Abstract
The diagnosis of tuberculosis (TB) is difficult in children, especially for smear-negative pulmonary and extrapulmonary TB, which are common at this age. We report an 11-year-old girl with TB otitis media with negative smear microscopy and Xpert MTB/RIF but positive Mycobacterium tuberculosis-specific transrenal DNA (Tr-MTB-DNA) test results and culture for M. tuberculosis.
CASE REPORT
An 11-year-old girl was admitted to the Department of Otolaryngology of the S. Orsola-Malpighi University Hospital, Bologna, Italy, with a 1-year history of bilateral chronic otitis media associated with conductive hearing loss; she had received several courses of broad-spectrum antibiotic therapy without significant improvement. A myringotomy was planned to aspirate the exudate and decompress the tympanic cavity, but instead of the expected effusion, a diffuse tympanic granulomatosis was noted. The child was therefore referred to the Pediatric Respiratory Service for further investigation to exclude a diagnosis of tubercular otitis media (TBOM). The child had a medical history of asthma and dust allergy but was otherwise healthy. Her parents had originated in Bangladesh, but she was born and had grown up in Italy. She had traveled to Bangladesh for 6 months the year before the beginning of the symptoms but had no known contact with persons having tuberculosis (TB) or chronic cough.
On admission, the child was in good condition, her general examination was normal, and there was no evidence of other disease localizations or disseminated TB. A chest X-ray was unremarkable. A tuberculin skin test (TST) (25-mm induration) and Quantiferon-TB Gold in-tube (QFT-IT; Qiagen, Germany; TBAg-Nil = 1.46 IU interferon gamma/ml) test were positive. In addition to the auricular secretion collected during micro-otoscopy, a multiple-sampling approach was performed: nasopharyngeal aspirates (NPA), gastric aspirates (GA), and sputum and urine samples were collected on consecutive days. Each sample underwent direct smear microscopy after Ziehl-Neelsen staining and testing with an Xpert MTB/RIF assay (Cepheid, France), and all were negative. All the specimens were also cultured on liquid (Bactec MGIT 960; Becton Dickinson, USA) and solid (Lowenstein-Jensen; Heipha, Germany) media.
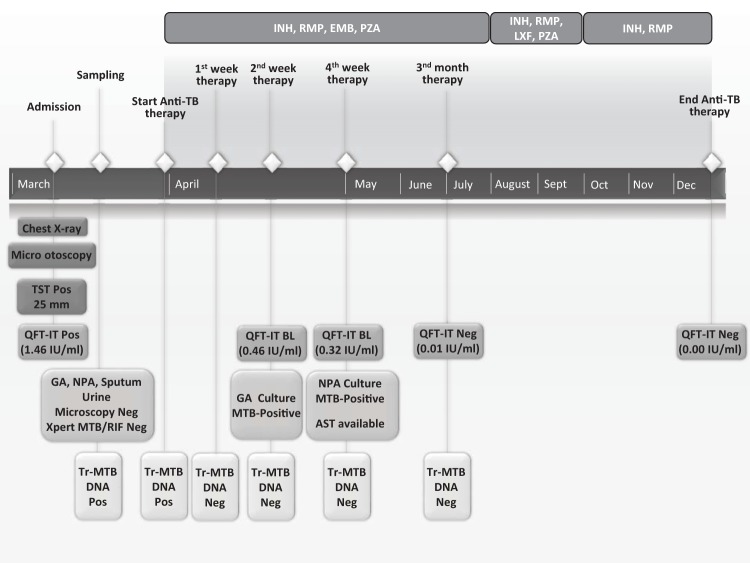
In order to detect Mycobacterium tuberculosis-specific transrenal DNA (Tr-MTB-DNA), as described by Cannas et al. (1), 6 urine samples (50 ml each) were collected during the study period, as depicted in Fig. 1, and quickly added to 1 ml of a stabilization buffer (0.5 M EDTA–0.5 M Tris-HCl, pH 8.5) to a final concentration of 10 mM, in order to minimize degradation of soluble DNA by nucleases. The stabilized urine specimens were then stored in 1-ml aliquots at −80°C. DNA was extracted from 1 ml of whole urine and eluted in 25 μl with an automated system based on magnetic silica particle technology (NucliSENS easyMAG; bioMérieux, France); urine specimens had previously been lysed off-board at 95°C for 30 min. Tr-MTB-DNA was amplified by in-house seminested PCR, targeting the M. tuberculosis IS6110 region. Primer sequences (F-785, 5′-ACCAGCACCTAACCGGCTGTGG-3′; R-913, 5′-CATCGTGGAAGCGACCCGCCAG-3′; Rn-851, 5′-GTAGGCGAACCCTGCCCAGGTC-3′) used in our study were designed by Cannas et al. (1), and their specificity has been already assessed against the complete sequences of human and several bacterial genomes. The first PCR amplification was performed using 5 μl of eluted DNA added to a mixture (final volume of 25 μl) containing 0.2 μM concentrations of the outer primers F-785 and R-913, a 0.2 mM concentration of each deoxynucleoside triphosphate (dNTP), 1× PCR buffer, 1.5 mM MgCl2, and 2 U AmpliTaq Gold (Applied Biosystems, USA), and denatured at 94°C for 10 min, followed by 20 cycles at 94°C for 30 s, 62°C for 30 s, and 72°C for 1 min and 1 cycle at 72°C for 5 min. The second PCR amplification was performed on 1 μl of the product from the first amplification (129 bp), using primers F-785 and Rn-851 for 35 cycles under the same condition as in the first reaction. The final amplification product (67 bp) was visualized on 3% agarose gel electrophoresis.
FIG 1.
Case report timeline describing sampling, treatment schedule and microbiological results. GA, gastric aspiration; NPA, nasopharyngeal aspirate; AST, anti-tubercular susceptibility test; QFT-IT, Quantiferon-TB Gold in-tube test; Tr-MTB-DNA, Mycobacterium tuberculosis transrenal DNA; INH, isoniazid; RMP, rifampin; EMB, ethambutol; PZA, pyrazinamide; LXF, levofloxacin.
The presence of Tr-MTB-DNA was detected in the first 2 urine samples collected before the start of treatment (Fig. 2). In order to evaluate the specificity of this approach, urine samples from 10 healthy individuals were also processed and were negative for Tr-MTB-DNA (data not shown).
FIG 2.
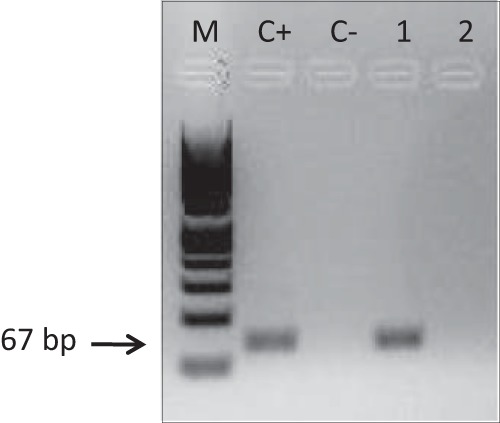
Detection of Tr-MTB-DNA in urine by seminested PCR, as described by Cannas et al. (1). Lanes: M, molecular weight standard (Marker VIII; Roche Diagnostics, Germany); C+, genomic DNA from M. tuberculosis H37RV; C−, no-template control; 1, urine collected before treatment; 2, urine collected 2 weeks after initiation of treatment. The Tr-MTB-DNA amplification product was 67 bp.
All family members (mother, father, and two older sisters) underwent a TST, a QFT-IT test, and a chest X-ray and were diagnosed as having latent TB infection. Given the clinical presentation, test results, and family history, the child underwent a 9-month anti-TB treatment: combined therapy with isoniazid, rifampin, ethambutol (replaced by levofloxacin after 4 months), and pyrazinamide for the first 6 months, followed by 3 months of maintenance with isoniazid and rifampin.
Follow-up testing of urine samples for Tr-MTB-DNA at 1, 2 (Fig. 2), 4, and 12 weeks after initiation of treatment was negative. Liquid culture confirmation for M. tuberculosis was obtained from 1 out of 3 GA after 3 weeks of incubation, while few colonies (<10) were detected on solid culture of NPA 2 weeks later. In contrast, all other samples (2 GA, 3 sputum samples, 3 urine samples, and the auricular secretion) were culture negative. An anti-TB susceptibility test was available 1 month after the start of therapy; the strain was susceptible to all first-line anti-tubercular drugs.
The child underwent a strict clinical and laboratory follow-up, including QFT-IT tests, which yielded results around the cutoff value after 2 and 4 weeks of treatment (TBAg-Nil = 0.46 IU/ml and 0.32 IU/ml, respectively), reverted to negative after 3 months of therapy, and remained stable during the study period.
Anti-TB treatment was well tolerated, and the signs of otitis media progressively improved, but the child had persistent conductive hearing loss. One year later, the girl was in good condition and her hearing loss was corrected with a hearing aid.
The patient's caregivers gave informed consent for the publication of this case report.
The diagnosis of TB in children is often challenging, especially in extrapulmonary TB (EPTB), where the collection of specimens for microbiological confirmation is difficult and the yield of the tests is low (2, 3). Otitis media is a rare presentation of EPTB, but sporadic cases are occasionally reported from countries where TB has low endemicity, especially in migrant populations. Tubercular otitis media is difficult to diagnose. Its presentation is often mistaken for other types of chronic suppurative otitis media, and the diagnosis relies on a high index of suspicion and confirmation by microbiological tests, which, however, perform poorly in this situation (4). The early detection of TBOM is essential because a delay in treatment is associated with profound hearing loss and serious complications, such as peripheral facial palsy, postauricular fistulae, acute mastoiditis, tuberculous osteomyelitis of the petrous pyramid, labyrinthitis, and, more rarely, meningitis, intracranial tuberculoma, or abscesses (5).
Recent research has focused on new diagnostic tools that could indirectly confirm active disease regardless of the site of presentation, possibly based on biomarkers detectable in biological specimens obtained without invasive procedures (6).
Tr-MTB-DNA is a marker of TB, and the assay is based on the detection of small (<300-bp), cell-free, M. tuberculosis-specific DNA sequences in urine. These DNA sequences are released in the bloodstream from the breakdown of dying M. tuberculosis cells and filtered by the kidneys when passing the glomerular barrier (7). Although several studies have evaluated this approach in the last decade, the procedure has not yet been standardized, and the performance of Tr-MTB-DNA is poorly defined. Several authors have reported high specificity (98% to 100%) but sensitivities ranging from 7% to 79% (for a review, see reference 8). Most of these discrepancies have been attributed to differences in the methods used for collection and storage of urine specimens and in the extraction and amplification of Tr-DNA. It has been suggested that improvement of extraction techniques, targeting Tr-DNA fragments smaller than 150 to 200 bp, and the use of real-time PCR might increase sensitivity and specificity of the test (8). In our study, we employed a magnetic-bead-based extraction method which is effective for extracting 200-bp fragments and therefore suitable for detecting Tr-DNA (9).
There are no available data on the performance of Tr-MTB-DNA for the diagnosis of EPTB in children, and the case here reported demonstrates its utility as an adjunct marker to standard microbiological diagnosis. Moreover, as the assay became negative after 1 week of treatment, it can be used as a marker to monitor treatment response. Urine is a product readily collected from children, and Tr-MTB-DNA has the potential to be developed as a dipstick diagnostic, which could be rapid and easy to use. The standardization of the technique, in particular steps regarding urine collection and stabilization (e.g., use of a Copan liquid Amies elution swab), DNA extraction (e.g., spin column-based or magnetic-bead-based extraction systems), and detection methodology (real-time PCR), should be encouraged.
REFERENCES
- 1.Cannas A, Goletti D, Girardi E, Chiacchio T, Calvo L, Cuzzi G, Piacentini M, Melkonyan H, Umansky SR, Lauria FN, Ippolito G, Tomei LD. 2008. Mycobacterium tuberculosis DNA detection in soluble fraction of urine from pulmonary tuberculosis patients. Int J Tuberc Lung Dis 12:146–151. [PubMed] [Google Scholar]
- 2.Perez-Velez CM, Marais BJ. 2012. Tuberculosis in children. N Engl J Med 367:348–361. doi: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- 3.Cuevas LE. 2011. The urgent need for new diagnostics for symptomatic tuberculosis in children. Indian J Pediatr 78:449–455. doi: 10.1007/s12098-010-0354-0. [DOI] [PubMed] [Google Scholar]
- 4.Dale OT, Clarke AR, Drysdale AJ. 2011. Challenges encountered in the diagnosis of tuberculous otitis media: case report and literature review. J Laryngol Otol 125:738–740. doi: 10.1017/S0022215111000971. [DOI] [PubMed] [Google Scholar]
- 5.Abes GT, Abes FL, Jamir JC. 2011. The variable clinical presentation of tuberculosis otitis media and the importance of early detection. Otol Neurotol 32:539–543. doi: 10.1097/MAO.0b013e3182117782. [DOI] [PubMed] [Google Scholar]
- 6.Tuuminen T. 2012. Urine as a specimen to diagnose infections in twenty-first century: focus on analytical accuracy. Front Immunol 3:45. doi: 10.3389/fimmu.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peter J, Green C, Hoelscher M, Mwaba P, Zumla A, Dheda K. 2010. Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Curr Opin Pulm Med 16:262–270. doi: 10.1097/MCP.0b013e328337f23a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green C, Huggett JF, Talbot E, Mwaba P, Reither K, Zumla AI. 2009. Rapid diagnosis of tuberculosis through the detection of mycobacterial DNA in urine by nucleic acid amplification methods. Lancet Infect Dis 9:505–511. doi: 10.1016/S1473-3099(09)70149-5. [DOI] [PubMed] [Google Scholar]
- 9.Huijsmans CJ, Damen J, van der Linden JC, Savelkoul PH, Hermans MH. 2010. Comparative analysis of four methods to extract DNA from paraffin-embedded tissues: effect on downstream molecular applications. BMC Res Notes 3:239. doi: 10.1186/1756-0500-3-239. [DOI] [PMC free article] [PubMed] [Google Scholar]