Abstract
Alpha-toxin is a major Staphylococcus aureus virulence factor. This study evaluated potential relationships between in vitro alpha-toxin expression of S. aureus bloodstream isolates, anti-alpha-toxin antibody in serum of patients with S. aureus bacteremia (SAB), and clinical outcomes in 100 hemodialysis and 100 postsurgical SAB patients. Isolates underwent spa typing and hla sequencing. Serum anti-alpha-toxin IgG and neutralizing antibody levels were measured by using an enzyme-linked immunosorbent assay and a red blood cell (RBC)-based hemolysis neutralization assay. Neutralization of alpha-toxin by an anti-alpha-toxin monoclonal antibody (MAb MEDI4893) was tested in an RBC-based lysis assay. Most isolates encoded hla (197/200; 98.5%) and expressed alpha-toxin (173/200; 86.5%). In vitro alpha-toxin levels were inversely associated with survival (cure, 2.19 μg/ml, versus failure, 1.09 μg/ml; P < 0.01). Both neutralizing (hemodialysis, 1.26 IU/ml, versus postsurgical, 0.95; P < 0.05) and IgG (hemodialysis, 1.94 IU/ml, versus postsurgical, 1.27; P < 0.05) antibody levels were higher in the hemodialysis population. Antibody levels were also significantly higher in patients infected with alpha-toxin-expressing S. aureus isolates (P < 0.05). Levels of both neutralizing antibodies and IgG were similar among patients who were cured and those not cured (failures). Sequence analysis of hla revealed 12 distinct hla genotypes, and all genotypic variants were susceptible to a neutralizing monoclonal antibody in clinical development (MEDI4893). These data demonstrate that alpha-toxin is highly conserved in clinical S. aureus isolates. Higher in vitro alpha-toxin levels were associated with a positive clinical outcome. Although patients infected with alpha-toxin-producing S. aureus exhibited higher anti-alpha-toxin antibody levels, these levels were not associated with a better clinical outcome in this study.
INTRODUCTION
Staphylococcus aureus is a leading cause of bacterial infections (1–4), including skin and soft tissue infections (5), pneumonia (6), bacteremia (7), endocarditis (8–10), and bone and joint infections (11). The risk of invasive S. aureus infections is significantly higher among certain subgroups, including hemodialysis-dependent patients and postoperative patients (12–14).These high-risk subpopulations are potential candidates for novel forms of prevention or treatment against invasive S. aureus infections.
Alpha-toxin, a β-barrel pore-forming exotoxin encoded by hla (15, 16), is a key virulence factor produced by most S. aureus isolates (17, 18). It binds to ADAM10 (the A disintegrin and metalloproteinase domain-containing protein 10) on target cell membranes and then heptamerizes to generate a transmembrane pore, resulting in cell lysis (19). Hyperproduction of alpha-toxin is associated with enhanced virulence in strains of both epidemic (USA300 and USA500) and endemic (ST93) community-associated methicillin-resistant S. aureus (CA-MRSA) isolates (20, 21). Studies with a number of animal models have also suggested that alpha-toxin is a key virulence factor in the pathogenesis of S. aureus infections, including pneumonia (22–25), skin and soft tissue infections (26, 27), and bloodstream infections (28).
Alpha-toxin represents an attractive target for novel immunotherapeutic approaches for prevention or treatment of invasive S. aureus infections (29–31). In murine S. aureus skin and soft tissue infection models, both active immunization with a nontoxigenic alpha-toxin mutant and passive immunization with alpha-toxin-specific antiserum or IgG significantly improved survival and reduced disease severity (27, 32–34). Antibodies against alpha-toxin were both necessary and sufficient for protection against soft tissue infection and were inversely associated with bacterial counts (32) and had a protective role in keratitis (35). Also, a recent report suggested that higher antibody (Ab) levels against alpha-toxin have a protective role against sepsis in patients with invasive S. aureus infections (36). Despite these important insights, the complex interplay between alpha-toxin production by bloodstream S. aureus isolates, anti-alpha-toxin antibody production by bacteremic patients, and the clinical outcomes for such patients is incompletely understood.
In the current study, we assessed in vitro alpha-toxin expression in S. aureus isolates and anti-alpha-toxin antibodies in patients with S. aureus bacteremia (SAB). Using acute-phase serum and the associated bloodstream bacterial isolates from a cohort of prospectively ascertained, clinically well-characterized patients with SAB, we evaluated potential relationships between in vitro alpha-toxin production and anti-alpha-toxin-specific IgG and neutralizing antibody levels to clinical outcome. We also sequenced hla genes from the bloodstream isolates to explore relationships between variations among hla genes and the clinical severity of infection. Finally, we tested the effectiveness of MEDI4893, a broadly reactive anti-alpha-toxin monoclonal antibody (MAb), in neutralizing alpha-toxin expressed by all of the hla variants identified in this investigation.
MATERIALS AND METHODS
Patient selection and ethics statement.
The Duke University Institutional Review Board approved this investigation. Eligible patients for the current study met all of the following criteria: (i) unique adult patients hospitalized at Duke University Medical Center with monomicrobial S. aureus bacteremia; (ii) no neutropenia (absolute neutrophil count of >100 neutrophils/μl); (iii) availability of (a) clinical data, (b) a bloodstream S. aureus isolate, and (c) acute-phase serum in the Staphylococcus aureus Bacteremia Group (SABG) Biorepository; (iv) presence of either (a) end-stage renal disease necessitating chronic hemodialysis or (b) a surgical procedure in the preceding 30 days. Samples from 100 hemodialysis patients and 100 postsurgical patients meeting these criteria and collected from July 2001 to December 2009 were included in this study. Serum samples from healthy adults ages 18 to 60 years were purchased from Bioreclamation, Westbury, NY, and used as controls.
Clinical outcomes and definitions.
Clinical outcomes were established by site investigators. Outcomes were defined as either “cure” or “failure.” A patient's outcome was defined as a cure if he or she was alive with no evidence of recurrent S. aureus infection at 12 weeks after the initial positive blood culture. An outcome of failure was defined as a patient who died for any reason or experienced culture-confirmed recurrent S. aureus infection within 12 weeks of the initial positive blood culture. Hemodialysis patients were defined as patients with end-stage renal disease necessitating chronic hemodialysis. Postsurgical patients were defined as patients who had undergone a surgical procedure in the preceding 30 days.
Staphylococcus aureus isolates.
S. aureus isolates were identified from the bloodstreams of patients who provided written informed consent, and samples were stored at −80°C until use. Isolates were linked to the clinical details relevant to the study subject's case by way of a unique study number. For patients with multiple positive blood cultures, the initial isolate was used for all characterizations.
spa typing and identification of clonal complexes.
S. aureus genomic DNA was extracted as described previously (37) by using an ultraclean microbial DNA kit (MO BIO Laboratories, Inc., Carlsbad, CA) in accordance with the manufacturer's instructions. spa typing was performed as previously described (38, 39). Briefly, eGenomics software was used to scan the primary sequences and to determine the spa types. The spa type number is representative of the repeat organization. Clonal complexes (CCs) for the isolates were identified via repeats pattern recognition from existing spa type and CC databases.
hla PCR and sequencing.
S. aureus genomic DNA extracted for spa typing was also used for hla PCR and sequencing. Two microliters of each sample DNA was PCR amplified under the following conditions using Fast Cycling PCR master mix (Qiagen, Valencia, CA) and 0.5 μM each forward and reverse primer (Hla-PCR-F1, 5′-TGTCTCAACTGCATTATTCTAAATTG-3′, and Hla-PCR-R1, 5′-CATCATTTCTGATGTTATCGGCTA-3′): an initial denaturation step of 95°C for 2 min, 35 cycles of 96°C for 30 s, 55°C for 30 s, and 68°C for 90 s, followed by 72°C for 2 min. Five to 20 ng of each PCR amplicon was sequenced with the BigDye Terminator cycle sequencing kit v3.1 (Applied Biosystems, Grand Island, NY) using the following primers: Hla-PCR-F1 and Hla-PCR-R1 (sequences above), Hla-Fr (5′-TGCAAATGTTTCAATTGGTCATAC-3′), AT_2F (5′-GCAGATTCTGATATTAATATTAAAAC-3′), and Hla-Re (5′-TCCCCAATTTTGATTCACCA-3′) (40).The consensus DNA sequences were translated to protein sequences, and amino acid variants were noted based on comparison to the published USA 300 hla sequence CP000255_SAUSA300_1058, used as a reference (41).
Monoclonal anti-alpha-toxin antibody MEDI4893.
MEDI4893 is a human immunoglobulin G1 kappa (IgG1κ) MAb with an extended half-life that binds alpha-toxin with high affinity and effectively blocks alpha-toxin pore formation in target cell membranes. MEDI4893 is composed of 2 identical light chains and 2 identical heavy chains and was derived from the parent progenitor molecule LC10 (34) by three amino acid substitutions (M252Y/S254T/T256E; also referred to as YTE) introduced into the heavy-chain CH2 constant region of the fragment crystallizable (Fc) domain to increase binding to the neonatal Fc receptor (FcRn) and consequently increase the serum half-life (42). MEDI4893 was expressed by a Chinese hamster ovary cell line and has an overall molecular mass of approximately 148 kDa.
Rabbit erythrocyte lysis assay.
Assessment of in vitro alpha-toxin production was done by quantitative analysis of rabbit red blood cell (RBC) lysis as previously described by our group (43) and others (44, 45). Briefly, 10 ml of tryptic soy broth (TSB; Becton Dickinson, Sparks, MD) in a 50-ml Falcon tube was inoculated with a loopful of culture from a fresh plate of each strain and incubated at 37°C and 220 rpm for overnight culture. The appropriate amount of overnight culture was inoculated into either 10 ml of Mueller-Hinton broth 2 (Sigma, St. Louis, MO) or 10 ml of TSB in a 50-ml Falcon tube to normalize the starting optical density at 600 nm (OD600) to 0.1 and incubated at 37°C and 220 rpm for 20 h. After 20 h of incubation, the culture was spun down at 3,100 rpm at 4°C for 10 min to remove the pellets. The supernatant was then filter sterilized and transferred to a sterile tube and stored at −80°C until further use.
The ability of the culture supernatant to lyse rabbit erythrocytes (RBC) was tested in a 96-well format. Specifically, 100 μl of 1:5-diluted culture supernatant in 1× phosphate-buffered saline (PBS) from each isolate was loaded into the first well and then serially diluted 2-fold, up to 1:80, in duplicate. After the dilution of each sample, 100 μl of 1% rabbit RBC (Innovative Research, Novi, MI) in 1× PBS was added to each well and incubated at 37°C for 1 h. Following incubation, plates were centrifuged for 5 min, 100 μl of the supernatant was removed gently to a new microtiter plate, and absorbance was read at 550 nm. The alpha-toxin level (in hemolytic units per milliliter [HU/ml]) was defined as the inverse of the dilution causing 50% hemolysis. Sterile distilled water served as the 100% hemolysis control (positive control), and 1× PBS was a negative control. All experiments were performed in triplicate, and the results were averaged.
ELISA alpha-toxin detection.
In vitro alpha-toxin expression was also determined using a capture enzyme-linked immunosorbent assay (ELISA) and Western blotting. The Δhla S. aureus strain was kindly provided by Binh An Diep at the University of California, San Francisco. S. aureus strains were incubated in 10 ml of TSB overnight at 37°C (250 rpm). Overnight cultures were diluted 1:100 into 20 ml TSB and grown for an additional 16 h at 37°C. Culture supernatants were harvested, frozen at −80°C, and used for all further testing. For the ELISA, Nunc Maxisorp 96-well plates (Thermo Fisher Scientific, Pittsburgh, PA) were coated with MEDI4893 at 0.1 μg/ml at 4°C. Following overnight incubation, the plates were washed three times with 1× PBS containing 0.1% Tween 20 (wash buffer). Plates were blocked with 2% bovine serum albumin (BSA) in 1× PBS for 1 h at room temperature and then washed three times with wash buffer. Sample supernatants and an alpha-toxin control (alpha-toxin purified from S. aureus Wood) were serially diluted on the plates and incubated for 1 h at room temperature, followed by three washings. Captured alpha-toxin was detected using 1 μg/ml rabbit anti-alpha-toxin polyclonal IgG (46) for 1 h followed by washing and incubation with goat anti-rabbit IgG–horseradish peroxidase (HRP) conjugate, Fc fragment specific (Jackson ImmunoResearch) at a 1:10,000 dilution for 1 h. The plates were again washed three times with wash buffer and antibody binding was detected using 100 μl of SureBlue Reserve 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (KPL), followed by neutralization with 50 μl of 0.2 N sulfuric acid. Absorbance was determined for each plate at 450 nm by using an Envision plate reader (PerkinElmer, Waltham, MA). Two standard deviations above the background OD values for the hla-negative strain were used to define the limit of detection of 0.173 μg/ml.
Alpha-toxin Western blot assay.
Western blot analysis was performed by concentrating 500 μl of culture supernatant to a 50-μl volume by using an Amplicon-10 concentrator (Millipore, Billerica, MA). To the concentrated supernatant was added 15 μl of lithium dodecyl sulfate sample buffer (Invitrogen, Grand Island, NY) and 5 μl of sample-reducing agent (Invitrogen, Grand Island, NY), and the mixture was heated at 95°C for 5 min. Samples were then resolved on a 4-to-13% bis-Tris gel (Invitrogen, Grand Island, NY) and blotted onto a polyvinylidene difluoride membrane by using the iBlot system (Invitrogen, Grand Island, NY), and alpha-toxin was detected using rabbit anti-alpha-toxin polyclonal IgG (2 μg/ml) as a primary antibody and goat anti-rabbit IgG conjugated to an infrared dye as a secondary antibody (1:15,000 dilution; Li-Cor, Lincoln, NE). Western blots were then imaged by using an Odyssey 2000 imager (Li-Cor, Lincoln, NE).
Alpha-toxin-specific neutralizing antibodies.
The concentrations of anti-alpha-toxin neutralizing antibody in human serum samples were determined using a rabbit RBC-based functional assay. An equine serum anti-alpha-toxin reference standard was acquired from the National Institute for Biological Standards and Control (NIBSC) in the United Kingdom (47). The standard (STD) and quality control (QC) used in the assay were calibrated to the NIBSC reference standard, and antibody levels for samples were reported in international units per ml (IU/ml). The STD, QC, and diluted human serum samples were mixed with an equal volume of 10 ng/ml alpha-toxin in a 96-well deep plate with shaking at 450 rpm for 2 h ± 5 min. Rabbit RBCs were washed three times with PBS, and the total viable cell number was determined by using a Vi-CELL viability analyzer prior to use. The sample–alpha-toxin mixtures were added to a microtiter plate and incubated with 5 × 106 rabbit RBCs with shaking at 450 rpm for 2 h ± 5 min. Following incubation, the microtiter plates were centrifuged at 500 × g for 5 min at 4°C. Intact RBCs formed a pellet in the bottom of each well. The supernatant from each well was transferred to a half-area polystyrene plate with a nonbinding surface, either manually or by using the PerkinElmer JANUS automation work station. The degree of hemolysis was determined by measuring hemoglobin absorbance at 415 nm, which is inversely related to the amount of neutralizing antibodies present in a sample. The conversion of OD to IU/ml was performed using a 4-parameter regression model: y = (A − D)/[1 + (x/C)(B + D)] without weighting (SoftMax Pro v5.4; Sunnyvale, CA), for comparison to a standard curve, assayed on each plate. The lower limit of quantitation (LLOQ) of the assay was 0.0007 IU/ml.
Alpha-toxin-specific IgG antibody.
The concentrations of anti-alpha-toxin IgG antibody in human serum samples were determined via an ELISA. The STD and QC used in the assay were calibrated to the NIBSC reference standard, and antibody levels for samples were reported in IU/ml. Microtiter plates were first coated with 1.5 μg/ml native alpha-toxin in carbonate/bicarbonate buffer and incubated at 4°C overnight. Plates were washed and blocked with blocking buffer (1× DPBS with 0.1% Tween 20, 5% BSA) at room temperature for 1 h. During this time, serum sample dilutions were prepared in dilution buffer.
The blocked plates were washed and STDs, QCs, and diluted human serum samples were added to plates in duplicate. Plates were incubated for 1 h with shaking at 600 rpm in a 20°C incubator and washed, and then an HRP-conjugated secondary mouse anti-human IgG Fc fragment (HP6043; EMD Millipore, Billerica, MA) was added, after 1:7,500 dilution in dilution buffer (1× DPBS with 0.1% Tween 20, 0.5% BSA). Plates were incubated for 1 h with shaking at 600 rpm in a 20°C incubator. Following incubation, plates were washed and TMB substrate was added to plates and incubated in the dark in a 20°C incubator without shaking. The reaction was stopped with sulfuric acid, the plate was read on a spectrophotometer at 450 nm, and data were analyzed with SoftMax Pro (SMP), version 5.4, using a 4-parameter logistical curve fit model without weighting. The LLOQ of the assay was 0.0001 IU/ml.
Statistical analysis.
Patient demographic and clinical variables were evaluated with counts and percentages in contingency tables or with medians and absolute ranges for continuous measures. Distributions of antibody and alpha-toxin levels for groups defined by infection type or outcome were summarized using scatter plots. For anti-alpha-toxin antibody and neutralizing antibody, the mixed-effect model was applied to evaluate the confidence interval of the group geometric mean titer and statistical significance of differences of geometric means between groups. For alpha-toxin levels, given the proportion of subjects with alpha-toxin levels lower than the assay detection limit, the bootstrap method was used to evaluate the geometric mean confidence interval and statistical significance of differences of geometric means between groups. The statistical significance of the association parameters by infection type or outcome was assessed using the Kruskal-Wallis test for continuous measures and Fisher's exact test for cross-classifications of categorical data. Pearson correlation coefficients were used to measure the strengths of association between continuous measures. Analyses were performed using SAS version 9.4 (Cary, NC) and with GraphPad Prism 5.0 (La Jolla, CA).
RESULTS
Study population and baseline characteristics.
Demographic characteristics of the 200 patients (100 hemodialysis and 100 postsurgical) are summarized in Table 1. Hemodialysis patients were more frequently African-American than white (76.8% versus 23.2%; P < 0.0001). Cured patients were significantly younger than patients who were failures (median age, 55 years versus 63 years; P = 0.009). The APACHE II score was significantly higher in hemodialysis than postsurgical patients (median APACHE II score, 16 versus 13; P < 0.0001) and in patients with a failure outcome versus patients who were cured (median APACHE II score, 16 versus 14; P = 0.001).
TABLE 1.
Baseline characteristics of the study population
| Parameter | Value for population analysis groupa |
||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 200)c | SAB infection source |
Outcome |
|||||
| Hemodialysis (n = 100) | Postsurgical (n = 100) | P valueb | Failure (n = 60) | Cured (n = 133) | P valueb | ||
| Sex | |||||||
| Male | 89 (44.5) | 45 (45) | 44 (44) | 1.00 | 23 (38.3) | 64 (48.1) | 0.22 |
| Female | 111 (55.5) | 55 (55) | 56 (56) | 37 (61.7) | 69 (51.9) | ||
| Race | |||||||
| White | 96 (49.0) | 23 (23.2) | 73 (75.3) | <0.0001 | 35 (59.3) | 59 (45.4) | 0.086 |
| African-American | 100 (51.0) | 76 (76.8) | 24 (24.7) | 24 (40.7) | 71 (54.6) | ||
| Age (yrs) | 58 [18, 84] | 55 [23, 84] | 60 [18, 83] | 0.06 | 63 [26, 84] | 55 [19, 83] | 0.009 |
| Route of acquisition | |||||||
| Hospital | 60 (30.3) | 9 (9.0) | 51 (52.0) | <0.0001 | 22 (37.3) | 36 (27.3) | 0.18 |
| Community | 129 (65.2) | 85 (85.0) | 44 (44.9) | 33 (55.9) | 91 (68.9) | ||
| Nursing home | 9 (4.5) | 6 (6.0) | 3 (3.1) | 4 (6.8) | 5 (3.8) | ||
| APACHE II score | 14 [1, 27] | 16 [8, 27] | 13 [1, 26] | <0.0001 | 16 [1, 26] | 14 [3, 27] | 0.001 |
| Reported duration of symptoms (days) | 2 [0,30] | 2 [1, 26] | 1 [0, 30] | 0.61 | 2 [0, 26] | 1 [1, 30] | 0.71 |
| Persistent fever at 72 h | 113 (65.3) | 70 (78.7) | 43 (51.2) | 0.0002 | 25 (49.0) | 82 (70.7) | 0.009 |
| Methicillin-resistant S. aureus | 116 (58) | 57 (57) | 59 (59) | 0.89 | 44 (73.3) | 68 (51.1) | 0.004 |
| Metastatic diseased | |||||||
| Any | 62 (31.2) | 41 (41.0) | 21 (21.2) | 0.004 | 24 (40.0) | 36 (27.3) | 0.09 |
| Osteoarticular | 23 (11.6) | 14 (14) | 9 (9.1) | 0.38 | 12 (20.0) | 11 (8.3) | 0.03 |
| Endocarditis | 26 (15.0) | 20 (22) | 6 (7.3) | 0.010 | 12 (21.4) | 14 (12.6) | 0.17 |
| Others | 34 (17.1) | 24 (24) | 10 (10.1) | 0.014 | 10 (16.7) | 22 (16.7) | 1.00 |
| Positive blood culture on follow-upe | 76 (47.5) | 55 (59.8) | 21 (30.9) | 0.0004 | 24 (52.2) | 49 (44.1) | 0.38 |
| Alpha-toxin ELISA negative | 27 (13.5) | 11 (11.0) | 16 (16.0) | 0.4222 | 12 (20.0) | 15 (11.3) | 0.0482 |
| Alphta-toxin ELISA positive | 173 (86.5) | 89 (89.0) | 84 (84.0) | 48 (80.0) | 118 (88.7) | ||
| Anti-alpha-toxin-neutralizing antibody seronegative | 156 (78.0) | 77 (77.0) | 79 (79.0) | 0.8646 | 48 (80.0) | 104 (78.2) | 0.8508 |
| Anti-alpha-toxin-neutralizing antibody seropositive | 44 (22.0) | 23 (23.0) | 21 (21.0) | 12 (20.0) | 29 (21.8) | ||
Data are reported as the number (with the percentage of the total in parentheses) for categorical variables; continuous variables are reported as means [with the minimimum and maximum values in brackets].
P values were calculated using Fisher's exact test for categorical variables and the Kruskal-Wallis test for continuous variables.
Not all patient-related information was available for all subjects.
The total number of study subjects with metastatic disease is less than the sum for the specific metastatic sites because some patients had >1 metastatic infection sites.
Follow-up blood cultures were not available for all study subjects.
Clonal complex types of the SAB isolates and association with clinical outcome.
All 200 isolates underwent spa genotyping. Of these 200 isolates, a clonal complex (CC) was successfully inferred from the spa type of 122 (61%) isolates. Of the isolates with an identifiable CC type, the majority belonged to CC5 (35.2%), followed by CC8 (13.5%) and CC30 (7.2%) (Table 2). Interestingly, 21 of 26 (81%) of the CC8 isolates were from hemodialysis patients, and 12 of 14 (86%) of the CC30 isolates were from postsurgical patients (see Table S1 in the supplemental material). There was no correlation between CC type and clinical outcome.
TABLE 2.
Clonal complex types of the S. aureus isolates
| CC type | No. (%) of subjects with CC type isolates in outcome group |
||
|---|---|---|---|
| Failure (n = 60) | Cure (n = 133) | Total (n = 193a) | |
| 5 | 26 (43.3) | 42 (31.6) | 68 (35.2) |
| 8 | 5 (8.3) | 21 (15.8) | 26 (13.5) |
| 30 | 7 (11.7) | 7 (5.3) | 14 (7.2) |
| 1 | 2 (3.3) | 2 (1.5) | 4 (2.1) |
| 45 | 1 (1.7) | 2 (1.5) | 3 (1.6) |
| Otherb | 19 (31.7) | 59 (44.4) | 78 (40.4) |
For 7 of the 200 study subjects, no outcome data were available.
Other CCs identified among the isolates included CC9 (2 samples) and CC59 (two samples); 74 isolates were nontypeable.
hla gene presence and alpha-toxin production in vitro by the SAB isolates.
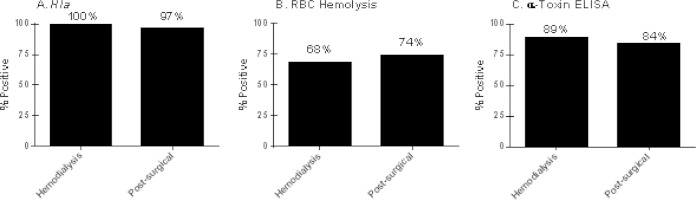
Ninety-eight percent (197/200) of the S. aureus isolates were found to possess the hla gene based on PCR results (Fig. 1A). We evaluated alpha-toxin production via a rabbit RBC lysis assay (Fig. 1B) and an alpha-toxin capture ELISA with confirmatory Western blotting (Fig. 1C). Alpha-toxin ELISA/Western blotting was significantly more sensitive than the RBC lysis assay at detecting alpha-toxin production in S. aureus isolates from both hemodialysis patients (89% versus 68%; P = 0.0004) and postsurgical patients (84% versus 74%; P = 0.117). Only 3 of the 197 isolates encoding the hla gene had RBC lytic activity without detectable alpha-toxin protein, whereas 32 of the 197 isolates had detectable alpha-toxin protein by ELISA without in vitro RBC lytic activity. These data demonstrated that alpha-toxin was expressed in vitro by a majority of these SAB isolates and that the ELISA was more sensitive in detecting alpha-toxin expression than the RBC lysis assay.
FIG 1.
hla gene prevalence, hemolytic activity, and alpha-toxin expression in S. aureus isolates. Assessment of hla gene prevalence by PCR (A), in vitro hemolytic activity by quantitative analysis of rabbit RBC lysis (B), and alpha-toxin expression measurement via a capture ELISA and Western blotting (C) was performed for S. aureus isolates obtained from SAB subjects with either end-stage renal disease requiring chronic hemodialysis or who had had a surgical procedure in the preceding 30 days.
Correlation of alpha-toxin presence or expression levels in vitro and clinical outcomes.
We next determined whether there was a correlation between the presence or absence of alpha-toxin or protein expression levels in vitro and clinical outcomes in the SAB hemodialysis and postsurgical populations. There were no differences in the number of alpha-toxin-positive isolates or the alpha-toxin levels expressed in vitro between hemodialysis and postsurgical patients (Fig. 2 and Table 1). Interestingly, subjects with alpha-toxin-expressing isolates had a better outcome than subjects with a nonexpressing isolate (Table 1; P = 0.0482). Levels of in vitro alpha-toxin production among the S. aureus isolates obtained from cured patients were also higher than among isolates from patients who were failures (Fig. 2; cure versus failure, P < 0.05 for hemodialysis patients and P < 0.01 for postsurgical patients).
FIG 2.
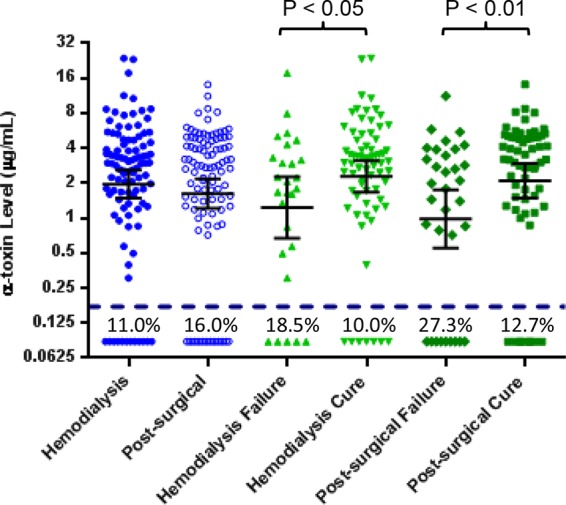
Analysis of alpha-toxin expression levels in S. aureus isolates based on infection type and clinical outcome. In vitro alpha-toxin expression levels (in μg/ml) were determined by a capture ELISA for the S. aureus isolates obtained from S. aureus bacteremia subjects; comparisons included hemodialysis versus postsurgical patients and patients with cure versus failure. Each symbol represents one S. aureus isolate, and the error bars represent the geometric means and 95% confidence intervals. The dashed line at 0.173 μg/ml represents the lower level of detection, and numbers below the dashed line represent the percentages of isolates with nondetectable alpha-toxin in each category.
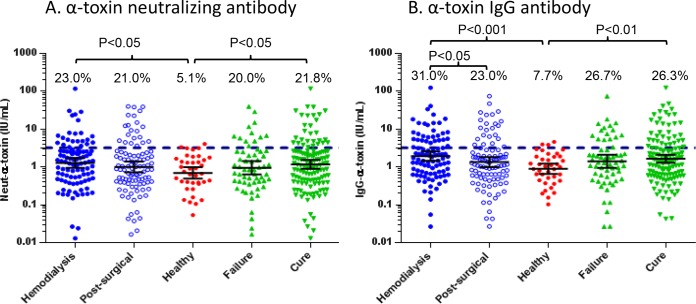
Anti-alpha-toxin antibody and neutralizing antibody levels.
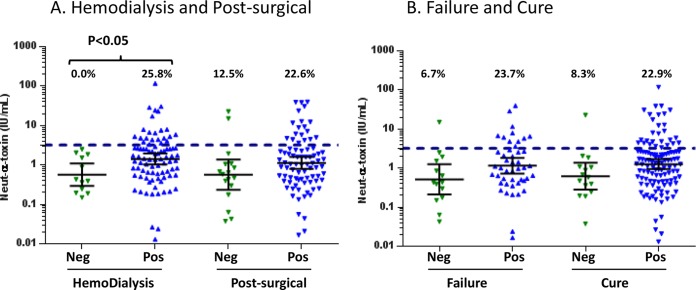
Next, we measured both alpha-toxin-neutralizing and alpha-toxin-specific IgG levels in serum of the hemodialysis and postsurgical patient groups. There was a strong correlation (r2 = 0.84) between anti-alpha-toxin IgG and alpha-toxin-neutralizing antibody levels in both patient groups. The levels of both anti-alpha-toxin IgG and alpha-toxin-neutralizing antibody were higher in the hemodialysis and postsurgical SAB subjects than in healthy control sera (P < 0.05) (Fig. 3A and B). The geometric mean levels of both neutralizing alpha-toxin antibody (1.17 IU/ml versus 0.95 IU/ml) (Fig. 3A) and anti-alpha-toxin IgG (1.65 IU/ml versus 1.41 IU/ml) (Fig. 3B) were higher in patients who were cured compared to patients who were failures, but these differences were not statistically different. Also, the levels of both anti-alpha-toxin IgG and alpha-toxin-neutralizing antibody were higher in the hemodialysis subjects than in postsurgical subjects (Fig. 3A and B). We also examined alpha-toxin-neutralizing antibody levels from subjects infected with S. aureus isolates that were either alpha-toxin positive or alpha-toxin negative in vitro (Fig. 4A and B). As expected, the levels of anti-alpha-toxin-neutralizing antibody were higher in patients infected with alpha-toxin-positive S. aureus isolates than in those infected with alpha-toxin-negative isolates in both hemodialysis and postsurgical SAB patients (Fig. 4A). For both the hemodialysis and postsurgical analyses and outcome analyses, the number of subjects with anti-alpha-toxin-neutralizing Ab levels greater than 3.2 IU/ml, the serological status cutoff value reported in previous studies (48), was higher for patients infected with alpha-toxin-expressing S. aureus (Fig. 4A and B). These data suggest that there is active immune surveillance during SAB and that the host makes a neutralizing Ab response against alpha-toxin during an infection.
FIG 3.
Anti-alpha-toxin antibody levels in the three different subject populations (including healthy controls). Anti-alpha-toxin-neutralizing antibody levels (in IU/ml) (A) and anti-alpha-toxin IgG antibody levels (in IU/ml) (B) in human serum samples are shown. Anti-alpha-toxin-neutralizing Ab levels were measured in an RBC-based toxin neutralization assay, and anti-alpha-toxin IgG levels were measured in an ELISA. Hemodialysis samples (n = 100) were from end-stage renal disease patients requiring hemodialysis. Postsurgical samples (n = 100) were from patients who had undergone a surgical procedure in the preceding 30 days. Healthy samples (n = 39) were from healthy donors, ages 18 to 45 years. Failure samples (n = 60) were from patients who died for any reason or experienced culture-confirmed recurrent S. aureus infection within 12 weeks of the initial positive blood culture. Cure samples (n = 133) were from patients who were alive with no evidence of recurrent S. aureus infection at 12 weeks after the initial positive blood culture. Each symbol represents one subject, and the bars represent the geometric means and 95% confidence intervals. The dashed line at 3.2 IU/ml represents a seropositive antibody level. The values above the dashed line represent the percentages of subjects with Ab levels greater than 3.2 IU/ml.
FIG 4.
Anti-alpha-toxin-neutralizing antibody levels in subjects infected with alpha-toxin-positive or -negative S. aureus strains. Serum levels of anti-alpha-toxin-neutralizing Ab (in IU/ml) are shown for hemodialysis versus postsurgical subjects (A) and for subjects with cure versus failure (B) who were infected with alpha-toxin-positive or -negative S. aureus isolates. S. aureus isolates were considered positive if they produced ≥0.173 μg/ml of alpha-toxin in vitro as measured in a capture ELISA. Each symbol represents one subject, and the bars represent the geometric means and 95% confidence intervals. The dashed line at 3.2 IU/ml represents a seropositive antibody level. The values above the dashed line represent the percentages of subjects with Ab levels greater than 3.2 IU/ml.
Alpha-toxin gene (hla) variants of the SAB isolates and association with clinical outcome.
To understand the genetic diversity among the alpha-toxin produced by these isolates and the impact on outcomes, we sequenced the hla gene from the 197 isolates and identified a total of 12 hla genotypic variants. Of the 12 hla variants, the five most common types comprised 93.9% of the 197 isolates (Table 3). Consistent with other reports (49), all of the isolates harboring a premature stop codon at amino acid 113 (variant 3) (Table 3) for which a clonal complex type was determined belonged to CC30 (see Table S2 in the supplemental material). The majority (18/27) of the isolates that did not express alpha-toxin belonged to the variant 3 group (see Table S2). There was no correlation between hla variant type and clinical outcome. Interestingly, 81% (17/21) of the variant 3 isolates came from surgery-associated bacteremia subjects, and 72% (23/32) of the variant 1 isolates came from hemodialysis-associated bacteremia subjects.
TABLE 3.
hla genotypes associated with S. aureus bacteremia among hemodialysis and postsurgery patients
| hla genotype | n | Amino acid residue or codon found at positiona: |
Neutralized by MEDI4893 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V6 | I40 | L78 | Q113 | T155 | D234 | S265 | D272 | H285 | T294 | I301 | S304 | K314 | |||
| 1 (USA300) | 32 | V | I | L | Q | T | D | S | D | H | T | I | S | K | Yes |
| 2 | 114 | E | T | Yes | |||||||||||
| 3 | 21 | I | Stop | S | E | T | T | NTb | |||||||
| 4 | 11 | T | Yes | ||||||||||||
| 5 | 7 | I | S | T | T | Yes | |||||||||
| 6 | 4 | E | Yes | ||||||||||||
| 7 | 3 | F | T | Yes | |||||||||||
| 8 | 1 | A | I | S | E | T | N | T | Yes | ||||||
| 9 | 1 | I | S | T | N | T | Yes | ||||||||
| 10 | 1 | I | S | T | T | C | Yes | ||||||||
| 11 | 1 | I | S | Y | T | Yes | |||||||||
| 12 | 1 | E | T | N | Yes | ||||||||||
| Total | 197c | 1 | 3 | 32 | 21 | 32 | 141 | 31 | 1 | 1 | 1 | 161 | 1 | 1 | |
The amino acid numbering system employed starts at the first methionine and includes the whole open reading frame.
NT, not tested. Isolates with a stop codon at Q113 did not produce alpha-toxin and were not tested in the toxin neutralization assay.
Of the 200 isolates, 197 had a detectable hla gene.
Susceptibility of hla variants to MEDI4893 neutralization.
To gain a better understanding about strain coverage of the anti-AT MAb clinical candidate MEDI4893 (29, 34), its neutralization capacity was assessed against all of the isolates producing functional alpha-toxin. MEDI4893 effectively neutralized each of the different toxin variants. These data confirm that hla is highly conserved in S. aureus and that multiple variants of alpha-toxin are neutralized by MEDI4893.
DISCUSSION
The impacts of the presence of hla and the expression of alpha-toxin on the severity of S. aureus bloodstream infections in hemodialysis and postsurgical patients are largely unknown. Using matched S. aureus isolates and serum from a large collection of contemporary, clinically well-characterized SAB patients, the current study found that virtually all clinical S. aureus isolates contain hla, and most express alpha-toxin in vitro. The study also found that SAB subjects had higher antibody levels to alpha-toxin than did healthy controls. These findings underscore the complex interplay between toxin production by S. aureus and the host's immune response to those toxins and provide important insights into any therapeutic attempt to interrupt that interaction.
The results of this study further establish alpha-toxin as a highly conserved virulence factor in S. aureus that is highly accessible to the immune system. This observation is vital for alpha-toxin to be considered a target for an active or passive immunotherapeutic agent. Most of the S. aureus isolates (98.5%) and all of the CCs encountered in this investigation encoded hla. Interestingly, all three S. aureus isolates lacking hla and 17/21 (81%) of the variant 3 isolates with the stop codon were from patients in the postsurgical group. We hypothesize that the compromised tissue in a surgical wound might enhance the ability of less-virulent S. aureus to establish infection.
Several others have examined the prevalence and role of anti-alpha-toxin antibodies in patients with S. aureus disease. High antibody levels to alpha-toxin have been described in S. aureus subjects with various dermatoses and especially in atopic dermatitis subjects (50). A more recent study evaluating the risk of sepsis in S. aureus-infected patients showed that the incidence of sepsis was significantly lower in patients with higher IgG levels against alpha-toxin, Hld, Panton-Valentine leukocidin, SEC-1, and PSM-α3 (36). Data suggesting that high levels of anti-alpha-toxin antibodies are protective in preventing S. aureus endocarditis were reported from a study by Ruotsalainen et al. (48). Intravenous drug users without endocarditis were more frequently antibody positive (>3.2 IU/ml; 44%) than were those with endocarditis (6%; P = 0.01) (48). The most compelling data that anti-alpha-toxin antibodies correlate with protection from S. aureus disease came from a recent report by Fritz et al. (51). The data from that study showed that symptomatic subjects colonized with S. aureus exhibited the highest mean baseline Ab titers against alpha-toxin (P = 0.002) and that patients with invasive S. aureus infections had the lowest preexisting Ab titers against alpha-toxin or LukF. High convalescent anti-alpha-toxin Ab levels correlated with protection against subsequent S. aureus infections during 12 months of follow-up.
S. aureus alpha-toxin expression is thought to be highly regulated by environmental factors, and its in vitro versus in vivo regulation has been shown to vary greatly between strains (52, 53) The results of our study demonstrate that the alpha-toxin ELISA was considerably more sensitive than the traditional RBC lysis assay in detecting alpha-toxin. Only 3 of the 197 isolates encoding hla had RBC lysis activity with no detectable alpha-toxin detected by ELISA, whereas 32 of the 197 hla-positive isolates had detectable alpha-toxin by ELISA but not by the RBC lysis assay. These data suggest that previous reports using the RBC lysis assay may have underreported the prevalence of alpha-toxin in different S. aureus isolates (43). We observed that 89% of hemodialysis and 84% of postsurgical isolates produced alpha-toxin in vitro, suggesting that the potential for alpha-toxin expression is highly conserved. Only 3 of the 24 isolates that lacked hla or contained the Q113 nonsense stop mutation, in which alpha-toxin production is known to be dysfunctional (49), had alpha-toxin detectable by ELISA. Consistent with other findings (54), we also observed larger amounts of alpha-toxin expressed from the CC8 isolates (see Table S1 in the supplemental material). Interestingly, in vitro alpha-toxin expression levels were approximately 2-fold higher in S. aureus isolates derived from cured hemodialysis patients (P < 0.05) and cured postsurgical patients (P < 0.01) versus those that were failures (Fig. 2). There are several possible explanations for this unanticipated finding. First, in vitro expression may not correlate with in vivo expression. Second, S. aureus can differentially express alpha-toxin at the initial site of infection versus in the blood (55). Third, other virulence factors may play a significant role in SAB. Finally, the proinflammatory properties of alpha-toxin may help elicit a stronger overall anti-S. aureus immune response.
This study identified a total of 12 different alpha-toxin sequence types in the 197 hla-positive clinical SAB isolates. Among these variants was the USA300 reference sequence, variant 1 (41), as well as variant 3, with the Q113 nonsense stop mutation previously described by Deleo and colleagues (49). The different mutations in alpha-toxin may be random in nature or may be the result of selective pressure by the immune system following the generation of antibodies that bind alpha-toxin. Importantly, all of the alpha-toxin variants were neutralized by MEDI4893 with the exception of variant 3 isolates, which were not tested due to a stop codon preventing in vitro expression. The MEDI4893 epitope has been identified in a crystal structure of the Fab bound to alpha-toxin and is a conformational, discontinuous epitope comprised of different amino acids from positions 177 to 200 and 261 to 271 (56). The results presented here suggest that the epitope is highly conserved. In summary, molecular and microbiological characterization of these S. aureus isolates show that the hla gene, alpha-toxin expression, and the MEDI4893 epitope are conserved in a diverse set of SAB isolates from both hemodialysis and postsurgical patients.
Levels of alpha-toxin IgG and alpha-toxin-neutralizing Ab levels varied over a 1,000-fold range (0.1 to greater than 100 IU/ml) and were consistent with a recent report showing a broad range of alpha-toxin-specific IgG levels in healthy adults (57). In comparison to alpha-toxin Ab levels in healthy controls, alpha-toxin Ab levels in the SAB patients in this study were significantly higher in all comparisons. Previous reports have used a serological status cutoff of 3.2 IU/ml to define a subject as seropositive (48). In the current study, only 5% of the healthy control samples were seropositive in the neutralization assay, compared to 21% and 23% of the postsurgical and hemodialysis samples, respectively. These data suggest that some of the infected patients in our study had adequate time to mount a measurable immune response from the time of infection to the time of presentation and that there was an active antibody response to alpha-toxin during SAB. The IgG and neutralizing antibody levels were highly correlated for healthy controls and hemodialysis and postsurgical subjects, suggesting that the polyclonal IgG response to alpha-toxin is neutralizing. Both neutralizing and IgG geometric mean antibody levels were higher in the patients that were cured versus the patients that were failures among both hemodialysis and postsurgical patients, but these differences were not statistically significant.
We also examined whether alpha-toxin-specific antibody levels correlated with alpha-toxin expression by the S. aureus isolates in vitro. Subjects that were infected with alpha-toxin-positive isolates had higher Ab levels than subjects infected with isolates that were alpha-toxin negative in vitro, which is consistent with other reports showing that anti-alpha-toxin IgG and neutralizing antibody levels were higher among the individuals infected with S. aureus isolates producing alpha-toxin in vitro (58). Taken together, these results suggest that alpha-toxin is an important, conserved virulence factor involved in bacteremia and that the immune systems detects and responds to alpha-toxin by developing neutralizing antibodies.
Our study has limitations. First, the type and duration of the antibiotic used among the patients likely had an effect on duration and severity of disease, outcome, and potentially on serum levels of antibodies. This is an important factor to consider, because the antibiotic used could have a direct impact on virulence factor expression, including alpha-toxin. For example, studies have demonstrated that protein synthesis inhibitor antibiotics suppress expression of virulence factors like TSST-1, alpha-toxin, and coagulase in S. aureus isolates (59–61), while the cell wall-acting antibiotics have either no effect or enhance production of extracellular virulence factors (44, 62). Second, the matched S. aureus isolates and serum samples were collected only after the subjects had experienced clinical symptoms for several days (average of 2 days [range, 0 to 30 days]) and had presented to the attending physician. Therefore, many of the subjects had time to mount an immune response to alpha-toxin, and this hindered us from correlating preexisting alpha-toxin Ab levels with the risk of infection or disease severity. A planned epidemiological study will examine antibody levels in samples collected and banked from patients undergoing elective surgeries or prior to being placed on a ventilator to examine whether preexisting Ab levels correlate with risk of infection. We have included high-risk S. aureus bacteremia subjects with either end-stage renal disease requiring chronic hemodialysis or who had had a surgical procedure in the preceding 30 days in this study. To establish the generalizability of the findings from this study, we need to extend the study to other disease settings, like pneumonia and skin and soft tissue infections, where the role of alpha-toxin is better established in animal models.
Despite these limitations, we were able to gain insight into the prevalence of alpha-toxin-expressing S. aureus, IgG, and neutralizing antibody levels against alpha-toxin and the susceptibility of different alpha-toxin variants expressed by S. aureus to MEDI4893. To our knowledge, this is the first study to correlate clinical outcomes in hemodialysis and postsurgical patients with hla gene presence, alpha-toxin expression, genotypic variation of the hla gene encoding alpha-toxin, and IgG and neutralizing antibody levels to alpha-toxin. Alpha-toxin was expressed by the majority (98.5%) of SAB isolates, and antibody levels were higher in sera from patients with S. aureus isolates that expressed alpha-toxin in vitro, suggesting that alpha-toxin is exposed to the immune system during SAB. Twelve distinct alpha-toxin sequence types were identified from the 197 hla-positive isolates, and MEDI4893 neutralized alpha-toxin produced by all 12 different variants. Based on these observations, the correlation of higher anti-alpha-toxin antibody levels to better clinical outcomes in epidemiological studies (36, 48, 51) and the efficacy of alpha-toxin MAbs or vaccines in animal models (27, 32–34), we have initiated a phase 2 clinical trial with MEDI4893 to prevent ventilator-associated S. aureus pneumonia. Further studies are under way to verify these observations among S. aureus pneumonia patients.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Weiyi Liu for testing serum samples in the serology assays and Xu Liu, Jia J. Li, Ahmad Akhgar, and Weiyi Liu for bioanalytical support.
This work was supported by a grant from MedImmune. Vance G. Fowler, Jr., was supported by NIH K24-AI093969.
Potential conflicts of interest include the following: V.G.F. served as Chair of the V710 Scientific Advisory Committee (Merck), has received grant support or has grants pending from Cerexa, Pfizer, Advanced Liquid Logic, MedImmune, and Cubist, has been a paid consultant for Merck, Astellas, Affinium, Theravance, Cubist, Cerexa, Durata, Pfizer, NovaDigm, Novartis, Medicines Company, Biosynexus, MedImmune, and Inimex, Bayer, and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance, and Novartis. B.K.S.-K. and L.P.P. were supported with a grant from MedImmune. T.H.R., F.R., Q.Y., J.T.T., J.A.M., and W.A.S. have no conflicts of interest to declare. All MedImmune authors were employees of MedImmune at the time that the work was performed and the manuscript was conceived and written and may or may not own stock in Astra Zeneca, MedImmune's parent company.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02023-14.
REFERENCES
- 1.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association. Endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434. [DOI] [PubMed] [Google Scholar]
- 2.Fluit AC, Jones ME, Schmitz FJ, Acar J, Gupta R, Verhoef J. 2000. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin Infect Dis 30:454–460. doi: 10.1086/313710. [DOI] [PubMed] [Google Scholar]
- 3.Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Pare C, Almirante B, Munoz P, Rizzi M, Naber C, Logar M, Tattevin P, Iarussi DL, Selton-Suty C, Jones SB, Casabe J, Morris A, Corey GR, Cabell CH. 2007. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 297:1354–1361. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 5.Hersh AL, Chambers HF, Maselli JH, Gonzales R. 2008. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 168:1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 6.Parker D, Prince A. 2012. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin Immunopathol 34:281–297. doi: 10.1007/s00281-011-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 8.Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 9.Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, Raoult D, Bayer A, Fowler VG Jr. 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 41:507–514. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- 10.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen AG, Espersen F, Skinhoj P, Rosdahl VT, Frimodt-Moller N. 1997. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980–1990. J Infect 34:113–118. doi: 10.1016/S0163-4453(97)92395-1. [DOI] [PubMed] [Google Scholar]
- 12.Klevens RM, Edwards JR, Andrus ML, Peterson KD, Dudeck MA, Horan TC. 2008. Dialysis surveillance report: National Healthcare Safety Network (NHSN) data summary for 2006. Semin Dial 21:24–28. doi: 10.1111/j.1525-139X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DJ, Arduino JM, Reed SD, Sexton DJ, Kaye KS, Grussemeyer CA, Peter SA, Hardy C, Choi YI, Friedman JY, Fowler VG Jr. 2010. Variation in the type and frequency of postoperative invasive Staphylococcus aureus infections according to type of surgical procedure. Infect Control Hosp Epidemiol 31:701–709. doi: 10.1086/653205. [DOI] [PubMed] [Google Scholar]
- 14.Tracy LA, Furuno JP, Harris AD, Singer M, Langenberg P, Roghmann MC. 2011. Staphylococcus aureus infections in US veterans, Maryland, USA, 1999–2008. Emerg Infect Dis 17:441–448. doi: 10.3201/eid1703.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DR, Pattee PA. 1980. Identification of a chromosomal determinant of alpha-toxin production in Staphylococcus aureus. Infect Immun 30:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattee PA, Glatz BA. 1980. Identification of a chromosomal determinant of enterotoxin A production in Staphylococcus aureus. Appl Environ Microbiol 39:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairweather N, Kennedy S, Foster TJ, Kehoe M, Dougan G. 1983. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun 41:1112–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto M. 2010. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol 64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 19.Wilke GA, Bubeck Wardenburg J. 2010. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A 107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chua KY, Monk IR, Lin YH, Seemann T, Tuck KL, Porter JL, Stepnell J, Coombs GW, Davies JK, Stinear TP, Howden BP. 2014. Hyperexpression of alpha-hemolysin explains enhanced virulence of sequence type 93 community-associated methicillin-resistant Staphylococcus aureus. BMC Microbiol 14:31. doi: 10.1186/1471-2180-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 106:5883–5888. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 23.Bubeck Wardenburg J, Patel RJ, Schneewind O. 2007. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bubeck Wardenburg J, Schneewind O. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaulding AR, Salgado-Pabon W, Merriman JA, Stach CS, Ji Y, Gillman AN, Peterson ML, Schlievert PM. 2014. Vaccination against Staphylococcus aureus pneumonia. J Infect Dis 209:1955–1962. doi: 10.1093/infdis/jit823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel AH, Nowlan P, Weavers ED, Foster T. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun 55:3103–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. 2012. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis 206:352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, Sellman BR. 2014. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58:1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLeo FR, Otto M. 2008. An antidote for Staphylococcus aureus pneumonia? J Exp Med 205:271–274. doi: 10.1084/jem.20080167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohlsen K, Lorenz U. 2010. Immunotherapeutic strategies to combat staphylococcal infections. Int J Med Microbiol 300:402–410. doi: 10.1016/j.ijmm.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Mocca CP, Brady RA, Burns DL. 2014. Role of antibodies in protection elicited by active vaccination with genetically inactivated alpha-hemolysin in a mouse model of Staphyloccocus aureus skin and soft tissue infections. Clin Vaccine Immunol 21:622–627. doi: 10.1128/CVI.00051-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragle BE, Bubeck Wardenburg J. 2009. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 77:2712–2718. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, Stover CK, Sellman BR. 2012. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 19:377–385. doi: 10.1128/CVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caballero A, Foletti D, Bierdeman M, Tang A, Arana A, Hasa-Moreno A, Sangalang E, O'Callaghan RJ. 2014. Effectiveness of alpha-toxin Fab monoclonal antibody therapy in limiting the pathology of Staphylococcus aureus keratitis. Ocul Immunol Inflamm 9:1–7. doi: 10.3109/09273948.2014.920035. [DOI] [PubMed] [Google Scholar]
- 36.Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, Johnson JK, Nguyen C, Chen WH, Roghmann MC. 2012. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 206:915–923. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 37.Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, Federspiel JJ, Tonthat GT, Rude TH, Barriere SL, Corey R, Fowler VG Jr. 2008. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol 46:678–684. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fowler VG Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, Federspiel J, Naidich S, Remortel B, Rude T, Brown P, Reller LB, Corey GR, Gill SR. 2007. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis 196:738–747. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 39.Mathema B, Mediavilla J, Kreiswirth BN. 2008. Sequence analysis of the variable number tandem repeat in Staphylococcus aureus protein A gene: spa typing. Methods Mol Biol 431:285–305. [DOI] [PubMed] [Google Scholar]
- 40.Ewing B, Hillier L, Wendl MC, Green P. 1998. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185. [DOI] [PubMed] [Google Scholar]
- 41.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 42.Dall'Acqua WF, Kiener PA, Wu H. 2006. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 43.Sharma-Kuinkel BK, Ahn SH, Rude TH, Zhang Y, Tong SY, Ruffin F, Genter FC, Braughton KR, Deleo FR, Barriere SL, Fowler VG Jr. 2012. Presence of genes encoding Panton-Valentine leukocidin is not the primary determinant of outcome in patients with hospital-acquired pneumonia due to Staphylococcus aureus. J Clin Microbiol 50:848–856. doi: 10.1128/JCM.06219-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kernodle DS, McGraw PA, Barg NL, Menzies BE, Voladri RK, Harshman S. 1995. Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis 172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 45.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 46.Tkaczyk C, Hamilton MM, Datta V, Yang XP, Hilliard JJ, Stephens GL, Sadowska A, Hua L, O'Day T, Suzich J, Stover CK, Sellman BR. 2013. Staphylococcus aureus alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis model. PLoS One 8:e75103. doi: 10.1371/journal.pone.0075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.NIBSC-HPA. 2010. The 3rd international standard for Staphylococcus alpha antitoxin, equine NIBSC code: STA. National Institute for Biological Standards and Control, p 1–4. National Institute for Biological Standards and Control. Potters Bar, Hertfordshire, United Kingdom. [Google Scholar]
- 48.Ruotsalainen E, Karden-Lilja M, Kuusela P, Vuopio-Varkila J, Virolainen-Julkunen A, Sarna S, Valtonen V, Jarvinen A. 2008. Methicillin-sensitive Staphylococcus aureus bacteraemia and endocarditis among injection drug users and nonaddicts: host factors, microbiological and serological characteristics. J Infect 56:249–256. doi: 10.1016/j.jinf.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Deleo FR, Kennedy AD, Chen L, Wardenburg JB, Kobayashi SD, Mathema B, Braughton KR, Whitney AR, Villaruz AE, Martens CA, Porcella SF, McGavin MJ, Otto M, Musser JM, Kreiswirth BN. 2011. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A 108:18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larinkari U. 1982. Serum antibody to staphylococcal teichoic acid and alpha-haemolysin in dermatological patients. Br J Dermatol 107:53–58. doi: 10.1111/j.1365-2133.1982.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 51.Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA. 2013. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 56:1554–1561. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bronner S, Monteil H, Prevost G. 2004. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev 28:183–200. doi: 10.1016/j.femsre.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Delaune A, Dubrac S, Blanchet C, Poupel O, Mader U, Hiron A, Leduc A, Fitting C, Nicolas P, Cavaillon JM, Adib-Conquy M, Msadek T. 2012. The WalKR system controls major staphylococcal virulence genes and is involved in triggering the host inflammatory response. Infect Immun 80:3438–3453. doi: 10.1128/IAI.00195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monecke S, Muller E, Buchler J, Stieber B, Ehricht R. 2014. Staphylococcus aureus in vitro secretion of alpha toxin (hla) correlates with the affiliation to clonal complexes. PLoS One 9:e100427. doi: 10.1371/journal.pone.0100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. 2004. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis 190:1498–1505. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- 56.Oganesyan V, Peng L, Damschroder MM, Cheng L, Sadowska A, Tkaczyk C, Sellman BR, Wu H, Dall'Acqua WF. 2014. Mechanisms of neutralization of a human anti-alpha toxin antibody. J Biol Chem 289:29874–29880. doi: 10.1074/jbc.M114.601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colque-Navarro P, Jacobsson G, Andersson R, Flock JI, Mollby R. 2010. Levels of antibody against 11 Staphylococcus aureus antigens in a healthy population. Clin Vaccine Immunol 17:1117–1123. doi: 10.1128/CVI.00506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verkaik NJ, Dauwalder O, Antri K, Boubekri I, de Vogel CP, Badiou C, Bes M, Vandenesch F, Tazir M, Hooijkaas H, Verbrugh HA, van Belkum A, Etienne J, Lina G, Ramdani-Bouguessa N, van Wamel WJ. 2010. Immunogenicity of toxins during Staphylococcus aureus infection. Clin Infect Dis 50:61–68. doi: 10.1086/648673. [DOI] [PubMed] [Google Scholar]
- 59.Bernardo K, Pakulat N, Fleer S, Schnaith A, Utermohlen O, Krut O, Muller S, Kronke M. 2004. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother 48:546–555. doi: 10.1128/AAC.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doss SA, Tillotson GS, Amyes SG. 1993. Effect of sub-inhibitory concentrations of antibiotics on the virulence of Staphylococcus aureus. J Appl Bacteriol 75:123–128. doi: 10.1111/j.1365-2672.1993.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 61.Gemmell CG, Ford CW. 2002. Virulence factor expression by Gram-positive cocci exposed to subinhibitory concentrations of linezolid. J Antimicrob Chemother 50:665–672. doi: 10.1093/jac/dkf192. [DOI] [PubMed] [Google Scholar]
- 62.Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, Hacker J. 1998. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 42:2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.