To the Editor
Inflammatory Breast Cancer (IBC) is a rare but aggressive form of breast cancer that is diagnosed clinically (1). IBC has characteristic clinical and biologic features suggestive of differences between IBC and non-IBC. Egypt has about a five times higher proportion of IBC patients than the USA (2–4) with distinct molecular features of IBC tumors that may reflect a more aggressive nature of the disease (5,6). Within Egypt, there have been no past comparisons between IBC and non-IBC patients with respect to survival. Therefore, as a part of this study, we decided to compare survival of IBC and non-IBC patients while taking into account, the epidemiologic, pathologic, and treatment characteristics of these two groups of patients from the National Cancer Institute of Cairo University (NCI-Cairo) in Egypt.
Patients included in this study were diagnosed and treated at NCI-Cairo from 2000 to 2005. The patients included two groups, 65 IBC patients and 52 non-IBC patients. IBC diagnosis was based on the presence of erythema, edema, and peau d’orange and some of the patients in this study were included in our previous studies (5,6). We reviewed the medical records, pathology reports and radiation records, and abstracted demographic, clinical and survival information (presence of metastases, treatment variables, and past medical history that is associated with overall survival). We applied the same exact procedure for abstracting information from the medical records of IBC and non-IBC patients. Survival variables included distant metastases, chemotherapy, radiation dose and frequency, type of surgical procedure, hormonal therapy, history of hypertension, pulmonary disease, type II diabetes, other cancers, and presence of chronic granulomatous mastitis. Duration of disease was the difference between the date of reported onset of clinical symptoms to the date of death or last date of follow- up of the patient, if lost to follow-up. Survival status was identified using a standard survey to elicit information on dead/alive status, place of death, and date of last visit to the treating physician. Patients’ relatives provided information on patients who were no longer living. Trained interviewers who participated in our previous studies from the Social Work Department of NCI-Cairo contacted the patients or next of kin by phone to elicit the survival information. When patients or next of kin were not available by phone because of changed phone numbers or relocation, home visits were conducted to complete the survival survey information. Death records were obtained to validate the date and cause of death. Four patients were lost to follow-up so death status was unknown. The study was approved by both Institutional Review Boards of NCI-Cairo in Egypt and the University of Michigan.
Statistical analysis was performed using SAS statistical package version 9.1.3 (SAS Inc. Cary, NC) to obtain adjusted and unadjusted hazard ratios. Cox proportional hazards were used for analysis of variables that were significant in the univariate analysis. These variables included the diagnosis of IBC, lymphatic vessel invasion, edema, peau d’orange, erythema, warmth, thickening, pain, itching, distant metastasis, chemotherapy, radiation, surgery, and hormonal therapy. Statistical analysis of the overall survival for each group (Kaplan–Meier curve for univariate analysis) was conducted. Comparison of statistical significance between survival curves was performed by the log rank Mantel-Cox.
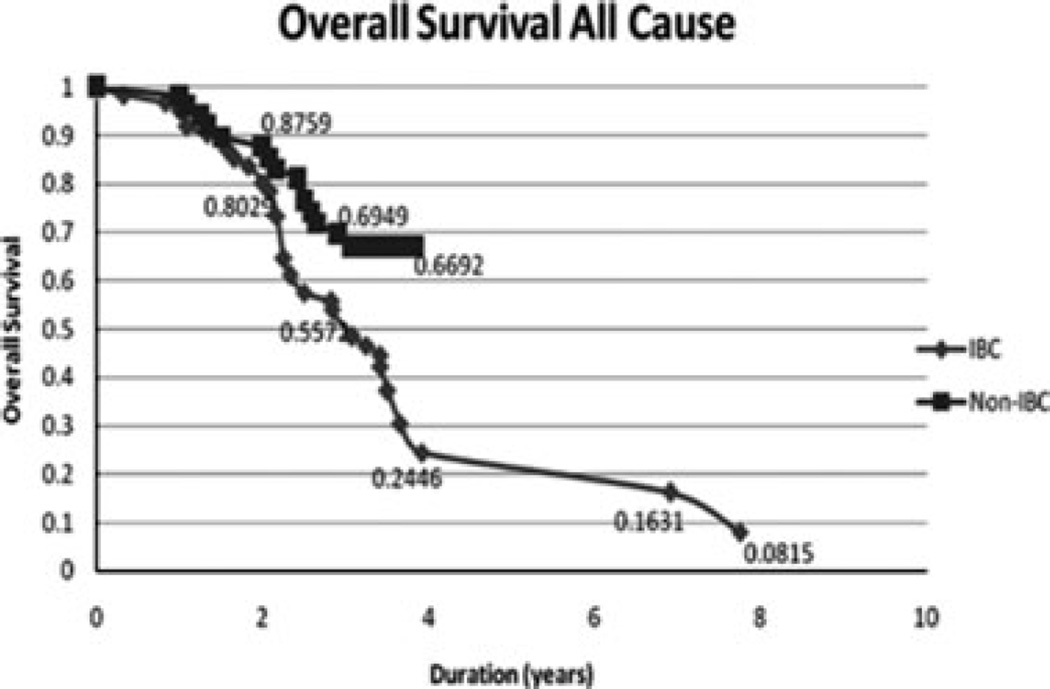
The mean age of both IBC and non-IBC patients was 51 years (SD ± 12) with a range of 28–75 years for IBC patients and 32–79 years for non-IBC patients. The Kaplan–Meier analysis showed a 2-year overall survival of 88% for non-IBC patients compared to 80% survival for IBC patients. The 4-year overall survival rate for non-IBC patients was 67% compared to 24% for IBC patients (p = 0.02) (Fig. 1).
Figure 1.
Comparison of the Kaplan–Meier specific survival curves comparing IBC patients to non-IBC patients in Egypt. The dotted line represents non-IBC patients and the solid line represents IBC patients. Log rank test value is 5.5 (p = 0.02).
This study showed that IBC patients had a significantly lower overall survival than non-IBC patients at 4 years. Our reported survival of IBC patients from Egypt at 4 years in this study (24%) is lower than the trends for overall survival of IBC patients in the USA in the same decade of 40% at 4 years (7) and 40% survival at 5 year (8). To our knowledge, this is the first study to report on the survival of IBC patients in Egypt where IBC proportions are high and tumors exhibit distinct molecular pathologic features (2,3,5,6). However, two studies from Tunisia, where IBC is also reported at a high proportion, showed a slightly poorer overall survival at 4–5 years of 19–20% (9,10).
The study has several strengths. First, this is one of the very few reports on survival difference between IBC and non-IBC from a developing country. Second, the availability of detailed information on pathologic, clinical and treatment data on both groups of patients added for better understanding of factors influencing survival. Third, the stable population in Egypt and the uncommon limited relocation allowed for contacting the patients and their next of kin to confirm the survival status, when patients discontinued their followup with the treating physicians. Also, the ability of our trained team to conduct home visits to track survival information when patients were not available by other sources was a significant asset for this study. The study also has a few limitations. One was the relatively small sample size which may have limited our ability to estimate the effect of some covariates in the comparison of survival between the IBC and non-IBC patients. Another limitation of this study was the follow-up period of only 4 years for the non-IBC patients. Also, as we were not able to collect consistent staging data for the patients in the study we did not add this to the analysis.
In summary, the most important finding of this study was that IBC patients had a significantly lower overall survival than non-IBC patients. Future studies should continue to follow the patients over an extended period past the 4-years included in this study.
REFERENCES
- 1.Anderson W, Schairer C, Chen B. Epidemiology of inflammatory breast cancer. Breast Dis. 2005;22:9–23. doi: 10.3233/bd-2006-22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omar S, Khaled H, Gaafar R. Breast cancer in Egypt: a review of disease presentation and detection strategies. East Mediterr Health J. 2003;9:448–463. [PubMed] [Google Scholar]
- 3.Soliman A, Lo A, Banerjee M, et al. High proportion of inflammatory breast cancer in the Population-based cancer registry of Gharbiah, Egypt. Breast J. 2009;15:432–434. doi: 10.1111/j.1524-4741.2009.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson W, Chu K, Chang S. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:1128–1135. [PubMed] [Google Scholar]
- 5.Lo A, Kleer C, Banerjee M, et al. Molecular epidemiologic features of inflammatory breast cancer: a comparison between Egyptian and US patients. Breast Cancer Res Treat. 2008;112:141–147. doi: 10.1007/s10549-007-9833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo A, Georgopoulos A, Kleer C, et al. Analysis of rho C expression and lymphovascular emboli inflammatory vs non-inflammatory breast cancers in Egyptian patients. Breast. 2009;18:55–59. doi: 10.1016/j.breast.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez A, Hennessey B, Broglio K. Trends for inflammatory breast cancer: is survival improving? Oncologist. 2007;12:904–912. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- 8.Hance K, Anderson W, Devesa S. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maalej M, Frikha H, Ben Salem S. Breast cancer in Tunisia: clinical and epidemiological study. Bull Cancer. 1999;86:302–306. [PubMed] [Google Scholar]
- 10.Boussen H, Bouzaiene H, Ben Hassouna J. Inflammatory breast cancer in Tunisia: reassessment of incidence and clinicopathological features. Semin Oncol. 2008;35:17–24. doi: 10.1053/j.seminoncol.2007.11.017. [DOI] [PubMed] [Google Scholar]