Abstract
Successful retrieval of an event includes an initial search phase in which the information is accessed and a subsequent elaboration phase in which an individual expands on event details. Traditionally, functional neuroimaging studies examining episodic memory retrieval either have not made a distinction between these two phases or have focused on the initial search process. The current study used an extended retrieval trial to compare the neural correlates of search and elaboration and to examine the effects of emotion on each phase. Prior to scanning, participants encoded positive, negative, and neutral images paired with neutral titles. After a thirty-minute delay, participants engaged in a scanned recognition task in which they viewed the neutral titles and indicated whether the title had been presented with an image during the study phase. Retrieval was divided into an initial memory search and a subsequent five-second elaboration phase. The current study identified neural differences between the search and elaboration phases, with search being associated with widespread bilateral activations across the entire cortex and elaboration primarily being associated with increased activity in the medial prefrontal cortex. The emotionality of the retrieval target was more influential during search relative to elaboration. However, valence influenced when the effect of emotion was greatest, with search engaging many more regions for positive events than negative ones, but elaboration engaging the dorsomedial prefrontal cortex more for negative events than positive events.
1. Introduction
Successful retrieval of an event includes two distinct phases: an initial search phase in which information is accessed and a subsequent elaboration phase in which additional event details are retrieved. Traditionally, functional neuroimaging studies examining episodic memory retrieval either have not distinguished these two aspects of retrieval or have focused on regions recruited during the initial search process, identifying a largely bilateral memory network that includes prefrontal (PFC), medial-temporal (MTL), medial-parieto-occipital, lateral parietal, anterior cingulate, occipital, and cerebellar regions (see Cabeza and Nyberg, 2000 and Spaniol et al., 2009 for reviews).
Although the processes supporting elaboration of material presented in an earlier laboratory session have rarely been examined, a number of recent studies have examined the neural correlates of search and elaboration phases during autobiographical retrieval (e.g., Addis et al., 2007; Daselaar et al., 2008; Holland et al., 2011), finding a number of regions that are differentially recruited during these two phases. Compared to the elaboration phase, autobiographical memory search has been associated with increased activity in the hippocampus (Daselaar et al., 2008), right dorsolateral and medial PFC regions (Daselaar et al., 2008), and bilateral occipital gyrus (Addis et al., 2007). Elaboration has been associated with greater activity in the left PFC ( Addis et al., 2007; Daselaar et al., 2008), right ventral PFC (Addis et al., 2007); left precuneus (Addis et al., 2007; Daselaar et al., 2008), and bilateral visual cortex (Daselaar et al., 2008). Despite some remaining ambiguity in the processes that distinguish search from elaboration, the extant data suggest a dissociation between these phases within autobiographical memory.
It is currently unclear whether the search and elaboration phases during the retrieval of laboratory-learned information will also dissociate. Previous research has demonstrated significant differences in the neural and cognitive processes supporting the search for autobiographical compared to other episodic event information. The initial search phase of a voluntary autobiographical memory is a complex, iterative process that relies on memory search and controlled retrieval processes involving left lateral PFC, intuitive monitoring processes supported by ventromedial PFC, and self-referential processes supported by medial PFC (see Cabeza & St. Jacques, 2007 for review). Episodic search for laboratory-learned information, by contrast, tends to involve a more deliberate monitoring supported by dorsolateral PFC (Gilboa, 2004; See McDermott, Szpunar, & Christ, 2009 for a meta-analysis) and is less likely to recruit the medial PFC for either intuitive monitoring or self-referential processing (Cabeza et al., 2004). Due to the substantial differences between episodic and autobiographical memory search, it is likely that the distinctions between search and elaboration that exist for autobiographical memory may not extend to other forms of episodic retrieval. Because the majority of research examining the neuropsychology and cognitive neuroscience of memory has assessed memory for stimuli presented in the laboratory, it is critical to understand the time course of the processes that support memory for these stimuli.
It is also likely that characteristics of the remembered stimuli, such as emotional valence, may differentially influence the search and elaboration phases of episodic memory retrieval. Identifying the specific timing of emotion’s effects on memory processes may be important, as it allows researchers to better understand circumstances in which the presence of emotion should alter memory performance. Although a number of recent studies have examined the effects of emotion on neural activity during retrieval (see Buchanan, 2007 for review), the emotion-phase interaction has not been examined. Previous studies have shown increased activity in the amygdala (e.g., Dolan et al., 2000; Dolcos et al., 2005; Murty et al., 2009; but see, Taylor et al., 1998), lateral temporal lobes (Murty et al., 2009), and lateral frontal lobes (Murty et al., 2009) during retrieval of emotional relative to neutral events, however these studies have not examined whether valence predominantly plays a role during search or elaboration of episodic information. Daselaar and colleagues (2008) examined the effect of emotion on neural recruitment in both phases of autobiographical memory retrieval. This study found that activity in a number of regions, including the hippocampus and amygdala, was correlated with emotional intensity during search. However, intensity was not related to activity in any regions during elaboration, suggesting an important role of emotion in selecting autobiographical information, but not in remembering the details of the event. Emotional valence was not examined in this prior study, leaving the question open as to the effect of valence on search and elaboration.
The current study uses an extended retrieval period to compare the search and elaboration phases of episodic memory retrieval. Although this comparison has previously been studied in autobiographical memory, examining these differences in episodic memory retrieval allows for more controlled comparisons across conditions. In an episodic memory study, researchers know the precise encoding conditions of every trial, reducing the number of potential confounds at retrieval. In addition, episodic memories can be evaluated on accuracy, a judgment that is very difficult in autobiographical memory studies. Therefore, episodic memory studies provide a unique opportunity to examine search and elaboration processes during accurate retrieval only. Based on prior literature, we hypothesize that the search phase will be associated with increased lateral PFC, MTL, and parietal activity relative to the elaboration phase. We expect that elaboration will be associated with increased sensory and medial PFC activity, as participants attempt to elaborate on both the physical stimulus as well as their own feelings about the image. However, this network of activity will not be as widespread and strong as that typically seen in autobiographical memory studies, as visual images are substantially less complex.
The second goal of the current study is to examine the emotion by phase interaction during episodic memory retrieval to help us better understand the precise role emotion plays in the retrieval process. We will first examine the effect of emotion by collapsing across positive and negative events, and comparing them to neutral events. In a second model, we will directly compare positive and negative events to isolate the effect of emotional valence. We hypothesize that emotion will play a greater role in search relative to elaboration, as has been seen in the autobiographical memory literature (Daselaar et al., 2008). However it is unclear how positive and negative valence will differentially influence search and elaboration processes.
To parallel the typical autobiographical memory retrieval design, participants encoded positive, negative, and neutral images presented with neutral titles. During a scanned retrieval session, participants viewed the neutral titles and were asked to retrieve the related emotional or neutral image. They pressed a button to indicate whether the neutral title had been studied and then elaborated on their memory for the encoding trial. This approach allows for the isolation of search and elaboration processes, and the use of a simple verbal cue to trigger a complex visual memory also parallels the typical autobiographical retrieval paradigm. In addition, this design prevents any confounds that may be associated with an emotional retrieval cue. One potential difficulty with examining the neural activity associated with emotional episodic memory retrieval is that re-presenting participants with studied emotional and neutral stimuli could lead to neural differences stemming from the processing of the retrieval cues, in addition to those related to remembering the encoding event. A number of recent fMRI studies have avoided this potential confound of on-line, emotional processing by having participants encode a neutral item in a neutral or emotional study context and using the neutral item as the retrieval cue (Maratos et al., 2001; Smith et al., 2004; Smith et al., 2005; Sterpenich et al., 2006). This methodology helps ensure that any valence differences at retrieval are related to the mnemonic content and not the retrieval cue.
2. Methods
2.1 Participants
Data from twenty-seven healthy young adults (mean age = 28, sd = 6.37, ages 18–39; mean education = 17.15, sd = 2.4; 15 male)1 are reported here, taken from a larger sample of participants, ranging in age from 18 to 85. Participants were all right-handed native English speakers without a history of psychiatric illness or neurological disorder and were recruited from the greater Boston area. All participants were paid for their participation and gave written informed consent in accordance with the requirements of the Institutional Review Board at Boston College.
2.2 Materials
Stimuli for the current study were 480 pictures (160 positive, 160 negative, and 160 neutral) selected from the IAPS database, Geneva Affective Picture Database (GAPED) and images used in Waring & Kensinger (2011). Images were selected so that arousal ratings were equated for positive and negative images, while positive and negative images were significantly higher in arousal than neutral images. However, arousal ratings given by participants in the current study were higher for negative relative to positive images (see behavioral results section). Neutral titles were selected for each picture to describe the picture as closely as possible. To confirm title neutrality, five pilot participants (4 female; M age = 21.6) were asked to view all titles and determine whether they were neutral, positive, or negative. Titles were replaced if 2 or more participants rated them as either positive or negative (e.g., “Medical Examination” was changed to “Rubber Gloves” and “Alleyway” was changed to “Concrete Arches”). The 480 title-picture pairs were randomly divided into 4 sets of 120 pictures each (40 positive, 40 negative, and 40 neutral).
2.3 Procedure
Following instruction and a short practice, participants encoded one of the sets of 120 title-image pairs that included a neutral title (e.g., “Lettuce”) with a positive, negative, or neutral image (e.g., a piece of rotting lettuce with bugs crawling on it as a negative image). Participants were given 3 seconds to make a decision regarding the appropriateness of the title as a description of the image (1 = poor description, 2 = acceptable description, and 3 = very good description) and were told to use this time to learn the title-image pairs.
After a half-hour delay (M = 34.4 minutes, sd = 7.5), participants took part in a scanned retrieval task. Participants were presented with the 120 neutral titles that were studied during the encoding phase and 120 unstudied neutral titles. These 240 titles were randomly presented to each participant across 6 retrieval runs of equal length. Participants were given up to 4 seconds to decide whether the title was “old” (i.e., seen previously) or “new” (i.e., not seen previously). The screen was removed following the participant’s button press. Across participants, it was varied which items were studied and which were reserved as foils on the recognition test.
Immediately following an “old” response, 80% of the time, participants were asked to “Elaborate” on the old item (i.e., think about the image presented with the title and the experience with that title and image at encoding). This 5-second “Elaborate” phase was followed by two ratings. Participants were asked to rate (on a 1–5 scale) how well they remembered both the image associated with the old item and their own personal thoughts and feelings while encoding the old item. Each rating was presented for 3 seconds and the order of the ratings was alternated across participants. To discourage participants from beginning their elaboration earlier in the search phase and to better allow for the separation of search and elaboration phases, 20% of trials were catch trials, on which participants were not asked to elaborate and instead moved on to the next trial.
Following a “new” response, 80% of the time, participants moved on to the next trial. To minimize the likelihood that participants would automatically begin preparing for the next trial after a “new” response, on 20% of the trials, participants were asked to “Imagine” an image that could have accompanied the new item. This “Imagine” phase was designed to mirror the “Elaboration” phase structure. It lasted for 5 sec and was followed by two ratings. Participants were asked to rate (on a 1–5 scale) the vividness of the image they generated for the new item and the vividness of their own personal thoughts and feelings. Each rating was presented for 3 seconds and the order of rating presentation was varied across participants. Following both ratings, participants were presented with a fixation cross for 0–6 seconds to introduce a random jitter.
After being removed from the scanner, participants were re-presented with the images from the encoding phase. They were asked to rate each image’s valence and arousal on a 1–7 scale and to indicate which specific emotions they associated with each image. Participants were given as much time as needed for each response, but were encouraged to respond based on their initial reaction.
2.3 Data Acquisition
Magnetic resonance images were acquired using a Siemens Tim Trio 3 Tesla scanner. Participants’ heads were held in place using cushions and a headrest. An initial localizing scan and auto-align scout were followed by a high resolution multi-echo T1 structural scan for anatomical visualization (176 1mm slices, TR = 2200 ms, TE1 = 1.64 ms, TE2 = 3.5 ms, TE3 = 5.36 ms, TE4 = 7.22 ms) and six runs of functional scans collected during memory retrieval. Whole brain, gradient-echo, echo planar images (31 3 mm slices aligned along the line between the anterior and posterior commissures, 20% skip, TR = 2 s, TE = 30 ms, Flip angle = 90) were acquired using interleaved acquisition. Participants also engaged in a diffusion weighted scan (68 2 mm slices, TR = 8450 ms, TE = 86 ms, diffusion weightings= 2, b-value1 = 0, b-value2 = 700, directions = 30), which will not be discussed in the current manuscript. All response data were collected using a magnet-safe button response box.
2.4 Preprocessing and Data Analysis
Images were preprocessed and analyzed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB. Images were co-registered, realigned, normalized (resampled at 3 mm at the segmentation stage and written at 2mm at the normalization stage) and smoothed using a Gaussian 8 mm kernel. The initial search phase was modeled as an event-related response at cue-onset. The elaboration phase was modeled as a 5-second epoch beginning at the old/new button press. The fMRI analysis examined the effect of emotion (i.e., positive, negative, or neutral events) and phase (i.e., search or elaboration) on neural activity during accurate “old” responses to studied items (i.e., “hits”). Hits were modeled in six conditions: neutral search, positive search, negative search, neutral elaboration, positive elaboration, and negative elaboration. Incorrect responses and correct “new” responses to unstudied items, although not relevant for the current analysis, were included in each model as two separate nuisance variables. Importantly, all search trials were included in the analysis, even those that were not followed by elaboration. Inclusion of such “catch” trials reduced the influence of collinearity between search and elaboration trials on our results.
Two full-factorial designs were used at the random-effects level to directly test the effects of emotion and phase, as well as the emotion-by-phase interaction. In the first, emotion (emotional and neutral events) and phase (search and elaboration) were included as 2-level within-subject factors. The results focus on three types of regions: regions showing a main effect of phase (i.e., search > elaboration or elaboration > search), regions showing a main effect of emotion, and regions showing differential phase differences based on emotion (i.e., phase-by-emotion interactions; e.g., regions showing greater emotion-related differences in the search phase relative to the elaboration phase). The directionality of all interaction effects was determined by examining the effect of emotion on neural activity in the search and elaboration phases separately.
The second model included only positive and negative events and included emotional valence (positive and negative events) and phase as 2-level within-subject factors. As in the first design, the results focus on the main effects and valence-by-phase interaction and the directionality of all interaction effects were determined by examining the effect of emotion on neural activity in the search and elaboration phases separately.
The significance threshold for all analyses was set at p < .005 (uncorrected). Monte Carlo simulations (Slotnick et al., 2003), run with the normalized voxel size of 2×2×2, determined that a 29-voxel extent corrected results to p < .05. Therefore, we discuss all clusters that reach this threshold. However, because this relatively large voxel extent may put us at risk for type 2 error (see Lieberman & Cunningham, 2009), we report all clusters with a voxel extent of 10 or more in the tables, as these results may be relevant for the purposes of future reviews and meta-analyses. Clusters reaching significance were overlaid on anatomical images from MRICron. For visualization purposes, activity within a 5 mm sphere around peak voxels was extracted using the REX (downloaded from http://web.mit.edu/swg/software.htm) toolbox. For all analyses, reported coordinates reflect the peak activity within active regions. These coordinates were converted from MNI coordinates to Talairach space, localized using the Talairach Client, and confirmed with the Talairach and Tournoux atlas (Talairach & Tournoux, 1988).
3. Results
3.1 Behavioral Results
The appropriateness of each image’s verbal title was evaluated during the encoding phase of the current task. Participants rated the titles for negative images as less appropriate than positive (t(26) = 7.61, p < .001) and neutral (t(26) = 10.02, p < .001) titles.
At retrieval, memory accuracy (hits minus false alarms) was high for positive (Macc = .65, SE = .03), negative (Macc = .66, SE = .02), and neutral (Macc = .64, SE = .02) events and equivalent across emotion conditions (t < 1 for all contrasts). Participants were faster responding to retrieval cues for neutral relative to positive (t(26) = 2.77, p < .05) and negative events (t(26) = 4.41, p < .001), and to positive relative to negative events (t(26) = 2.15, p < .05).
Following each memory decision, participants rated external vividness (i.e., vividness of their memory for the actual image) and internal vividness (i.e., vividness of their memory for their own thoughts and feelings at the time of encoding). Positive and negative events did not differ for either measure (t < 1 for both contrasts), but neutral events had significant lower ratings for internal vividness (t(26) = 4.11, p < .001 and t(26) = 4.58, p < .001 compared to positive and negative events, respectively) as well as external vividness (t(26) = 2.27, p < .05 and t(26) = 3.10, p < .01 compared to positive and negative events, respectively).
Ratings collected after the memory test confirmed that positive images were judged as more positive than neutral images (t(26) = 7.19, p < .001) which were judged as more positive than negative images (t(26) = 9.07, p < .001). Additionally, negative and positive images were judged as more arousing than neutral images (p < .001 for both contrasts), and negative images were more arousing than positive (t(26) = 2.91, p < .01). All behavioral results are presented in Table 1.
Table 1.
Behavioral results as a function of stimulus valence
| Negative Images | Positive Images | Neutral Images | |
|---|---|---|---|
| Appropriateness | 2.07a (0.06) | 2.40b (0.04) | 2.42b (0.04) |
| Accuracy (Hits-FAs) | 0.66a (0.02) | 0.65a (0.03) | 0.64a (0.02) |
| Response Time (Hits) | 1823.15a (46.2) | 1772.39b (48.3) | 1713.23c (47.9) |
| Valence | 2.96a (0.1) | 4.98b (0.08) | 4.31c (0.06) |
| Arousal | 4.90a (0.09) | 4.41b (0.09) | 3.93c (0.07) |
| Internal Vividness | 2.57a (0.12) | 2.50a (0.11) | 2.21b (0.13) |
| External Vividness | 2.89a (0.12) | 2.87a (0.12) | 2.73b (0.13) |
Note: Values represent the average for stimuli of that particular valence. Standard deviations are presented in parentheses. Means with different superscripts are significantly different at p<.05.
3.2 Imaging Results
3.2.1 Main effects of phase and emotion
All regions showing a main effect of phase are presented in Table 2 along with the results of follow-up analyses examining the directionality of this effect. The initial search phase was associated with increased posterior activity relative to the elaboration phase (see green regions of Figure 1). In addition, memory search was associated with increased activity in ventrolateral PFC and bilateral MTL regions during this initial search phase than in the subsequent elaboration phase. Compared to the search phase, elaboration was associated with increased activity primarily in the medial PFC (mPFC), including dorsal and ventral regions (see orange regions of Figure 1).
Table 2.
Results of a 2×2 Full-Factorial ANOVA with Emotion (Emotional v. Neutral) and Phase (Search v. Elaboration) as within-subject factors.
| MNI Coordinates
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Region of Interest | Hemisphere | BA | x | y | z | p-value | k | Directionality of Effect |
| Main Effect of Phase | ||||||||
| Claustrum | L | na | −30 | 22 | −2 | 0.000 | 66461 | Search > Elaboration |
| Insula | R | 13 | 30 | 24 | −2 | 0.000 | Search > Elaboration | |
| Superior Parietal Lobe | L | 7 | −30 | −58 | 46 | 0.000 | Search > Elaboration | |
| Fusiform Gyrus | L | 20 | −34 | −42 | −22 | 0.000 | Search > Elaboration | |
| R | 37 | 36 | −46 | −20 | 0.000 | Search > Elaboration | ||
| Dorsolateral Prefrontal Cortex | L | 9 | −40 | 10 | 26 | 0.000 | Search > Elaboration | |
| Ventrolateral Prefrontal Cortex | R | 10 | 18 | 52 | −8 | 0.000 | 58 | Search > Elaboration |
| 10 | 34 | 54 | 0 | 0.001 | 29 | Search > Elaboration | ||
| Dorsolateral Prefrontal Cortex | R | 8 | 22 | 32 | 48 | 0.000 | 5287 | Elaboration > Search |
| Anterior Cingulate | L | 24 | −4 | 22 | −6 | 0.000 | Elaboration > Search | |
| Medial Prefrontal Cortex | L | 10 | −8 | 60 | 10 | 0.000 | Elaboration > Search | |
| Parahippocampal Gyrus | L | 19 | −34 | −48 | 0 | 0.000 | 116 | Elaboration > Search |
| Fusiform Gyrus | R | 20 | 52 | −6 | −32 | 0.000 | 50 | Elaboration > Search |
| Inferior Temporal Gyrus | R | 20 | 56 | −22 | −26 | 0.000 | Elaboration > Search | |
| Precentral Gyrus | R | 4 | 38 | −20 | 54 | 0.000 | 279 | Elaboration > Search |
| Postcentral Gyrus | R | 3 | 28 | −36 | 58 | 0.004 | Elaboration > Search | |
| Inferior Temporal Gyrus | L | 20 | −56 | −24 | −22 | 0.000 | 68 | Elaboration > Search |
| Superior Temporal Gyrus | R | 22 | 58 | −6 | 0 | 0.000 | 338 | Elaboration > Search |
| Claustrum | R | na | 36 | −12 | 6 | 0.000 | Elaboration > Search | |
| Insula | R | 13 | 40 | −12 | 18 | 0.001 | Elaboration > Search | |
| Paracentral Lobe | R | 31 | 6 | −20 | 46 | 0.000 | 561 | Elaboration > Search |
| Premotor Cortex | R | 6 | 16 | −14 | 62 | 0.000 | Elaboration > Search | |
| Premotor Cortex | L | 6 | −12 | −14 | 60 | 0.000 | Elaboration > Search | |
| Inferior Parietal Lobe | R | 40 | 58 | −30 | 32 | 0.000 | 42 | Elaboration > Search |
| Claustrum | L | na | −38 | −24 | 0 | 0.000 | 313 | Elaboration > Search |
| Superior Temporal Gyrus | L | 22 | −54 | −8 | 2 | 0.000 | Elaboration > Search | |
| Insula | L | 13 | −40 | 4 | −6 | 0.000 | Elaboration > Search | |
| Superior Temporal Gyrus | L | 39 | −42 | −58 | 26 | 0.000 | 48 | Elaboration > Search |
| Posterior Cingulate | R | 31 | 22 | −44 | 22 | 0.001 | 26T | Elaboration > Search |
| Superior Temporal Gyrus | R | 42 | 60 | −24 | 10 | 0.001 | 12T | Elaboration > Search |
| Postcentral Gyrus | R | 4 | 12 | −36 | 68 | 0.001 | 23T | Elaboration > Search |
| Caudate | R | na | 22 | 4 | 26 | 0.001 | 29 | Elaboration > Search |
| Postcentral Gyrus | L | 5 | −14 | −48 | 64 | 0.002 | 10T | Elaboration > Search |
| Caudate | L | na | −22 | −6 | 30 | 0.002 | Elaboration > Search | |
| Main Effect of Emotion | ||||||||
| Caudate | L | na | −32 | −36 | 10 | 0.000 | 48 | Neutral > Emotional |
| Cerebellum | R | na | 26 | −48 | −46 | 0.000 | 17T | Neutral > Emotional |
| Culmen | L | na | −12 | −32 | −26 | 0.001 | 27T | Neutral > Emotional |
| Superior Temporal Gyrus | L | 38 | −44 | 10 | −20 | 0.000 | 136 | Emotional > Neutral |
| Hypothalamus | L | na | −6 | −2 | −18 | 0.002 | 11T | Emotional > Neutral |
| Phase-by-Emotion Interaction | ||||||||
| Caudate | L | na | −32 | −36 | 10 | 0.000 | 42 | Neutral>Emotional Search; Neutral≈Emotional Elaboration |
| Cerebellum | R | na | 28 | −48 | −44 | 0.000 | 10T | Neutral>Emotional Search; Neutral≈Emotional Elaboration |
| Fusiform Gyrus | L | 37 | −48 | −42 | −18 | 0.001 | 18T | Neutral>Emotional Search; Neutral≈Emotional Elaboration |
| Temporoparietal Junction | R | 40 | 58 | −24 | 20 | 0.003 | 12T | Neutral>Emotional Search; Neutral≈Emotional Elaboration |
| Superior Temporal Gyrus | L | 38 | −44 | 10 | −16 | 0.000 | 111 | Emotional>Neutral Search; Emotional≈Neutral Elaboration |
| Hypothalamus | L | na | −6 | −2 | −18 | 0.000 | 41 | Emotional>Neutral Search; Emotional≈Neutral Elaboration |
| Hippocampus | L | na | −26 | −18 | −18 | 0.001 | 32 | Emotional>Neutral Search; Emotional≈Neutral Elaboration |
| Anterior Cingulate | L | 24 | −4 | 16 | 22 | 0.002 | 17T | Emotional>Neutral Search; Emotional≈Neutral Elaboration |
| Caudate | R | na | 4 | 14 | 20 | 0.004 | Emotional>Neutral Search; Emotional≈Neutral Elaboration | |
| Caudate | R | na | 14 | −34 | 26 | 0.002 | 16T | Emotional>Neutral Search; Emotional≈Neutral Elaboration |
| Insula | R | 13 | 32 | 2 | 24 | 0.004 | 11T | Emotional>Neutral Search; Emotional≈Neutral Elaboration |
Clusters significant at an uncorrected threshold of p<.005; k ≥ 10 voxels. Clusters presented in order of significance.
Up to 8 local maxima that are at least 8mm apart reported for each cluster.
“≈”Indicates that activity was not significantly different across conditions.
Indicates a cluster size smaller than the designated cluster size of 29
BA= approximate Brodmann Area; L=left, R=right
Figure 1.

Regions showing a main effect of memory phase. Follow-up contrasts identified directionality of the effect in each region. Regions showing increased activity for search relative to elaboration are depicted in green whereas regions showing increased activity for elaboration are depicted in orange.
A small network of regions showed a main effect of emotion (see Table 2). The left superior temporal gyrus (BA 38) was engaged more during retrieval of emotional relative to neutral events. However the left caudate showed the opposite pattern, preferentially activated during retrieval of neutral events.
3.2.2 Phase-by-Emotion interactions
A number of regions, including lateral and medial temporal lobe, showed an emotion-by-phase interaction (See Table 2). Follow-up contrasts were conducted to determine the directionality of these interactions. These contrasts determined that the effect of emotion on activity was greater in the search phase relative to the elaboration phase. For the left caudate, this interaction was driven by decreased deactivation during search for neutral stimuli. However, the interaction in the left lateral temporal lobe (Figure 2a) and MTL (Figure 2b) was driven by increased activation during search for emotional stimuli than neutral stimuli.2
Figure 2.
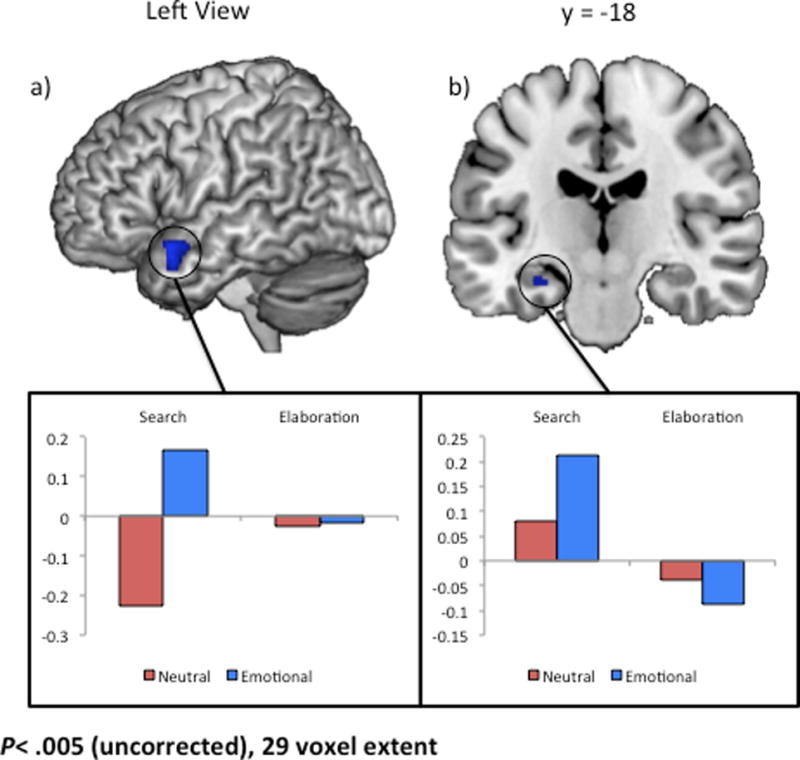
Regions showing an emotion-by-phase interaction. Follow-up contrasts identified directionality of the effect in each region. Regions showing a greater emotional > neutral effect in search relative to elaboration are depicted in blue. Signal was extracted from a 5 mm sphere surrounding the peak voxel in clusters in (a) left lateral temporal lobe, and (b) left hippocampus.
3.2.3 Main effects of phase and valence
The second model replicated the main effect of phase using only positive and negative images. As in the prior model, search engaged posterior regions to a greater extent and elaboration primarily engaged a network of frontal regions extending from ventromedial to dorsolateral regions (See Table 3 and Figure 3). The main effect of valence was significant in a number of medial regions, such as the left hippocampus, right uncus, and bilateral putamen (See Table 3). As revealed in Table 3, the effect was mainly driven by increased activity during retrieval of positive relative to negative events.
Table 3.
Results of a 2×2 Full-Factorial ANOVA with Valence (Positive v. Negative) and Phase (Search v. Elaboration) as within-subject factors.
| MNI Coordinates
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Region of Interest | Hemisphere | BA | x | y | z | p-value | k | Directionality of Effect |
| Main Effect of Phase | ||||||||
| Claustrum | L | na | −30 | 22 | −2 | 0.000 | 56979 | Search > Elaboration |
| Insula | R | 13 | 30 | 24 | −2 | 0.000 | Search > Elaboration | |
| Superior Parietal Lobe | L | 7 | −30 | −58 | 46 | 0.000 | Search > Elaboration | |
| Fusiform Gyrus | L | 20 | −34 | −42 | −22 | 0.000 | Search > Elaboration | |
| R | 37 | 36 | −46 | −20 | 0.000 | Search > Elaboration | ||
| Dorsolateral Prefrontal Cortex | L | 9 | −40 | 10 | 26 | 0.000 | Search > Elaboration | |
| Cerebellum | L | na | −20 | −38 | −44 | 0.000 | 27T | Search > Elaboration |
| Cerebellum | R | na | 22 | −38 | −42 | 0.000 | 20T | Search > Elaboration |
| Superior Temporal Gyrus | L | 22 | −52 | −8 | −12 | 0.001 | 19T | Search > Elaboration |
| Superior Temporal Gyrus | L | 38 | −44 | 8 | −22 | 0.001 | 15T | Search > Elaboration |
| Ventrolateral Prefrontal Cortex | R | 10 | 18 | 54 | −6 | 0.002 | 11T | Search > Elaboration |
| Somatosensory Cortex | L | 10 | −4 | 58 | 6 | 0.000 | 2279 | Elaboration > Search |
| Anterior Cingulate | R | 32 | 4 | 30 | −8 | 0.000 | Elaboration > Search | |
| Anterior Cingulate | L | 32 | −6 | 40 | −6 | 0.000 | Elaboration > Search | |
| Medial Prefrontal Cortex | R | 11 | 2 | 50 | −16 | 0.000 | Elaboration > Search | |
| L | 11 | .4 | 42 | .20 | 0.000 | Elaboration > Search | ||
| Dorsolateral Prefrontal Cortex | R | 8 | 20 | 30 | 50 | 0.000 | 361 | Elaboration > Search |
| Inferior Temporal Gyrus | L | 20 | −56 | −24 | −22 | 0.000 | 79 | Elaboration > Search |
| Caudate | L | na | −20 | −10 | 32 | 0.000 | 281 | Elaboration > Search |
| Fusiform Gyrus | R | 20 | 52 | −6 | −32 | 0.000 | 27T | Elaboration > Search |
| Paracentral Lobe | R | 31 | 2 | −20 | 46 | 0.000 | 208 | Elaboration > Search |
| Parahippocampal Gyrus | L | 19 | −34 | −48 | −2 | 0.000 | 61 | Elaboration > Search |
| Inferior Parietal Lobe | R | 40 | 58 | −30 | 34 | 0.000 | 113 | Elaboration > Search |
| Superior Temporal Gyrus | R | 42 | 60 | −24 | 10 | 0.001 | Elaboration > Search | |
| Dorsolateral Prefrontal Cortex | L | 9 | −20 | 42 | 38 | 0.000 | 92 | Elaboration > Search |
| Superior Temporal Gyrus | R | 22 | 58 | −8 | 0 | 0.000 | 152 | Elaboration > Search |
| Precentral Gyrus | R | 4 | 32 | −22 | 52 | 0.000 | 134 | Elaboration > Search |
| Insula | R | 13 | 40 | −12 | 18 | 0.001 | 40 | Elaboration > Search |
| Claustrum | R | na | 32 | 6 | 12 | 0.001 | 20T | Elaboration > Search |
| Insula | R | 13 | 42 | 4 | −6 | 0.001 | 22T | Elaboration > Search |
| Claustrum | R | na | 36 | −12 | 6 | 0.002 | 11T | Elaboration > Search |
| Claustrum | L | na | −38 | −20 | −2 | 0.003 | 13T | Elaboration > Search |
| Main Effect of Valence | ||||||||
| Hippocampus | L | na | −26 | −14 | −14 | 0.000 | 89 | Positive > Negative |
| Insula | L | 13 | −38 | −10 | 22 | 0.000 | 62 | Positive > Negative |
| Putamen | R | na | 24 | 2 | 14 | 0.000 | 159 | Positive > Negative |
| Putamen | L | na | −24 | 2 | 10 | 0.000 | 251 | Positive > Negative |
| Culmen | R | na | 10 | −44 | −24 | 0.000 | 38 | Positive > Negative |
| Ventrolateral Prefrontal Cortex | R | 11 | 28 | 44 | −2 | 0.001 | 20T | Positive > Negative |
| Premotor Cortex | L | 6 | −6 | −26 | 70 | 0.001 | 37 | Positive > Negative |
| Uncus | R | 34 | 16 | −2 | −24 | 0.002 | 41 | Negative > Positive |
| Phase-by-Valence Interaction | ||||||||
| Hippocampus/BA27/BA30 | L | 27/30 | −20 | −40 | 4 | 0.001 | 15T | Negative>Positive Search; Negative≈Positive Elaboration |
| Culmen | R | na | 10 | −44 | −24 | 0.000 | 216 | Positive>Negative Search; Positive≈Negative Elaboration |
| L | na | −6 | −44 | −24 | 0.001 | Positive>Negative Search; Positive≈Negative Elaboration | ||
| Putamen | L | na | −24 | 2 | 12 | 0.000 | 286 | Positive>Negative Search; Positive≈Negative Elaboration |
| Hippocampus | L | na | −26 | −12 | −16 | 0.000 | 98 | Positive>Negative Search; Positive≈Negative Elaboration |
| Putamen | R | na | 24 | 2 | 14 | 0.000 | 101 | Positive>Negative Search; Positive≈Negative Elaboration |
| Insula | L | 13 | −40 | −8 | 24 | 0.000 | 33 | Positive>Negative Search; Positive≈Negative Elaboration |
| Cerebellum | R | na | 22 | −76 | −32 | 0.000 | 67 | Positive>Negative Search; Positive≈Negative Elaboration |
| Subcallosal Gyrus | L | 34 | −10 | 0 | −14 | 0.000 | 21T | Positive>Negative Search; Positive≈Negative Elaboration |
| Superior Temporal Gyrus | R | 38 | 38 | 0 | −24 | 0.000 | 26T | Positive>Negative Search; Positive≈Negative Elaboration |
| Premotor Cortex | L | 6 | −12 | −18 | 58 | 0.001 | 65 | Positive>Negative Search; Positive≈Negative Elaboration |
| Anterior Prefrontal Cortex | R | 11 | 28 | 46 | −4 | 0.001 | 14T | Positive>Negative Search; Positive≈Negative Elaboration |
| Putamen | R | na | 24 | 14 | 6 | 0.002 | 10T | Positive>Negative Search; Positive≈Negative Elaboration |
| Culmen | R | na | 10 | −42 | −22 | 0.000 | 18T | Positive>Negative Search; Negative>Positive Elaboration |
| Inferior Temporal Gyrus | L | 20 | −46 | −16 | −26 | 0.001 | 21T | Positive>Negative Search; Negative>Positive Elaboration |
| Dorsomedial Prefrontal Cortex | L | 9 | −18 | 30 | 32 | 0.001 | 32 | Positive>Negative Search; Negative>Positive Elaboration |
Clusters significant at an uncorrected threshold of p<.005; k ≥ 10 voxels. Clusters presented in order of significance.
Up to 8 local maxima that are at least 8mm apart reported for each cluster.
“≈”Indicates that activity was not significantly different across conditions.
Indicates a cluster size smaller than the designated cluster size of 29
BA= approximate Brodmann Area; L=left, R=right
Figure 3.

Regions showing a main effect of memory phase in an analysis including only the emotional items. Follow-up contrasts identified directionality of the effect in each region. Regions showing increased activity for search relative to elaboration are depicted in green whereas regions showing increased activity for elaboration are depicted in orange.
3.2.4 Phase-by-Valence interactions
The valence-by-phase interaction was significant in a network of regions that included PFC and MTL regions, specifically the hippocampus, and putamen (See Table 3 and Figure 4). This interaction was primarily driven by regions showing a greater positive > negative effect in search relative to elaboration (e.g., left hippocampus; see Figure 4a), although the dorsomedial prefrontal cortex showed both a greater positive > negative effect in search relative to elaboration as well as a greater negative > positive effect in elaboration relative to search (Figure 4b).3
Figure 4.

Regions showing a valence-by-phase interaction. Follow-up contrasts identified directionality of the effect. Both the left hippocampus (a)and dorsomedial prefrontal cortex (b) were significantly more activity during positive relative to negative search. However in the dorsomedial prefrontal cortex, activity was also greater during negative relative to positive elaboration. Signal was extracted from a 5 mm sphere surrounding the peak voxel in each region.
4. Discussion
The current study identified the neural correlates of two phases of episodic memory retrieval: the initial search phase in which participants access the mnemonic information, and the following elaboration phase in which participants expand on event details. Relative to elaboration, memory search relied more heavily on a widespread, bilateral network including lateral and dorsomedial PFC, medial and lateral temporal, parietal, and occipital regions. Elaboration, on the other hand, was more strongly associated with activity in the medial PFC. Importantly, although these networks are quite similar to those identified in autobiographical memory studies, search of episodic events in the current study was not associated with ventromedial PFC activity and elaboration was not associated with increased visual activity. These differences support prior research suggesting that the cognitive processes and neural substrates that contribute to retrieval of episodic and autobiographical memories are not identical (Cabeza et al., 2004; Gilboa, 2004; See McDermott, Szpunar, & Christ, 2009 for a meta-analysis).
The second goal of the present study was to identify the effects of emotion and emotional valence on episodic memory search and elaboration. Even when the retrieval cue itself lacks emotional content, the emotionality of the retrieval target was still influential during the initial search processes of retrieval, and in fact had more effect on neural recruitment during search than elaboration. These results suggest that the emotion associated with an episodic memory plays a primary role in early retrieval processes. However, valence can further affect when the effect of emotion is greatest, with positive valence playing a larger role during search than elaboration.
4.1 Neural correlates of episodic memory search and elaboration
During episodic memory search, participants recruited a widespread, bilateral network including lateral and dorsomedial PFC, medial and lateral temporal, parietal, and occipital regions. This network is consistent with previous functional neuroimaging studies examining episodic memory retrieval (see Cabeza and Nyberg, 2000 for review) and studies examining the search phase of autobiographical memory (e.g., Addis et al., 2007; Daselaar et al., 2008; Holland et al., 2011). Importantly, search was associated primarily with bilateral lateral PFC activity, distinguishing it from autobiographical memory search, which is associated with left lateral and ventromedial PFC activity. Previous studies have argued that these differences in PFC activation reflect distinctions in monitoring processes, where episodic memory retrieval relies on deliberate monitoring to judge memory accuracy, a process that is unnecessary for most autobiographical memory tasks (Gilboa, 2004; See McDermott, Szpunar, & Christ, 2009 for a meta-analysis). Because episodic memory tasks require participants to monitor for accuracy prior to making their response, it is possible that participants fully access the mnemonic content during search, even if they are not explicitly instructed to do so. Increased activity in visual regions during search relative to elaboration supports this prediction.
Although we hypothesized that elaboration would be associated with increased sensory and medial PFC activity relative to search, elaboration was actually associated with reduced visual activity relative to search. Retrieval of laboratory-learned stimuli, such as a single image or word, often encountered relatively recently, is usually much quicker than retrieval of an autobiographical event and does not require extensive elaboration to recall all sensory information. In addition, it is possible that the memory decision in the present task – absent in most studies of autobiographical memory – encouraged participants to recall the image prior to deciding whether the title had been studied. As such, it may not have been necessary for participants to continue elaborating on these details once the image had been accessed, leading to decreased visual activity during the elaboration phase.
Relative to search, the elaboration phase was associated with increased activity in the mPFC, a region of the brain that has been linked to self-referential processing (Gusnard et al., 2001; Kelley et al., 2002). During elaboration, participants were encouraged to think about the image in as much detail as possible, including their memory of the encoding event and their personal reaction to it. The increased mPFC activity during elaboration may relate to participants’ reflection on their reaction to the stimulus at encoding.
4.2 Effect of emotion on episodic memory search and elaboration
The current study was also designed to examine the effects of emotion and emotional valence on episodic memory search and elaboration. By dividing memory retrieval into two distinct memory phases, researchers are better able isolate the specific mechanisms that allow stimulus characteristics, or other external factors, to improve or impair memory performance. For instance, one can determine whether a characteristic, such as emotion, facilitates the search of an emotional item (as we see here) or the elaboration of specific details. Identifying these specific cognitive mechanisms is important when attempting to identify particular memory deficits in populations with decreased memory ability, and can highlight distinct approaches for improving these impairments, depending on the population’s specific deficit. In the first analysis, we examined the effect of emotion on neural recruitment, irrespective of valence. Although the neural networks associated with search for emotional and neutral events overlapped substantially, there were a number of regions in which emotion led to increased (e.g., left lateral temporal lobe and left hippocampus) or decreased (e.g., right temporoparietal junction) activity during search. Increased activity in the left medial and lateral temporal lobes suggests that successful retrieval of an emotional image might be richer than retrieval of a neutral image, as these regions have been implicated in memory recollection (e.g., Giovanello, Schnyer, & Verfaellie, 2004; Henke, Buck, Weber, & Wieser, 1997; Yonelinas, Hopfinger, Buonocore, Kroll, & Baynes, 2001) and semantic knowledge (e.g., Dennis et al., 2008), respectively. This explanation is supported by slower reaction times for negative and positive events, relative to neutral events, suggesting that participants spend more time generating these emotional images. It is also consistent with participants’ self-reported memory vividness ratings, which were higher for the emotional memories than the neural ones. Importantly, due to our use of a neutral retrieval cue, any neural differences associated with neutral and emotional events is driven by differences in the mnemonic content, not in the cue, itself.
All regions identified in the phase by emotion interaction were driven by differences during the search phase, with greater overlap in the networks associated with elaboration of emotional and neutral events. Therefore, as in autobiographical memory retrieval (Daselaar et al., 2008), emotion may play a primary role in directing retrieval search and in initially accessing the relevant event. As nearly complete memory representations are required prior to the memory decision, it is possible that emotion does not play any additional role on neural activity after this point. However, it is possible that there are sustained effects of emotion and emotional valence that are not identified in the current analysis; for instance, for false memories, emotion may continue to effect the way the memory for the purported event is elaborated, leading to a greater sense of vividness for those events than for purported neutral events (e.g., Phelps & Sharot, 2008).
4.3 Effect of emotional valence on episodic memory search and elaboration
To better understand the effects of emotion on memory search and elaboration, the next analysis directly compared memory for positive and negative events. Once again, many regions identified in the phase by valence interaction were driven by differences during memory search rather than elaboration. Specifically, a majority of the identified regions were recruited to a greater extent during search for positive images relative to negative, including PFC and MTL regions. Although a majority of regions showed greater differences during the initial search phase, emotional valence did have an effect on neural recruitment during elaboration. Specifically, elaboration of negative events was associated with increased activity in the dorsomedial PFC relative to positive events. These results suggest that positive and negative emotion influence memory retrieval in different ways, with positive emotion primarily affecting processes involved in memory search and negative emotion associated with processes during elaboration. The paragraphs below describe potential mechanisms by which positive and negative emotion might influence memory retrieval.
Positive memory search was associated with increased activity in PFC, lateral temporal, and MTL activity relative to negative search. Previous studies of autobiographical memory retrieval have reported similar PFC and MTL increases during retrieval of positive autobiographical events (Markowitsch et al., 2003; Piefke et al., 2003). Such neural differences may emerge due to underlying differences in positive and negative retrieval. For instance, the increased frontoparietal activity associated with positive memory retrieval may reflect the tendency to broaden attention during cognitive tasks involving positive information, as proposed in the broaden-and-build theory of positive emotions (Fredrickson, 2001). The results of the present study support this broadening of attention during the retrieval of positive information in an episodic memory task.
Although positive event search was associated with increased activity in the anterior hippocampus, it will be important for future research to examine whether this pattern extends to all regions of the hippocampus or whether there may be differences along the posterior-anterior access. Previous work with both animals and humans has demonstrated that the anterior and posterior hippocampus differ in their connections to other brain regions, leading to more gist-based, global representations supported by the anterior hippocampus and more local, detail-focused representations supported by the posterior hippocampus (see Poppenk et al., 2013 for review). This distinction parallels literature showing more gist-based processing for positive event retrieval and detail-focused processing for negative event retrieval (reviewed by Kensinger, 2009). We identified a small cluster of posterior hippocampus, extending into BA27 and BA30, in which negative event search was associated with increased activity. This region did not reach our cluster extent for significance (p< .005, k=15), but it suggests an intriguing finding that may benefit from further investigation, especially via high-resolution imaging that could clearly separate anterior from posterior hippocampus and posterior hippocampus from surrounding temporal cortices.
Although many regions were more active during the search for positive memories compared to negative ones, this valence difference was no longer present during elaboration. In fact, there was some evidence for a valence reversal, with increased activity during the elaboration of negative compared to positive events within the dorsomedial PFC. These results suggest an interesting time-course difference for the retrieval of positive and negative memories. Future research is required to investigate whether this increased engagement for negative memories may be associated with particular qualities that are being elaborated. Although we did not find differences in subjective vividness ratings between positive and negative memories in this study, valence may still affect the time spent elaborating on the image as a whole, the emotional features within the image, or the participant’s personal reaction to the image.
4.4 Limitations
When examining the behavioral and neural effects associated with emotion and emotional valence, it is important to consider other potential differences unrelated to the factors of interest. The retrieval cues in the current study were neutral titles that had been paired with positive, negative, and neutral images during encoding. The encoding task required participants to evaluate the appropriateness of each title for the associated image. Although positive and neutral titles were rated as equally appropriate, negative titles were rated as less appropriate, overall, than the other two conditions. It is possible that these differences across retrieval cues could lead to differences in memory performance. However, ratings of title appropriateness were not correlated with memory accuracy in any emotion condition (p > .1 for all relationships). In addition, inclusion of individual subjects’ appropriateness ratings for positive and negative events did not significantly alter the results of the current analysis, with all reported peaks retaining significance with the added covariate.
Although the positive and negative images selected for the present study were matched in terms of pre-study normed arousal ratings, the participants in this study rated the negative images as more arousing than the positive. When arousal is confounded with valence, as it is in this study, it is possible that valence-related neural differences are actually driven by arousal. Prior studies comparing retrieval of emotional and neutral events suggest that emotional arousal is associated with increased activity in the amygdala (e.g., Dolan et al., 2000; Dolcos et al., 2005; Murty et al., 2009; but see, Taylor et al., 1998), lateral temporal lobes (Murty et al., 2009), and lateral frontal lobes (Murty et al., 2009). These studies suggest that the more arousing events (i.e., negative events in this study) would be associated with increased activity in the search phase. This pattern is the opposite of our present results, which may suggest that the effects seen here are driven more by valence differences than arousal differences. It is also possible, however, that the higher arousal associated with negative events could have caused sufficient narrowing of attention to reduce the scope of activity relative to the less arousing positive events. To examine whether arousal directly influenced our results, we re-ran the valence model with individual subjects’ arousal ratings for positive and negative events included as covariates. Doing so did not significantly alter the results of the current analysis, suggesting that arousal differences did not drive the reported valence differences.
The current study examined the influence of emotion and emotional valence on the neural correlates of memory retrieval in the context of a task that showed no behavioral effect of emotion on memory performance. A benefit of this matched performance is that differences in memory strength across the emotion categories are unlikely to have confounded the results. A limitation is that it makes it impossible to link the neural differences to behavioral outcomes. Null effects of emotion on behavioral measures of memory often occur; some of these null findings may reflect a lack of sensitivity in the behavioral measures while others may reflect instances when emotion facilitates one stage of processing but impedes another, leaving no noticeable effect on the final outcome measures (see Bennion et al., in press for further discussion). The neural differences identified in the current study suggest that fMRI can capture effects of emotion on memory retrieval, even when behavioral measures cannot.
It is worth noting that at a less conservative threshold (p < .05, k ≥ 10) emotional retrieval was associated with increased right amygdala activity (20, −2, −12) relative to neutral retrieval, but there were no amygdala regions preferentially recruited during neutral retrieval. A phase by emotion interaction was also identified at this reduced threshold in bilateral amygdala (20, −4, −16 and −20, −6, −14). Further analysis determined that activity in the regions identified by the interaction was greater for emotional relative to neutral search, but not elaboration, providing additional evidence that the emotional content of a studied item plays an important role during the initial search for that item, but may not influence neural activity after the information has been acquired.
4.5 Conclusions
The current study used an extended retrieval trial to identify neural differences between the search and elaboration phases of episodic memory retrieval. The distinct patterns of activity in the two phases suggest that episodic memory search is associated with accessing the full memory representation, whereas elaboration is primarily associated with recalling additional information about the participant’s personal experience studying the image. The second goal of the present study was to identify the effects of emotion and emotional valence on episodic memory search and elaboration. The results suggest that the emotionality of the retrieval target can still be influential during the initial search processes of retrieval even when the retrieval cue itself lacks emotional content. However, valence can influence when the effect of emotion is greatest, with positive emotion playing a larger role during search and negative emotion playing a larger role during elaboration. Future research should examine the precise processes positive and negative emotion influence during search and elaboration, and how such influences might lead to differences in the memory representation.
Acknowledgments
The authors would like to thank Katherine Mickley Steinmetz for her assistance designing the current study and Halle Zucker for her assistance creating presentation scripts and running participants. Magnetic resonance data were collected at the Harvard Center for Brain Science. We thank the staff there, particularly Tammy Moran and Ross Mair, for their assistance with data collection and quality assurance. This work was supported by a Memory and Cognitive Disorders grant from the McKnight Endowment Fund for Neuroscience (EAK), and NIH grant MH080833 (EAK).
Footnotes
This sample is slightly older than the young adult sample for a typical fMRI study. As such, all analyses were also run with age as a covariate. All reported clusters remained significant with this addition.
Due to differences in response times across conditions, we also ran this model with average response time as a covariate. Inclusion of response time did not significantly alter the results of this analysis.
Due to differences in ratings of arousal and title appropriateness between negative and positive valence conditions, we also ran this model with these ratings as covariates. All reported peaks were significant at p < .005 with the added covariates. However, two reported clusters in the main effect of valence (the ventrolateral PFC and the premotor cortex) as well as one cluster in the interaction (the putamen) do not reach the established criteria for cluster size (10 voxels).
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennion KA, Ford JH, Murray BD, Kensinger EA. Misconceptions in the study of emotional memory. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617713000945. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW. Retrieval of Emotional Memories. Psychological Bulletin. 2007;133:761–779. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition: II. An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. TRENDS in Cognitive Sciences. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Hayers SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher PC. Dissociable temporal lobe activations during emotional episodic memory retrieval. NeuroImage. 2000;11:203–209. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences, USA. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology: The broaden-and build theory of positive emotions. American Psychologist. 2001;56:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory – one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Giovanello K, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Holland AC, Addis DR, Kensinger EA. The neural correlates of specific versus general autobiographical memory construction and elaboration. Neuropsychologia. 2011;49:3164–3177. doi: 10.1016/j.neuropsychologia.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. Emotion Review. 2009;1:99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhove MM, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39:643–665. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Szupnar KK, Christ SE. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47:2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, et al. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J Cogn Neurosci. 2009;21(10):1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Sharot How (and Why) Emotion Enhances the Subjective Sense of Recollection. Current Directions in Psychological Science. 2008;17:147–152. doi: 10.1111/j.1467-8721.2008.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends in Cognitive Science. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Smith AP, Henson RN, Dolan RJ, Rugg MD. fMRI correlates of the episodic retrieval of emotional contexts. NeuroImage. 2004;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Smith AP, Henson RN, Rugg MD, Dolan RJ. Modulation of retrieval processing reflects accuracy of emotional source memory. Learning & Memory. 2005;12:472–479. doi: 10.1101/lm.84305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analysis using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, D’Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewall G, Degueldre C, Luxen A, Collete F, Maquet P. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. Journal of Neuroscience. 2006;26:7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Taylor SF, Liberzon I, Fig LM, Decker LR, Minoshima S, Koeppe RA. The effect of emotional content on visual recognition memory: A PET activation study. NeuroImage. 1998;8:188–197. doi: 10.1006/nimg.1998.0356. [DOI] [PubMed] [Google Scholar]
- Waring JD, Kensinger EA. How emotion leads to selective memory: Neuroimaging evidence. Neuropsychologia. 2011;49:1831–1842. doi: 10.1016/j.neuropsychologia.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NEA, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: An fMRI study. NeuroReport. 2001;12:359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]


