Abstract
Twelve Burkholderia pseudomallei isolates collected over a 32-month period from a patient with chronic melioidosis demonstrated identical multilocus sequence types (STs). However, whole-genome sequencing suggests a polyclonal infection. This study is the first to report a mixed infection with the same ST.
TEXT
Burkholderia pseudomallei is a Gram-negative bacterium that causes the potentially fatal disease melioidosis (1). Melioidosis is the most common cause of community-acquired bacteremic pneumonia in the tropical Top End of the Northern Territory, Australia (2), with annual infection rates in recent years of up to 50 per 100,000 people (3). Multilocus sequence typing (MLST) is a widely adopted genotyping method for characterizing bacterial pathogens, including B. pseudomallei (4–6). MLST has been used to demonstrate that B. pseudomallei is among the most recombinogenic bacterial species studied to date, with a recombination-to-mutation ratio more than twice that of Streptococcus pneumoniae (7). Although useful for identifying strain relatedness, MLST is insensitive to genome-wide variation. To investigate genome-wide variation and better detect polyclonal infections, whole-genome sequencing is essential.
Case history.
The Darwin Prospective Melioidosis Study (DPMS) has documented all known Top End melioidosis cases since October 1989 (2). The 103rd enrolled DPMS patient, P103, was a 49-year-old male who had a history of asthma and chronic lung disease and who had received intermittent therapy with oral prednisolone. Although P103 had positive B. pseudomallei serological titers since he was first tested in August 1990, serology itself is not an accurate diagnostic tool, particularly in regions where melioidosis is endemic; culture confirmation is required for a definitive melioidosis diagnosis (8). Given his positive serology, P103's sputum was repeatedly tested for B. pseudomallei, but it remained culture negative (9, 10) until September 1994, when B. pseudomallei isolate MSHR338 was obtained. Upon diagnosis, standard therapy comprising 2 weeks of intravenous ceftazidime followed by 3 months of oral doxycycline was commenced. Despite an improvement in symptoms, the sputum was again culture positive in December 1994. An additional round of intravenous ceftazidime followed by oral therapy with various combinations of doxycycline, trimethoprim-sulfamethoxazole, and chloramphenicol was administered. However, P103 still remained culture positive, with isolates retrieved from sputum or throat specimens 4, 7, 15, 21, and 30 months after obtaining MSHR338 (Table 1). All samples collected after March 1997 were B. pseudomallei negative. MIC testing of MSHRs 338 and 346 demonstrated that these isolates remained sensitive to ceftazidime, doxycycline, trimethoprim-sulfamethoxazole, and chloramphenicol. From the 888 melioidosis cases enrolled in the DPMS to August 2014, only 1 other case, P314, demonstrated long-term B. pseudomallei persistence. P314 has ongoing B. pseudomallei respiratory tract colonization that was first diagnosed in 2000 (11); in contrast, B. pseudomallei was eventually eradicated in P103 32 months after the initial diagnosis and the commencement of therapy.
TABLE 1.
Bacterial strains collected from P103
| Strain | Collection date |
|---|---|
| MSHR338 | September 1994 |
| MSHR338-5a (MSHR338) | September 1994 |
| MSHR346Ab | January 1995 |
| MSHR346Bb | January 1995 |
| MSHR376 | April 1995 |
| MSHR2844a (MSHR376) | April 1995 |
| MSHR2845a (MSHR376) | April 1995 |
| MSHR391 | December 1995 |
| MSHR443 | June 1996 |
| MSHR487 | March 1997 |
| MSHR2848a (MSHR487) | March 1997 |
| MSHR2849a (MSHR487) | March 1997 |
An additional subculture from the original stock (denoted in parentheses) to identify mixed genotypes.
Morphological variants that were observed within the same clinical specimen.
MLST analysis.
MLST was initially performed on seven isolates derived from six clinical specimens over a 32-month period (Table 1). DNA was extracted from single purified colonies as previously detailed (12). All seven isolates were sequence type 243 (ST-243), which has only been identified in isolates from this patient (http://bpseudomallei.mlst.net/).
Genomic analysis.
Illumina GAIIx or HiSeq 2000 (Illumina, Inc., San Diego, CA) whole-genome sequencing was performed on 12 P103 B. pseudomallei isolates, including 5 isolates derived from three clinical specimens to more thoroughly sample within-host B. pseudomallei diversity (Table 1). Comparative genomic analyses were performed using SPANDx v2.3 (13), and maximum parsimony phylogenetic reconstruction of orthologous core single-nucleotide polymorphism (SNP) variants was carried out using PAUP* v4.0b (14). 454 GS FLX+ sequencing (454 Life Sciences, Branford, CT) was also performed on MSHR338. The 454 reads were combined with Illumina data for de novo genome assembly, as previously described (11), and checked for errors with iterative correction of reference nucleotides (iCORN2) (15) using the Illumina reads. The final MSHR338 hybrid assembly (GenBank accession no. ATJY00000000.1) is in 59 high-quality contigs totaling 7,317,227 bp.
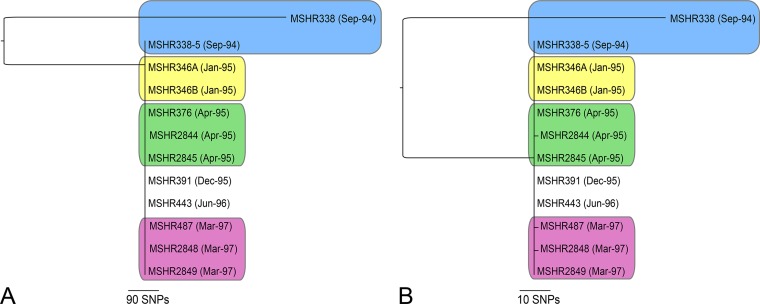
Phylogenetic reconstruction of the P103 genomes using default (i.e., permissive) SNP density filtering parameters in SPANDx (whereby only regions containing ≥3 SNPs within 10 bp are excluded [16]) demonstrated that MSHRs 346A, 346B, 376, 391, 443, 2845, and 2849 were identical. MSHRs 487, 2844, and 2848 each differed by one SNP (Fig. 1A). In contrast, 842 SNPs separated MSHR338 from the dominant genotype. No large deletions (>50 bp) were observed in any P103 strains compared with MSHR338, although 36 unique insertions or deletions (indels) were found in MSHR338 (Table 2). No indels were found among the other 11 strains. Given the relatively large genetic distance between MSHR338 and the subsequent P103 isolates, the potential for additional genotypes within the original MSHR338 culture stock was explored further. This stock was plated onto chocolate agar, and 10 individual colonies were screened using allele-specific real-time PCR (17) interrogation of an SNP at position 332 of the MSHR338 Seq0033 contig, which differentiates MSHR338 from other P103 isolates. All 10 colonies matched the dominant genotype. One colony (MSHR338–5) was fully sequenced (Table 1) and was identical to the dominant genotype, confirming the presence of this genotype in the original clinical specimen.
FIG 1.
Maximum parsimony phylogenetic analyses of P103 Burkholderia pseudomallei isolates over a 32-month infection. All isolates are ST-243; however, MSHR338 is an outlier according to whole-genome sequencing. Colored shading indicates isolates that were derived from the same clinical specimen. (A) Default single-nucleotide polymorphism (SNP) density filtering in SPANDx (excluding regions with ≥3 SNPs per 10 bp). Single SNPs in MSHR487, MSHR2844, and MSHR2489 are present but not visible due to scale. (B) Moderate recombination SNP filtering in SPANDx (excluding regions with ≥3 SNPs per 300 bp). Only SNPs on the MSHR338 branch were removed with the recombinogenic filter. SNPs in MSHR487, MSHR2844, and MSHR2489 are visible. The consistency index for both trees is 1.0.
TABLE 2.
Indels between MSHR338 and other P103 strains
| Locationa | Protein | MSHR338, othersb | Effect | Amino acid change |
|---|---|---|---|---|
| M218_20600: Seq0023, 74246 | Methyltransferase | GGCC, G | Upstream | |
| M218_20665: Seq0023, 99625 | Polyketide synthase | A, ACCGAAGCGACCGAAGTGG | Insertion | T3810 → TEATEVA |
| M218_20685: Seq0024, 19320 | Polyketide synthase | ACGG, A | Deletion | DG1463 → D |
| Seq0025, 264 | CTT, C | Intergenic | ||
| Seq0026, 155 | G, GC | Intergenic | ||
| Seq0026, 19908 | C, CG | Intergenic | ||
| Seq0026, 23627 | AAGTGC, A | Intergenic | ||
| Seq0026, 23632 | CGAAGTGG, C | Intergenic | ||
| Seq0026, 23634 | AAG, A | Intergenic | ||
| Seq0026, 24939 | G, GGCC | Intergenic | ||
| M218_20910: Seq0026, 40186 | 2-Keto-4-pentenoate hydratase | CCG, C | Frameshift | P110: 254 → 257 amino acids |
| Seq0026, 40687 | CCG, C | Intergenic | ||
| Seq0026, 40707 | GCAT, G | Intergenic | ||
| Seq0026, 46450 | CGCGCG, C | Intergenic | ||
| Seq0026, 48033 | G, GT | Intergenic | ||
| M218_20940: Seq0026, 49266 | Hypothetical protein | CGGCGCGCGGGCGCGCG, C | Deletion and frameshift | PARPRA140: 188 → 204 amino acids |
| M218_20945: Seq0026, 50476 | Membrane protein | TGGCGC, T | Upstream | |
| M218_21440: Seq0026, 166557 | Serine transporter | GTC, G | Frameshift | |
| M218_21485: Seq0026, 181817 | Nonribosomal peptide synthase | GTCGACA, G | Deletion | DVD576 → D |
| M218_21485: Seq0026, 181833 | Nonribosomal peptide synthase | ACATCG, A | Frameshift | VDV571: 1354 → 1352 amino acids |
| M218_21530: Seq0026, 198738 | Entericidin | C, CG | Upstream | |
| Seq0026, 204399 | AG, A | Intergenic | ||
| Seq0026, 204633 | TTG, T | Intergenic | ||
| M218_21560: Seq0026, 205821 | Transposase | CGTCA, C | Upstream | |
| Seq0027, 10298 | G, GT | Intergenic | ||
| M218_21660: Seq0027, 22185 | 3-Demethyl-ubiquinone-9 3-methyltransferase | G, GA | Upstream | |
| M218_22155: Seq0027, 148292 | Aminopeptidase | C, CCCCGTGTGT | Insertion | G282 → GTHG |
| Seq0028, 89723 | CG, C | Intergenic | ||
| Seq0028, 90812 | T, TG | Intergenic | ||
| Seq0028, 90857 | GC, G | Intergenic | ||
| M218_22960: Seq0028, 93133 | Membrane protein | TGC, T | Frameshift | AQ212: 481 → 538 amino acids |
| Seq0030, 121 | AGGCGC, A | Intergenic | ||
| M218_23840: Seq0031, 84459 | Hypothetical protein | G, GCCGCATCACCCGCATCAC | Insertion | A822 → GDAGDAA |
| M218_23840: Seq0031, 85558 | Hypothetical protein | C, CCGGCGTTGT | Insertion | P455 → PTTP |
| M218_23840: Seq0031, 87016 | Hypothetical protein | GCC, G | Upstream | |
| M218_23880: Seq0031, 94561 | Hypothetical protein | T, TA | Upstream |
Nucleotide position in the MSHR338 genome (GenBank accession no. ATJY00000000).
All other strains from P103 besides MSHR338.
Given the ST-243 diversity in P103, the relative roles of recombination and mutation were investigated. A recombination filtering parameter (i.e., excluding regions with ≥3 SNPs within 300 bp) was first applied to the 12 P103 genomes. Using this filter, the majority of SNP differences (n = 778; 94%) in MSHR338 were removed (Fig. 1B). Closer examination of these SNPs in Integrative Genomics Viewer 2.3.34 (18) showed that the vast majority (99.9%) of SNPs in MSHR338 were colocated within a 1.3-Mbp region (Fig. 2). These results strongly suggest that one or more recent recombination events led to the ST-243 diversity in P103. Given that no other isolates with similar variants were found in P103, this recombination event probably occurred prior to infection, although the possibility of within-host recombination with an unrelated ST that was not sampled or that became extinct cannot be ruled out.
FIG 2.

Contribution of recombination to ST-243 diversity in P103. Comparison of MSHR338 relative to other P103 isolates shows that 99.9% of SNPs found in MSHR338 are located within a single ∼1.3-Mbp region on chromosome 2, indicating that one large (or several small) recombination events likely led to the recent divergence of MSHR338 from other P103 isolates. Two single-nucleotide polymorphism (SNP) mutations, one each in MSHR0487 and MSHR2848, are also visible. MSHR338 contigs were reordered relative to MSHR305 (23) using progressiveMauve (24) prior to visualization in Integrative Genomics Viewer (18).
To the best of our knowledge, this study is the first to report a polyclonal infection with the same ST, and it demonstrates that MLST can be insensitive for detecting polyclonality. Two previous studies of mixed B. pseudomallei infections using various genotyping methods estimated a B. pseudomallei polyclonal infection rate of between 1.5 and 28% (19, 20); however, the true rate remains unknown. Within-host evolution in P103 is unlikely given the relatively low diversity in other reported melioidosis cases (20–22), including a 12-year chronic-carriage case (11). We therefore conclude that the genotypes recovered from P103 resulted from inoculation with two or more genotypes. The MSHR338 genotype was only detected in the first culture specimen from this patient and at low frequency (<10%) and might have been missed with less intensive sampling. Increased adoption of whole-genome sequencing, in combination with greater sampling efforts, will unveil further instances of polyclonality in clinical specimens. We speculate that polyclonal infection may have led to the difficulties in pathogen eradication observed in this case. However, a greater understanding of pathogen evolution and adaptation within the human host is needed to better inform the precise clinical ramifications of polyclonal infections.
Nucleotide sequence accession number.
The sequence determined in this study has been deposited in GenBank under accession no. ATJY00000000.1.
ACKNOWLEDGMENTS
We thank our colleagues in the microbiology laboratory at Royal Darwin Hospital for expertise in identifying B. pseudomallei isolates. We also thank Leisha Richardson (Menzies School of Health Research), James Cook, and Kevin Drees (Northern Arizona University) for laboratory and genomics assistance.
This study was funded by the Australian National Health and Medical Research Council via project grant award 1046812 and by the U.S. Department of Homeland Security S&T CB Division Bioforensics R&D Program (HSHQDC-10-C-00139).
MSHR338 454 sequencing data were kindly provided by Henry S. Gibbons at the U.S. Army Edgewood Chemical Biological Center.
We declare no conflicts of interest.
REFERENCES
- 1.Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N Engl J Med 367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 2.Currie BJ, Fisher DA, Howard DM, Burrow JN, Lo D, Selva-Nayagam S, Anstey NM, Huffam SE, Snelling PL, Marks PJ, Stephens DP, Lum GD, Jacups SP, Krause VL. 2000. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis 31:981–986. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 3.Parameswaran U, Baird RW, Ward LM, Currie BJ. 2012. Melioidosis at Royal Darwin Hospital in the big 2009–2010 wet season: comparison with the preceding 20 years. Med J Aust 196:345–348. doi: 10.5694/mja11.11170. [DOI] [PubMed] [Google Scholar]
- 4.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vesaratchavest M, Tumapa S, Day NP, Wuthiekanun V, Chierakul W, Holden MT, White NJ, Currie BJ, Spratt BG, Feil EJ, Peacock SJ. 2006. Nonrandom distribution of Burkholderia pseudomallei clones in relation to geographical location and virulence. J Clin Microbiol 44:2553–2557. doi: 10.1128/JCM.00629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Spratt BG. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol 41:2068–2079. doi: 10.1128/JCM.41.5.2068-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson T, Giffard P, Beckstrom-Sternberg S, Auerbach R, Hornstra H, Tuanyok A, Price EP, Glass MB, Leadem B, Beckstrom-Sternberg JS, Allan GJ, Foster JT, Wagner DM, Okinaka RT, Sim SH, Pearson O, Wu Z, Chang J, Kaul R, Hoffmaster AR, Brettin TS, Robison RA, Mayo M, Gee JE, Tan P, Currie BJ, Keim P. 2009. Phylogeographic reconstruction of a bacterial species with high levels of lateral gene transfer. BMC Biol 7:78. doi: 10.1186/1741-7007-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dance DAB. 2002. Melioidosis. Curr Opin Infect Dis 15:127–132. doi: 10.1097/00001432-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AC, Wuthiekanun V, Limmathurosakul D, Wongsuvan G, Day NP, Peacock SJ. 2006. Role of selective and nonselective media for isolation of Burkholderia pseudomallei from throat swabs of patients with melioidosis. J Clin Microbiol 44:2316. doi: 10.1128/JCM.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashdown LR. 1979. An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology 11:293–297. doi: 10.3109/00313027909061954. [DOI] [PubMed] [Google Scholar]
- 11.Price EP, Sarovich DS, Mayo M, Tuanyok A, Drees KP, Kaestli M, Beckstrom-Sternberg SM, Babic-Sternberg JS, Kidd TJ, Bell SC, Keim P, Pearson T, Currie BJ. 2013. Within-host evolution of Burkholderia pseudomallei over a twelve-year chronic carriage infection. mBio 4(4):e00388–13. doi: 10.1128/mBio.00388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currie BJ, Gal D, Mayo M, Ward L, Godoy D, Spratt BG, LiPuma JJ. 2007. Using BOX-PCR to exclude a clonal outbreak of melioidosis. BMC Infect Dis 7:68. doi: 10.1186/1471-2334-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarovich DS, Price EP. 2014. SPANDx: a genomics pipeline for comparative analysis of large haploid whole genome re-sequencing datasets. BMC Res Notes 7:618. doi: 10.1186/1756-0500-7-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swofford DL. 2002. PAUP*: phylogenetic analysis using parsimony (and other methods), version 4.0 beta. Sinauer Associates, Sunderland, MA. [Google Scholar]
- 15.Otto TD, Sanders M, Berriman M, Newbold C. 2010. Iterative correction of reference nucleotides (iCORN) using second generation sequencing technology. Bioinformatics 26:1704–1707. doi: 10.1093/bioinformatics/btq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germer S, Holland MJ, Higuchi R. 2000. High-throughput SNP allele-frequency determination in pooled DNA samples by kinetic PCR. Genome Res 10:258–266. doi: 10.1101/gr.10.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. 2011. Integrative genomics viewer. Nat Biotechnol 29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitt TL, Trakulsomboon S, Dance DA. 2007. Recurrent melioidosis: possible role of infection with multiple strains of Burkholderia pseudomallei. J Clin Microbiol 45:680–681. doi: 10.1128/JCM.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limmathurotsakul D, Wuthiekanun V, Chantratita N, Wongsuvan G, Thanwisai A, Biaklang M, Tumapa S, Lee S, Day NP, Peacock SJ. 2007. Simultaneous infection with more than one strain of Burkholderia pseudomallei is uncommon in human melioidosis. J Clin Microbiol 45:3830–3832. doi: 10.1128/JCM.01297-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden HS, Lim R, Brittnacher MJ, Sims EH, Ramage ER, Fong C, Wu Z, Crist E, Chang J, Zhou Y, Radey M, Rohmer L, Haugen E, Gillett W, Wuthiekanun V, Peacock SJ, Kaul R, Miller SI, Manoil C, Jacobs MA. 2012. Evolution of Burkholderia pseudomallei in recurrent melioidosis. PLoS One 7:e36507. doi: 10.1371/journal.pone.0036507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limmathurotsakul D, Holden MT, Coupland P, Price EP, Chantratita N, Wuthiekanun V, Amornchai P, Parkhill J, Peacock SJ. 2014. Micro-evolution of Burkholderia pseudomallei during an acute infection. J Clin Microbiol 52:3418–3421. doi: 10.1128/JCM.01219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone JK, Johnson SL, Bruce DC, Detter JC, Mayo M, Currie BJ, Gelhaus HC, Keim P, Tuanyok A. 2013. Complete genome sequence of the encephalomyelitic Burkholderia pseudomallei strain MSHR305. Genome Announc 1(4):e00656–13. doi: 10.1128/genomeA.00656-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]