Abstract
We developed an immunochromatographic assay kit that uses fluorescent silica nanoparticles bound to anti-Acanthamoeba antibodies (fluorescent immunochromatographic assay [FICGA]) and evaluated its efficacy for the detection of Acanthamoeba and diagnosis of Acanthamoeba keratitis (AK). The sensitivity of the FICGA kit was evaluated using samples of Acanthamoeba trophozoites and cysts diluted to various concentrations. A conventional immunochromatographic assay kit with latex labels (LICGA) was also evaluated to determine its sensitivity in detecting Acanthamoeba trophozoites. To check for cross-reactivity, the FICGA was performed by using samples of other common causative pathogens of infectious keratitis, such as Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, and Candida albicans. Corneal scrapings from patients with suspected AK were tested with the FICGA kit to detect the presence of Acanthamoeba, and the results were compared with those of real-time PCR. The FICGA kit detected organisms at concentrations as low as 5 trophozoites or 40 cysts per sample. There were no cross-reactivities with other pathogens. The FICGA was approximately 20 times more sensitive than the LICGA for the detection of Acanthamoeba trophozoites. The FICGA kit yielded positive results for all 10 patients, which corresponded well with the real-time PCR results. The FICGA kit demonstrated high sensitivity for the detection of Acanthamoeba and may be useful for the diagnosis of AK.
INTRODUCTION
Acanthamoeba keratitis (AK) is a severe and sight-threatening ocular infection which usually occurs in the context of soft contact lens wear or trauma. It is caused by Acanthamoeba spp. which inhabit various environments, such as lakes, oceans, soil, and tap water (1–3). Acanthamoeba can assume two different morphological forms: the trophozoite, which can utilize nutrition and proliferate, and the dormant protective cyst, which can withstand high temperatures, desiccation, and pharmacologic insults. Acanthamoeba can transform between the trophozoite and cyst forms to adjust to various environments (3–6). The incidence of AK has increased dramatically in recent years, a trend which has been attributed to the increasing prevalence of soft contact lens wear and usage of contact lens disinfectant solutions that do not prevent the growth of Acanthamoeba (7, 8). Since the clinical manifestations of AK are similar to those of herpes simplex keratitis, the condition can often be misdiagnosed (9–11). Therefore, reliable detection of Acanthamoeba is essential for an accurate diagnosis of AK. As delayed diagnosis has been associated with poor visual outcomes (12, 13), it is important to identify a method for the rapid and specific diagnosis of AK.
Microscopic examination and culture of corneal scrapings are the diagnostic procedures conventionally used to detect Acanthamoeba (14). Microscopic examination of corneal smears stained with Fungiflora Y, calcofluor white stain, and acridine orange stain has been reported to be an effective method of diagnosing AK (15–17), but these tests require technical expertise, and a false negative can occur if there is an insufficient sample from the corneal scraping. Culturing live Acanthamoeba isolates is time-consuming, and a long incubation time is needed to confirm Acanthamoeba growth. This results in decreased sensitivity of the test and delays in starting treatment (14, 18).
Recently, highly sensitive PCR procedures which amplify Acanthamoeba DNA have been used in the diagnosis of AK (19–22). Real-time PCR can also provide quantitative values for Acanthamoeba DNA copy numbers, enabling clinicians to estimate the efficacy of AK treatment (23, 24). However, these genetic procedures require expensive specialized equipment and technical expertise. Moreover, these tests are available only in certain facilities, such as academic centers.
Immunochromatographic assays (ICGA) are useful for antigen detection, and they can generally be completed within 30 min and do not require specialized equipment or expertise (25–29). Because of its rapidity and simplicity, the ICGA is utilized in many clinical tests, such as pregnancy tests and tests which detect antigens from causative pathogens, such as viruses and bacteria (25–27). In the field of ophthalmology, it is used for the diagnosis of adenoviral conjunctivitis and herpetic keratitis (28, 29). Colloidal gold and latex, each of which serves as a label when coupled to an antibody, are used to visualize antigens in ICGA kits for adenovirus and herpesvirus, respectively (28, 29). ICGA kits that use colloidal gold or latex labels usually show results using a detection line which appears on the membrane in either blue or red. However, checking for this detection line with only the naked eye is not ideal, as it may reduce the test's sensitivity or lead to false positives (30).
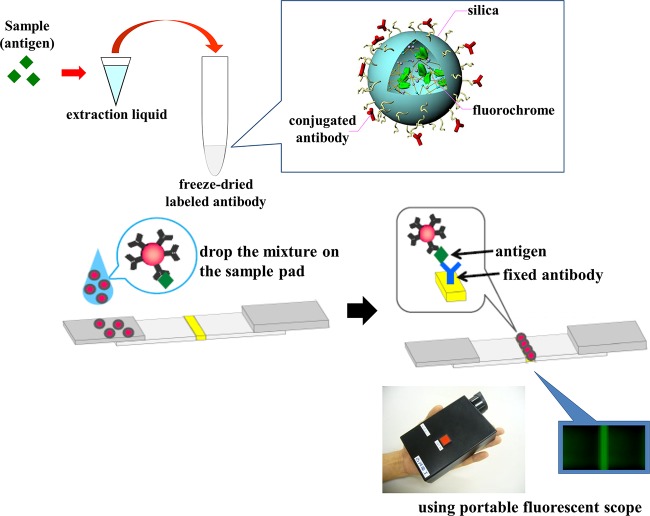
In this study, an ICGA kit using an anti-Acanthamoeba antibody was developed to detect Acanthamoeba. A fluorescent substance, instead of colloidal gold or latex, was used to label the anti-Acanthamoeba antibody, and the detection line was visualized using a portable fluorescence microscope. The ICGA kit used in this study (the fluorescent immunochromatographic assay [FICGA]) consists of a test strip, extraction liquid containing surfactant, and fluorescent silica nanoparticles (Quartz Dot; Furukawa Electric Co., Ltd.), each coupled with an antibody for Acanthamoeba castellanii. Quartz Dot is an amorphous silica nanoparticle with a diameter of approximately 290 nm and is harmless to the human body. Since the surface of the particle is covered by highly hydrophilic hydroxyl groups, the particles disperse uniformly in the sample solution or buffer. Nonspecific adsorption due to hydrophobic interactions is infrequent, thereby minimizing fluorescent noise and maximizing sensitivity. Moreover, the antibody-fluorescent label complex is stabilized by strong covalent modifications on its surface and has high luminance since it contains a high concentration of the fluorochrome rhodamine 6G. Rhodamine 6G is an ideal fluorescent marker because it fluoresces at 555 nm, a wavelength to which the eye is extremely sensitive under light-adapted conditions (31). We used mouse monoclonal antibodies to Acanthamoeba castellanii, which were produced as previously described (32). These antibodies recognize pathogenic Acanthamoeba spp. but not any other amoebas (32). The aim of this study was to investigate the efficacy of a FICGA for the detection of Acanthamoeba and diagnosis of AK.
MATERIALS AND METHODS
Immunochromatographic assay for detection of Acanthamoeba.
To perform the assay, a sample was treated with 200 μl of extraction liquid and freeze-dried fluorescent silica nanoparticles, and 80 μl of this mixture was placed on the edge of a test strip which had been previously sprayed with anti-Acanthamoeba antibodies. Thirty minutes after applying the sample mixture, the fluorescent emission was observed with a portable fluorescence microscope (Immuno Chromato-Reader; Furukawa Electric Co., Ltd.) (Fig. 1). The fluorescent intensity of the test line was also measured with a specialized fluorescent scanner 60 min after application of the sample. The fluorescence microscope can detect signals at an intensity of approximately 100 arbitrary units.
FIG 1.
Principle of the fluorescent immunochromatographic assay kit. The sample is treated with extraction liquid and mixed with anti-Acanthamoeba monoclonal antibodies, which are conjugated to fluorescent silica nanoparticles. Acanthamoeba antigens, if present in the sample, form complexes with antibodies which are fixed in place on a strip. Fluorescent emission is observed by using a portable fluorescence microscope. If there are no Acanthamoeba antigens in the sample, no fluorescent band is observed.
A second ICGA kit was developed with the same mouse-derived anti-Acanthamoeba antibodies labeled with latex markers (the latex-labeled immunochromatographic assay [LICGA]). For this kit, equal parts of sample fluid and antibody solution were mixed, and 100 μl of this mixture was applied to a test strip with an anti-Acanthamoeba antibody test line and a mouse IgG control antibody line. The test result was confirmed within 30 min after sample application. A red band at both the test and control antibody line positions was considered a positive result, whereas a negative result consisted of a red band at the control position only.
In vitro examination.
Acanthamoeba castellanii strain ATCC 30011 was purchased from the American Type Culture Collection and used to examine the sensitivity of the FICGA and LICGA kits.
Trophozoites were grown axenically in peptone-yeast extract-glucose (PYG) medium at 25°C in a tissue culture flask (Becton Dickinson, Tokyo, Japan). Encystment was induced by transferring the trophozoites from the PYG medium to Neff's constant-pH encystment medium and incubating the trophozoites for at least 2 weeks at 25°C (33). The number of trophozoites or cysts in suspension was counted using a hemocytometer and diluted using extraction liquid.
For the LICGA, a diluted trophozoite culture was applied to the test strip, and the test line was checked for a reaction. Three independent assays were performed with different concentrations of Acanthamoeba solution. For the FICGA, culture media of trophozoites and cysts were applied to the FICGA kit. The test strips were evaluated for the presence or absence of fluorescence via microscopic examination. The fluorescent intensity of each test line was also measured to determine whether signal intensity was correlated with the Acanthamoeba concentration in the samples. In examinations with both the LICGA and the FICGA, the person assessing the positive or negative result was not informed of the Acanthamoeba concentration in the samples.
Cross-reactivity tests were conducted for Pseudomonas aeruginosa (strain PAO-1), Staphylococcus aureus (strain Newman), Staphylococcus epidermidis (clinical isolate), and Candida albicans (clinical isolate), which are common causative pathogens of infectious keratitis. The culture medium of each pathogen was diluted to a concentration of 107 CFU/ml using extraction liquid and then applied to the FICGA test strip.
Clinical evaluation. (i) Subjects and samples.
Clinical samples were collected from 10 patients (5 men and 5 women, ranging from 18 to 57 years of age) with suspected AK based on the clinical presentation. In order to collect a sample, we scraped corneal lesions liberally to obtain a sufficient amount of tissue and to maximize the amount of Acanthamoeba collected. The samples were divided into equal parts for each of the following examinations: FICGA, real-time PCR for Acanthamoeba DNA, direct microscopic examination, and culture. In three cases where a sufficient amount of sample was not obtained, the samples were examined by only the FICGA and real-time PCR. All of these examinations were conducted by an investigator who was not involved in the care of the patients. Informed consent was obtained from each patient.
This study was approved by the institutional review board of Ehime University Hospital, and the procedures we performed followed the Declaration of Helsinki.
(ii) Microbial examination and real-time PCR.
Scrapings were smeared onto glass slides, and some were Gram stained (in 6 cases) or stained with Fungiflora Y (in 1 case). The slides were examined by light and fluorescence microscopy. Samples were inoculated directly onto 1.5% nonnutrient agar (NNA) overlaid with Escherichia coli and cultured for at least 7 days at room temperature (25°C to 30°C). The samples were prepared for real-time PCR analysis for Acanthamoeba DNA as described previously (22).
RESULTS
In vitro examination.
The aim of this study was to evaluate the sensitivity of the LICGA and the FICGA for the detection of Acanthamoeba. The thresholds for detecting Acanthamoeba trophozoites with the LICGA are shown in Fig. 2. Three independent assays showed similar results, namely, that trophozoites were detected via this method at concentrations of >100 trophozoites per sample. The thresholds for detecting Acanthamoeba trophozoites and cysts with the FICGA kit are shown in Table 1. The FICGA kit detected trophozoites at concentrations as low as 5 organisms per sample and cysts at concentrations as low as 40 organisms per sample. The FICGA was approximately 20 times more sensitive than the LICGA for detecting Acanthamoeba trophozoites. There was a strong positive correlation between the intensities of the fluorescent test lines (as measured by a specialized fluorescent scanner) and the Acanthamoeba concentrations in the trophozoite and cyst forms (Fig. 3).
FIG 2.
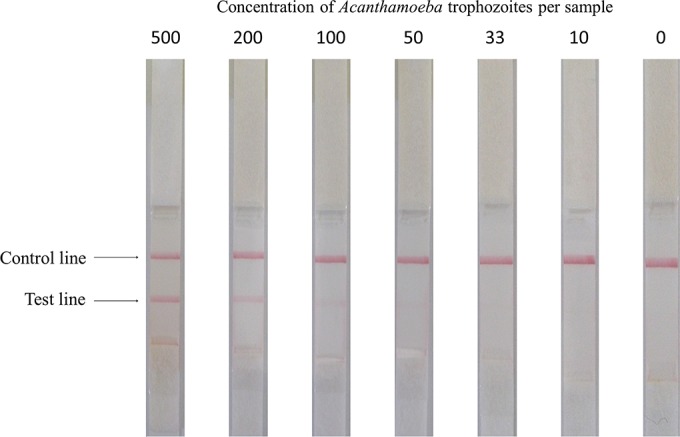
Photograph of test strips used for an immunochromatographic assay with latex labels. The Acanthamoeba castellanii concentration (number of trophozoites per sample) is shown above each strip. The test line was seen at concentrations of at least 100 trophozoites per sample.
TABLE 1.
Detection of Acanthamoeba by fluorescent immunochromatographic assay
| Morphological form | Result at an Acanthamoeba concentration (no. of organisms) ofa: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 8 | 10 | 20 | 40 | 80 | 800 | 8,000 | |
| Trophozoites | − | + | NT | + | + | + | + | + | NT |
| Cysts | − | NT | − | NT | − | + | + | + | + |
+, positive; −, negative; NT, not tested.
FIG 3.

Correlation between fluorescent intensity of the test line measured by a specialized fluorescent scanner and concentration of Acanthamoeba trophozoites and cysts in the samples. There was a significant positive correlation between fluorescent intensity and the Acanthamoeba concentration in the trophozoite and cyst forms. au, arbitrary units.
When samples of P. aeruginosa, S. aureus, S. epidermidis, and C. albicans were used, the FICGA yielded negative results, and there were no cross-reactivities.
Clinical evaluation.
Samples from all 10 patients tested positive for Acanthamoeba DNA when evaluated by real-time PCR (Table 2). The maximum copy number was 4.0 × 105 copies per sample, and the minimum was <25 copies per sample. The FICGA kit detected Acanthamoeba in all 10 patients, consistent with the results of real-time PCR. In 7 patients who were evaluated by smear analysis and culture, the smears and cultures were both positive in 2 patients. Either the culture or the smear was positive in 2 patients, and both studies were negative in the remaining 3 patients. All patients responded well to treatment with antiamoebic therapy with topical biguanides and antifungals.
TABLE 2.
Profiles of patients and results of various diagnostic tests for Acanthamoeba
| Case no. | Age (yr) | Sexa | Smear result | Culture result | Real-time PCR result (DNA copy no.) | FICGA resultb |
|---|---|---|---|---|---|---|
| 1 | 19 | F | Negativec | Negative | Positive (1.1 × 105) | Positive |
| 2 | 18 | M | Negativec | Positive | Positive (6.8 × 10) | Positive |
| 3 | 19 | F | NTd | NT | Positive (1.2 × 105) | Positive |
| 4 | 57 | F | NT | NT | Positive (<25) | Positive |
| 5 | 32 | F | Negativec | Negative | Positive (1.0 × 102) | Positive |
| 6 | 50 | M | NT | NT | Positive (4.0 × 105) | Positive |
| 7 | 24 | F | Negativec | Negative | Positive (<25) | Positive |
| 8 | 29 | M | Positivec | Positive | Positive (2.5 × 104) | Positive |
| 9 | 36 | M | Positivec | Negative | Positive (2.3 × 103) | Positive |
| 10 | 30 | M | Positivee | Positive | Positive (3.2 × 104) | Positive |
M, male; F, female.
FICGA, fluorescent immunochromatographic assay.
Gram stain.
NT, not tested.
Fungiflora Y stain.
DISCUSSION
In recent years, amplification of Acanthamoeba DNA by PCR has become the principal procedure for detecting Acanthamoeba and enabling sensitive diagnosis of AK (19–24). However, PCR has some limitations, as previously stated. A rapid and readily available procedure would help to ensure an accurate diagnosis at the first visit and reduce the risk of inappropriate treatments that complicate the clinical picture and make diagnosis more difficult. In the current study, we succeeded in developing an ICGA kit for detecting Acanthamoeba antigens. This kit was able to detect Acanthamoeba within just 30 min and was extremely simple to use compared with other diagnostic procedures. This test may enable clinicians to diagnose AK in the outpatient examination room. Although the ICGA is quick and easy to use, most existing kits have relatively low sensitivities. For instance, the sensitivity of the ICGA kit for detecting adenovirus has been reported as approximately 60%, whereas the specificity is >90% (29). In order to improve sensitivity, we used antibodies conjugated with fluorescent silica nanoparticles. In our novel FICGA kit, we examined the test line for the presence or absence of fluorescence using a portable fluorescence microscope. We expected that this detection method would be more sensitive than conventional ICGA tests, which are read by direct examination with the naked eye. Our current results demonstrate that the FICGA kit was 20 times more sensitive for detecting Acanthamoeba than the conventional LICGA kit, indicating that the FICGA is a promising approach for improving the accuracy of pathogen detection in a variety of infectious diseases. Although a specialized fluorescence microscope is required to read the test strips, it is a small device which is easy to acquire and is relatively inexpensive, and therefore it can be made available in nearly any local clinic.
In the in vitro portion of the examination, Acanthamoeba cysts were detected at concentrations as low as 40 organisms per sample, whereas trophozoites were detected at concentrations as low as 5 organisms per sample. It is unclear why the assay was less sensitive for detecting cysts than for detecting trophozoites, but one possibility is that unlike trophozoites, cysts may not reliably lyse when exposed to the surfactant in the extraction liquid. This would weaken the signal from the antigen-antibody reaction. Acanthamoeba cysts have been reported to be resistant to some DNA extraction methods used for PCR (34), which would support this theory. Therefore, improvements in the extraction method may increase the sensitivity of assays for Acanthamoeba cysts. As the extraction liquid is not able to isolate antigens from solid specimens, the current FICGA kit is available for the analysis of only liquid or semisolid specimens, such as cultures on gel medium, ocular discharge, or corneal scrapings. To further expand the applications of this assay, the extraction method should be improved in further experiments.
The fluorescent intensity of the test line was strongly correlated with the Acanthamoeba concentration in the sample. This result suggests that the FICGA kit may have the potential to provide a rough quantitative value of Acanthamoeba disease burden, similar to real-time PCR. This information would help clinicians to estimate prognosis and make appropriate treatment decisions (23, 24), although a calibration curve would need to be made. In the current study, the fluorescent intensity of the clinical samples was not quantified because the specialized fluorescent scanner was not available at our facility, and measurements need to be performed within 60 min of the assay. In future studies, it would be of great clinical interest to compare the fluorescent intensity with DNA copy numbers obtained by real-time PCR to determine whether they are correlated in clinical samples. To improve the convenience of our assay, we are now developing a handheld quantitative reader.
In the clinical cases included in this study, the diagnosis of AK was confirmed in all 10 patients by the detection of Acanthamoeba DNA with real-time PCR. The FICGA kit detected Acanthamoeba antigens in all the samples, including those which tested negative by smear or culture and even in cases with low DNA copy numbers. This suggests that the FICGA can detect Acanthamoeba even when there are only a few organisms in the corneal scrapings.
In conclusion, we developed a novel ICGA kit using fluorescent silica nanoparticles for the rapid diagnosis of AK. Our in vitro studies suggest that this kit is highly sensitive for the detection of Acanthamoeba castellanii, and when the test was applied to clinical specimens, the FICGA results corresponded closely with the results of real-time PCR. Although further evaluation of this technique is needed, the FICGA kit seems to be useful for the diagnosis of AK as it is more rapid and simpler to use than conventional diagnostic procedures, including PCR.
ACKNOWLEDGMENTS
This study was supported in part by a grant for research on emerging and reemerging infectious diseases from the Ministry of Health, Labor and Welfare of Japan (H23-Shinkosaiko-ippan-014).
Hideki Aizawa, Kazutomi Miyoshi, and Michio Ohkubo are employees of Furukawa Electric Co., Ltd. (Tokyo, Japan). The other authors declare no conflicts of interest.
REFERENCES
- 1.Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mergeryan H. 1991. The prevalence of Acanthamoeba in the human environment. Rev Infect Dis 13(Suppl 5):S390–S391. [DOI] [PubMed] [Google Scholar]
- 3.Illingworth CD, Cook SD. 1998. Acanthamoeba keratitis. Surv Ophthalmol 42:493–508. doi: 10.1016/S0039-6257(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 4.Penland RL, Wilhelmus KR. 1997. Comparison of axenic and monoxenic media for isolation of Acanthamoeba. J Clin Microbiol 35:915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byers TJ, Akins RA, Maynard BJ, Lefken RA, Martin SM. 1980. Rapid growth of Acanthamoeba in defined media; induction of encystment by glucose-acetate starvation. J Protozool 27:216–219. doi: 10.1111/j.1550-7408.1980.tb04684.x. [DOI] [PubMed] [Google Scholar]
- 6.Chagla AH, Griffiths AJ. 1974. Growth and encystation of Acanthamoeba castellanii. J Gen Microbiol 85:139–145. doi: 10.1099/00221287-85-1-139. [DOI] [PubMed] [Google Scholar]
- 7.Thebpatiphat N, Hammersmith KM, Rocha FN, Rapuano CJ, Ayres BD, Laibson PR, Eagle RC Jr, Cohen EJ. 2007. Acanthamoeba keratitis: a parasite on the rise. Cornea 26:701–706. doi: 10.1097/ICO.0b013e31805b7e63. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2007. Acanthamoeba keratitis—multiple states, 2005-2007. MMWR Morb Mortal Wkly Rep 56:532–534. [PubMed] [Google Scholar]
- 9.Bacon AS, Frazer DG, Dart JK, Matheson M, Ficker LA, Wright P. 1993. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984–1992. Eye (London, England) 7(Pt 6):719–725. [DOI] [PubMed] [Google Scholar]
- 10.Johns KJ, O'Day DM, Head WS, Neff RJ, Elliott JH. 1987. Herpes simplex masquerade syndrome: Acanthamoeba keratitis. Current Eye Res 6:207–212. doi: 10.3109/02713688709020092. [DOI] [PubMed] [Google Scholar]
- 11.Tay-Kearney ML, McGhee CN, Crawford GJ, Trown K. 1993. Acanthamoeba keratitis: a masquerade of presentation in six cases. Aust N Z J Ophthalmol 21:237–245. doi: 10.1111/j.1442-9071.1993.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 12.Claerhout I, Goegebuer A, Van Den Broecke C, Kestelyn P. 2004. Delay in diagnosis and outcome of Acanthamoeba keratitis. Graefes Arch Clin Exp Ophthalmol 242:648–653. doi: 10.1007/s00417-003-0805-7. [DOI] [PubMed] [Google Scholar]
- 13.Bacon AS, Dart JK, Ficker LA, Matheson MM, Wright P. 1993. Acanthamoeba keratitis: the value of early diagnosis. Ophthalmology 100:1238–1243. [DOI] [PubMed] [Google Scholar]
- 14.Radford CF, Minassian DC, Dart JK. 2002. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol 86:536–542. doi: 10.1136/bjo.86.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiraishi A, Kobayashi T, Hara Y, Yamaguchi M, Uno T, Ohashi Y. 2009. Rapid detection of Acanthamoeba cysts in frozen sections of corneal scrapings with Fungiflora Y. Br J Ophthalmol 93:1563–1565. doi: 10.1136/bjo.2009.158113. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelmus KR, Osato MS, Font RL, Robinson NM, Jones DB. 1986. Rapid diagnosis of Acanthamoeba keratitis using calcofluor white. Arch Ophthalmol 104:1309–1312. doi: 10.1001/archopht.1986.01050210063026. [DOI] [PubMed] [Google Scholar]
- 17.Hahn TW, O'Brien TP, Sah WJ, Kim JH. 1998. Acridine orange staining for rapid diagnosis of Acanthamoeba keratitis. Jpn J Ophthalmol 42:108–114. doi: 10.1016/S0021-5155(97)00127-5. [DOI] [PubMed] [Google Scholar]
- 18.Hammersmith KM. 2006. Diagnosis and management of Acanthamoeba keratitis. Curr Opin Ophthalmol 17:327–331. doi: 10.1097/01.icu.0000233949.56229.7d. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann OJ, Green SM, Morlet N, Kilvington S, Keys MF, Matheson MM, Dart JK, McGill JI, Watt PJ. 1998. Polymerase chain reaction analysis of corneal epithelial and tear samples in the diagnosis of Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 39:1261–1265. [PubMed] [Google Scholar]
- 20.Pasricha G, Sharma S, Garg P, Aggarwal RK. 2003. Use of 18S rRNA gene-based PCR assay for diagnosis of Acanthamoeba keratitis in non-contact lens wearers in India. J Clin Microbiol 41:3206–3211. doi: 10.1128/JCM.41.7.3206-3211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yera H, Zamfir O, Bourcier T, Ancelle T, Batellier L, Dupouy-Camet J, Chaumeil C. 2007. Comparison of PCR, microscopic examination and culture for the early diagnosis and characterization of Acanthamoeba isolates from ocular infections. Eur J Clin Microbiol Infect Dis 26:221–224. doi: 10.1007/s10096-007-0268-6. [DOI] [PubMed] [Google Scholar]
- 22.Kandori M, Inoue T, Takamatsu F, Kojima Y, Hori Y, Maeda N, Tano Y. 2010. Two cases of Acanthamoeba keratitis diagnosed only by real-time polymerase chain reaction. Cornea 29:228–231. doi: 10.1097/ICO.0b013e3181a39020. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda Y, Miyazaki D, Yakura K, Kawaguchi A, Ishikura R, Inoue Y, Mito T, Shiraishi A, Ohashi Y, Higaki S, Itahashi M, Fukuda M, Shimomura Y, Yagita K. 2012. Assessment of real-time polymerase chain reaction detection of Acanthamoeba and prognosis determinants of Acanthamoeba keratitis. Ophthalmology 119:1111–1119. doi: 10.1016/j.ophtha.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Itahashi M, Higaki S, Fukuda M, Mishima H, Shimomura Y. 2011. Utility of real-time polymerase chain reaction in diagnosing and treating Acanthamoeba keratitis. Cornea 30:1233–1237. doi: 10.1097/ICO.0b013e3182032196. [DOI] [PubMed] [Google Scholar]
- 25.Battaglioli G, Nazarian EJ, Lamson D, Musser KA, St George K. 2012. Evaluation of the RIDAQuick norovirus immunochromatographic test kit. J Clin Virol 53:262–264. doi: 10.1016/j.jcv.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Hotomi M, Togawa A, Takei S, Sugita G, Sugita R, Kono M, Fujimaki Y, Kamide Y, Uchizono A, Kanesada K, Sawada S, Okitsu N, Tanaka Y, Saijo Y, Yamanaka N. 2012. Evaluation of a rapid immunochromatographic ODK-0901 test for detection of pneumococcal antigen in middle ear fluids and nasopharyngeal secretions. PLoS One 7:e33620. doi: 10.1371/journal.pone.0033620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitamura K, Shimizu H, Yamazaki M, Ichikawa M, Nagai K, Katada J, Wada A, Kawakami C, Sugaya N. 2013. Clinical evaluation of highly sensitive silver amplification immunochromatography systems for rapid diagnosis of influenza. J Virol Methods 194:123–128. doi: 10.1016/j.jviromet.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Inoue Y, Shimomura Y, Fukuda M, Miyazaki D, Ohashi Y, Sasaki H, Tagawa Y, Shiota H, Inada N, Okamoto S, Araki-Sasaki K, Kimura T, Hatano H, Nakagawa H, Nakamura S, Hirahara A, Tanaka K, Sakuma H. 2013. Multicentre clinical study of the herpes simplex virus immunochromatographic assay kit for the diagnosis of herpetic epithelial keratitis. Br J Ophthalmol 97:1108–1112. doi: 10.1136/bjophthalmol-2012-302254. [DOI] [PubMed] [Google Scholar]
- 29.Uchio E, Aoki K, Saitoh W, Itoh N, Ohno S. 1997. Rapid diagnosis of adenoviral conjunctivitis on conjunctival swabs by 10-minute immunochromatography. Ophthalmology 104:1294–1299. doi: 10.1016/S0161-6420(97)30145-6. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino T, Takanashi T, Okada M, Uchida S. 2002. Oxybuprocaine induces a false-positive response in immunochromatographic SAS Adeno Test. Ophthalmology 109:808–809. doi: 10.1016/S0161-6420(01)01028-4. [DOI] [PubMed] [Google Scholar]
- 31.Hough EA, Ruddock KH. 1969. The Purkinje shift. Vision Res 9:313–315. doi: 10.1016/0042-6989(69)90009-1. [DOI] [PubMed] [Google Scholar]
- 32.Hiwatashi E, Tachibana H, Kaneda Y, Obazawa H. 1997. Production and characterization of monoclonal antibodies to Acanthamoeba castellanii and their application for detection of pathogenic Acanthamoeba spp. Parasitol Int 46:197–205. doi: 10.1016/S1383-5769(97)00029-9. [DOI] [Google Scholar]
- 33.Mito T, Suzuki T, Kobayashi T, Zheng X, Hayashi Y, Shiraishi A, Ohashi Y. 2012. Effect of photodynamic therapy with methylene blue on Acanthamoeba in vitro. Invest Ophthalmol Vis Sci 53:6305–6313. doi: 10.1167/iovs.12-9828. [DOI] [PubMed] [Google Scholar]
- 34.Goldschmidt P, Degorge S, Saint-Jean C, Yera H, Zekhnini F, Batellier L, Laroche L, Chaumeil C. 2008. Resistance of Acanthamoeba to classic DNA extraction methods used for the diagnosis of corneal infections. Br J Ophthalmol 92:112–115. doi: 10.1136/bjo.2007.125898. [DOI] [PubMed] [Google Scholar]