Abstract
Thyroid carcinoma is the most common endocrine malignancy, and the incidence of thyroid carcinoma has been progressively increasing. Most thyroid carcinomas contain one of a small number of mutually exclusive driver mutations, such as BRAFV600E, RAS mutations, RET gene fusions, or PAX8/PPARG gene fusions. The PAX8/PPARG gene fusion results in production of a PAX8-PPARγ fusion protein, denoted PPFP, and is found in ~30 – 35% of follicular thyroid carcinomas as well as a subset of follicular variant of papillary thyroid carcinomas. In vitro and in vivo evidence indicate that PPFP can act as an oncoprotein. Although the specific mechanism of PPFP action is yet to be defined, PPFP is considered to act as a dominant negative inhibitor of wild type PPARγ and/or as a unique transcriptional activator of subsets of PPARγ and PAX8 responsive genes. Detection of the fusion transcript in thyroid nodule biopsy specimens can aid clinical decision-making when cytological analyses are indeterminate. The PPARγ agonist pioglitazone is highly therapeutic in a transgenic mouse model of PPFP thyroid carcinoma, suggesting that PPARγ agonists may be therapeutic in patients with PPFP thyroid carcinomas.
Introduction
Thyroid carcinoma is the most common endocrine malignancy, and the incidence of thyroid carcinoma has more than doubled since 1990. This increased diagnosis may be a result of the increased use of imaging techniques (computed tomography, ultrasound, etc.) that enable the incidental detection of small non-palpable thyroid nodules, as well as the increased use of ultrasound-guided fine needle aspiration (FNA) biopsy of these nodules. Still, at least part of the increase is from finding more large tumors.1 The American Cancer Society estimates that in the United States in 2014 there will be about 62,980 new cases of thyroid carcinoma (47,790 in women and 15,190 in men) and about 1,890 deaths from thyroid carcinoma (1,060 women and 830 men).2
Although thyroid carcinomas rarely produce thyroid hormone, at least 95% of these tumors arise from thyroid hormone-producing follicular epithelial cells. These carcinomas are broadly categorized as papillary thyroid carcinomas (PTC, ~85% prevalence), follicular thyroid carcinomas (FTC, ~10% prevalence) and anaplastic (undifferentiated) thyroid carcinomas (ATC, 1 – 2% prevalence) based upon histological characteristics. PTCs and FTCs usually are well differentiated, although some are more poorly differentiated. Histological subtypes of PTC include classical, follicular variant, tall cell and others. ATC is the rarest of all the thyroid carcinomas but the most aggressive type, with a median survival of only ~6 months. In the future, the classification of follicular cell-derived thyroid carcinomas may be based upon underlying genetic changes as well as histological features. In addition to carcinomas, thyroid nodules may be due to benign hyperplasia or benign follicular adenomas. It is unclear whether adenomas have malignant potential. In addition to the above tumors, ~ 3–5% of thyroid carcinomas originate from the calcitonin-producing parafollicular C cells (medullary thyroid carcinomas).
Population studies suggest that 3 – 8% of asymptomatic adults have thyroid nodules.3-10 These thyroid nodules can be detected by palpation or more commonly imaging, especially in adults of increased age.3, 4, 7-9, 11, 12 Approximately 95% of thyroid nodules are benign, and an important clinical challenge is to accurately identify the ~5% that are malignant. Presently, FNA biopsy of thyroid nodules followed by cytological examination provides an accurate diagnosis of malignant or benign in most cases, but about 25% of all nodules cannot be accurately diagnosed by FNA cytology.3-10 As our understanding of the molecular pathology of thyroid cancer improves, this is translating into new molecular diagnostic tests that have the potential to improve the cytological interpretation of FNA biopsies. In addition, advances in our understanding of the molecular pathology of thyroid cancer are leading to the development of better prognostic markers and novel targeted therapies.
Gene mutations in thyroid carcinoma
The vast majority of thyroid cancers contain one of a small number of driver mutations, such as BRAFV600E, RAS mutations, RET gene fusions, or PAX8/PPARG gene fusions. BRAFV600E is the most common driver mutation in thyroid cancer,13-15 and approximately 40 – 45% of PTCs contain this mutation. It is especially common in tall cell PTC and also is found in classic PTC, but it is uncommon in follicular variant PTC.16,17 RET gene fusions also are found in PTC and are particularly common in radiation-induced cancers.18-21 RAS mutations are found primarily in follicular variant PTCs, FTCs and benign follicular adenomas.22-24
The PAX8/PPARG gene fusion is found in 30 – 35% of FTCs25-27 as well as a substantially smaller fraction of follicular variant PTCs.25, 28, 29 This rearrangement also is occasionally found in follicular adenomas.26, 27, 30 Thus, RAS mutations and PAX8/PPARG gene fusions are found in the same histological types of thyroid tumors, although RAS mutations are substantially more common in follicular adenomas.
Another chromosomal rearrangement involving PPARG, in this case resulting in a gene fusion with CREB3L2, has been found in a very small number of FTCs.31 The existence of two different fusion partners with PPARG in FTCs suggests that the PPARγ portion of the resulting fusion proteins is important to the mechanism of carcinogenesis.
Additional mutations accumulate as thyroid carcinomas become less differentiated. Anaplastic carcinomas probably arise from preexisting well differentiated carcinomas through the acquisition of abnormalities such as p53 mutations32-35 or abnormalities in β-catenin signaling.36-38 Activation of the phosphatidylinositide 3-kinase (PI3K)/AKT pathway also is common, and can occur through a number of mechanisms such as activating mutations in PIK3CA or AKT1, or loss of PTEN expression through genetic or epigenetic mechanisms.39-43 PI3K/AKT pathway activation also is frequent in FTCs.42, 44, 45
This review will focus on the PAX8/PPARG rearrangement. The mechanism of oncogenesis, the role of fusion transcript detection in the diagnostic evaluation of thyroid nodules, and the potential of the resulting PAX8/PPARγ fusion protein (PPFP) as a therapeutic target will be discussed.
Function and structure of PAX8
PAX8 belongs to the paired box family of transcription factors. It is necessary for normal thyroid development, and in the mature thyrocyte PAX8 drives the expression of many thyroid-specific genes such as those encoding thyroglobulin, thyroid peroxidase and the sodium iodide symporter.46 Although PAX8 also is expressed in the developing brain and kidney, the only abnormality in Pax8−/− mice is the absence of a thyroid gland. PAX8 mutations also are a known cause of congenital hypothyroidism in humans.47
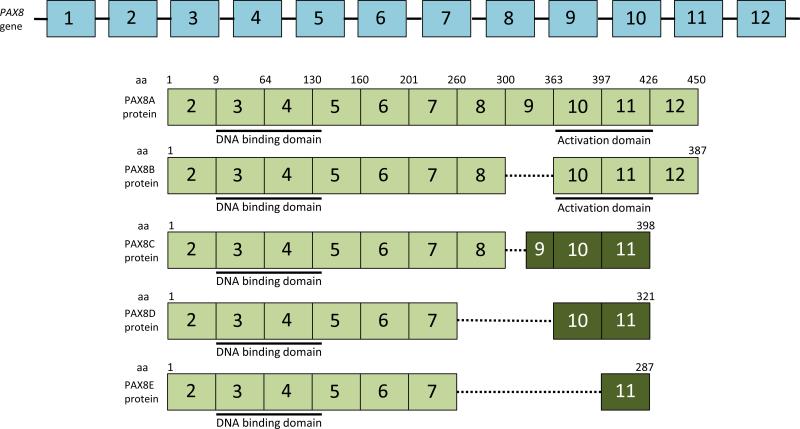
The structure of the PAX8 gene and the resulting mRNA and proteins are shown schematically in Figure 1. PAX8 has 12 exons, with the translational start codon being derived from exon 2. The DNA binding domain (paired domain) of PAX8 is found at the amino terminus of the protein, encoded by exons 3, 4 and the beginning of exon 5. Alternative splicing of exons 8-10 results in the production of 4 or 5 RNA transcript and protein isoforms, denoted PAX8A, B, C, D and E. PAX8A is the longest isoform and includes all codons from exons 2-12. PAX8B is deleted in exon 9, but its sequence (NM_013951) has been suppressed from GenBank due to insufficient evidence of its existence. However, as will be noted below, one of the known splice variants of PPFP is of the PAX8B type (i.e., deleted in exon 9). Deletion of exon 9 does not alter the reading frame of the downstream exons. PAX8C utilizes an internal exon 9 5’ splice site, resulting in a smaller exon 9 and a reading frame shift that alters and shortens the carboxyl terminal end of the protein, with the stop codon now being in exon 11. PAX8D is deleted in exons 8 and 9, and PAX8E is deleted in exons 8, 9 and 10. Both of these result in truncated proteins shorter than PAX8C but with a reading frame shift identical to that of PAX8C. PAX8A and PAX8B contain a serine, threonine and tyrosine-rich transcriptional activation domain encoded by exons 10-11 that is not present in the other isoforms.48 Accordingly, transfection data indicate that PAX8A and PAX8B have greater transcriptional activity than PAX8C.49
Figure 1.
Schematic representation of the PAX8 gene, mRNA and protein isoforms. The exons are numbered 1 -12 (not drawn to scale). PAX8A mRNA is depicted below the gene and contains all 12 exons. Coding exons in the mRNA are shown in green, noncoding in white. The PAX8A protein is depicted below its mRNA. The DNA binding domain (paired domain) is found at the amino terminus of the protein, encoded by exons 3, 4 and the 5’ end of exon 5. A carboxyl terminal transcriptional activation domain is encoded by exons 10 and 11 and is rich in serine, threonine and tyrosine. Alternative splicing results in the isoforms PAX 8B, 8C, 8D and 8E. In PAX8B, exon 9 is deleted but this deletion does not alter the reading frame downstream. PAX8C utilizes an internal exon 9 5’ splice site, resulting in a smaller exon 9 and a reading frame shift that alters and shortens the carboxyl terminal end of the protein, resulting in a stop codon in exon 11. PAX8D is deleted in exons 8 and 9, and PAX8E is deleted in exons 8, 9 and 10. Both of these result in truncated proteins shorter than PAX8C but with a reading frame shift identical to that of PAX8C. The reading frame shift is depicted by a darker shade of green within the exons. aa, amino acid.
Function and structure of PPARγ
PPARγ belongs to the nuclear receptor family of transcription factors. It is the master regulator of adipogenesis as well as a potent modulator of whole-body lipid metabolism and insulin sensitivity.50, 51 Fatty acids and eicosanoids such as 15 deoxy Δ12,14 prostaglandin J2 can act as endogenous ligands for PPARγ.52-56 Synthetic ligands such as thiazolidinediones are potent activators of PPARγ with robust insulin sensitizing activity and are used in the treatment of type 2 diabetes mellitus.57 PPARγ has additional functions, such as anti-inflammatory activity.58 There is evidence that PPARγ may be a tumor suppressor and this will be discussed subsequently.
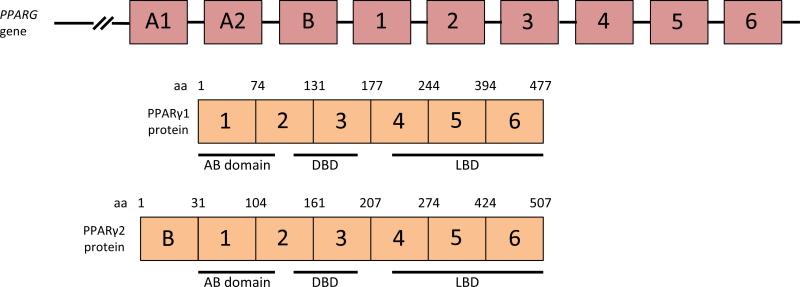
The structure of the relevant portion of the PPARG gene and the resulting protein are shown schematically in Figure 2. PPARγ has the classic structure of a nuclear hormone receptor with an amino terminal regulatory “AB” domain, a centrally located zinc finger DNA binding domain, and a ligand binding/transcriptional regulatory domain that occupies the carboxyl terminal half of the protein. The PPARG gene has two promoters that drive the transcription of two different first exons, resulting in the production of the protein isoforms PPARγ1 and PPARγ2. PPARγ2 is identical to PPARγ1 except that it has a unique 30 amino acid addition at its amino terminus. The proteins appear to be functionally similar in transfection experiments,59 but their expression patterns differ. PPARγ1 is broadly expressed, whereas PPARγ2 is expressed specifically in adipocytes.
Figure 2.
Schematic representation of the PPARG gene and PPARγ1 protein. Only the PPARG exons that contribute to the PPARγ1 protein are shown, and for convenience they are numbered starting at 1 (the gene has several additional upstream exons). The exons are not drawn to scale. The PPARγ1 protein has an N-terminal regulatory “AB” domain, a zinc finger DNA binding domain (DBD) and C-terminal ligand binding domain/transcriptional regulatory domain (LBD).
Structure of the PAX8/PPARG fusion gene, transcript and protein
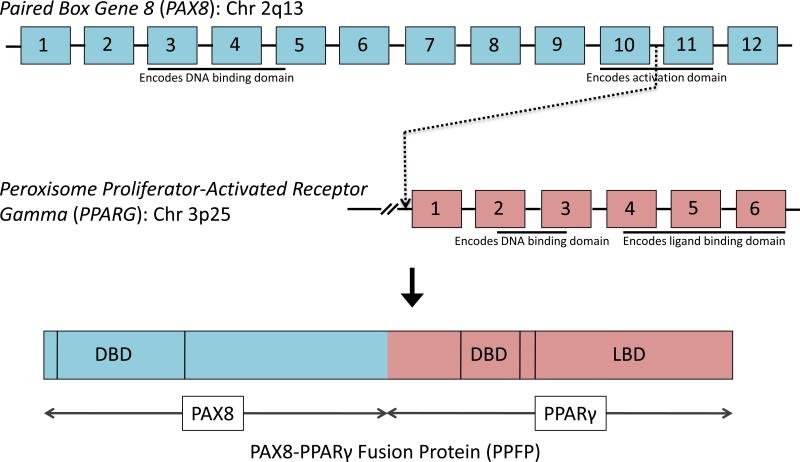
The PAX8/PPARG rearrangement is created by a translocation between chromosomal regions 2q13 and 3p25.60 This translocation results in a fusion transcript wherein most of the coding sequence of PAX8 (2q13) is fused in frame with the entire coding exons of PPARγ1 (3p25). The PAX8 promoter, which is highly active in thyroid follicular cells, drives the expression of the fusion transcript, resulting in high level expression of the fusion transcript and protein (PPFP).61
The fusion gene, transcript and protein are represented schematically in Figure 3. Typically, the translocation fuses PAX8 intron 10 with the intron immediately preceding the first coding exon of PPARγ1. The resulting PPFP consists of an N-terminal PAX8 fragment encoded by exons 2-10 fused in frame to full length PPARγ1. The PAX8 portion is essentially a truncated version of PAX8A, missing much of the C-terminal activation domain. However, alternative splicing has resulted in the detection of multiple RNA isoforms within the same tumor.60 Specifically, transcripts with PAX8 exons 1-8, 1-9, and 1-10 with exon 9 deleted have been detected fused to the first coding exon of PPARγ1, and all of these maintain the PPARγ1 reading frame. Some studies have reported expression of one of these shorter isoforms without expression of isoforms containing exon 10. In these cases it is not known if the actual chromosomal translocation is still in PAX8 intron 10, or in intron 8 or 9.
Figure 3.
The PAX8/PPARG rearrangement is created by a translocation between chromosomal regions 2q13 and 3p25. This translocation results in a fusion transcript wherein most of the coding sequence of PAX8 (2q13) is fused in frame with the entire coding exons of PPARγ1 (3p25). The resulting fusion protein, denoted as PPFP, contains the DNA binding domain and part of the C-terminal activation domain of PAX8, as well as the DNA and ligand binding domains (DBD, LBD) of PPARγ. Not shown, PAX8 variants result in alternative isoforms of PPFP that only include PAX8 exons 1-8, or 1-9, or 1-10 without exon 9.
Detection of PPFP in thyroid tumors
Since PPARγ is expressed at very low levels in the normal thyroid, high-level expression of PPARγ by immunohistochemistry provides evidence for the presence of PPFP. However, this is not entirely specific, and definitive testing for PPFP requires either fluorescent in situ hybridization (FISH) or RT-PCR. Based upon these techniques, PAX8/PPARγ rearrangements have been found in about 1/3 of FTCs and substantially less frequently in other thyroid tumors.26-30, 60, 62-75 Although the sample size is small, a comparison of FTCs with and without PPFP showed no significant differences in the extent of capsular or vascular invasion, disease-free survival or overall survival.75
PPFP as an oncoprotein
Several in vitro studies have provided evidence that PPFP can act as an oncoprotein. Both transient and stable transfection of PPFP in Nthy-ori 3-1 cells (human SV40 large T antigen-immortalized thyrocytes) have shown accelerated growth rates and lower numbers of cells in the G0/G1 resting state compared to empty vector-transfected cells.76 PPFP also decreased the rate of apoptosis, suggesting that the reduced apoptosis may be partially responsible for the accelerated growth.76 In addition, PPFP expression in FRTL-5 rat thyroid cells resulted in increased DNA synthesis as evidenced by 3H-thymidine incorporation.77 Nthy-ori 3-1 cells76 and rat thyroid PCCL3 cells78 that stably express PPFP exhibit increased anchorage-independent growth (colony formation in soft agar), a hallmark of cellular transformation. Expression of either PPFP or CREB3L2/PPARγ in primary human thyroid cells resulted in an increased number of cells and increased DNA synthesis,31 suggesting that the common PPARγ moiety is at least partially responsible for the oncogenic actions of these fusion proteins. Although little else is known regarding the function of CREB3L2/PPARγ, further comparison of this fusion protein with PPFP has the potential to reveal important insights into the mechanisms of oncogenesis. In the osteosarcoma U2OS cell line, transfected PPARγ transactivated PPARγ response elements whereas PPFP was ineffective.60 Furthermore, coexpression of PPFP with PPARγ abrogated PPARγ-mediated reporter gene expression in a dominant negative fashion. This is important because there is evidence that PPARγ may have anti-tumor or tumor suppressor properties, which would imply that PPFP may be oncogenic by inhibiting the activity of endogenous PPARγ. For example, addition of a PPARγ agonist to several PPARγ-positive ATC cell lines led to an increased portion of cells in G0/G1 with a reduced number of cells in G2/M and S phase, suggesting decreased cell proliferation.79 In addition, DNA synthesis was slowed down, evident from decreased 3H-thymidine incorporation in these cells while expression of cell-cycle progression inhibitors p21cip1 and p27kip1 was increased.79 Overexpression of PPARγ by transfection into PPARγ-positive or –negative cell lines similarly decreased colony formation in soft agar and triggered nuclear condensation, fragmentation of chromatin and apoptosis, with G0/G1 cell cycle arrest in several human thyroid cancer cell lines.62, 79-81 These data provide evidence that PPARγ has a tumor suppressive effect in some thyroid cell lines, and similar data have been obtained in several nonthyroidal cell lines.82-87
There also is in vivo evidence that PPARγ may have anti-tumor properties. For example, mice with a homozygous knock in mutation in thyroid hormone receptor beta were unexpectedly found to develop thyroid carcinoma, and the disease became more aggressive in the presence of a single allele deletion of Pparg.88
Although the above data suggest a simple story that endogenous PPARγ is a thyroid cancer tumor suppressor and that PPFP is oncogenic by virtue of dominant negative activity against PPARγ, the actual situation is likely more complex. Firstly, PPARγ is expressed at extremely low levels in the normal thyroid89 and it is not known if it has any function in that organ. Second, microarray data from human thyroid carcinomas strongly suggest that PPFP has PPARγ-like activity in these tumors.72, 90 For example, compared to non-PPFP thyroid carcinomas, two of the most highly induced genes in PPFP carcinomas are AQP7 and ANGPTL4, both of which are well known to be induced by PPARγ in adipocytes. Furthermore, PPFP activates the AQP7 promoter in a PPARγ-like manner in thyroid and non-thyroid cell types based on transfection data. In addition, high level expression of endogenous PPARγ appears to contribute to the aggressive behavior of human ATCs, at least when assessed in cell culture and orthotopic injections in mice.91 Finally, PPARγ antagonists have antiproliferative effects on a wide variety of cancer cell lines.92
The expression of PPFP in transgenic mice is apparently not sufficient to cause thyroid cancer, which is consistent with the observation that benign thyroid adenomas occasionally express PPFP. However, transgenic mice with thyroid-specific expression of PPFP and thyroid-specific homozygous deletion of Pten develop metastatic thyroid carcinoma.93 Deletion of Pten was added to the PPFP mouse model because it results in increased phosphorylated (activated) AKT (pAKT), as observed in human PPFP carcinomas.94 Increased pAKT is common in FTCs in general and is not specific for PPFP carcinomas,42 and can result from a variety of genetic or epigenetic events.
Importantly, the PPARγ agonist pioglitazone dramatically decreased the thyroid carcinoma size and completely prevented metastatic disease in this mouse model of PPFP thyroid carcinoma.93 Most remarkably, pioglitazone caused an adipogenic response in the remaining thyroid cancer cells as manifested by lipid accumulation and induction of a broad array of adipocyte PPARγ target genes (PPARγ is the master regulator of adipogenesis). This indicates that, in the presence of pioglitazone, PPFP is very strongly PPARγ-like. It should be noted that mice with Pten deletion alone (without PPFP expression) develop benign thyroid hyperplasia that is unaffected by pioglitazone.93
Interestingly, there also is evidence that PPFP may inhibit tumorigenesis in vivo by inhibiting angiogenesis.95 Although ectopic PPFP expression caused immortalized thyrocytes to exhibit increased growth and decreased apoptosis in vitro, these cells showed decreased mouse xenograft tumor growth in vivo. Further investigation of the xenograft tumors identified reduced CD31 staining and VEGF expression, suggesting that PPFP inhibits neovascularization. Expression of tissue inhibitor of metalloproteinase 3 (TIMP3), an inhibitor of angiogenesis, was increased, and this was considered a potential explanation for the findings. However, TIMP3 is repressed, not induced, in PPFP FTCs obtained from patients.72, 90
Mechanisms of cellular transformation and oncogenesis by PPFP
Although the specific mechanism of PPFP action is yet to be defined, PPFP can act as a dominant negative inhibitor of wild type PPARγ and/or as a unique transcriptional activator of a subset of PPARγ responsive genes. It is expressed in thyroid carcinomas 10-50 fold above endogenous PPARγ.72, 90 PPFP also has mixed actions on PAX8 target genes in transfection experiments. It is important to note that PPFP has the DNA binding domains of both PAX8 and PPARγ. Since it contains transcriptional regulatory domains of both PAX8 and PPARγ, it has the potential to bring inappropriate transcriptional coregulatory proteins to PAX8 and PPARγ target genes. Therefore, a plausible mechanism of oncogenesis is the modulation of the downstream pathways of PAX8 or PPARγ.
PAX8 is required for normal thyroid development and is important in the maintenance of differentiated follicular cell function. When PPFP was expressed in human thyroid cancer cell lines, PAX8 responsive genes (sodium iodide symporter (SLC5A5), thyroid peroxidase (TPO), thyroid stimulating hormone receptor (TSHR), and thyroglobulin (TG)) were variably stimulated or inhibited. SLC5A5 gene expression was stimulated in response to PPFP alone in one study77 whereas this stimulatory effect required cotransfection of PPFP with wild type PAX8 in another study.96 TSHR expression was inhibited96 while TPO transcription was increased by PPFP.77 Repression of the TG promoter was observed in response to PPFP77 but cotransfection with PPFP and PAX8 was required for this inhibitory effect in another study.96 In both these studies, PPFP inhibited PAX8 mediated transcription of TG in a dominant negative fashion, while the addition of a PPARγ agonist did not reverse this dominant negative effect.77, 96 The molecular basis for the variable effects of PPFP on PAX8-responsive genes is not known. Other PAX transcription factors are associated with cancers (reviewed in 97). For example, gene fusions of PAX3 or PAX7 with FKHR underlie alveolar rhabdomyosarcomas, gene fusions of PAX5 with IGH are found in lymphomas, and over expression of PAX2 is found in a variety of cancers. Therefore it is possible that the structurally-related PAX8 portion of PPFP is actively involved in its oncogenic action.
PPARγ is a nuclear hormone receptor that is expressed at very low levels in the normal thyroid and has no as-yet-identified function in that organ. In vitro studies indicate that PPFP can inhibit wild type PPARγ function, and the concept that PPFP may be oncogenic by inhibition of postulated tumor suppressor activities of endogenous PPARγ remains attractive, as discussed above. However, it also is clear that PPFP can act in a PPARγ-like manner.72, 90 In vitro, PPFP stimulates the promoters of some PPARγ target genes and represses others.72, 76, 77
Analysis of gene expression profiling data of FTCs that express PPFP versus those that do not demonstrated that PPFP cancers have a distinct transcriptional signature.72, 90, 98 The studies by Giordano et al72 and Lacroix et al90 yielded highly concordant data. These studies identified numerous adipocyte PPARγ target genes such as AQP7 and ANGPTL4 as being upregulated in PPFP carcinomas, and gene ontology pathways such as fatty acid metabolism and beta oxidation were highly enriched. In addition, a number of genes known to be associated with cancer were identified in these data sets. For example, both studies found the cancer-associated genes MCYL1 and NRG1 to be induced in PPFP tumors, and found the angiogenic factors FGFBP1, PGF and ANGPTL4 to be induced and the anti-angiogenic factor TIMP3 to be repressed. However, for unclear reasons, the PPFP thyroid carcinoma gene expression profile identified by Lui et al98 has virtually no overlap with the above two studies. Lui et al98 found that PPFP tumors are associated with upregulation of genes associated with signal transduction, cell growth and translational control, and repression of ribosomal protein and translational associated genes. A limitation in the interpretation of gene expression profiling data from PPFP thyroid carcinomas is that the specific genes that are essential for oncogenesis are not known.
Stable expression of PPFP in the rat thyroid cell line PCCL3 was found to activate the Wnt/TCF pathway in a subset of cells.78 This TCF-active fraction was enriched in the ability to grow under anchorage independent conditions and to invade through Matrigel, a synthetic basement membrane. In addition, the thyroids from the previously described mouse model of PPFP carcinoma were found to have induction of a number of Wnt/TCF target genes. These are potentially important observations because the Wnt/TCF pathway plays a central role in the cell biology of normal stem cells as well as in cancer stem cells from a variety of organs such as the colon. In fact, the PCCL3/PPFP cells were hierarchically organized such that TCF-active cells gave rise to both TCF-active and –inactive cells, whereas TCF-inactive cells only gave rise to TCF–inactive cells. This hierarchical organization is typical of stem cells. Thus, it is possible that PPFP activates thyroid cancer stem cells via the induction of TCF-responsive genes.
PPFP as a diagnostic tool and a therapeutic target
PPFP is primarily found in FTCs and follicular variant PTCs. It is not possible to diagnose FTCs by cytological analysis of FNA biopsies because the diagnosis of FTC requires evidence of invasion. This is a major reason why ~20 to 25% of thyroid FNA biopsies are considered cytologically indeterminate. Evidence is accumulating that the use of molecular analyses in this situation results in better discrimination of benign versus malignant thyroid nodules. For example, in one study, 5 nodules with indeterminate cytology were found to contain PPFP RNA in the biopsy specimens, and subsequent surgical pathology indicated all 5 were malignant.99 The implication is that the expression of PPFP in a thyroid nodule biopsy with indeterminate cytology indicates the need for surgery. Thus, analysis of PPFP along with other driver mutations is a promising approach to enhance clinical decision making in patients with indeterminate thyroid nodule biopsies.
As described above, the PPARγ agonist pioglitazone is highly therapeutic in a mouse model of PPFP thyroid carcinoma, greatly reducing the size of the primary tumor and eliminating metastatic disease.93 These data suggest that pioglitazone may be therapeutic in patients with PPFP thyroid carcinoma, a hypothesis that is being tested in a phase II clinical trial in patients with advanced disease not amenable to cure by surgery or radioiodine (clinicaltrials.gov identifier NCT01655719). If successful this would be especially notable because pioglitazone is FDA-approved for the long-term therapy of type 2 diabetes and has very little toxicity compared with other targeted chemotherapies such as tyrosine kinase inhibitors. As a ligand for PPFP, pioglitazone turns this fusion protein into a strongly PPARγ-like transcription factor, and this results in trans-differentiation of the mouse PPFP thyroid cancer cells into adipocyte-like cells. It is presumed that the cancer cells lose malignant character as they gain differentiated character. This is supported by limited observations that a non-adipogenic PPARγ agonist with full insulin sensitizing properties has no anti-tumor effect in the mouse model of PPFP carcinoma.78 Thus, although the proadipogenic actions of pioglitazone are considered an unwanted side effect in the therapy of diabetes, this action probably is integral to the therapeutic action of pioglitazone in PPFP thyroid carcinoma.
Conclusions
The t(2;3)(q13;p25) chromosomal translocation that produces PPFP is a driver mutation found in ~30-35% of FTCs as well as a subset of follicular variant PTCs. PPFP can act as a dominant negative inhibitor of PPARγ or as a PPARγ-like transcription factor depending on the target gene and cellular context, and it also has variable effects on PAX8 target genes. It is presumed that modulation of subsets of these pathways underlies the oncogenic actions of PPFP. The identification of PPFP as an oncoprotein has important clinical implications. Cytological criteria are insufficient to differentiate FTCs from benign lesions in thyroid biopsy specimens. The molecular detection of PPFP in thyroid biopsies with indeterminate cytology provides evidence that the nodule is highly likely to be malignant and that surgery is indicated. Although the majority of thyroid cancer patients are cured by surgery with or without radioiodine therapy, patients with locally recurrent or distant metastatic disease can be difficult or impossible to cure. Therefore, novel therapeutic approaches are necessary for the management of these patients. Since pioglitazone results in a highly therapeutic effect in a mouse model of PPFP thyroid carcinoma, this drug may be therapeutic in patients with PPFP thyroid carcinoma. This currently is being tested in clinical trial NCT01655719. It is likely that the pro-adipogenic actions of pioglitazone as a PPFP ligand underlie its therapeutic efficacy, a point which needs to be kept in mind if additional PPARγ agonists are tested in this disease.
Key points.
PPFP is a consequence of a chromosomal translocation found in ~1/3 of follicular carcinomas and substantially smaller subsets of follicular variant papillary carcinomas and benign follicular adenomas.
In vitro and in vivo evidence indicate that PPFP acts as an oncoprotein.
PPFP can act as a dominant negative inhibitor of wild type PPARγ and/or as a PPARγ-like transcription factor, and similarly can activate or repress PAX8-responsive genes.
The PPARγ agonist pioglitazone has a powerful therapeutic effect in a mouse model of PPFP thyroid carcinoma, and is currently being tested in a phase II clinical trial.
Acknowledgements
This work was supported by NIH grants R01CA151842 and R01CA166033.
Footnotes
Competing Interests:
The authors declare no competing interests.
References
- 1.Enewold L, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol. Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Facts and Figures 2014. American Cancer Society; Atlanta, GA, USA.: 2014. [Google Scholar]
- 3.Wiest PW, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17:487–496. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 4.Tomimori E, Pedrinola F, Cavaliere H, Knobel M, Medeiros-Neto G. Prevalence of incidental thyroid disease in a relatively low iodine intake area. Thyroid. 1995;5:273–276. doi: 10.1089/thy.1995.5.273. [DOI] [PubMed] [Google Scholar]
- 5.Greaves TS, et al. Follicular lesions of thyroid: a 5-year fine-needle aspiration experience. Cancer. 2000;90:335–341. [PubMed] [Google Scholar]
- 6.Sclabas GM, et al. Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary series of 240 patients. Am. J. Surg. 2003;186:702–9. doi: 10.1016/j.amjsurg.2003.08.015. discussion 709-10. [DOI] [PubMed] [Google Scholar]
- 7.Papini E, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J. Clin. Endocrinol. Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 8.Carroll BA. Asymptomatic thyroid nodules: incidental sonographic detection. AJR Am J Roentgenol. 1982;138:499–501. doi: 10.2214/ajr.138.3.499. [DOI] [PubMed] [Google Scholar]
- 9.Yassa L, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–516. doi: 10.1002/cncr.23116. [DOI] [PubMed] [Google Scholar]
- 10.Brander A, Viikinkoski P, Nickels J, Kivisaari L. Thyroid gland: US screening in a random adult population. Radiology. 1991;181:683–687. doi: 10.1148/radiology.181.3.1947082. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferri EL. Thyroid cancer in thyroid nodules: finding a needle in the haystack. Am. J. Med. 1992;93:359–362. doi: 10.1016/0002-9343(92)90163-6. [DOI] [PubMed] [Google Scholar]
- 12.Frates MC, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J. Clin. Endocrinol. Metab. 2006;91:3411–3417. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 13.Namba H, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. 2003;88:4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Kang DW, Kim SH, Seong IO, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med. J. 2004;45:818–821. doi: 10.3349/ymj.2004.45.5.818. [DOI] [PubMed] [Google Scholar]
- 15.Cohen Y, et al. BRAF mutation in papillary thyroid carcinoma. J. Natl. Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 16.Adeniran AJ, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am. J. Surg. Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 17.Trovisco V, et al. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J. Pathol. 2004;202:247–251. doi: 10.1002/path.1511. [DOI] [PubMed] [Google Scholar]
- 18.Rabes HM, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin. Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 19.Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–1694. [PubMed] [Google Scholar]
- 20.Bounacer A, et al. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene. 1997;15:1263–1273. doi: 10.1038/sj.onc.1200206. [DOI] [PubMed] [Google Scholar]
- 21.Leeman-Neill RJ, et al. RET/PTC and PAX8/PPARγ chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer. 2013;119:1792–1799. doi: 10.1002/cncr.27893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esapa CT, Johnson SJ, Kendall-Taylor P, Lennard TW, Harris PE. Prevalence of Ras mutations in thyroid neoplasia. Clin. Endocrinol. (Oxf) 1999;50:529–535. doi: 10.1046/j.1365-2265.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- 23.Motoi N, et al. Role of ras mutation in the progression of thyroid carcinoma of follicular epithelial origin. Pathol. Res. Pract. 2000;196:1–7. doi: 10.1016/S0344-0338(00)80015-1. [DOI] [PubMed] [Google Scholar]
- 24.Namba H, Rubin SA, Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol. Endocrinol. 1990;4:1474–1479. doi: 10.1210/mend-4-10-1474. [DOI] [PubMed] [Google Scholar]
- 25.French CA, et al. Genetic and biological subgroups of low-stage follicular thyroid cancer. Am. J. Pathol. 2003;162:1053–1060. doi: 10.1016/S0002-9440(10)63902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikiforova MN, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab. 2003;88:2318–2326. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 27.Marques AR, et al. Expression of PAX8-PPAR gamma 1 rearrangements in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 2002;87:3947–3952. doi: 10.1210/jcem.87.8.8756. [DOI] [PubMed] [Google Scholar]
- 28.Castro P, et al. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006;91:213–220. doi: 10.1210/jc.2005-1336. [DOI] [PubMed] [Google Scholar]
- 29.Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am. J. Surg. Pathol. 2002;26:1016–1023. doi: 10.1097/00000478-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Dwight T, et al. Involvement of the PAX8/peroxisome proliferator-activated receptor gamma rearrangement in follicular thyroid tumors. J. Clin. Endocrinol. Metab. 2003;88:4440–4445. doi: 10.1210/jc.2002-021690. [DOI] [PubMed] [Google Scholar]
- 31.Lui W-O, et al. CREB3L2-PPARgamma fusion mutation identifies a thyroid signaling pathway regulated by intramembrane proteolysis. Cancer Res. 2008;68:7156–7164. doi: 10.1158/0008-5472.CAN-08-1085. [DOI] [PubMed] [Google Scholar]
- 32.Fagin JA, et al. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J. Clin. Invest. 1993;91:179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donghi R, et al. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J. Clin. Invest. 1993;91:1753–1760. doi: 10.1172/JCI116385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobashi Y, et al. Stepwise participation of p53 gene mutation during dedifferentiation of human thyroid carcinomas. Diagn. Mol. Pathol. 1994;3:9–14. doi: 10.1097/00019606-199403010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Ito T, et al. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res. 1992;52:1369–1371. [PubMed] [Google Scholar]
- 36.Garcia-Rostan G, et al. Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am. J. Pathol. 2001;158:987–996. doi: 10.1016/s0002-9440(10)64045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Rostan G, et al. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999;59:1811–1815. [PubMed] [Google Scholar]
- 38.Kurihara T, et al. Immunohistochemical and sequencing analyses of the Wnt signaling components in Japanese anaplastic thyroid cancers. Thyroid. 2004;14:1020–1029. doi: 10.1089/thy.2004.14.1020. [DOI] [PubMed] [Google Scholar]
- 39.Ricarte-Filho JC, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Rostan G, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65:10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- 41.Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2008;93:278–284. doi: 10.1210/jc.2007-1076. [DOI] [PubMed] [Google Scholar]
- 42.Hou P, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin. Cancer Res. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 43.Dahia PL, et al. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res. 1997;57:4710–4713. [PubMed] [Google Scholar]
- 44.Kim CS, et al. AKT activation promotes metastasis in a mouse model of follicular thyroid carcinoma. Endocrinology. 2005;146:4456–4463. doi: 10.1210/en.2005-0172. [DOI] [PubMed] [Google Scholar]
- 45.Saito J, et al. Regulation of FRTL-5 thyroid cell growth by phosphatidylinositol (OH) 3 kinase-dependent Akt-mediated signaling. Thyroid. 2001;11:339–351. doi: 10.1089/10507250152039073. [DOI] [PubMed] [Google Scholar]
- 46.Pasca di Magliano M, Di Lauro R, Zannini M. Pax8 has a key role in thyroid cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13144–13149. doi: 10.1073/pnas.240336397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macchia PE, et al. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat. Genet. 1998;19:83–86. doi: 10.1038/ng0598-83. [DOI] [PubMed] [Google Scholar]
- 48.Poleev A, et al. Determination of functional domains of the human transcription factor PAX8 responsible for its nuclear localization and transactivating potential. Eur. J. Biochem. 1997;247:860–869. doi: 10.1111/j.1432-1033.1997.00860.x. [DOI] [PubMed] [Google Scholar]
- 49.Poleev A, et al. Distinct functional properties of three human paired-box-protein, PAX8, isoforms generated by alternative splicing in thyroid, kidney and Wilms tumors. Eur. J. Biochem. 1995;228:899–911. doi: 10.1111/j.1432-1033.1995.tb20338.x. [DOI] [PubMed] [Google Scholar]
- 50.Rosen ED, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 51.Yamauchi T, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J. Biol. Chem. 2001;276:41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- 52.Xu HE, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 53.Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 54.Kliewer SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raman P, Kaplan BLF, Thompson JT, Vanden Heuvel JP, Kaminski NE. 15-Deoxy-delta12,14-prostaglandin J2-glycerol ester, a putative metabolite of 2-arachidonyl glycerol, activates peroxisome proliferator activated receptor gamma. Mol. Pharmacol. 2011;80:201–209. doi: 10.1124/mol.110.070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raman P, Kaplan BLF, Kaminski NE. 15-Deoxy-Δ12,14-prostaglandin J2-glycerol, a putative metabolite of 2-arachidonyl glycerol and a peroxisome proliferator-activated receptor γ ligand, modulates nuclear factor of activated T cells. J. Pharmacol. Exp. Ther. 2012;342:816–826. doi: 10.1124/jpet.112.193003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehmann JM, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 58.Corzo C, Griffin PR. Targeting the Peroxisome Proliferator-Activated Receptor-γ to Counter the Inflammatory Milieu in Obesity. Diabetes Metab J. 2013;37:395–403. doi: 10.4093/dmj.2013.37.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mueller E, et al. Genetic analysis of adipogenesis through peroxisome proliferator-activated receptor gamma isoforms. J. Biol. Chem. 2002;277:41925–41930. doi: 10.1074/jbc.M206950200. [DOI] [PubMed] [Google Scholar]
- 60.Kroll TG, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science. 2000;289:1357–1360. doi: 10.1126/science.289.5483.1357. [DOI] [PubMed] [Google Scholar]
- 61.Mascia A, Nitsch L, Di Lauro R, Zannini M. Hormonal control of the transcription factor Pax8 and its role in the regulation of thyroglobulin gene expression in thyroid cells. J. Endocrinol. 2002;172:163–176. doi: 10.1677/joe.0.1720163. [DOI] [PubMed] [Google Scholar]
- 62.Martelli ML, et al. Inhibitory effects of peroxisome proliferator-activated receptor gamma on thyroid carcinoma cell growth. J. Clin. Endocrinol. Metab. 2002;87:4728–4735. doi: 10.1210/jc.2001-012054. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am. J. Clin. Pathol. 2003;120:71–77. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 64.Aldred MA, et al. Peroxisome proliferator-activated receptor gamma is frequently downregulated in a diversity of sporadic nonmedullary thyroid carcinomas. Oncogene. 2003;22:3412–3416. doi: 10.1038/sj.onc.1206400. [DOI] [PubMed] [Google Scholar]
- 65.Lacroix L, et al. PAX8 and peroxisome proliferator-activated receptor gamma 1 gene expression status in benign and malignant thyroid tissues. Eur. J. Endocrinol. 2004;151:367–374. doi: 10.1530/eje.0.1510367. [DOI] [PubMed] [Google Scholar]
- 66.Hibi Y, et al. Is thyroid follicular cancer in Japanese caused by a specific t(2; 3)(q13; p25) translocation generating Pax8-PPAR gamma fusion mRNA? Endocr. J. 2004;51:361–366. doi: 10.1507/endocrj.51.361. [DOI] [PubMed] [Google Scholar]
- 67.Marques AR, et al. Underexpression of peroxisome proliferator-activated receptor (PPAR)gamma in PAX8/PPARgamma-negative thyroid tumours. Br. J. Cancer. 2004;91:732–738. doi: 10.1038/sj.bjc.6601989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahin M, et al. PPARgamma staining as a surrogate for PAX8/PPARgamma fusion oncogene expression in follicular neoplasms: clinicopathological correlation and histopathological diagnostic value. J. Clin. Endocrinol. Metab. 2005;90:463–468. doi: 10.1210/jc.2004-1203. [DOI] [PubMed] [Google Scholar]
- 69.Castro P, Roque L, Magalhães J, Sobrinho-Simões M. A subset of the follicular variant of papillary thyroid carcinoma harbors the PAX8-PPARgamma translocation. Int. J. Surg. Pathol. 2005;13:235–238. doi: 10.1177/106689690501300301. [DOI] [PubMed] [Google Scholar]
- 70.Cheung L, et al. Detection of the PAX8-PPAR gamma fusion oncogene in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 2003;88:354–357. doi: 10.1210/jc.2002-021020. [DOI] [PubMed] [Google Scholar]
- 71.Foukakis T, et al. The Ras effector NORE1A is suppressed in follicular thyroid carcinomas with a PAX8-PPARgamma fusion. J. Clin. Endocrinol. Metab. 2006;91:1143–1149. doi: 10.1210/jc.2005-1372. [DOI] [PubMed] [Google Scholar]
- 72.Giordano TJ, et al. Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8-PPARG translocation. Clin. Cancer Res. 2006;12:1983–1993. doi: 10.1158/1078-0432.CCR-05-2039. [DOI] [PubMed] [Google Scholar]
- 73.Klemke M, et al. On the prevalence of the PAX8-PPARG fusion resulting from the chromosomal translocation t(2;3)(q13;p25) in adenomas of the thyroid. Cancer Genet. 2011;204:334–339. doi: 10.1016/j.cancergen.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Jenkins RB, et al. Frequent occurrence of cytogenetic abnormalities in sporadic nonmedullary thyroid carcinoma. Cancer. 1990;66:1213–1220. doi: 10.1002/1097-0142(19900915)66:6<1213::aid-cncr2820660622>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 75.Boos LA, et al. Diagnostic and prognostic implications of the PAX8-PPARγ translocation in thyroid carcinomas-a TMA-based study of 226 cases. Histopathology. 2013;63:234–241. doi: 10.1111/his.12150. [DOI] [PubMed] [Google Scholar]
- 76.Gregory Powell J, et al. The PAX8/PPARgamma fusion oncoprotein transforms immortalized human thyrocytes through a mechanism probably involving wild-type PPARgamma inhibition. Oncogene. 2004;23:3634–3641. doi: 10.1038/sj.onc.1207399. [DOI] [PubMed] [Google Scholar]
- 77.Au AYM, et al. PAX8-peroxisome proliferator-activated receptor gamma (PPARgamma) disrupts normal PAX8 or PPARgamma transcriptional function and stimulates follicular thyroid cell growth. Endocrinology. 2006;147:367–376. doi: 10.1210/en.2005-0147. [DOI] [PubMed] [Google Scholar]
- 78.Vu-Phan D, et al. The thyroid cancer PAX8-PPARG fusion protein activates Wnt/TCF-responsive cells that have a transformed phenotype. Endocr. Relat. Cancer. 2013;20:725–739. doi: 10.1530/ERC-13-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aiello A, et al. Peroxisomal proliferator-activated receptor-gamma agonists induce partial reversion of epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology. 2006;147:4463–4475. doi: 10.1210/en.2005-1610. [DOI] [PubMed] [Google Scholar]
- 80.Ohta K, Endo T, Onaya T. The mRNA levels of thyrotropin receptor, thyroglobulin and thyroid peroxidase in neoplastic human thyroid tissues. Biochem. Biophys. Res. Commun. 1991;174:1148–1153. doi: 10.1016/0006-291x(91)91540-s. [DOI] [PubMed] [Google Scholar]
- 81.Park J-W, et al. Troglitazone, the peroxisome proliferator-activated receptor-gamma agonist, induces antiproliferation and redifferentiation in human thyroid cancer cell lines. Thyroid. 2005;15:222–231. doi: 10.1089/thy.2005.15.222. [DOI] [PubMed] [Google Scholar]
- 82.Kitamura S, et al. Peroxisome proliferator-activated receptor gamma induces growth arrest and differentiation markers of human colon cancer cells. Jpn. J. Cancer Res. 1999;90:75–80. doi: 10.1111/j.1349-7006.1999.tb00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keelan JA, et al. 15-Deoxy-Delta(12,14)-prostaglandin J(2), a ligand for peroxisome proliferator-activated receptor-gamma, induces apoptosis in JEG3 choriocarcinoma cells. Biochem. Biophys. Res. Commun. 1999;262:579–585. doi: 10.1006/bbrc.1999.1257. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi N, et al. Activation of PPARgamma inhibits cell growth and induces apoptosis in human gastric cancer cells. FEBS Lett. 1999;455:135–139. doi: 10.1016/s0014-5793(99)00871-6. [DOI] [PubMed] [Google Scholar]
- 85.Tontonoz P, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kubota T, et al. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 87.Elstner E, et al. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kato Y, et al. PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene. 2006;25:2736–2747. doi: 10.1038/sj.onc.1209299. [DOI] [PubMed] [Google Scholar]
- 89.Karger S, et al. Evaluation of peroxisome proliferator-activated receptor-gamma expression in benign and malignant thyroid pathologies. Thyroid. 2005;15:997–1003. doi: 10.1089/thy.2005.15.997. [DOI] [PubMed] [Google Scholar]
- 90.Lacroix L, et al. Follicular thyroid tumors with the PAX8-PPARgamma1 rearrangement display characteristic genetic alterations. Am. J. Pathol. 2005;167:223–231. doi: 10.1016/s0002-9440(10)62967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wood WM, et al. PPARγ Promotes Growth and Invasion of Thyroid Cancer Cells. PPAR Res. 2011;2011:171765. doi: 10.1155/2011/171765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burton JD, Goldenberg DM, Blumenthal RD. Potential of peroxisome proliferator-activated receptor gamma antagonist compounds as therapeutic agents for a wide range of cancer types. PPAR Res. 2008;2008:494161. doi: 10.1155/2008/494161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dobson ME, et al. Pioglitazone induces a proadipogenic antitumor response in mice with PAX8-PPARgamma fusion protein thyroid carcinoma. Endocrinology. 2011;152:4455–4465. doi: 10.1210/en.2011-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diallo-Krou E, et al. Paired box gene 8-peroxisome proliferator-activated receptor-gamma fusion protein and loss of phosphatase and tensin homolog synergistically cause thyroid hyperplasia in transgenic mice. Endocrinology. 2009;150:5181–5190. doi: 10.1210/en.2009-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reddi HV, et al. Expression of the PAX8/PPARγ Fusion Protein Is Associated with Decreased Neovascularization In Vivo: Impact on Tumorigenesis and Disease Prognosis. Genes Cancer. 2010;1:480–492. doi: 10.1177/1947601910373545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Espadinha C, Cavaco BM, Leite V. PAX8PPARgamma stimulates cell viability and modulates expression of thyroid-specific genes in a human thyroid cell line. Thyroid. 2007;17:497–509. doi: 10.1089/thy.2006.0263. [DOI] [PubMed] [Google Scholar]
- 97.Robson EJD, He S-J, Eccles MR. A PANorama of PAX genes in cancer and development. Nat. Rev. Cancer. 2006;6:52–62. doi: 10.1038/nrc1778. [DOI] [PubMed] [Google Scholar]
- 98.Lui W-O, et al. Expression profiling reveals a distinct transcription signature in follicular thyroid carcinomas with a PAX8-PPAR(gamma) fusion oncogene. Oncogene. 2005;24:1467–1476. doi: 10.1038/sj.onc.1208135. [DOI] [PubMed] [Google Scholar]
- 99.Nikiforov YE, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]