Abstract
Weak-positive Neisseria gonorrhoeae nucleic acid amplification test results are difficult to interpret. We show that the frequency of unconfirmed N. gonorrhoeae results from the cobas 4800 test rises exponentially after 38.0 cycles, where the likelihood of an unconfirmed result exceeds 29%. Supplementary testing of such samples should be avoided; instead, treatment should be based on clinical pretest probability.
TEXT
The accurate diagnosis of gonorrhea demands laboratory tests that are sensitive, specific, reproducible, and robust because of variable clinical symptoms and the consequences of missed or incorrect diagnoses. The use of nucleic acid amplification tests (NAATs) for diagnosis has been a significant advance because of improved sensitivity and ease of specimen collection and transport (1).
International guidelines recommend that N. gonorrhoeae-positive NAAT results be confirmed by using supplementary assays with different targets if the positive predictive value (PPV) is <90% (2). The need for supplemental testing of urogenital samples is debated because of the high PPV of these samples (3, 4).
Labtests Auckland and Aotea Pathology are two large community laboratories in Auckland and Wellington, New Zealand, respectively. Both employ the cobas 4800 CT/NG assay, which targets the direct repeat 9 region of the N. gonorrhoeae genome, for testing in low-prevalence populations (5, 6). Confirmation of N. gonorrhoeae-positive cobas 4800 results is routinely performed with a duplex porA and opa assay (7) of samples from extragenital sites (not Communauté Européenne approved for use as an in vitro diagnostic medical device in the cobas 4800 CT/NG test) and of urogenital specimens with late threshold cycle (CT) values. There are limitations to the use of the porA target alone, as it has been previously reported that there is a possibility of false-negative results due to the acquisition of a meningococcal porA sequence (8). Concurrent culture is now performed only in certain clinical situations.
Between September 2012 and January 2014, a total of 134 patient samples (73 urogenital, 42 pharyngeal, 19 rectal) were positive for N. gonorrhoeae in the cobas 4800 test and met the criteria for supplementary testing. Samples were referred from general practice, sexual health clinics, and other community health providers. Twenty-two of the samples were from females, 73 were from males, and no gender or date-of-birth information was available for 39 of them. The median age of the patients at the time of testing was 27 years with a range of 3 to 64 years.
Of the134 samples in this study, 120 (90%) were positive for at least two N. gonorrhoeae targets. The remaining 14 could not be confirmed with either the porA or the opa assay and produced a significantly higher mean cobas 4800 CT value (30.6 versus 38.4, P < 0.01). The majority of the unconfirmed samples (71%) were from extragenital sites.
Concern about the interpretation of these results arises from issues with false-positive results in low-prevalence populations, test reproducibility when there are low genome copy numbers in the specimen, and the possibility of cross-reaction with commensal Neisseria species at extragenital sites (9, 10). In order to address these issues, we undertook an investigation to determine the analytical sensitivity of the cobas 4800 CT/NG test and correlate this with the reproducibility of the results of porA and opa supplementary assays. Following this, we determined whether the CT value in the cobas 4800 CT/NG assay was correlated with the results of supplementary assays of clinical samples.
A quantified N. gonorrhoeae DNA standard (Vircell) was reconstituted according to the manufacturer's instructions to yield a stock concentration of 12,920 copies/μl (11). The stock DNA was then diluted in cobas collection kit buffer to achieve standards representing 1,000, 100, 50, 10, 5, 1, 0.5, 0.1, and 0.05 copies/μl. The standards were tested with the cobas 4800 instrument in accordance with the manufacturer's instructions for swabs (12). Standards with high N. gonorrhoeae concentrations (5 to 1,000 copies/μl) were tested in duplicate, while 1- and 0.5-copy/μl standards were tested on 10 consecutive occasions and the mean CT value and coefficient of variation (CV) were calculated. As expected, an increasing variability of CT values was seen at ≤1 copy/μl (mean CT, >38.0; CV, 1.75 to 1.94%). When the standards were tested by supplementary assays, the porA target performed similarly to the cobas 4800 CT/NG test. The opa target produced earlier CT values but without an increase in analytical sensitivity (data not shown).
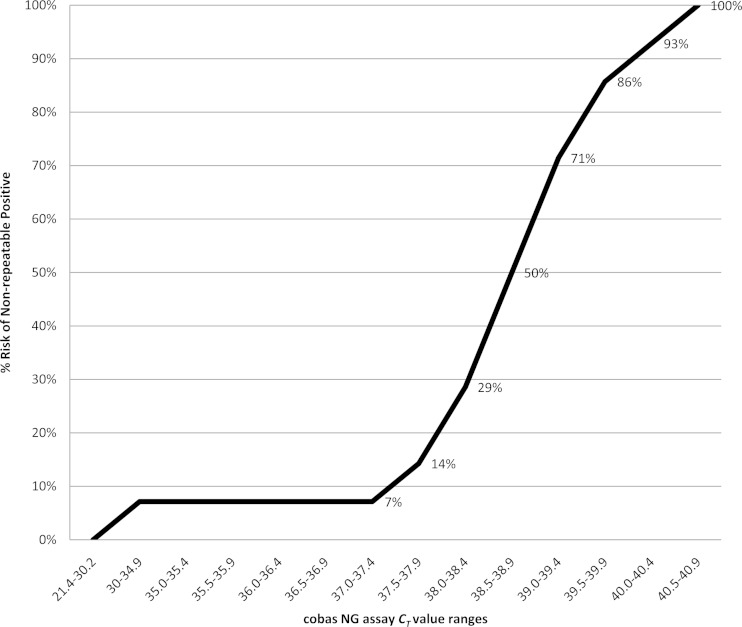
The second part of the analysis determined whether cobas 4800 CT/NG test CT values were related to the likelihood of confirmation by porA and opa supplementary assays. The frequency of negative supplementary testing associated with cobas N. gonorrhoeae CT values was calculated from the 134 samples that were tested by all three assays (Fig. 1). The frequency of N. gonorrhoeae results unconfirmed by porA and opa begins to rise after a CT of 37.5 in the cobas 4800 CT/NG test and rises exponentially after 38.0 cycles. A cobas 4800 CT/NG test CT of ≥38 represents less than one copy of the N. gonorrhoeae genome in the sample. At this level, the likelihood of a nonconfirmable result exceeds 29%.
FIG 1.
The percentage of samples positive by cobas that are negative for porA and opa supplementary targets increases as the cobas N. gonorrhoeae threshold cycle (CT) rises (n = 134).
We therefore propose that supplementary testing of cobas 4800 CT/NG test samples with CTs of ≥38 has limited utility. For urogenital specimens, where the PPV is high, we recommend reporting these results as indeterminate or equivocal. Treatment should be based on clinical pretest probability.
Where specimens are taken from low-risk patients for screening purposes (including testing protocols used at cervical smear or insertion of an intrauterine contraceptive device), we suggest repeat testing if concern remains. Correlation not just with the clinical presentation (symptomatic or asymptomatic) but also with social history and epidemiological risks is advised, and further testing may be warranted.
ACKNOWLEDGMENT
We have no conflicts of interest to declare.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2014. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 2.Bignell C, Ison C, FitzGerald M. 2012. United Kingdom national guideline for gonorrhoea testing—2012. British Association for Sexual Health and HIV, Clinical Effectiveness Group, Macclesfield, United Kingdom: http://www.bashh.org/documents/4490.pdf. [Google Scholar]
- 3.Bromhead C, Miller A, Jones M, Whiley D. 2013. Comparison of the cobas 4800 CT/NG test with culture for detecting Neisseria gonorrhoeae in genital and nongenital specimens in a low-prevalence population in New Zealand. J Clin Microbiol 51:1505–1509. doi: 10.1128/JCM.03223-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry MD, Jones RN, Corden SA. 2014. Is confirmatory testing of Roche cobas 4800 CT/NG test Neisseria gonorrhoeae positive samples required? Comparison of the Roche cobas 4800 CT/NG test with an opa/pap duplex assay for the detection of N gonorrhoeae. Sex Transm Infect 90:303–308. doi: 10.1136/sextrans-2013-051410. [DOI] [PubMed] [Google Scholar]
- 5.The Institute of Environmental Science and Research Ltd. 2012. Sexually transmitted infections in New Zealand: annual surveillance report 2012. The Institute of Environmental Science and Research Ltd, Porirua, New Zealand. [Google Scholar]
- 6.Rockett R, Goire N, Limnios A, Turra M, Higgens G, Lambert SL, Bletchly C, Nissen MD, Sloots TP, Whiley DM. 2010. Evaluation of the cobas 4800 CT/NG test for detecting Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Infect 86:470–473. doi: 10.1136/sti.2010.042812. [DOI] [PubMed] [Google Scholar]
- 7.Goire N, Nissen MD, LeCornec GM, Sloots TP, Whiley DM. 2008. A duplex Neisseria gonorrhoeae real-time polymerase chain reaction assay targeting the gonococcal porA pseudogene and mulitcopy opa genes. Diagn Microbiol Infect Dis 61:6–12. doi: 10.1016/j.diagmicrobio.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Whiley DM, Limnios A, Moon NJ, Gehrig N, Goire N, Hogan T, Lam A, Jacob K, Lambert SB, Nissen MD, Sloots TP. 2011. False-negative results using the Neisseria gonorrhoeae porA pseudogene PCR—a clinical gonococcal isolate with an N. meningitides porA sequence. Euro Surveill 16:19874 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19874. [PubMed] [Google Scholar]
- 9.Upton A, Bromhead C, Whiley DM. 2013. Neisseria gonorrhoeae false-positive result obtained from a pharyngeal swab by using the Roche cobas 4800 CT/NG assay in New Zealand in 2012. J Clin Microbiol 51:1609–1610. doi: 10.1128/JCM.00485-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabrizi SN, Unemo M, Limnios AE, Hogan TR, Hjelmevoll S, Garland SM, Tapsall J. 2011. Evaluation of six commercial nucleic acid amplification tests for the detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol 49:3610–3615. doi: 10.1128/JCM.01217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vircell Microbiologists. 2014. Amplirun Neisseria gonorrhoeae DNA control product insert. Vircell Microbiologists, Granada, Spain. [Google Scholar]
- 12.Roche Molecular Systems. 2011. Roche cobas 4800 CT/NG test product insert, V5.0. Roche Molecular Systems, Inc, Branchburg, NJ. [Google Scholar]