Abstract
There is an urgent need for simple, rapid, and affordable diagnostic tests for tuberculosis (TB) to combat the great burden of the disease in developing countries. The microscopic observation drug susceptibility assay (MODS) is a promising tool to fill this need, but it is not widely used due to concerns regarding its biosafety and efficiency. This study evaluated the automated MODS (Auto-MODS), which operates on principles similar to those of MODS but with several key modifications, making it an appealing alternative to MODS in resource-limited settings. In the operational setting of Chiang Rai, Thailand, we compared the performance of Auto-MODS with the gold standard liquid culture method in Thailand, mycobacterial growth indicator tube (MGIT) 960 plus the SD Bioline TB Ag MPT64 test, in terms of accuracy and efficiency in differentiating TB and non-TB samples as well as distinguishing TB and multidrug-resistant (MDR) TB samples. Sputum samples from clinically diagnosed TB and non-TB subjects across 17 hospitals in Chiang Rai were consecutively collected from May 2011 to September 2012. A total of 360 samples were available for evaluation, of which 221 (61.4%) were positive and 139 (38.6%) were negative for mycobacterial cultures according to MGIT 960. Of the 221 true-positive samples, Auto-MODS identified 212 as positive and 9 as negative (sensitivity, 95.9%; 95% confidence interval [CI], 92.4% to 98.1%). Of the 139 true-negative samples, Auto-MODS identified 135 as negative and 4 as positive (specificity, 97.1%; 95% CI, 92.8% to 99.2%). The median time to culture positivity was 10 days, with an interquartile range of 8 to 13 days for Auto-MODS. Auto-MODS is an effective and cost-sensitive alternative diagnostic tool for TB diagnosis in resource-limited settings.
INTRODUCTION
Tuberculosis (TB) is an infectious disease of major concern worldwide, with the majority burden borne by developing countries (1). Multidrug resistant tuberculosis (MDR-TB) is a variant strain of TB that is resistant to at least rifampin (RIF) and isoniazid (INH), the two most common TB treatments. Thailand is among the highest TB-burdened countries with an estimated 110,000 TB cases (159 cases/100,000 population) in 2012 (1). Thailand also has one of the highest burdens of MDR-TB among Southeast Asian countries (1, 2). Mycobacterial laboratories in Thailand are faced with challenges of meeting the growing demands of the TB and MDR-TB epidemics, and simple, rapid, and affordable tests are urgently needed.
In the province of Chiang Rai as well as the other provinces in Thailand, the acid-fast bacillus (AFB) sputum-smear test is used in combination with chest X-ray and observation of clinical symptoms to diagnose TB in the hospital setting. Patients diagnosed in this way are registered as TB patients. This is followed by solid mycobacterial culture, which requires a long waiting period (approximately 1 month), to make a more conclusive diagnosis. Mycobacterium tuberculosis and nontuberculous mycobacteria (NTM) are not distinguished in the current practice. Liquid mycobacterial culture, which is faster but much more expensive, is used only for highly suspected TB cases in order to obtain a more definite diagnosis or for drug susceptibility testing (DST). Mycobacterial growth indicator tube (MGIT) 960 is an industry gold standard liquid mycobacterial culture apparatus, but due to its higher operational cost, it has limited use in resource-limited settings such as Chiang Rai, Thailand (3).
The microscopic observation drug susceptibility (MODS) assay is a rapid, economical, and highly sensitive/specific method for the detection of M. tuberculosis and DST directly from a sputum sample which was developed by a research team in Lima, Peru (4). The test uses 24-well plates with four wells for a single patient specimen: two wells are drug free, while the other two wells contain RIF and INH. After inoculation, the plates are sealed in zip-lock bags and then incubated. M. tuberculosis from sputum is rapidly grown in a liquid medium, and a diagnosis is made using morphological characterization patterns specific to M. tuberculosis following inoculation under an inverted light microscope (4). MODS has also been recommended by the World Health Organization (WHO) as an affordable and highly effective alternative to existing gold standard liquid mycobacterial culture methods for testing sputum samples of TB-suspected individuals (5).
Despite this, MODS is not widely used in resource-limited settings due to concerns regarding biosafety and efficiency for handling large numbers of samples (6). With respect to biosafety, the MODS assay uses liquid wells; manipulation of these liquids for inoculation carries the risk of aerosolization, spillage, cross-contamination, and occupational infection, though sealed plates may minimize this risk (6, 7). MODS also requires manual reading of each individual well by trained laboratory professionals, which requires both time and human resources given that a large number of sputum samples may need to be tested in high-TB-burdened settings. Moreover, there is also concern about MODS' ability to differentiate M. tuberculosis and NTM (7).
In this study, we evaluated the automated MODS (Auto-MODS), which was developed by the TB/HIV Research Foundation (THRF) in Chiang Rai, Thailand, in collaboration with University of Alberta, Canada, to address these concerns. We compared the performance of Auto-MODS for TB diagnosis and DST in reference to the gold standard method used in Thailand, i.e., MGIT 960 plus the SD Bioline TB Ag MPT64 (SD Bioline) test, in the real clinical setting of Chiang Rai, Thailand.
MATERIALS AND METHODS
Ethics.
The protocol of this study was approved by the Chiang Rai Regional Hospital Ethics Committee, Thailand, and University of Alberta's Health Research Ethics Board, Canada. The study was approved by the Department of Disease Control, Ministry of Public Health, Thailand.
Study patients and sample collection.
In the current clinical practice of Chiang Rai, Thailand, patients are diagnosed and registered as TB patients based on the AFB sputum smear test, chest X-ray, and clinical presentation. Since the diagnosis is made by physicians without mycobacterial culture results, we refer to these patients as “clinically diagnosed,” to indicate that the clinical diagnosis does not necessarily reflect the true TB status (which is defined by the gold standard, MGIT 960 plus SD Bioline).
Since 1996, clinically diagnosed TB patients in all 17 hospitals across Chiang Rai, Thailand, have been registered and required to send sputum samples to the mycobacterial laboratory of Chiang Rai Regional Hospital. Sputum samples routinely received from May 2011 to March 2012 were consecutively recruited into our study. Additionally, we collected samples from individuals who visited the same 17 hospitals between June 2012 to September 2012 and who were clinically diagnosed as non-TB patients.
The following inclusion criteria were used: (i) incident TB, defined by a clinical diagnosis; (ii) clinical diagnosis of non-TB with negative AFB smear test results; (iii) age of 18 years or older at clinic visit; and (iv) sputum samples collected prior to, or up to 2 weeks after, treatment initiation. Sputum samples that had a volume of less than 1 ml, that arrived at the laboratory without proper packaging, or that were cultured more than 1 week after arrival and those from extrapulmonary-TB patients were excluded from the study.
Laboratory methods. (i) Specimen processing.
To enhance the reliability of sampling and to obtain the most biologically active samples, each patient was asked for 3 sputum samples at three different times within 1 week. Only one sample was kept for culture in our study: if all 3 sputum samples were AFB smear negative, then the most active AFB smear-negative sputum sample (usually the most recently collected one) was kept. Otherwise, the most active AFB smear-positive sputum sample was chosen.
Sputum samples were immediately and safely stored after sampling in a refrigerator between 2°C and 8°C and were cultured within 7 days. All sputum samples were decontaminated and inoculated using the sodium hydroxide–N-acetyl-l-cysteine method (8) and were subsequently split into four aliquots, 500 μl, 500 μl, 300 μl, and 100 μl, to be used for MGIT 960 culture, Auto-MODS culture, Ogawa culture, and AFB smear slides, respectively. The detailed sample processing procedure is shown in Fig. S1 in the supplemental material.
(ii) MGIT 960 culture and SD Bioline test techniques.
MGIT 960, which was endorsed in 2007 by the WHO (9), served as the gold standard reference method in this study in identifying culture-positive samples (either TB or NTM) and drug-resistant TB. The SD Bioline test, an immunochromatographic assay using mouse monoclonal antibodies to detect MPT64 antigen/protein, which is specific for M. tuberculosis complex, was used in combination with the MGIT 960 to distinguish TB and NTM in the National Tuberculosis Reference Laboratory (NTRL) as a routine reference method (10). Our goal was to evaluate the performance of Auto-MODS against MGIT 960 plus the SD Bioline test.
Mycobacterial culture by MGIT 960 was performed at the mycobacterial laboratory of the Chiang Rai Regional Hospital. Culture-positive samples were subcultured to obtain mycobacteria isolates and were then sent to the NTRL in Thailand, where the SD Bioline TB test was used to distinguish TB and NTM and DST by MGIT 960 was conducted to identify drug-resistant TB cases. This is a routine process in Thailand, as mycobacterial culture can be done in mycobacterial laboratories of regional hospitals, while DST can be done only at the NTRL to ensure biosafety. The MGIT 960 culture testing and DST adhered strictly to the guidelines outlined in the official MGIT 960 manual (11).
A culture result was determined by the MGIT 960 instrument and then confirmed by visual observation of cord formation followed by an AFB smear test. A culture-positive result was reported only if the MGIT 960 instrument and AFB smear test both showed a positive result. A culture-negative result was reported if a positive culture result was not observed for 42 days. Contaminated culture results were confirmed by visual observation of rapid overgrowth and clouding of the MGIT 960 tube.
(iii) Auto-MODS culture techniques.
The techniques of the original MODS assay have been described previously (4, 12). Briefly, broth cultures are prepared in a 24 well-plate apparatus with 4 wells per sputum sample (2 well with culture, 1 well with INH, 1 well with RFP). To minimize cross-contamination and occupational exposure, it is recommended that plates be permanently sealed inside plastic zip-lock bags after inoculation. The cultures in each well are examined under an inverted light microscope.
Four modifications to the original MODS assay (4) were made for Auto-MODS.
(a) Modification 1.
To reduce the risk of occupational exposure to and cross-contamination between samples, 1.5-ml screw-cap tubes were used instead of the well plates.
(b) Modification 2.
To differentiate TB and NTM, a p-nitrobenzoic acid (PNB)-containing tube was used. The growth of M. tuberculosis is inhibited by PNB, whereas NTM (including species such as Mycobacterium chelonae, M. kansasii, the M. avium complex, and M. marinum) are resistant (4, 13, 14). Auto-MODS therefore uses 5 tubes for each sputum sample (2 tubes with culture, 1 tube with INH, 1 tube with RFP, and 1 tube with PNB) (Fig. 1).
FIG 1.
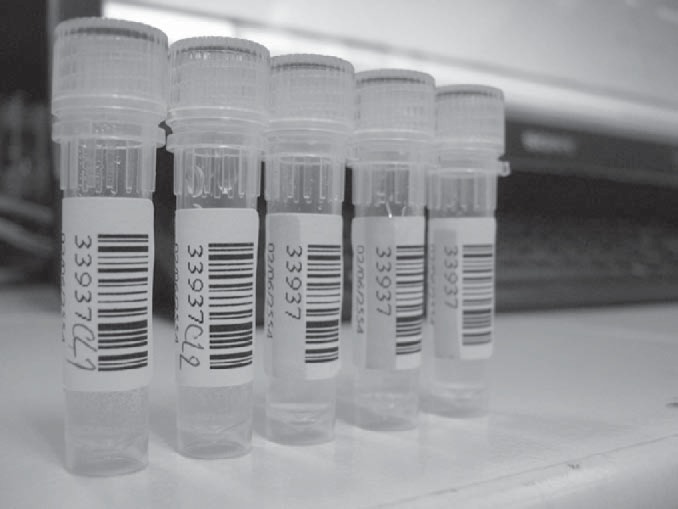
Five-tube design of Auto-MODS.
(c) Modification 3.
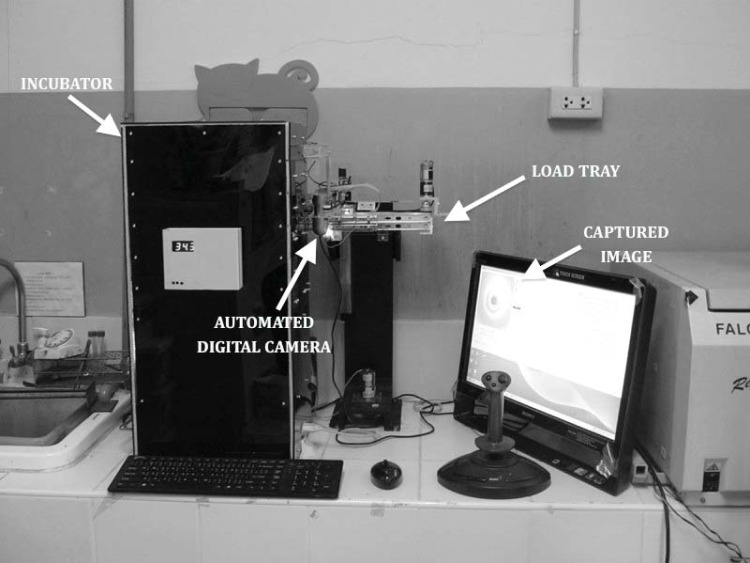
To enhance biosafety and to reduce the human-resource need for frequent reading, a computer-assisted digital camera to automatically and consecutively take images of each tube was used in Auto-MODS (Fig. 2). Images taken by the digital camera once per day were automatically transferred to the computer and available for daily visual reading by the laboratory technician directly on the computer screen.
FIG 2.
Automated image-capturing system.
(d) Modification 4.
To enhance the digital image quality by reducing background noise, a low-speed centrifuge was applied to remove large particles in the liquid before inoculation of each sample into the Auto-MODS tube (see Fig. S1 in the supplemental material).
The identification of TB and testing of drug susceptibility were performed simultaneously using Auto-MODS. The Middlebrook 7H9 broth culture was used (15). The concentrations of PNB, INH, and RFP used in each tube were 0.5 mg/ml, 0.1 μg/ml, and 1 μg/ml, respectively. Tubes were incubated at 37°C in an incubator and images were captured by a computer-assisted digital camera daily from each tube automatically. Both the incubator and the digital camera were integrated into the Auto-MODS system.
The criteria for interpretation of Auto-MODS results were the same as those for MODS (15), except for one change: if one of the two tubes with culture was positive and the other was contaminated, the result was interpreted as positive. The remaining three tubes were examined only if the culture result was found to be positive. In addition to the culture-positive result, a negative PNB (no growth of TB) tube was interpreted as TB positive and a positive PNB tube was interpreted as NTM. Growth in a drug-containing tube indicated resistance against the corresponding drug.
For the purpose of quality control, three positive-control tubes were used for each run of Auto-MODS, containing (i) M. tuberculosis H37Rv; (ii) an INH-resistant strain; and (iii) an RFP-resistant strain. In addition, we used a tube with NTM as a non-TB control in each run.
(iv) Ogawa solid culture.
The Ogawa solid culture method is a highly sensitive and specific method and is routinely used in Chiang Rai, Thailand, for the majority of TB sputum samples due to a relatively low cost. The Ogawa solid culture was also performed in this study for the purpose of evaluating the speed of Auto-MODS.
(v) Result reading.
All readings of the Auto-MODS cultures and MGIT 960 cultures conducted in the mycobacterial laboratory of the Chiang Rai Regional Hospital were performed by the same technician directly on the computer screen after a digital image of the sample tube was taken. The technician, B.C., is a certified medical technologist and the laboratory coordinator for the TB/HIV Foundation. He has over 20 years of experience working as a laboratory technician, and he had worked with the original MODS for over 6 months and handled over 300 samples during that time. He was blinded to the culture results of one test when he read the results of the other test. For Auto-MODS, only the captured digital images, not the actual tubes, were read.
Statistical analysis.
Sensitivity, specificity, and positive and negative likelihood ratios were calculated to evaluate the performance of Auto-MODS in reference to MGIT 960. The proportion of contamination and the median time to culture positivity of both Auto-MODS and MGIT 960 were calculated. McNemar's test was used to compare the contamination rates of Auto-MODS and MGIT 960. For samples that were culture positive by both Auto-MODS and MGIT 960, the times to culture positivity in Auto-MODS and MGIT 960 were compared using the Wilcoxon signed-rank test. A P value less than 0.05 was used to indicate statistical significance. The 95% exact confidence intervals (CIs) were calculated under the binomial assumption. Stata 12.0 software was used for statistical analysis.
RESULTS
A total of 380 sputum samples from clinically diagnosed TB patients and 138 from clinically diagnosed non-TB subjects were collected. Among clinically diagnosed TB patients, 39 were excluded due to nonincident cases, 7 were excluded due to age under 18, 53 were excluded because they had already been receiving treatment for more than 2 weeks, 18 were excluded because samples were cultured more than 1 week after collection, and 6 were excluded because the site of TB was extrapulmonary. Among clinically diagnosed non-TB subjects, 3 were excluded due to age under 18. Therefore, a total of 257 clinically diagnosed TB patients and 135 clinically diagnosed non-TB subjects were included in the study. The characteristics of these 392 patients are shown in Table 1.
TABLE 1.
Characteristics of 392 patients at sample collection
| Characteristic | No. (%) clinically diagnosed with: |
|
|---|---|---|
| TB | Non-TB | |
| Sex | ||
| Male | 177 (68.9) | 80 (59.3) |
| Female | 80 (31.1) | 55 (40.7) |
| Age | ||
| Median [IQR] | 48 [36–62] | 54 [39–64] |
| Race | ||
| Thai | 169 (65.8) | 101 (74.8) |
| Non-Thai | 37 (14.4) | 7 (5.2) |
| Hill tribe | 33 (12.8) | 27 (20.0) |
| Unknown | 18 (7.0) | 0 (0) |
| Patient type | ||
| New | 245 (95.3) | |
| Relapse | 12 (4.7) | |
| AFB smear result | ||
| Positive | 212 (82.5) | 0 (0) |
| Negative | 45 (17.5) | 135 (100) |
| HIV status | ||
| Negative | 195 (75.9) | 0 (0) |
| Positive | 45 (17.5) | 0 (0) |
| Unknown | 17 (6.6) | 135 (100) |
A total of 392 sputum samples were cultured by both MGIT 960 and Auto-MODS. Among these, 6 sputum samples were contaminated during both the MGIT 960 and Auto-MODS culture process; 11 samples were contaminated during the MGIT 960 culture process only; and 12 samples were contaminated during the Auto-MODS culture process only. There was no statistically significant difference in the contamination rate: 4.3% for MGIT 960 and 4.6% for Auto-MODS (P = 0.83). Both culture methods conducted in the Mycobacterial Laboratory in Chiang Rai Regional Hospital meet the contamination rate criterion of less than 8% (11).
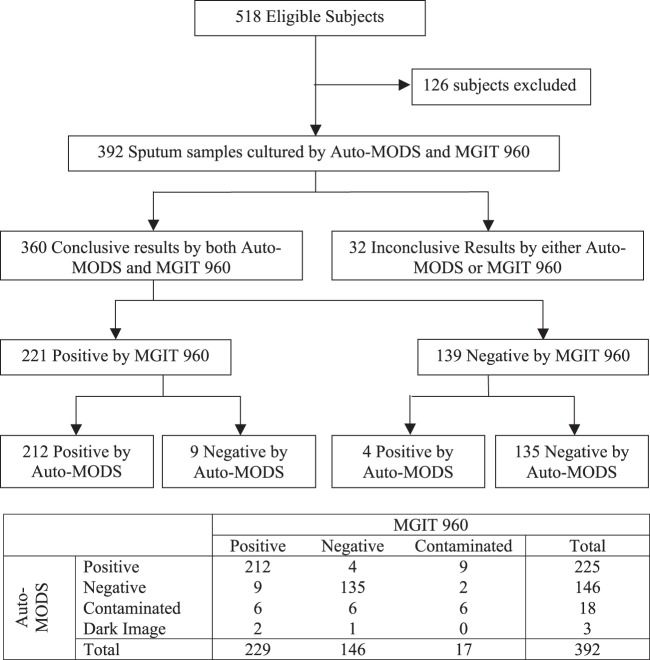
In addition, three clinically diagnosed TB samples were excluded from the analysis because the image taken by the Auto-MODS digital camera was too dark to lead to a confirmed result. In total, this left us with 360 sputum samples for comparative analysis. The recruitment of subjects and culture results is shown in a flowchart (Fig. 3).
FIG 3.
STARD flowchart showing recruitment of subjects and culture results.
Auto-MODS performance in reporting culture results.
The validity and speed of Auto-MODS in reporting the culture positivity were evaluated in reference to MGIT 960 culture: that is, we took MGIT 960 culture results as true in distinguishing culture-positive samples (either TB or NTM) from culture-negative samples (non-TB).
Validity.
Of the 360 samples, 221 (61.4%) were positive and 139 (38.6%) were negative by MGIT 960 for mycobacterial cultures. Of the 221 true-positive samples, Auto-MODS reported 212 positives and 9 negatives, yielding a sensitivity of 95.9% (95% CI, 92.4% to 98.1%). Of the 139 true-negative samples, Auto-MODS reported 135 negatives and 4 positives, yielding a specificity of 97.1% (95% CI, 92.8% to 99.2%). The positive and negative likelihood ratios for Auto-MODS in reference to MGIT 960 were 33.3 (95% CI, 12.7 to 87.6) and 0.04 (95% CI, 0.02 to 0.08), respectively. Sensitivity analysis, carried out by splitting the samples into those with a 0- to 3-day culture delay and those with a 4- to 7-day culture delays, did not affect the reported results (see Tables S1 and S2 in the supplemental material). The culture results, overall analysis results, and sensitivity and specificity estimates based on AFB smear-positive and AFB smear-negative samples separately are shown in Tables 2 and 3.
TABLE 2.
Culture results of Auto-MODS in reference to MGIT 960
| Auto-MODS result | No. with MGIT 960 result |
|||||
|---|---|---|---|---|---|---|
| Overall |
AFB+ |
AFB− |
||||
| + | − | + | − | + | − | |
| + | 212 | 4 | 187 | 3 | 25 | 1 |
| − | 9 | 135 | 3 | 3 | 6 | 132 |
| Total | 221 | 139 | 190 | 6 | 31 | 133 |
TABLE 3.
Performance measurements of Auto-MODS in reference to MGIT 960a
| Samples | % (95% CI) |
|||
|---|---|---|---|---|
| Sensitivity | Specificity | LR+ | LR− | |
| Overall | 95.9 (92.4–98.1) | 97.1 (92.8–99.2) | 33.3 (12.7–87.6) | 0.04 (0.02–0.08) |
| AFB+ | 98.4 (95.5–99.7) | 50.0 (11.8–88.2) | 1.97 (0.88–4.38) | 0.03 (0.01–0.13) |
| AFB− | 80.6 (62.5–92.5) | 99.2 (95.9–100) | 107.3 (15.1–760) | 0.20 (0.10–0.40) |
LR+, positive likelihood ratio; LR−, negative likelihood ratio.
Speed.
For the 221 true-culture-positive samples, the median time to culture positivity was 10 days (interquartile range [IQR], 8 to 13 days) for Auto-MODS. Ninety percent of those samples were culture positive within 3 weeks. This is statistically and clinically meaningfully faster than the Ogawa solid culture method, which has a median time to positive culture of 30 days (IQR, 23 to 40 days) (P < 0.0001). Auto-MODS was statistically slower than the gold standard method, MGIT 960, which has a median of 6 days (IQR, 5 to 9 days) (P < 0.0001) (Fig. 4).
FIG 4.
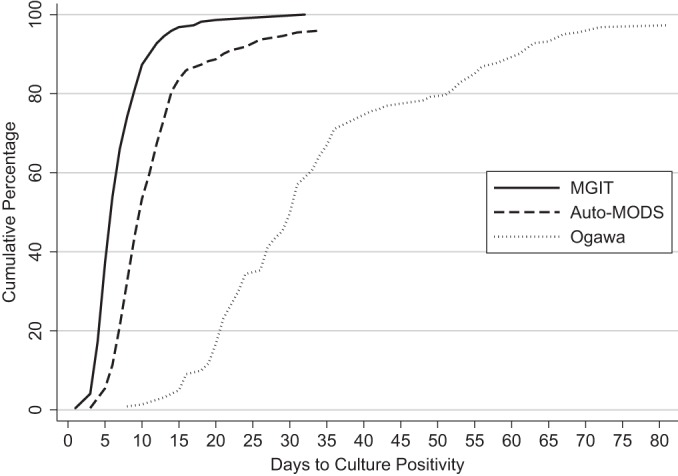
Cumulative probability of culture positivity of Auto-MODS, MGIT 960, and Ogawa by days since the start of assay.
Reporting NTM and drug susceptibility testing (DST).
The performance of Auto-MODS in distinguishing TB and NTM and drug susceptibility testing was evaluated based on the 212 samples that were culture positive by both MGIT 960 and Auto-MODS. Of the 212 positive culture samples, 188 remained for further analysis: 8 samples were excluded due to contamination by Auto-MODS in either the PNB tube or drug-containing tubes, and 16 samples were excluded because of the limitations of conducting DST by MGIT 960 (the subculturing process resulted in contaminated mycobacterial isolates or it did not yield sufficient mycobacterial isolates).
Among these 188 samples, both the SD Bioline test and Auto-MODS identified the same 183 TB samples and the same 5 NTM samples. DST results for the 183 TB samples in MGIT 960 and Auto-MODS are presented in Table 4.
TABLE 4.
Drug susceptibility testing results from Auto-MODS compared to MGIT 960
| Auto-MODS result | No. of samples with MGIT result |
|||||
|---|---|---|---|---|---|---|
| Rifampin |
Isoniazid |
MDR-TB |
||||
| Resistant (+) | Susceptible (−) | Resistant (+) | Susceptible (−) | Resistant (+) | Susceptible (−) | |
| Resistant (+) | 3 | 0 | 12 | 3 | 3 | 0 |
| Susceptible (−) | 0 | 180 | 1 | 167 | 0 | 180 |
| Total | 3 | 180 | 13 | 170 | 3 | 180 |
Of the 3 rifampin-resistant TB samples and 13 isoniazid-resistant TB samples, Auto-MODS reported 3 (100%) and 12 (92.3%), respectively. Among these, there were 3 MDR-TB samples (both rifampin and isoniazid resistant), all of which were reported by Auto-MODS (100%). The sensitivity is not reported here due to the limitation of small sample size for drug-resistant TB. The specificity values for detection by Auto-MODS of rifampin-susceptible TB, isoniazid-susceptible TB, and non-MDR-TB were 100% (95% CI, 98.0% to 100%), 98.2% (95% CI, 94.9% to 99.6%), and 100% (95% CI, 97.9% to 100%). Thus, Auto-MODS is a highly specific test for DST.
DISCUSSION
Missed and misdiagnosed TB cases are key concerns preventing effective control of TB; 2.9 million missed TB cases occurred worldwide in 2012 (1). Given the high disease burden and constrained financial and technical resources in developing countries, conventional tools for rapid TB detection and DST, such as the gold standard MGIT 960, are not practical because of high operational costs and advanced laboratory requirements.
The MODS is a noncommercial laboratory method which is feasible for use in any laboratory setting that is equipped with an incubator, a centrifuge, and an inverted light microscope (4, 12). Numerous studies have demonstrated MODS to be a valid and cost-effective alternative tool for TB diagnosis in resource-limited settings (4, 6, 12). However, the MODS requires a greater workforce and has biosafety concerns, which restricts its use in hospital settings (12).
The samples used in this study came from actual patients who were seeking a TB diagnosis in the highly TB-burdened and resource-limited setting of Chiang Rai, Thailand. Sputum sample collection, transportation, storage, and processing were conducted through the routine system currently used in Chiang Rai, Thailand. Thus, the strength of our study is rooted in its clinically relevant, real-world design. One limitation of our study is that no back-up samples were stored, so we were unable to report results for the approximately 5% of the samples that were contaminated. Another limitation is that the reference methods, MGIT 960 and the SD Bioline TB test, may not fully correspond to the truth, but the potential degree of misclassification should be minimal.
We found Auto-MODS to be both a highly sensitive and specific test for TB detection. When a culture-positive result is reported by Auto-MODS, we can effectively rule in the diagnosis of TB. When a culture-negative result is reported by Auto-MODS, we can effectively rule out the diagnosis of TB. This means that Auto-MODS has a low risk of missing TB cases, which qualifies it as a highly sensitive screening assay for an infectious disease like TB. Also, the first priority in the 2013 WHO global TB report for combatting TB is to reach the missed cases (1). In practice, if the culture results by Auto-MODS are to be used in combination with AFB smear results to draw a diagnosis conclusion, patients with negative AFB smear results should be diagnosed as TB patients if the Auto-MODS culture result is positive.
The high specificity of Auto-MODS in performing DST means that once a positive drug-resistant result is reported by Auto-MODS, we have confidence in diagnosing a patient as having drug-resistant TB. However, due to the small sample size of MDR-TB in our study, the evaluation of the sensitivity of Auto-MODS in performing DST requires further study.
Auto-MODS presents several potential advantages for its use in hospitals. (i) All samples are cultured in screw-cap tubes, reducing the biohazard risk to laboratory operators. This is expected to also reduce cross-contamination after inoculation (although it is possible that cross-contamination may already have occurred prior to inoculation). In our study, among 392 cultured samples, there were 4 samples that were culture positive in one of the two tubes with culture and contaminated in another. Three of them were truly positive according to the gold standard MGIT 960. Thus, only one sample might be cross contaminated. (ii) The automatic image capturing system contributes to reduced human effort and increased biosafety. (iii) Digital copies of the culture results by an assisted computer enable sharing and review of the results, even remotely, at any time. (iv) The five-tube design simplifies the culturing and DST into a one-step process. (v) A PNB tube was used to differentiate between TB and NTM. The use of PNB in combination with liquid culture methods such as MGIT 960 and MODS to distinguish TB and NTM has been reported in other studies (13, 16). However, it is important to note that PNB may not be able to inhibit the growth of rapid growers other than NTM that have a cord factor. This is a limitation of both MGIT 960 and Auto-MODS.
Compared to MGIT, Auto-MODS might have a lower biohazard risk, because tubes were not opened during the culturing process. Meanwhile, MGIT is opened when there is a high TB count in the culture, samples are smeared on slides, and the tubes are subsequently reinoculated for DST; these processes carry significant biosafety concerns. However, caution should be exercised by technicians because both MGIT and Auto-MODS require decontamination of sputum samples, which also involves substantial biohazard risk.
The time to report DST results is the same as the time to report culture positivity in Auto-MODS, based on the rationale of our design. However, in addition to the time of reporting TB cases, approximately another 7 days is needed to report drug susceptibility results by MGIT 960, given that DST process is performed after the mycobacterial culture in MGIT 960 and subculture on solid media. Also, in Chiang Rai, Thailand, DST is routinely performed at the NTRL, which requires an additional subculture and waiting time to receive results; this also increases the likelihood of contamination.
However, compared to the gold standard, MGIT 960, which is able to test different sample types (i.e., urine and cerebrospinal fluid), Auto-MODS is currently limited to sputum samples from pulmonary-TB patients. Also, the automatic image-capturing system had two drawbacks: (i) compared to MODS, a slightly longer time to culture positivity was observed (4, 12), and (ii) of the 257 sputum samples from clinically diagnosed patients, Auto-MODS failed to report results for 3 samples because the captured images were too dark at the start and it was not possible to observe cord formation. Future work to fine-tune the automatic image capturing system should consider this point.
Recent work using an autofocus algorithm for automatic diagnosis through pattern recognition has already shown promise for TB detection in resource-limited settings, further reducing the need for manual labor (17, 18). Our team is also near completion of an image analysis algorithm for fully automated pattern recognition (19).
These modifications came at a slightly increased cost for initially setting up Auto-MODS ($7,000) compared to MODS ($5,000 [$3,000 for machine and $2,000 for incubator]). But the consumption costs are the same for Auto-MODS and MODS in terms of TB detection and two-drug DST, at approximately $2.00 per sample. As for labor costs, although they were not evaluated in this study, it is reasonable to expect lower labor costs of Auto-MODS given the automated image-capturing system. Compared to the gold standard, MGIT 960, Auto-MODS is cost-effective with regard to both initial set-up ($7,000 versus $85,000) and individual sample cost ($2.00 versus $20.00). These costs are estimates made by experienced medical technicians in the mycobacterial laboratory of Chiang Rai Regional Hospital and the NTRL.
As evidenced by this study, Auto-MODS holds significant potential as a cost-effective tool for TB diagnosis. Future work to evaluate Auto-MODS's capability for use in DST is needed to solidify its promise for use in resource-limited settings.
Supplementary Material
ACKNOWLEDGMENTS
The development of Auto-MODS and this study to evaluate it would not have been possible without the generous support of the Alberta Innovates Centre for Machine Learning (AICML), Canada.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01946-14.
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2012. Tuberculosis control in the South-East Asia region. World Health Organization, Regional Office for South-East Asia, New Delhi, India. [Google Scholar]
- 3.World Health Organization UNITAID. 2012. Tuberculosis diagnostic technology landscape. UNITAID Secretariat, World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Caviedes L, Lee T, Gilman RH, Sheen P, Spellman E, Lee EH, Berg DH, Montenegro-James S, the Tuberculosis Working Group in Peru . 2000. Rapid, efficient detection and drug susceptibility testing of mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J Clin Microbiol 38:1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. 2011. Noncommercial culture and drug-susceptibility testing methods for screening patients at risk for multidrug-resistant tuberculosis. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 6.Moore DAJ. 2007. Future prospects for the MODS assay in multidrug-resistant tuberculosis diagnosis. Future Microbiol 2:97–101. doi: 10.2217/17460913.2.2.97. [DOI] [PubMed] [Google Scholar]
- 7.Kam KM. 2014. Microscopic observation drug susceptibility (MODS): where are we going? Int J Tuberc Lung Dis 18:127. doi: 10.5588/ijtld.13.0777. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 1998. Laboratory services in TB controls: parts I, II and III. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.World Health Organization. 2007. Use of liquid TB culture and drug susceptibility testing (DST) in low and medium income settings: summary report of the expert group meeting on the use of liquid culture media. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 10.World Health Organization UNITAID. 2014. Tuberculosis diagnostic technology and market landscape, 3rd ed. UNITAID Secretariat, World Health Organization, Geneva, Switzerland. [Google Scholar]
- 11.Siddiqi S, Rüsch-Gerdes S. 2006. MGIT procedure manual. Foundation for Innovative New Diagnostics, Geneva, Switzerland. [Google Scholar]
- 12.Moore DAJ, Evans CAW, Gilman RH, Caviedes L, Coronel J, Vivar Aldo Sanchez E, Pinedo Y, Saravia JC, Salazar C, Oberhelman R, Hollm-Delgado M, LaChira D, Escombe AR, Friedland JS. 2006. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med 355:1539–1550. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma B, Pal N, Malhotra B, Vyas L. 2010. Evaluation of a rapid differentiation test for Mycobacterium tuberculosis from other mycobacteria by selective inhibition with p-nitrobenzoic acid using MGIT 960. J Lab Physicians 2:89. doi: 10.4103/0974-2727.72157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staropoli JF, Branda JA. 2008. Cord formation in a clinical isolate of Mycobacterium marinum. J Clin Microbiol 46:2814. doi: 10.1128/JCM.00197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coronel J, Roper M, Caviedes L, Moore D. 2008. MODS: a user guide. Universidad Peruana Cayetano Heredia, Lima, Peru: http://www.modsperu.org/MODS_user_guide.pdf. [Google Scholar]
- 16.Agarwal A, Dhole TN, Sharma YK. 2014. Evaluation of p-nitro benzoic acid (pnb) inhibition test to differentiate Mycobacterium tuberculosis from non-tuberculous mycobacteria using microscopic observation of drug susceptibility (MODS) methodology. Indian J Tuberc 61:232–235. [PubMed] [Google Scholar]
- 17.Alva A, Aquino F, Gilman RH, Olivares C, Requena D, Gutiérrez AH, Zimic M. 2013. Morphological characterization of Mycobacterium tuberculosis in a MODS culture for an automatic diagnostics through pattern recognition. PLoS One 8:e82809. doi: 10.1371/journal.pone.0082809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comina G, Mendoza D, Velazco A, Coronel J, Sheen P, Gilman RH, Zimic M. 2011. Development of an automated MODS plate reader to detect early growth of Mycobacterium tuberculosis. J Microsc 242:325–330. doi: 10.1111/j.1365-2818.2010.03477.x. [DOI] [PubMed] [Google Scholar]
- 19.Li Q. 2013. Development of statistical methods for the analysis of high-dimensional biological data. M.Sc. dissertation. University of Alberta, Edmonton, Alberta, Canada. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.