Abstract
The increasing burden of drug-resistant tuberculosis (TB) poses an escalating threat to national TB control programs. To assist appropriate treatment for TB patients, accurate and rapid detection of drug resistance is critical. The GeneChip test is a novel molecular tool for the diagnosis of TB drug resistance. Performance-related data on GeneChip are limited, and evaluation in new and previously treated TB cases has never been performed. We evaluated the diagnostic performance of GeneChip in detecting resistance to rifampin (RMP) and isoniazid (INH) and in detecting multidrug-resistant tuberculosis (MDR-TB) in comparison with standard drug susceptibility testing (DST) and compared the results in a group of previously treated and newly detected TB patients in an urban area in southeastern China. One thousand one hundred seventy-three (83.8%) new cases and 227 (16.2%) previously treated cases were collected between January 2011 and September 2013. The GeneChip showed a specificity of 97.8% and a sensitivity of 94.8% for detection of RMP resistance and 97.3% and 70.9%, respectively, for INH resistance in new cases. For previously treated cases, the overall sensitivity, specificity, and agreement rate are 94.6%, 91.3%, and 92.1%, respectively, for detection of RMP resistance and 69.7%, 95.4%, and 86.8%, respectively, for INH resistance. The sensitivity and specificity of MDR-TB were 81.8% and 99.0% in new cases and 77.8% and 93.4% in previously treated cases, respectively. The GeneChip system provides a simple, rapid, reliable, and accurate clinical assay for the detection of TB drug resistance, and it is a potentially important diagnostic tool in a high-prevalence area.
INTRODUCTION
Tuberculosis (TB) is an infectious disease caused by the bacillus Mycobacterium tuberculosis and remains a major global health problem. In 2012, the World Health Organization (WHO) estimated an incidence of 8.6 million new TB cases and 1.3 million TB deaths (just under 1.0 million among HIV-negative people and 0.3 million HIV-associated TB deaths) (1).
The emergence of drug-resistant TB (especially multidrug-resistant TB [MDR-TB], defined as resistance to both rifampin [RMP] and isoniazid [INH]) represents a serious threat to TB control efforts worldwide. Globally, 3.6% of new TB cases and 20.2% of previously treated cases are estimated to have MDR-TB (1, 2). Acid-fast bacillus (AFB) smear microscopy is the most common method for diagnosing TB. However, low sensitivity and an inability to detect smear-negative pulmonary cases limit the usefulness of this technique (3, 4). Diagnosis based on culture is the reference standard, but results take weeks to obtain. Conventional drug susceptibility testing (DST) can provide definitive results, but this approach is time-consuming and usually requires several weeks to produce susceptibility profiles, which can lead to inadequate treatment and further acquired drug resistance during this period (5, 6). Therefore, highly sensitive and specific, easy-to-apply, quick, and cost-effective methods are needed in the diagnosis of MDR-TB (7, 8).
Several methods have been developed that improve the speed of detecting MDR-TB based on encoding regions of the genes associated with drug resistance. Among them, the Xpert MTB/RMP assay was an integrated specimen processing and nucleic acid amplification-based test for detection of M. tuberculosis and RMP resistance-conferring mutations directly from sputum (9). The GeneChip MDR kit (CapitalBio, Beijing, China) is based on molecular analysis from multiple PCRs and reverse hybridization. The biochip test is designed to identify common mutations for RMP and INH resistance in the rpoB and katG genes and the promoter of the inhA gene. By uncovering mutations in these genes, GeneChip can detect TB and its multidrug-resistant form in M. tuberculosis isolates and sputum samples (5, 10). Previous evaluations of GeneChip for MDR-TB detection have shown promising results. The rate of concordance between the GeneChip assay and DNA sequencing results was 100%, and the rates of concordance with conventional DST results were 91.8% for isolates and 94.6% for sputum samples for RMP resistance and 70.2% for isolates and 78.1% for sputum samples for INH resistance (10, 11).
To our knowledge, GeneChip has never been evaluated in drug-resistant and -susceptible TB cases with differing treatment histories. Although historically MDR-TB was thought to be predominantly created through treatment mismanagement, primary transmission has been identified as a large problem both in China and elsewhere (12–14). As person-to-person spread of drug resistance increases, identifying the efficacy of diagnostic tools in both new and previously treated cases may become more important. In our study, we aimed to evaluate the feasibility and accuracy of GeneChip for diagnosing resistance to RMP and INH and diagnosing MDR-TB in patients with differing treatment histories.
MATERIALS AND METHODS
Design of study.
In four counties and one downtown area of Lianyungang City in Jiangsu Province, all newly registered patients with sputum smear-positive pulmonary TB were enrolled. From January 2011 through September 2013, a total of 1,816 clinical isolates were collected in this study. Each newly registered TB patient was interviewed by the clinician using medical records to obtain treatment history. When medical records were not available, we used self-reporting. The definitions of new and previously treated cases were derived from WHO guidelines (15). We collected two or three sputum specimens (2 ml each) from patients. Specimens were transported to corresponding prefectural or municipal laboratories within 3 days after collection and were divided into two aliquots for detection: one of the aliquots was subjected to species identification and detection of gene mutations by the GeneChip and the other aliquot was processed for culture and DST. Based on the result of the molecular test, treatment regimens were changed.
DNA preparation and sputum culture.
The DNA extraction of all sputum examples followed the manufacturer's protocol for the CapitalBio Universal kit as previously reported (10, 16). The sputum samples were cultured and isolated on Löwenstein-Jensen (LJ) culture medium. LJ culture media were incubated at 37°C and observed on the 3rd day to detect contamination. Subsequently, we recorded the growth on LJ media each week for 8 weeks.
GeneChip assay.
The GeneChip assay was performed according to the manufacturer's instructions (10, 17). Briefly, it includes a biochip kit; apparatus for sample preparation, hybridization, washing, and data acquisition; and dedicated software for automated diagnosis (10). Multiplex asymmetric PCR (MAPCR) was performed in a TC-96/G/H (b) thermal cycler. The PCR products were hybridized with a GeneChip in a BioMixer II three-dimensional tilting agitator (CapitalBio) and a hybridization oven. An automated SlideWasher-8 (also from CapitalBio) was then used to wash and dry the hybridized slides. The fluorescent signal on the slides that was emitted by the microarrays was detected using a GeneArray scanner (LuxScan-10K confocal laser scanner; CapitalBio), and the fluorescence intensities were quantified by the M. tuberculosis drug resistance detection array test system (CapitalBio). The drug resistance pattern for INH and RMP can be found in the previous report (5, 10). Ahead of the study, all technicians were trained by the National TB Reference Laboratory and confirmed by proficiency testing. All GeneChip results were compared with conventional DST results.
Drug susceptibility test.
The DST was performed according to the proportion method as recommended by the WHO/International Union against Tuberculosis and Lung Disease (IUATLD) (18). The concentrations of anti-TB drugs were 0.2 mg/ml for INH and 40 mg/ml for RMP. As a parallel test, p-nitrobenzoic acid (PNB) was utilized for nontuberculous mycobacterium (NTM) identification. Growth in LJ medium containing PNB indicates that the bacilli do not belong to the M. tuberculosis complex (19). For internal quality assurance of DST, a standard H37Rv strain was included with each new batch of LJ medium, and DST was also performed when reading was done after 4 and after 6 weeks. External quality control for culture and DST was conducted by the provincial TB reference laboratory, which participates in the annual proficiency review of DST organized by the Hong Kong Supranational Tuberculosis Reference Laboratory and has passed each review since 2010.
Analysis of data.
The laboratory result of the DST was used as the reference standard to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of GeneChip. All data were evaluated by the National Reference Laboratory of TB. Chi-square tests were used for statistical analysis, and the criterion for significance was set at a P value of <0.05 based on a two-sided test. Analyses were conducted with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement.
All patients gave written informed consent before they were included in this study. This project was approved by the Institutional Review Board of the Jiangsu Provincial Center for Disease Control and Prevention.
RESULTS
Study population.
During the study period, 1,816 sputum smear-positive TB cases were enrolled and tested by culture, DST, and GeneChip. Of the 1,816 TB cases, 416 cases were excluded (142 cultures were either negative or contaminated, 126 were distinguished as NTM cases, and 148 cases [123 new cases and 25 previously treated cases] could not be distinguished by GeneChip testing). Thus, 1,400 cases were included in the final analysis (Fig. 1). The age of the 1,400 patients ranged from 15 to 94 years (mean ± standard deviation [SD], 49.67 ± 20.57 years). New cases were significantly younger than previously treated cases (mean ± SD, 48.54 ± 21.00 versus 55.51 ± 17.07 years, P < 0.001). One thousand sixty-eight participants (76.3%) were male, and 332 (23.7%) were female. Of the 1,400 patients included in the study, 1,173 (83.8%) were new cases and 227 (16.2%) were previously treated cases (Table 1).
FIG 1.
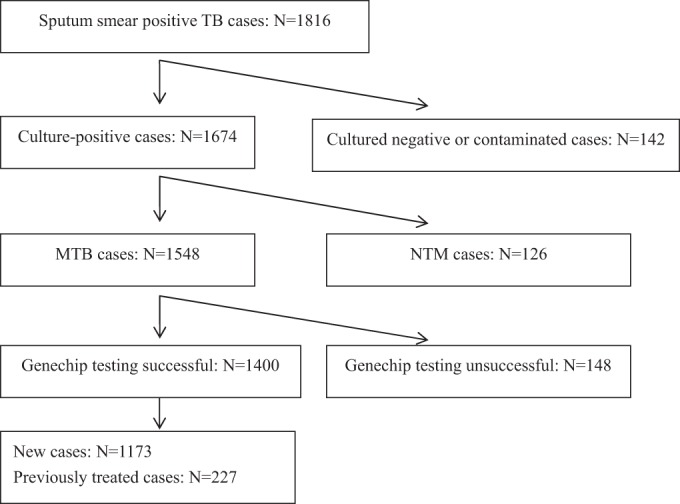
Flow chart of tuberculosis subjects included in this study Summarizing the results of two tests, the results for 1,400 specimens were usable for evaluation of the performance of GeneChip. TB, tuberculosis; MTB, Mycobacterium tuberculosis; NTM, nontuberculous mycobacterium.
TABLE 1.
Characteristics in patients with differing treatment histories
| Characteristic | New cases | Previously treated cases | P |
|---|---|---|---|
| Age, yr (mean ± SD) | 48.54 ± 21.00 | 55.51 ± 17.07 | <0.001 |
| Age group (no.) | <0.001 | ||
| <40 yr | 452 | 47 | |
| 40–59 yr | 301 | 83 | |
| ≥60 yr | 420 | 97 | |
| Sex (no.) | 0.244 | ||
| Male | 888 | 180 | |
| Female | 285 | 47 | |
| Community (no.) | 0.291 | ||
| Rural | 991 | 198 | |
| Urban | 182 | 29 |
Performance evaluation of GeneChip for RMP and INH resistance in new and previously treated TB cases.
For detecting M. tuberculosis RMP resistance in new cases, the overall sensitivity, specificity, agreement rate, positive predictive value (PPV), negative predictive value (NPV), and kappa values are 94.83%, 97.76%, 97.61%, 68.75%, 99.73%, and 0.78, respectively. In previously treated cases, the overall sensitivity, specificity, agreement rate, PPV, NPV, and kappa values are 94.55%, 91.28%, 92.07%, 77.61%, 98.13%, and 0.8, respectively. The sensitivity of GeneChip in new and previously treated TB cases showed no significant difference (P = 0.947). The specificity and total agreement rate of GeneChip were significantly higher in new TB cases than in previously treated cases (Table 2).
TABLE 2.
Performance evaluation of GeneChip for rifampin and isoniazid resistance in tuberculosis cases with different treatment historiesa
| GeneChip | Conventional drug susceptibility testingc |
Sensitivity (%) | Specificity (%) | AR | PPV (%) | NPV (%) | Kappa | PLR (%) | NLR (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. susceptible (%) | No. resistant (%) | Total no. | |||||||||
| New cases | |||||||||||
| Rifampin | 94.8 | 97.8b | 97.6b | 68.8 | 99.7b | 0.78 | 42.30 | 0.05 | |||
| Susceptible | 1,090 (97.8) | 3 (5.2) | 1,093 | ||||||||
| Resistant | 25 (2.2) | 55 (94.8) | 80 | ||||||||
| Total | 1,115 | 58 | |||||||||
| Isoniazid | 70.9 | 97.3 | 94.8b | 72.9 | 97.0 | 0.69 | 26.0 | 0.30 | |||
| Susceptible | 1,034 (97.3) | 32 (29.1) | 1,066 | ||||||||
| Resistant | 29 (2.7) | 78 (70.9) | 107 | ||||||||
| Total | 1,063 | 110 | |||||||||
| Previously treated cases | |||||||||||
| Rifampin | 94.6 | 91.3 | 92.1 | 77.6 | 98.1 | 0.8 | 10.8 | 0.06 | |||
| Susceptible | 157 (91.3) | 3 (5.5) | 160 | ||||||||
| Resistant | 15 (8.7) | 52 (94.5) | 67 | ||||||||
| Total | 172 | 55 | |||||||||
| Isoniazid | 69.7 | 95.4 | 86.8 | 88.3b | 86.2 | 0.69 | 15.0 | 0.32 | |||
| Susceptible | 144 (95.4) | 23 (30.3) | 167 | ||||||||
| Resistant | 7 (4.6) | 53 (69.7) | 60 | ||||||||
| Total | 151 | 76 | |||||||||
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AR, agreement rate; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Statistically significantly different from the opposing type of case with the same drug (P < 0.05).
Culture was used as the gold standard.
The evaluation of the diagnostic value of GeneChip for INH resistance in patients with differing treatment histories is shown in Table 2. For new cases, the overall sensitivity, specificity, agreement rate, PPV, NPV, and kappa values are 70.91%, 97.27%, 94.8%, 72.9%, 97%, and 0.69, respectively. For previously treated cases, the overall sensitivity, specificity, agreement rate, PPV, NPV, and kappa values are 69.74%, 95.36%, 86.78%, 88.33%, 86.23%, and 0.69, respectively. The kappa value between GeneChip and DST for INH resistance was 0.69 in both new TB cases and previously treated cases. There were no differences between new and previously treated TB cases in terms of their sensitivity and specificity. The total agreement rate between GeneChip and DST was significantly higher in new TB cases than in previously treated cases.
Performance evaluation of GeneChip for MDR in new and previously treated TB cases.
The GeneChip system displayed a sensitivity of 81.8% and a specificity of 99% compared with the results of DST in new cases, which is higher than that in previously treated cases. The overall correlation between the two tests showed excellent agreement, 98.4% in new cases and 93.4% in previously treated cases. In all cases, the sensitivity, specificity, agreement rate, positive predictive value (PPV), negative predictive value (NPV), and kappa values are 79.8%, 98.3%, 97.1%, 75.5%, 98.6%, and 0.76, respectively (Table 3).
TABLE 3.
Performance evaluation of GeneChip for multidrug-resistant and susceptible tuberculosis cases with different treatment historiesa
| GeneChip | No. of cases by conventional drug susceptibility testingc |
Sensitivity (%) |
Specificity (%) |
AR | PPV (%) | NPV (%) | Kappa | PLR (%) | NLR (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDR-TB (%) | Non-MDR (%) | Total | |||||||||
| New cases | 81.8 | 99.0b | 98.4b | 76.6 | 99.3b | 0.78 | 84.0 | 0.18 | |||
| MDR-TB | 36 (81.8) | 11 (1.0) | 47 | ||||||||
| Non-MDR | 8 (18.2) | 1,118 (99.0) | 1,126 | ||||||||
| Total | 44 | 1,129 | |||||||||
| Previously treated cases | 77.8 | 93.4 | 90.3 | 74.5 | 94.4 | 0.70 | 11.8 | 0.24 | |||
| MDR-TB | 35 (77.8) | 12 (6.6) | 47 | ||||||||
| Non-MDR | 10 (22.2) | 170 (93.4) | 180 | ||||||||
| Total | 45 | 182 | |||||||||
| All cases | 79.8 | 98.3 | 97.1 | 75.5 | 98.6 | 0.76 | 45.5 | 0.21 | |||
| MDR-TB | 71 (79.8) | 23 (1.8) | 94 | ||||||||
| Non-MDR | 18 (20.2) | 1,288 (98.2) | 1,306 | ||||||||
| Total | 89 | 1,311 | |||||||||
Abbreviations: TB, tuberculosis; MDR, multidrug resistant; PPV, positive predictive value; NPV, negative predictive value; AR, agreement rate; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Statistically significant (P < 0.05).
Culture was used as the gold standard.
DISCUSSION
Drug-resistant TB (DR-TB) has shifted the paradigm of global TB control and is a major public health concern in China. According to our 2011–2012 drug resistance survey, there is a high prevalence of MDR-TB in Lianyungang City, 4.2% in new cases and 27.6% in previously treated patients. This high prevalence of MDR-TB is alarming, and improved control efforts are necessary to both lower the current burden and prevent further drug resistance (20). An accurate and rapid diagnostic method is urgently needed in our area. The diagnosis of drug-resistant TB requires TB patients to be tested for susceptibility to drugs. Due to this, efforts should be made to increase diagnostic capabilities to detect resistance to the two first-line drugs, RMP and INH.
After development in 2009, GeneChip has been shown to be cost-effective and diagnostically satisfactory for detecting drug resistance (16). However, a performance evaluation of GeneChip for resistance in new and previously treated TB cases has never been performed. In this MDR epidemic setting, we found that the GeneChip system for DR-TB assay was in high concordance with DST in both new and previously treated TB cases. Kappa values (0.80, 0.78, 0.69, and 0.69) were also very high and showed strong concordance. Thus, the GeneChip system can be a potentially accurate and rapid alternative for MDR-TB detection in areas of high drug resistance. In our study, new cases were significantly younger than previously treated cases. One possible explanation is that older people assume more family responsibility, and they may tend to stop medication when they feel better or when they come under more pressing social obligations. It is also possible that older people gain less attention in the provision of health care, and they might be at higher risk for treatment default (21).
Using GeneChip diagnostic systems efficiently in MDR-TB control programs should be further investigated. In our study, all smear-positive TB cases in four counties and one downtown area of Lianyungang City were enrolled. This provided an excellent population-based sample. Our results indicate that GeneChip has especially high sensitivity (>94%) for detecting RMP resistance in both new and previously treated cases. Identifying RMP resistance accurately in TB patients is of critical importance because in many settings, especially in areas with a high burden of drug resistance such as China, it correlates strongly with MDR-TB (22). Due to this, the WHO currently recommends that individuals with confirmed resistance to RMP be treated as MDR-TB patients until INH resistance can also be confirmed reliably (23). These results are encouraging, as the major limitation of some rapid diagnostic tools in detecting resistance to RMP is low sensitivity (24, 25).
In addition, we found a higher PPV for detecting RMP and INH among previously treated cases than among new cases. Various factors can influence the PPV of diagnostic methods (26, 27), including a strong correlation between the prevalence rates. The MDR-TB prevalence rate in previously treated cases is higher than that in new TB cases around the world. In our study area, there are more than six times as many previously treated cases as new cases (19). The PPV usually reaches above 90% in drug resistance diagnostic assays only when the prevalence of resistance is above 15% (18, 28). Accordingly, in this study, we found a noticeable difference in the PPVs between patients with differing treatment histories. Therefore, GeneChip is perhaps most effectively used, at least currently, in previously treated cases (28). As primary transmission of TB drug resistance increases, we can also expect to see a rise above current rates locally (4.2%) among new cases. Therefore, the PPV of GeneChip for new cases may also increase and may need to be reevaluated in the future.
There are several limitations of this study. First, classification was based on patient history of prior TB treatment and reviewing of medical records. Potential misclassification of new and previously treated cases could occur when some cases were reported as new but received TB treatment in the past. Second, this study did not collect information on HIV status because patients with TB in China are not routinely tested for HIV. Therefore, we cannot know whether our results were influenced in some way by the effects of HIV comorbidity. Third, missed phenotypic RMP resistance for TB has been reported in a few recent papers, and we were not able to assess this in our sample (29, 30). Last, a small proportion of specimens failed to produce results. Future studies must investigate reasons for this irregularity.
Conclusions.
This is the first study investigating the diagnostic value of GeneChip for the detection of resistant M. tuberculosis in both new and previously treated TB cases. Our study shows that, when evaluating drug resistance, the GeneChip system was in high concordance with the DST in both new and previously treated TB cases and showed especially high sensitivity in the detection of RMP resistance. Given that detection of RMP resistance shows strong concordance with MDR-TB, GeneChip is a potentially important diagnostic tool in high-prevalence areas.
ACKNOWLEDGMENTS
This study was supported by the National Nature Science Foundation of China (81302480), Key Personnel of Science and Education Industrial Engineering (JKRC2011005), and the Ministry of Health (W201208). The sponsors had no role in the study design or data analysis.
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis report 2013. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Matteelli A, Migliori GB, Cirillo D, Centis R, Girard E, Raviglione M. 2007. Multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis: epidemiology and control. Expert Rev Anti Infect Ther 5:857–871. doi: 10.1586/14787210.5.5.857. [DOI] [PubMed] [Google Scholar]
- 3.Behr M, Warren S, Salamon H, Hopewell P, de Leon AP, Daley C, Small P. 1999. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353:444–449. doi: 10.1016/S0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 4.Moure R, Muñoz L, Torres M, Santin M, Martín R, Alcaide F. 2011. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol 49:1137–1139. doi: 10.1128/JCM.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Li L, Luo F, Cheng P, Wu F, Wu Z, Hou T, Zhong M, Xu J. 2012. Rapid and accurate detection of RMP-and INH-resistant Mycobacterium tuberculosis in spinal tuberculosis specimens by CapitalBio DNA microarray: a prospective validation study. BMC Infect Dis 12:303. doi: 10.1186/1471-2334-12-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rüsch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. 2006. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol 44:688–692. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah M, Chihota V, Coetzee G, Churchyard G, Dorman SE. 2013. Comparison of laboratory costs of rapid molecular tests and conventional diagnostics for detection of tuberculosis and drug-resistant tuberculosis in South Africa. BMC Infect Dis 13:352. doi: 10.1186/1471-2334-13-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C. 2011. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaich A, Frei R. 2014. Performance of the Xpert MTB/RIF assay on nonrespiratory specimens and accuracy of this assay for detection of rifampin resistance in a low-prevalence setting. J Clin Microbiol 52:706. doi: 10.1128/JCM.02591-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Zhou Y, Wang C, Zhu L, Wang S, Li Q, Jiang G, Zhao B, Huang H, Yu H. 2009. Rapid, accurate determination of multidrug resistance in M. tuberculosis isolates and sputum using a biochip system. Int J Tuberc Lung Dis 13:914–920. [PubMed] [Google Scholar]
- 11.Pang Y, Xia H, Zhang Z, Li J, Dong Y, Li Q, Ou X, Song Y, Wang Y, O'Brien R. 2013. Multicenter evaluation of GeneChip for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 51:1707–1713. doi: 10.1128/JCM.03436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He GX, Wang HY, Borgdorff MW, van Soolingen D, van der Werf MJ, Liu ZM, Li XZ, Guo H, Zhao YL, Varma JK, Tostado CP, van den Hof S. 2011. Multidrug-resistant tuberculosis, People's Republic of China, 2007–2009. Emerg Infect Dis 17:1831–1838. doi: 10.3201/eid1710.110546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Xu S, Wang L, Chin DP, Wang S, Jiang G, Xia H, Zhou Y, Li Q, Ou X. 2012. National survey of drug-resistant tuberculosis in China. N Engl J Med 366:2161–2170. doi: 10.1056/NEJMoa1108789. [DOI] [PubMed] [Google Scholar]
- 14.Devaux I, Kremer K, Heersma H, Van Soolingen D. 2009. Clusters of multidrug-resistant Mycobacterium tuberculosis cases, Europe. Emerg Infect Dis 15:1052–1060. doi: 10.3201/eid1507.080994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): global report on surveillance and response. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 16.Lu W, Chen C, Shao Y, Shi J, Zhong C, Yang D, Song H, Li G, Ding X, Peng H. 2012. Evaluation of biochip system in determining isoniazid and rifampicin resistances of Mycobacterium tuberculosis in sputum samples. PLoS One 7:e52953. doi: 10.1371/journal.pone.0052953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, Jiang G, Wang S, Wang C, Li Q, Yu H, Zhou Y, Zhao B, Huang H, Xing W. 2010. Biochip system for rapid and accurate identification of mycobacterial species from isolates and sputum. J Clin Microbiol 48:3654–3660. doi: 10.1128/JCM.00158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. 2012. Global tuberculosis control: WHO report 2012. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 19.Liu Q, Zhu L, Shao Y, Song H, Li G, Zhou Y, Shi J, Zhong C, Chen C, Lu W. 2013. Rates and risk factors for drug resistance tuberculosis in northeastern China. BMC Public Health 13:1171. doi: 10.1186/1471-2458-13-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao Y, Yang D, Xu W, Lu W, Song H, Dai Y, Shen H, Wang J. 2011. Epidemiology of anti-tuberculosis drug resistance in a Chinese population: current situation and challenges ahead. BMC Public Health 11:110. doi: 10.1186/1471-2458-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shargie EB, Lindtjørn B. 2007. Determinants of treatment adherence among smear-positive pulmonary tuberculosis patients in southern Ethiopia. PLoS Med 4:e37. doi: 10.1371/journal.pmed.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. 2008. Anti-tuberculosis drug resistance in the world: fourth global report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 23.Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D'Ambrosio L, Zignol M, Floyd K, Centis R, Cirillo DM, Tortoli E. 2013. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J 42:252–271. doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 24.Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam K, Rigouts L, Rüsch-Gerdes S, Wright A. 2009. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol 47:3501–3506. doi: 10.1128/JCM.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang CY, Van Deun A. 2013. Rapid diagnosis of rifampicin resistance: who needs confirmation? Int J Tuberc Lung Dis 17:2. doi: 10.5588/ijtld.12.0914. [DOI] [PubMed] [Google Scholar]
- 26.Xia Q, Braunstein SL, Stadelmann LE, Pathela P, Torian LV. 2014. The effect of case rate and coinfection rate on the positive predictive value of a registry data-matching algorithm. Public Health Rep 129(Suppl 1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K. 2014. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drobniewski F, Nikolayevskyy V, Balabanova Y, Bang D, Papaventsis D. 2012. Diagnosis of tuberculosis and drug resistance: what can new tools bring us? Int J Tuberc Lung Dis 16:860–870. doi: 10.5588/ijtld.12.0180. [DOI] [PubMed] [Google Scholar]
- 29.Rigouts L, Gumusboga M, de Rijk WB, Nduwamahoro E, Uwizeye C, de Jong B, Van Deun A. 2013. Rifampin resistance missed in automated liquid culture system for Mycobacterium tuberculosis isolates with specific rpoB mutations. J Clin Microbiol 51:2641–2645. doi: 10.1128/JCM.02741-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Deun A, Aung KJ, Bola V, Lebeke R, Hossain MA, de Rijk WB, Rigouts L, Gumusboga A, Torrea G, de Jong BC. 2013. Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol 51:2633–2640. doi: 10.1128/JCM.00553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


