, only 20 provider-initiated, unscheduled contacts to the treatment group occurred over the course of the study and only 14 contacts included a discussion of symptoms.
Exit interviews (n=167) were conducted with patients. Overall, 94.0 % found the automated system quite or very easy to use, 91.0 % found the call length acceptable, and 77.0 % said they were quite or very satisfied with using the system. Sixty-one percent of participants reported that the system was very much or quite helpful in keeping track of their symptoms, and 52.0 % reported that the system helped them feel like they were participating in their care. Within the treatment group (n=84), 79.0 % reported that they were quite or very confident that the automated system notified their oncology providers of their symptoms. In spite of this, only 25.0 % agreed that the system helped their doctor or nurse decrease their symptoms.
At the end of study, we also interviewed the participating oncology physicians and nurses (n=13). Eleven (85 %) were somewhat to very satisfied with the system and found the alert reports to be timely (100 %), easy to interpret (89 %), and useful (82 %). Ten (83 %) reported contacting the patient at least sometimes. Two (15 %) were not satisfied and stated that while they received the reports, they never read them. None of the providers reported having technical difficulties receiving reports.
Discussion
The aim of this study was to evaluate the efficacy of an automated telephone monitoring system utilized by cancer patients to communicate unrelieved symptoms to their oncology providers and whether symptom outcomes would be improved as a result. Despite patients’ willingness to use the system and providers’ admission of the usefulness of alert reports, symptom control was not significantly better in comparison to usual care. While oncology providers received hundreds of alert reports, they did not respond as anticipated. Symptom-related provider-initiated contacts in the treatment group were more common than in the control (14 vs. 4 contacts), but it was clinically insignificant in relation to the number of unrelieved symptoms reported. While infrequent, if a provider was to contact a patient it was more likely to occur during the earlier of the two cycles measured. Treatment participants evaluated the system favorably, but, consistent with our findings of infrequent calls initiated by providers, 75.0%of treatment participants believed that their physicians/nurses did not use the information to improve their care.
While providers rarely initiated contact, neither did patients. Symptom reports registered in the automated system documented thousands of days of moderate-to-severe symptoms in both groups, yet patients rarely called providers to address unrelieved symptoms even when patient-initiated contact is the standard approach to obtain further symptom care. Thus, patient-self-monitoring of symptoms and transmission of unrelieved symptoms to their oncology providers does not appear to be adequately used by patients. Automated monitoring systems offer an approach to overcome patient reluctance to call providers.
While the goal of implementing a remote symptom monitoring system was achieved, it did not change provider behavior or prompt symptom treatment intensification. Patients in this study received initial symptom care during office visits prior to beginning chemotherapy. Standard practice is to provide patients with information about symptom self-care and a variety of prescriptions to treat symptoms that might develop. The reasons that oncology providers did not initiate contacts with patients after receiving alerts are important to understand if symptoms outcomes are to improve. Lack of follow-up may happen because busy practices do not allow sufficient time for staff to interact with unscheduled patients. This constraint may be due to oncology work force shortage issues and/or the reimbursement climate that does not allow billing for telephone-based symptom management intensification. Provider as well as patient expectations about their ability to achieve symptom relief after initial symptom treatment also may influence follow-up. Further research is needed to explore system, provider, and patient factors that are barriers to symptom management intensification when initial symptom treatment is inadequate.
Other studies testing automated symptom reporting systems have primarily reported provider and patient satisfaction and usability and most have collected symptom data in the clinic setting during a visit [7, 11, 12, 19] or as a means to collect clinical trial outcomes data without the intent to make it actionable [9]. These studies, like our own, have generally found high levels of patient satisfaction and usability. Provider acceptability has also been reported [15, 16, 20]. There are few studies to date that have reported provider use of automated symptom reports and symptom outcomes. Some studies have found increased discussion of symptoms at clinic visits when automated symptom reports were provided as part of the visit, but unlike our study, these studies did not determine if this resulted in symptom reduction [11, 18, 19]. Given et al. studied metastatic breast cancer patients and found that an automated voice response system that assessed symptoms and directed patients to self-care strategies was more effective in achieving symptom reduction than a comparison group who received live telephone coaching [8]. In a more recent study that paralleled our own in design, Cleeland et al. compared a twice-weekly automated telephone monitoring symptoms after lung cancer thoracotomy with intervention alerts for moderate-to-severe symptoms emailed to the surgical team [16]. The control group reported symptoms but generated no alerts. The intervention group achieved both greater and more rapid symptom reduction and, in contrast to our study findings, 60 % of alerts resulted in a provider-initiated call. There may be differences in the interpretation and response to symptoms by surgeons, vigilant for postoperative complications, and medical oncology providers who may normalize symptoms as an expected part of chemotherapy treatment.
A potential limitation of our study was the relatively small number of oncology practices and providers involved. Provider attitudes could be shaped by the environment and may not be generalizable to symptom care in oncology overall. However, practices included both community and academic settings in two separate states with no observed difference in study results based on providers or practices. A second limitation is that the sample was predominantly female, and men were more likely than women to consent but never call after enrolling (18 % male vs 8 % female). However, when specific aim 1 was analyzed stratified by gender, there was no difference in the lack of symptom relief for either men or women. Another potential limitation is that data on unplanned patient– provider-initiated contacts and whether symptoms were discussed was a patient-reported variable. Recollection of the number and focus of calls and visits may not be accurate although the data were reported within 24 h to a few days of occurrence. Finally, aims 2 and 3 were based on the erroneous assumption that providers would respond to at least some of the alerts. The number of responses was less than expected and the comparative analyses of these data for aims 2 and 3 may be underpowered. However, the more important finding was that providers took no action whether they had automated symptom reports to assist them or not. This should be explored in future investigations.
In conclusion, despite the willingness of patients to use a daily automated telephone symptom monitoring system and providers’ receipt of the information, symptom management did not improve, providers did not intensify symptom treatment, and there were no gains in symptom relief. While the study provides support for the use of technology in monitoring patient-reported outcomes, further research is needed to identify and overcome system and provider barriers to symptom treatment intensification.
Acknowledgments
This research was funded by the National Institutes of Health, National Cancer Institute (R01 CA89474). The content is solely the responsibility of the authors.
Study funding is now complete. The first three authors have full control of all primary data and agree to allow the journal to review pertinent de-identified data if requested.
Footnotes
Conflict of interest The authors have no financial relationship with the National Cancer Institute other than they had salary and benefit support during the implementation of the study.
Contributor Information
Kathi H. Mooney, Email: kathi.mooney@nurs.utah.edu, University of Utah College of Nursing, 10 South 2000 East, Salt Lake City, UT 84112, USA; Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Susan L. Beck, University of Utah College of Nursing, 10 South 2000 East, Salt Lake City, UT 84112, USA Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Robert H. Friedman, Boston University School of Medicine, Boston, MA, USA Boston University School of Public Health, Boston, MA, USA; Medical Information Systems Unit, Boston Medical Center, Boston, MA, USA.
Ramesh Farzanfar, Boston University School of Medicine, Boston, MA, USA; Boston University School of Public Health, Boston, MA, USA; Medical Information Systems Unit, Boston Medical Center, Boston, MA, USA.
Bob Wong, University of Utah College of Nursing, 10 South 2000 East, Salt Lake City, UT 84112, USA.
References
- 1.Atherton P, Sloan J. Rising importance of patient-reported outcomes. Lancet Oncol. 2006;7(11):883–884. doi: 10.1016/S1470-2045(06)70914-7. [DOI] [PubMed] [Google Scholar]
- 2.Basch E, Abernethy A. Supporting clinical practice decisions with real-time patient-reported outcomes. J Clin Oncol. 2011;29(8):954–956. doi: 10.1200/JCO.2010.33.2668. [DOI] [PubMed] [Google Scholar]
- 3.Bren L. The importance of patient-reported outcomes. It’s all about the patients. FDA Consum. 2006;40(6):26–33. [PubMed] [Google Scholar]
- 4.Grenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res. 2009;18(1):115–123. doi: 10.1007/s11136-008-9430-6. [DOI] [PubMed] [Google Scholar]
- 5.Trotti A, et al. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–5127. doi: 10.1200/JCO.2007.12.4784. [DOI] [PubMed] [Google Scholar]
- 6.Wu A, et al. Adding the patient perspective to comparative effectiveness research. Health Aff. 2012;29(10):1863–1871. doi: 10.1377/hlthaff.2010.0660. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter JS, et al. Oncology outpatient and provider responses to a computerized symptom assessment system. Oncol Nurs Forum. 2008;35(4):661–669. doi: 10.1188/08/ONF.661-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Given C, et al. Managing symptoms among patients with breast cancer during chemotherapy: results of a two-arm behavioral trial. J Clin Oncol. 2008;26:5855–5862. doi: 10.1200/JCO.2008.16.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basch E, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23(15):3552–3561. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 10.Basch E, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol. 2005;23(15):3552–3561. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 11.Berry D, et al. Enhancing patient–provider communication with the electronic self-report assessment for cancer: a randomized trial. J Clin Oncol. 2011;29(8):1029–1035. doi: 10.1200/JCO.2010.30.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark T, Fortner B, Johnson G. Evaluation of a tablet PC technology to screen and educate oncology patients. Support Care Cancer. 2008;16(4):371–378. doi: 10.1007/s00520-007-0312-1. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein J, Friedman R. The potential role of telecommunications technologies in the management of chronic health conditions. Dis Manag Health Outcomes. 2000;8(2):57–63. [Google Scholar]
- 14.Main D, et al. A qualitative study of work and work return in cancer survivors. Psychooncology. 2005;14(11):992–1004. doi: 10.1002/pon.913. [DOI] [PubMed] [Google Scholar]
- 15.Mooney KH, et al. Telephone-linked care for cancer symptom monitoring: a pilot study. Cancer Pract. 2002;10(3):147–154. doi: 10.1046/j.1523-5394.2002.103006.x. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland C, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol. 2011;29(8):994–1000. doi: 10.1200/JCO.2010.29.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobak KA, et al. Computer-administered clinical rating scales. A review. Psychopharmacology (Berl) 1996;127(4):291–301. doi: 10.1007/s002130050089. [DOI] [PubMed] [Google Scholar]
- 18.Hilarius D, et al. Use of health-related quality-of-life assessments in daily clinical oncology nursing practice: a community hospital-based intervention study. Cancer. 2008;113(3):628–637. doi: 10.1002/cncr.23623. [DOI] [PubMed] [Google Scholar]
- 19.Velikova G, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 20.Williams E, et al. Randomised controlled trial of an automated, interactive telephone intervention (TLC Diabetes) to improve type 2 diabetes management: baseline findings and six-month outcomes. BMC Public Health. 2012;12:602–612. doi: 10.1186/1471-2458-12-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salminen E, et al. Needs of developing the skills of palliative care at the oncology ward: an audit of symptoms among 203 consecutive cancer patients in Finland. Support Care Cancer. 2008;16(1):3–8. doi: 10.1007/s00520-007-0252-9. [DOI] [PubMed] [Google Scholar]
- 22.Teunissen SC, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manag. 2007;34(1):94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Cleeland C, Mendoza T. Symptom measurement by patient report. In: Cleeland C, Fisch M, Dunn A, editors. Cancer symptom science: measurement, mechanisms and management. Cambridge: Cambridge University Press; 2011. pp. 271–289. [Google Scholar]
- 24.Stewart BJ, Archbold PG. Nursing intervention studies require outcome measures that are sensitive to change part 2. Res Nurs Health. 1993;16(1):77–81. doi: 10.1002/nur.4770160110. [DOI] [PubMed] [Google Scholar]
- 25.Youngblut JM, Caspar GR. Single item indicators in nursing research. Res Nurs Health. 1993;16:459–465. doi: 10.1002/nur.4770160610. [DOI] [PMC free article] [PubMed] [Google Scholar]