Abstract
An occurrence of Vibrio cholerae non-O1/O139 gastroenteritis in the U.S. Gulf Coast is reported here. Genomic analysis revealed that the isolate lacked known virulence factors associated with the clinical outcome of a V. cholerae infection but did contain putative genomic islands and other accessory virulence factors. Many of these factors are widespread among environmental strains of V. cholerae, suggesting that there might be additional virulence factors in non-O1/O139 V. cholerae yet to be determined. Phylogenetic analysis revealed that the isolate belonged to a phyletic lineage of environmental V. cholerae isolates associated with sporadic cases of gastroenteritis in the Western Hemisphere, suggesting a need to monitor non-O1/O139 V. cholerae in the interest of public health.
INTRODUCTION
Vibrio spp. are natural inhabitants of marine and estuarine environments, and they cause human infections that most commonly present as gastroenteritis or wound infections and/or septicemia (1, 2). The infection is generally acquired through the consumption of contaminated food or water or by the direct invasion of wounds (3). Vibrio cholerae is the causative agent of cholera, the severe watery diarrheal disease that has the potential to become pandemic. Based on variable somatic (outer membrane) O-antigen composition, more than 200 serogroups of V. cholerae have been recognized to date (4). Of them, toxigenic strains of V. cholerae typically belong to serogroups O1 or O139 and are rare in the United States (5). All other serogroups, notably the non-O1/O139 serogroup, are frequently isolated from environmental sources and have been associated with sporadic cases of gastroenteritis or extraintestinal infections. Although none has caused a pandemic yet (6), these serogroups have reportedly caused epidemics of cholera through the acquisition of genes carried on mobile elements (e.g., O antigens, vibrio pathogenicity island 1 [VPI-1], VPI-2, cholera toxin phage [CTXϕ], and heat-stable enterotoxin [NAG-ST]). Human illnesses caused by environmental V. cholerae non-O1/O139 serogroups are reported regularly (7). Over the past few decades, environmental studies have shown that nontoxigenic V. cholerae strains inhabit estuarine waters along the Atlantic and Gulf coasts (8–14). They are most commonly isolated from environmental sources, such as brackish water, oysters, and sewage, and have been reported in many countries, such as Bangladesh, Brazil, Guam, Great Britain, and the United States, even when cholera outbreaks had not been recorded for decades in those countries (2).
In the United States, the consumption of raw or undercooked seafood is the leading cause of non-O1/O139 V. cholerae-associated gastroenteritis, with isolated cases reported (8, 15). Outbreaks of intestinal illness caused by non-O1/O139 V. cholerae have been reported more commonly than would be expected (16–18). Since 2000, an average of 44 cases has been reported each year (http://www.cdc.gov/nationalsurveillance/cholera-vibrio-surveillance.html). The majority of infections to date have originated from the Gulf of Mexico, where the surface water is warm, often reaching 34°C in late summer (19), thereby providing optimal conditions for the growth of Vibrio spp. (8, 15, 20–24).
In this report, we describe the isolation of a nontoxigenic V. cholerae non-O1/O139 strain from a 30-year-old male with a history of hypertension who presented with a complaint of >30 episodes of watery nonbloody stools in a 48-h period. Using whole-genome sequencing technology, we sequenced the isolate to determine the presence of virulence factors and phylogenetic relatedness.
CASE REPORT
A 30-year-old male with a history of hypertension presented with a complaint of >30 episodes of watery nonbloody stools in a 48-h period. He had visited Lake Charles, LA, 2 days prior to the onset of symptoms and had eaten a seafood platter consisting of battered and fried catfish, shrimp, stuffed crab, and crawfish étouffée. The physical examination was unremarkable. The laboratory evaluation revealed a normal white blood cell count and normal renal function without electrolyte abnormalities. A stool culture was sent to the Keesler Air Force Base (AFB) laboratory, and only a V. cholerae isolate was obtained on blood and thiosulfate-citrate-bile salts-sucrose (TCBS) agars; it was identified as V. cholerae by the Keesler AFB laboratory using the Vitek 2 (bioMérieux, France). The patient was treated successfully with a one-time 300-mg dose of doxycycline, with complete resolution of symptoms.
MATERIALS AND METHODS
The Keesler AFB submitted a TCBS agar culture to the Mississippi Public Health Laboratory (MPHL) for confirmatory culture-based identification and to the University of Southern Mississippi Gulf Coast Research Laboratory (GCRL) for molecular identification. The isolate was also tested for motility, protease activity, hemolytic activity, and biofilm formation. The motility test was performed in motility indole urea (MIU), a semisolid medium in which a pure colony was inoculated through a stab with a sterile straight wire followed by incubation at 37°C overnight. Motility was shown by the spreading turbidity from the stab line or by turbidity throughout the medium. Protease activity was tested using a marine agar-skim milk agar double-layer plate onto which a layer of marine agar was overlaid on skim milk agar (25). The hemolytic activity was tested on commercially available blood agar (tryptic soy agar [TSA] plus 5% sheep blood; Hardy Diagnostic). Biofilm production was determined by a semiquantitative adherence assay on 96-well microtiter plates, as described previously (26). V. cholerae El Tor reference strain N16961 (ATCC 39315) was used as a positive control for the biofilm formation assay. Biofilm formation was quantified by the addition of 400 μl of 95% ethanol to each crystal violet-stained well of the microtiter plate followed by the determination of absorbance at 570 nm using a spectrophotometer (GENESYS 10S Bio UV/Vid spectrophotometer; Thermo Scientific).
Genomic DNA from the isolate was sent to the University of Maryland, where it was subjected to next-generation whole-genome Illumina and hybrid Ilumina/454 pyrosequencing; the libraries were constructed with target insert sizes of 3 kb and 100-bp paired-end reads. Hybrid and Illumina sequences were assembled using the Celera and Velvet assemblers, as described elsewhere (27). The combination of next-generation whole-genome Illumina and 454 pyrosequencing yielded good-quality draft assemblies (311 contigs) of the V. cholerae BJG-01 genome (PRJNA64001). The RAST subsystem annotation identified 3,467 predicted coding sequences and 97 noncoding RNAs. Approximately 23% of the predicted coding sequences were annotated as hypothetical proteins, including proteins conserved in other bacteria.
RESULTS AND DISCUSSION
The isolate was identified as non-O1/O139 V. cholerae by the Keesler AFB lab and the MPHL and was assigned the isolate designation BJG-01. Multiple yellow sucrose-fermenting colonies were subjected to confirmatory PCR at the GCRL with the V. cholerae transmembrane regulatory protein-producing gene (toxR) and the outer membrane protein W gene (ompW) (28). Subsequent molecular tests showed that the strain lacked the O biosynthetic genes wbe and wbf specific for serogroups O1 and O139, respectively, and the cholera toxin gene ctxA and toxin-coregulated pili tcpA gene, which confirmed that it was a nontoxigenic V. cholerae non-O1/O139 strain. The isolate BJG-01 exhibited motility on MIU medium, a typical trait of V. cholerae, and demonstrated protease activity, as evident by a zone of clear lysis around the bacterial growth on a double-layer plate containing marine agar overlay on skim milk agar. The isolate also showed beta hemolysis on blood agar (TSA plus 5% sheep blood; Hardy Diagnostic) and showed biofilm formation, a well-known survival strategy for V. cholerae under adverse conditions. The biofilm formation assay revealed that the isolate BJG-01 was strongly positive for biofilm formation (optical density at 570 nm [OD570], 1.07 ± 0.13, 1.9× higher than that of reference strain N16961 [ATCC 39315] [0.57 ± 0.17]).
Although V. cholerae non-O1/O139 strains typically lack the two major virulence factors, cholera toxin (CTX) and toxin-coregulated pili (TCP) (6), they often contain a battery of genes coding for extracellular products that collectively play an important role in pathogenesis. The V. cholerae isolate BJG-01 contained several putative virulence factors associated with hemolysis and proteolysis. The genome of V. cholerae BJG-01 encodes a predicted heat-labile El Tor hemolysin (polyethylene glycol [PEG] 3222) known to induce fluid accumulation in the infant mouse (29); a predicted heat-stable hemolysin (PEG 2480) similar to the thermostable direct hemolysin of Vibrio parahaemolyticus (30); cytolysin and hemolysin; a pore-forming toxin (PEG 2791) associated with lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans (31); and hemagglutinin/protease (PEG 2851). The genome of V. cholerae BJG-01 also encodes other putative virulence-related factors found in toxigenic and nontoxigenic V. cholerae, including toxR-toxS virulence regulators (PEG 1593 and 1594), the integron integrase IntI4 (PEG 841), RTX toxin (PEG 2870), multiple lipases, the outer membrane protein OmpU, and the type VI secretion system (T6SS). The widespread occurrence of many of these putative virulence factors in toxigenic and nontoxigenic V. cholerae isolates suggests that all V. cholerae strains have the potential to be virulent, but it is likely that these factors play an important but undetermined role in the ecology of V. cholerae. Hemolysin is an accessory virulence factor in V. cholerae believed to contribute to disease in humans; recently, hemolysin reportedly caused infection leading to death and developmental delay in a study using Caenorhabditis elegans (31). However, the presence of hemolysin genes in the genomes of pathogenic and nonpathogenic, as well as clinical and environmental, Vibrio spp. suggests that these genes may play a role in environmental fitness. Smith and Oliver (32) suggested that these hemolysins may have a function in the cold shock response. T6SS codes for a newly described mechanism for protein transport across the cell envelope of Gram-negative bacteria, and although first identified in V. cholerae V52 (33), T6SS gene clusters have been reported in Pseudomonas aeruginosa, Salmonella enterica, Yersinia pestis, and Escherichia coli O157:H7 (33). T6SS gene clusters are required for virulence and/or survival of a bacterium in the presence of a eukaryotic host (34–36) or other bacterium. Weber et al. (37) reported that T6SS in Vibrio anguillarum regulates the stress response, suggesting an ecological fitness function in addition to pathological consequences for the host. Thus, hemolysins and T6SS may serve a more basic purpose for V. cholerae in the environment, but they act as virulence factors when a human host is encountered.
Interestingly, we identified the osmoregulatory choline-glycine betaine locus, betABIT, in the genome of BJG-01; this trait was not previously reported in V. cholerae but has been described in both V. parahaemolyticus and Vibrio vulnificus (38, 39). Typically, the ectoine (ectABC) synthesis system is found in V. cholerae, and the glycine betaine system is found in V. vulnificus, whereas V. parahaemolyticus possesses both of these synthesis systems (38, 39). A BLAST search showed that the betABIT locus of V. cholerae shares a greater similarity with the betABIT locus of Pseudomonas spp. than with that of V. parahaemolyticus. The betABIT locus was interrogated among the available sequenced genomes of V. cholerae (>200) and was identified in three other V. cholerae strains, namely, V. cholerae bv. albensis VL426, V. cholerae RC385, and V. cholerae PS15.
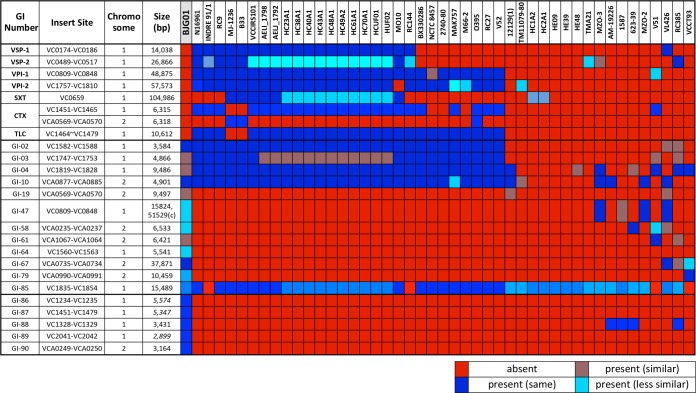
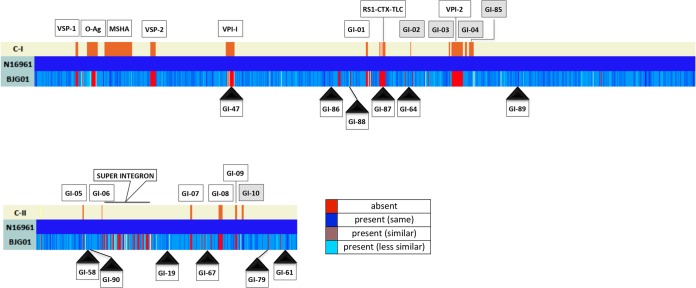
Genomic islands, notably pathogenicity islands, contribute to the evolution and diversification of microbial life (40). They are clusters of genes with a common history distinct and divergent from the histories of the organisms in which they reside. Furthermore, genomic islands encode multiple complex and potentially advantageous traits frequently acquired by lateral transfer and may be major contributors to the evolution and diversification of V. cholerae. Using a comparative genomic approach, described previously by Chun et al. (41), we observed that V. cholerae BJG-01 does not contain the pathogenicity islands frequently found in toxigenic V. cholerae O1, namely, Vibrio seventh pandemic islands (VSP-I and VSP-II), Vibrio pathogenicity islands (VPI-1 and VPI-2), a cholera toxin phage (CTXϕ), an SXT element, and a toxin-linked cryptic plasmid (TLC) (Fig. 1). However, V. cholerae BJG-01 contains several factors necessary for the complete CTXϕ life cycle and for CTX production and translocation, including TolQRA, inner membrane proteins involved in CTXϕ attachment to the cell, XerCD tyrosine recombinases (which catalyze recombination between CTXϕ and the host genome), and LexA (involved in CTXϕ expression). In addition, the isolate contains 4 (GI-02 to GI-04 and GI-10) of the 10 genomic islands (GI-01 to GI-10) with various nucleotide similarities that are frequently found in toxigenic V. cholerae O1 isolates (Fig. 1 and Fig. 2). Further, V. cholerae BJG-01 encodes 12 other genomic islands, 6 (GI-47, GI-64, GI-86, GI-87, GI-88, and GI-89) in chromosome I (C-I) (Fig. 1) and 6 (GI-19, GI-58, GI-61, GI-67, GI-79, and GI-90) in chromosome II (C-II). The relative locations of these genomic islands with reference to the V. cholerae N16961 genome are depicted in Fig. 2. Our comparative genomic analysis also showed that V. cholerae BJG-01 contains GI-85, frequently found in most toxigenic and nontoxigenic V. cholerae, and encodes part of the Tol-Pal protein system, which are reported to be critical cell envelope components and affect cell morphology and virulence (42). Functional characteristics of the genomic islands are illustrated in Table S1 of the supplemental material.
FIG 1.
Reciprocal BLASTN analysis of major virulence factors and other genomic islands in V. cholerae BJG-01. Colors represent the relatedness of the virulence factors and the genomic islands in terms of their gene contents.
FIG 2.
Chromosomal distribution of genomic islands in BJG-01 in reference to V. cholerae N16961. C-I, chromosome 1; C-II, chromosome 2. Rectangular boxes with solid black triangles represent the GIs and their relative positions (in reference to the N16961 genome) in the BJG-01 genome. The rectangular shaded boxes indicate the GIs that were present in N16961 and BJG-01. Colors represent the relatedness of the GIs in terms of gene content.
Multilocus variable-number tandem-repeat (VNTR) analysis (MLVA), a discriminating tool used to trace human V. cholerae infections (43) and resolve distinct populations of clinical isolates from different geographic regions (44), showed that V. cholerae BJG-01 is genotype 12.12.5.10, genetically distinct (see Table S2 in the supplemental material) from V. cholerae non-O1/O139 isolates of clinical and environmental origin, including representative 2010 Haitian V. cholerae isolates (27).
The core oligosaccharide (OS) and O antigens are major regions of the V. cholerae lipopolysaccharide (LPS) synthesized by the wav and wb* gene clusters, respectively. Analysis of the wav gene cluster (Fig. 3) revealed that the core OS of V. cholerae BJG-01 belongs to the type 3b core OS, identified previously in V. cholerae bv. albensis VL426 (41). However, the genetic organization of the O antigen appeared to be distinct, as the wb* gene cluster of BJG-01 showed that the cassettes comprised several smaller gene sets specific to the V. cholerae BJG-01 O antigen (Fig. 3), such as those under GenBank accession numbers AFOU01000268.1 (three genes), AFOU01000125.1 (three genes), and AFOU01000130.1 (four genes) and gene sets identified in other V. cholerae strains, such as VCA_002417 of V. cholerae VL426, VchoM_03542 to VchoM_03544 of V. cholerae MO10, and wbeW of V. cholerae N16961. Similar extensive diversity in the O-antigen gene clusters (wb*) was reported previously by Chun et al. (41) and Nesper et al. (45) and indicates that the O-antigen gene clusters are horizontally transferred, since they comprise several smaller gene sets of origin.
FIG 3.
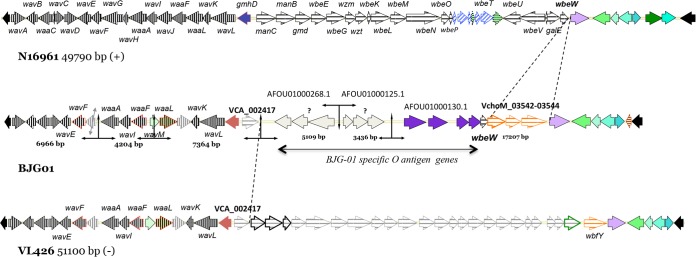
Genetic organization of wav and wb* gene clusters in V. cholerae BJG-01 compared to that of V. cholerae bv. EIT or N16961 and V. cholerae bv. albensis VL426. Homologous aspects are indicated by the same colors. BJG-01 has a variant of the type 3b core oligosaccharide similar to that of VL426, whereas the O-antigen (wb*) gene cluster appeared to be very distinct, with a set of BJG-01-specific gene sets.
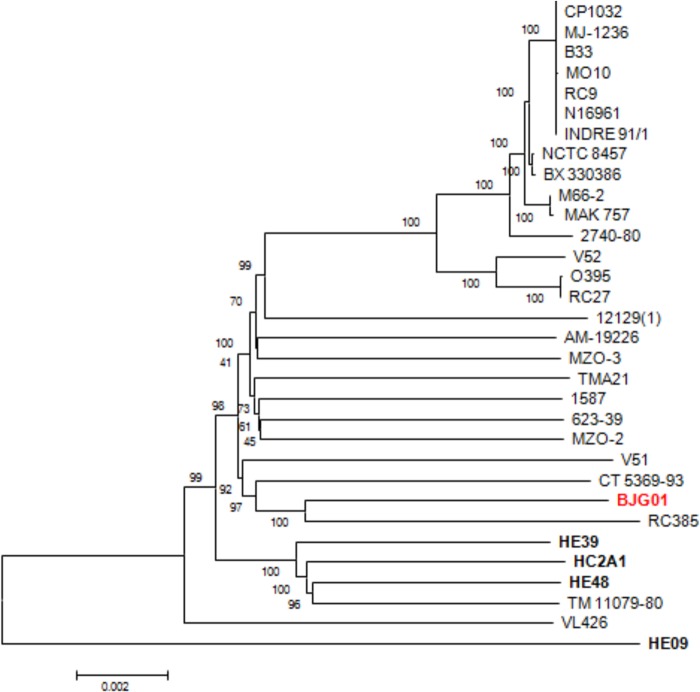
The phylogeny of V. cholerae BJG-01 was inferred by constructing a genome-relatedness neighbor-joining tree, which employed homologous alignment of 1,163 highly conserved orthologous protein-coding genes (∼1,182,674 bp) of 32 V. cholerae genomes. The deduced phylogenic tree (Fig. 4) shows that V. cholerae BJG-01occupies a non-O1/O139 phyletic lineage and forms a monophyletic clade with three V. cholerae strains (RC385, CT 5369–93, and V51) isolated from the Americas. V. cholerae RC385, an environmental strain belonging to serogroup O135, was isolated from the Chesapeake Bay. V. cholerae CT 5369–93 serotype O1 was isolated from Brazil in 1993, and V51 serotype O141 is a 1987 clinical isolate from the United States. Our conclusion is that the phyletic lineage of these V. cholerae non-O1/O139 strains represents a lineage resident in the Western Hemisphere.
FIG 4.
Phylogeny of V. cholerae BJG-01. The neighbor-joining tree was constructed based on 1,163 highly conserved orthologs representing 1,182,674 bp of the BJG-01 genome. The nucleotide substitution is the Kimura 2-parameter model. Numbers at the nodes indicate the bootstrap supports, and the bar indicates the number of substitutions per nucleotide position.
In conclusion, V. Cholerae non-O1/O139 isolates can be pathogenic for humans and are autochthonous to aquatic environments throughout the world. The mechanisms by which non-O1/O139 V. cholerae functions successfully in two very distinct habitats (i.e., as a human pathogen but native to the aquatic environment) have yet to be fully described. Toxigenic epidemic strains of V. cholerae O1 that carry major virulence factors conferring pathogenicity have been described, but in nontoxigenic V. cholerae strains, the underlying mechanism(s) causing diarrhea in humans is not clearly understood. Here, we investigated a nontoxigenic strain of V. cholerae isolated from a clinical case and found it to lack most of the major virulence-encoding regions of toxigenic V. cholerae and an important virulence factor of non-O1/O139 V. cholerae, the NAG-specific enterotoxin gene (stn), reported to be essential for pathogenesis in a human volunteer study (46). Therefore, it appears that additional virulence factors yet to be determined are present in nontoxigenic V. cholerae associated with human disease. In this study, V. cholerae BJG-01 was found to encode a large number of genomic islands and other putative virulence genes. However, the functionality of these genomic islands remains obscure, as the majority have been annotated as either hypothetical proteins or proteins of unknown function. Some of the accessory genes detected in V. cholerae BJG-01 have previously been identified in other environmental V. cholerae non-O1/O139 isolates. VNTR genotyping indicated that V. cholerae BJG-01 is a distinct genotype, whereas phylogenetic analysis shows that it belongs to a phyletic lineage of non-O1/O139 V. cholerae associated with sporadic cases of gastroenteritis in the Western Hemisphere.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by NSF RAPID grant 1043126, by NSF grant EF-0813285/EF-0813066 as part of the joint NSF-NIH Ecology of Infectious Diseases program, and by NIH grant 1RO1A1039129.
The views expressed in this material are those of the authors and do not reflect the official policy or position of the U.S. government, the Department of Defense, or the Department of the Air Force.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02187-14.
REFERENCES
- 1.Morris JG Jr, Black RE. 1985. Cholera and other vibrios in the United States. N Engl J Med 312:343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- 2.Thompson FL, Austin B, Swings J. 2006. The biology of the vibrios. ASM Press, Washington, DC. [Google Scholar]
- 3.Faraque SM, Nair GB. 2006. Epidemiology, p 385 In Thompson FL, Austin B, Swings J (ed), The biology of the vibrios. ASM Press, Washington, DC. [Google Scholar]
- 4.Chatterjee SN, Chaudhuri K. 2003. Lipopolysaccharides of Vibrio cholerae. I. Physical and chemical characterization. Biochim Biophys Acta 1639:65–79. doi: 10.1016/j.bbadis.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Weber JT, Levine WC, Hopkins DP, Tauxe RV. 1994. Cholera in the United States, 1965–1991. Risks at home and abroad. Arch Intern Med 154:551–556. [PubMed] [Google Scholar]
- 6.Kaper JB, Morris JG Jr, Levine MM. 1995. Cholera. Clin Microbiol Rev 8:48–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weis KE, Hammond RM, Hutchinson R, Blackmore CG. 2011. Vibrio illness in Florida, 1998–2007. Epidemiol Infect 139:591–598. doi: 10.1017/S0950268810001354. [DOI] [PubMed] [Google Scholar]
- 8.Morris JG Jr, Wilson R, Davis BR, Wachsmuth IK, Riddle CF, Wathen HG, Pollard RA, Blake PA. 1981. Non-O group 1 Vibrio cholerae gastroenteritis in the United States: clinical, epidemiologic, and laboratory characteristics of sporadic cases. Ann Intern Med 94:656–658. doi: 10.7326/0003-4819-94-5-656. [DOI] [PubMed] [Google Scholar]
- 9.Colwell RR, Seidler RJ, Kaper J, Joseph SW, Garges S, Lockman H, Maneval D, Bradford H, Roberts N, Remmers E, Huq I, Huq A. 1981. Occurrence of Vibrio cholerae serotype O1 in Maryland and Louisiana estuaries. Appl Environ Microbiol 41:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood MA, Ness GE. 1982. Survival of Vibrio cholerae and Escherichia coli in estuarine waters and sediments. Appl Environ Microbiol 43:578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaper J, Lockman H, Colwell RR, Joseph SW. 1979. Ecology, serology, and enterotoxin production of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol 37:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenyon JE, Gillies DC, Piexoto DR, Austin B. 1983. Vibrio cholerae (non-O1) isolated from California coastal waters. Appl Environ Microbiol 46:1232–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motes ML Jr, Zywno SR, DePaola A, Becker RE, Presnell MW. 1983. Isolation of Vibrio cholerae serotype Ogawa from a Florida estuary. Appl Environ Microbiol 45:321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffitt KJ, Grimes DJ. 2013. Abundance and distribution of Vibrio cholerae, V. parahaemolyticus, and V. vulnificus following a major freshwater intrusion into the Mississippi Sound. Microb Ecol 65:578–583. doi: 10.1007/s00248-013-0203-6. [DOI] [PubMed] [Google Scholar]
- 15.Wilson R, Lieb S, Roberts A, Stryker S, Janowski H, Gunn R, Davis B, Riddle CF, Barrett T, Morris JG Jr, Blake PA. 1981. Non-O group 1 Vibrio cholerae gastroenteritis associated with eating raw oysters. Am J Epidemiol 114:293–298. [DOI] [PubMed] [Google Scholar]
- 16.Aldová E, Laznickova K, Stepankova E, Lietava J. 1968. Isolation of nonagglutinable vibrios from an enteritis outbreak in Czechoslovakia. J Infect Dis 118:25–31. doi: 10.1093/infdis/118.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Dakin WP, Howell DJ, Sutton RG, O'Keefe MF, Thomas P. 1974. Gastroenteritis due to nonagglutinable (noncholera) vibrios. Med J Aust 2:487–490. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 1981. Outbreak of Vibrio cholerae non-O1gastroenteritis: Italy. Morb Mortal Wkly Rep 30:374–375. [PubMed] [Google Scholar]
- 19.Johnson CN, Flowers AR, Noriea NF 3rd, Zimmerman AM, Bowers JC, DePaola A, Grimes DJ. 2010. Relationships between environmental factors and pathogenic vibrios in the northern Gulf of Mexico. Appl Environ Microbiol 76:7076–7084. doi: 10.1128/AEM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hlady WG, Klontz KC. 1996. The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis 173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro RL, Altekruse S, Hutwagner L, Bishop R, Hammond R, Wilson S, Ray B, Thompson S, Tauxe RV, Griffin PM. 1998. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988–1996. Vibrio Working Group. J Infect Dis 178:752–759. [DOI] [PubMed] [Google Scholar]
- 22.Tamplin ML. 2001. Coastal vibrios: identifying relationships between environmental condition and human disease. Hum Ecol Risk Assess 7:1437–1445. doi: 10.1080/20018091095113. [DOI] [Google Scholar]
- 23.Lipp EK, Huq A, Colwell RR. 2002. Effects of global climate on infectious disease: the cholera model. Clin Microbiol Rev 15:757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huq A, Sack RB, Nizam A, Longini IM, Nair GB, Ali A, Morris JG Jr, Khan MN, Siddique AK, Yunus M, Albert MJ, Sack DA, Colwell RR. 2005. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl Environ Microbiol 71:4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sizemore RK, Stevenson LH. 1970. Method for the isolation of proteolytic marine bacteria. Appl Microbiol 20:991–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaieb K, Chehab O, Zmantar T, Rouabhia M, Mahdouani K, Bakhrouf A. 2007. In vitro effect of pH and ethanol on biofilm formation by clinical ica-positive Staphylococcus epidermidis strains. Ann Microbiol 57:431–437. doi: 10.1007/BF03175085. [DOI] [Google Scholar]
- 27.Hasan NA, Choi SY, Eppinger M, Clark PW, Chen A, Alam M, Haley BJ, Taviani E, Hine E, Su Q, Tallon LJ, Prosper JB, Furth K, Hoq MM, Li H, Fraser-Liggett CM, Cravioto A, Huq A, Ravel J, Cebula TA, Colwell RR. 2012. Genomic diversity of 2010 Haitian cholera outbreak strains. Proc Natl Acad Sci U S A 109:E2010–E2017. doi: 10.1073/pnas.1207359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nandi B, Nandy RK, Mukhopadhyay S, Nair GB, Shimada T, Ghose AC. 2000. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J Clin Microbiol 38:4145–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichinose Y, Yamamoto K, Nakasone N, Tanabe MJ, Takeda T, Miwatani T, Iwanaga M. 1987. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun 55:1090–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoh M, Honda T, Miwatani T. 1985. Production by non01 Vibrio cholerae of hemolysin related to thermostable direct hemolysin of Vibrio parahaemolyticus. FEMS Microbiol Lett 29:197–200. doi: 10.1111/j.1574-6968.1985.tb00861.x. [DOI] [Google Scholar]
- 31.Cinar HN, Kothary M, Datta AR, Tall BD, Sprando R, Bilecen K, Yildiz F, McCardell B. 2010. Vibrio cholerae hemolysin is required for lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans. PLoS One 5:e11558. doi: 10.1371/journal.pone.0011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith B, Oliver JD. 2006. In situ gene expression by Vibrio vulnificus. Appl Environ Microbiol 72:2244–2246. doi: 10.1128/AEM.72.3.2244-2246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yahr TL. 2006. A critical new pathway for toxin secretion? N Engl J Med 355:1171–1172. doi: 10.1056/NEJMcibr063931. [DOI] [PubMed] [Google Scholar]
- 34.Bingle LE, Bailey CM, Pallen MJ. 2008. Type VI secretion: a beginner's guide. Curr Opin Microbiol 11:3–8. doi: 10.1016/j.mib.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Cascales E. 2008. The type VI secretion toolkit. EMBO Rep 9:735–741. doi: 10.1038/embor.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filloux A, Hachani A, Bleves S. 2008. The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology 154:1570–1583. doi: 10.1099/mic.0.2008/016840-0. [DOI] [PubMed] [Google Scholar]
- 37.Weber B, Hasic M, Chen C, Wai SN, Milton DL. 2009. Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ Microbiol 11:3018–3028. doi: 10.1111/j.1462-2920.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- 38.Reen FJ, Almagro-Moreno S, Ussery D, Boyd EF. 2006. The genomic code: inferring Vibrionaceae niche specialization. Nat Rev Microbiol 4:697–704. doi: 10.1038/nrmicro1476. [DOI] [PubMed] [Google Scholar]
- 39.Naughton LM, Blumerman SL, Carlberg M, Boyd EF. 2009. Osmoadaptation among Vibrio species and unique genomic features and physiological responses of Vibrio parahaemolyticus. Appl Environ Microbiol 75:2802–2810. doi: 10.1128/AEM.01698-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hacker J, Kaper JB. 2000. Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol 54:641–679. doi: 10.1146/annurev.micro.54.1.641. [DOI] [PubMed] [Google Scholar]
- 41.Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, Taviani E, Jeon YS, Kim DW, Brettin TS, Bruce DC, Challacombe JF, Detter JC, Han CS, Munk AC, Chertkov O, Meincke L, Saunders E, Walters RA, Huq A, Nair GB, Colwell RR. 2009. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A 106:15442–15447. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubuisson JF, Vianney A, Hugouvieux-Cotte-Pattat N, Lazzaroni JC. 2005. Tol-Pal proteins are critical cell envelope components of Erwinia chrysanthemi affecting cell morphology and virulence. Microbiology 151:3337–3347. doi: 10.1099/mic.0.28237-0. [DOI] [PubMed] [Google Scholar]
- 43.Kendall EA, Chowdhury F, Begum Y, Khan AI, Li S, Thierer JH, Bailey J, Kreisel K, Tacket CO, LaRocque RC, Harris JB, Ryan ET, Qadri F, Calderwood SB, Stine OC. 2010. Relatedness of Vibrio cholerae O1/O139 isolates from patients and their household contacts, determined by multilocus variable-number tandem-repeat analysis. J Bacteriol 192:4367–4376. doi: 10.1128/JB.00698-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh R, Nair GB, Tang L, Morris JG, Sharma NC, Ballal M, Garg P, Ramamurthy T, Stine OC. 2008. Epidemiological study of Vibrio cholerae using variable number of tandem repeats. FEMS Microbiol Lett 288:196–201. doi: 10.1111/j.1574-6968.2008.01352.x. [DOI] [PubMed] [Google Scholar]
- 45.Nesper J, Kraiss A, Schild S, Blass J, Klose KE, Bockemuhl J, Reidl J. 2002. Comparative and genetic analyses of the putative Vibrio cholerae lipopolysaccharide core oligosaccharide biosynthesis (wav) gene cluster. Infect Immun 70:2419–2433. doi: 10.1128/IAI.70.5.2419-2433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris JG Jr, Takeda T, Tall BD, Losonsky GA, Bhattacharya SK, Forrest BD, Kay BA, Nishibuchi M. 1990. Experimental non-O group 1 Vibrio cholerae gastroenteritis in humans. J Clin Invest 85:697–705. doi: 10.1172/JCI114494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.