Summary
We demonstrate the importance of the CHIEF pathway and colon and rectal cancer risk. We identify key genes within the pathway that contribute to risk and summarize the magnitude of the risk associated with this pathway.
Abstract
Inflammation, hormones and energy-related factors have been associated with colorectal cancer (CRC) and it has been proposed that convergence and interactions of these factors importantly influence CRC risk. We have previously hypothesized that genetic variation in the CHIEF (convergence of hormones, inflammation and energy-related factors) pathway would influence risk of CRC. In this paper, we utilize an Adaptive Rank Truncation Product (ARTP) statistical method to determine the overall pathway significance and then use that method to identify the key elements within the pathway associated with disease risk. Data from two population-based case–control studies of colon (n = 1555 cases and 1956 controls) and rectal (n = 754 cases and 959 controls) cancer were used. We use ARTP to estimate pathway and gene significance and polygenic scores based on ARTP findings to further estimate the risk associated with the pathway. Associations were further assessed based on tumor molecular phenotype. The CHIEF pathway was statistically significant for colon cancer (P ARTP = 0.03) with the most significant interferons (P ARTP = 0.0253), JAK/STAT/SOCS (P ARTP = 0.0111), telomere (P ARTP = 0.0399) and transforming growth factor β (P ARTP = 0.0043) being the most significant subpathways for colon cancer. For rectal cancer, interleukins (P ARTP = 0.0235) and selenoproteins (P ARTP = 0.0047) were statistically significant although the pathway overall was of borderline significance (P ARTP = 0.06). Interleukins (P ARTP = 0.0456) and mitogen-activated protein kinase (P ARTP = 0.0392) subpathways were uniquely significant for CpG island methylator phenotype-positive colon tumors. Increasing number of at-risk alleles was significantly associated with both colon [odds ratio (OR) = 6.21, 95% confidence interval (CI): 4.72, 8.16] and rectal (OR = 7.82, 95% CI: 5.26, 11.62) cancer. We conclude that elements of the CHIEF pathway are important for CRC risk.
Introduction
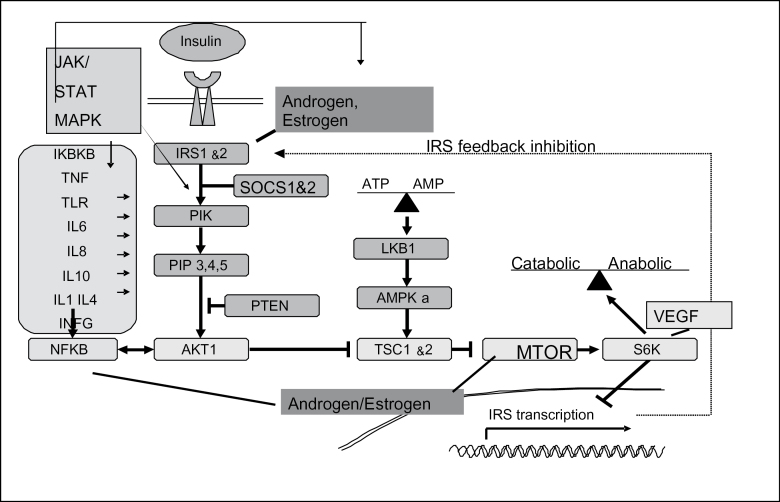
Inflammatory processes appear to be a key element in the underlying carcinogenic process for colorectal cancer (CRC). We proposed an integrated pathway where the convergence of hormones, inflammation and energy-related factors (CHIEF) are central to the etiology of CRC (1). The CHIEF pathway takes an integrated approach to CRC through its incorporation of elements of angiogenesis, hormones and energy-related factors within the underlying inflammatory state of the colon and rectum (see Figure 1).
Fig. 1.
Convergent signaling pathways where inflammation and metabolic signaling intersect along the CHIEF pathway.
Genes within the pathway function within multiple subpathways (a list of all of the genes included along with their alias and chromosome location is given in Supplementary Table 1, available at Carcinogenesis Online). For instance, the pathway core contains a serine/threonine protein kinase 11 (STK11 or LKB1) and is involved in the regulation of mammalian target of rapamycin (MTOR). STK11 responds to changes in cellular energy balance (ATP levels) (2,3) and governs whole body insulin sensitivity (4,5). A different portion of the pathway that responds to insulin, estrogen and androgen and certain proto-oncogene growth factors contain the tumor suppressor PTEN (Phosphatase TENsin homolog deleted on chromosome 10). PTEN acts as a metabolic regulator by modulating signaling via the phosphatidylinositol 3-kinase (PI3K; oncogene formal name PIK3CA) and the v-akt murine thymoma viral oncogene homolog 1 (Akt1 also known as protein kinase B or PKB) pathway. Nuclear factor kappa B (NF-κB) is an important nuclear transcription factor that regulates cytokines and is critical for the regulation of tumorigenesis, cell proliferation, apoptosis, response to oxidative stress and inflammation. Vascular endothelial growth factor (VEGF) regulates RPS6K and IRS-1 and plays an important role in regulation of cell growth signaling; it is a major mediator of tumor angiogenesis (6,7). Steroid hormones including estrogen, androgen and progesterone have been shown to have both anti- and proinflammatory properties (8,9). The receptors of the steroid hormones have been shown to interact with NF-κB in an antagonist manner (9–11). Estrogen also has been shown to repress IL-6 expression as well as IκB, potentially explaining its anti-inflammatory mechanism (9,12).
Cytokines are a key element of the inflammatory process in the CHIEF pathway since inflammation is initiated by the synthesis and secretion of proinflammatory cytokines [e.g. tumor necrosis factor (TNF) and IL-6 in macrophages] in response to an inflammation-provoking insult. The binding of proinflammatory cytokines to their receptors triggers the activation of NF-κB, which in turn activates the expression of a wide variety of genes including cytokines and cyclooxygenase-2 (COX-2). The transforming growth factor β (TGFβ) signaling pathway is involved in all aspects of tumorigenesis, including cell growth regulation, avoidance of apoptosis, promotion of inflammation and angiogenesis, immune response and stimulation of tumor invasion and metastasis (13). Key cytokine-related genes are interleukins, interferons and TNF-related genes and genes within the TGFβ signaling pathway.
Included in the CHIEF pathway are signal transduction and activation of transcription (STAT) and mitogen-activated kinases (MAPKs) genes that are involved in both inflammation and metabolic signaling associated with hormones and energy-related factors. The STAT protein family members are phosphorylated in response to cytokines and growth factors and involved in convergent areas of multiple pathways. MAPKs serve as an integration point for multiple biological signals and are involved in a variety of cellular processes such as proliferation, differentiation and transcription regulation and have three primary components—JNK, p38 and ERK. JNK-1 or MAPK-8 is activated by TNF-α and is necessary for apoptosis. NF-κB is required to terminate JNK signaling. P38, also known as MAPK-14, plays a role in multiple mechanisms including angiogenesis, the PI3K pathway and cytokines. The inflammatory loci are further influenced by interaction with epithelial and vascular endothelial cells and are closely linked with angiogenesis, another component of the CHIEF pathway; angiogenesis and inflammation are hallmark features of tumorigenesis (14).
In this paper, we summarize the significance of this pathway as it relates to colon and rectal cancer risk using Adaptive Rank Truncation Product (ARTP). This statistical method utilizes a permutation method that allows us to combine single nucleotide polymorphism (SNP) P values within a gene, gene ARTP P values within a subpathway and subpathway ARTP P values within a pathway in order to capture gene, subpathway and pathway level effects with colon and rectal cancer. To further estimate the magnitude of the association of this pathway on colon and rectal cancer risk, we utilize a polygenic risk score that is based on the ARTP findings. We evaluate associations overall as well as by tumor molecular phenotype.
Methods
Two study populations are included in these analyses. The first study, a population-based case–control study of colon cancer, included cases (n = 1555 with complete genotype data) and controls (n = 1956 with complete genotype data) identified between 1 October 1991 and 30 September 1994, living in the Twin Cities Metropolitan Area or a seven-county area of Utah or enrolled in the Kaiser Permanente Medical Care Program of Northern California (KPMCP) (15). The second study, with identical data collection methods, included cases with cancer of the rectosigmoid junction or rectum (n = 754 cases and n = 959 controls with complete genotype data) who were identified between May 1997 and May 2001 in Utah and at the KPMCP (16). Eligible cases were between 30 and 79 years of age at the time of diagnosis, living in the study geographic area, English speaking, mentally competent to complete the interview and with no previous history of CRC and no previous diagnosis of familial adenomatous polyposis, ulcerative colitis or Crohn’s disease. Cases who did not meet these criteria were ineligible as were individuals who were not Black, White or Hispanic (for the colon cancer study since diet history questionnaire was not adapted at that time for other ethnic groups). Controls were matched to cases by sex and by 5 year age groups. At KPMCP, controls were randomly selected from membership lists; in Utah, controls 65 years and older were randomly selected from the Health Care Financing Administration lists and controls younger than 65 years were randomly selected from driver’s license lists. In Minnesota, controls were selected from driver’s license and state-identification lists. Eligibility for controls was the same as those outlined for cases. Study details have been previously reported (15,16). All study participants provided informed consent prior to completing the study questionnaire; the study was approved by the Institutional Review Board on Human Subjects at all institutions.
TagSNPs and genetic assessment
TagSNPs were selected using the following parameters: r 2 = 0.8 defined linkage disequilibrium (LD) blocks using a Caucasian LD map, minor allele frequency >0.1, range = −1500 bp from the initiation codon to +1500 bp from the termination codon and one SNP/LD bin. All markers were genotyped using a custom multiplexed bead array assay format based on GoldenGate chemistry (Illumina, San Diego, CA). A genotyping call rate of 99.85% was attained. Blinded internal replicates represented 4.4% of the sample set. The duplicate concordance rate was 100.00%. Supplementary Table 1, available at Carcinogenesis Online, list all genes included in the subpathway, whereas Supplementary Table 2, available at Carcinogenesis Online, list number of SNPs assessed for each gene and the P ARTP value for each gene on the platform. A total of 155 genes and 1246 SNPs were included in the analysis. SNPs per gene ranged from 1 to 71.
Statistical methods
The LD measure, minor allele frequency and test for Hardy–Weinberg equilibrium were calculated among White controls using the ALLELE procedure within SAS version 9.3 (SAS Institute, Cary, NC). The goal of the analysis was to evaluate the overall associations between genes and pathways as they relate to colon and rectal cancer. To do this, we used ARTP which utilizes a highly efficient permutation algorithm to determine significance at the gene, subpathway and pathway level for colon and rectal separately (17). Case/control status was permuted 10 000 times within R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) and P values based on 1-degree of freedom (df) Wald chi-square tests were generated from logistic regression models adjusted for age, study center, race/ethnicity and sex. Associations with SNPs within ARTP were assessed assuming an additive model unless a preliminary check of the odds ratios (ORs) using the co-dominant model indicated a dominant or recessive mode of inheritance. For SNPs with P values <0.05 on genes that were associated with colon or rectal cancer using ARTP, we also report ORs and 95% confidence intervals (CIs) assessed from multiple logistic regression models in SAS, adjusting for study matching variables: age, center, race/ethnicity and sex to show the magnitude of the association between these SNPs and colon or rectal cancer risk. Genes were assigned to only one subpathway prior to the hierarchical analyses, although many genes could function in multiple subpathways.
Tumors were defined by specific molecular alterations: any TP53 mutation, any KRAS mutation, MSI+ and CpG island methylator phenotype (CIMP) which was defined as positive if at least two of five markers methylated. CIMP was based on the classic panel (18). Microsatellite instability (MSI) was based on BAT26, TGFβ RII and a panel of 10 tetranucleotide repeats that has been shown to correlate highly with the Bethesda Panel (19); our study was done prior to the Bethesda Panel development. As the proportion of MSI+ tumors in the rectal cases was <3% (20), we did not examine these tumor markers. We evaluated pathway associations using ARTP as described above for tumor molecular phenotypes relative to controls.
To summarize the risk associated with the CHIEF pathway, we calculated polygenic summary scores. To conservatively estimate risk, we included, in the risk models, SNPs from genes where the gene ARTP P values were ≤0.10 and the SNP P values within those genes were ≤0.10. Since genes are associated with multiple subpathways, we did not restrict to genes where the subpathway was significant. If SNPs within the same gene had r 2 values of ≥0.80, only one SNP was included in the model. Risk was modeled using at-risk alleles, using all genotypes with the low-risk genotype or referent group as zero. For the co-dominant or additive model, a score of 0, 1 or 2 was assigned relative to the number of at-risk alleles, whereas scores of 0 or 2 were assigned for the dominant and recessive models in order to capture the risk associated with the various genotypes. Polygenic scores were then used to summarize risk across the genes and SNPs to better capture the risk associated with the pathway. Individuals missing >5% of SNP data were dropped from the analysis. The continuous score variable was redefined as a categorical variable based on number of at-risk alleles and the distribution of the populations. Adjustments for body mass index, family history of CRC, use of aspirin/non-steroidal anti-inflammatory drugs, cigarette smoking status and dietary energy intake did not alter the observed risk, therefore adjustments were made for matching variables of age, sex, race and study center only. All of the genes assessed and their corresponding subpathway and gene P ARTP are included in Supplementary Table 2, available at Carcinogenesis Online.
Results
The majority of cases and controls were over 60 years of age, were non-Hispanic white and were male (Table I). TP53 mutations were slightly more prevalent among rectal cancer cases (49.64%) versus colon cancer cases (45.95%). MSI tumors were rare among rectal cancers, and CIMP+ tumors were present in 11.11% of rectal cancers and in 26.93% of colon cancers.
Table I.
Description of study population
| Colon | Rectal | |||
|---|---|---|---|---|
| Controls | Cases | Controls | Cases | |
| n (%) | n (%) | n (%) | n (%) | |
| Age | ||||
| 30–39 | 40 (2.04) | 23 (1.48) | 21 (2.19) | 19 (2.52) |
| 40–49 | 128 (6.54) | 102 (6.56) | 101 (10.53) | 96 (12.73) |
| 50–59 | 326 (16.67) | 290 (18.65) | 243 (25.34) | 196 (25.99) |
| 60–69 | 673 (34.41) | 538 (34.60) | 329 (34.31) | 250 (33.16) |
| 70–79 | 789 (40.34) | 602 (38.71) | 265 (27.63) | 193 (25.60) |
| Center | ||||
| Utah | 378 (19.33) | 249 (16.01) | 365 (38.06) | 274 (36.34) |
| KPMCP | 787 (40.24) | 744 (47.85) | 594 (61.94) | 480 (63.66) |
| Minnesota | 791 (40.44) | 562 (36.14) | ||
| Race/ethnicity | ||||
| Non-Hispanic white | 1828 (93.46) | 1428 (91.83) | 824 (85.92) | 625 (82.89) |
| Hispanics | 75 (3.83) | 59 (3.79) | 63 (6.57) | 61 (8.09) |
| Black | 53 (2.71) | 68 (4.37) | 43 (4.48) | 29 (3.85) |
| Asian | 29 (3.02) | 39 (5.17) | ||
| Sex | ||||
| Male | 1047 (53.53) | 870 (55.95) | 541 (56.41) | 451 (59.81) |
| Female | 909 (46.47) | 685 (44.05) | 418 (43.59) | 303 (40.19) |
| Tumor molecular phenotypes | ||||
| CIMP+ | 272 (26.93) | 59 (11.11) | ||
| KRAS2 Mutation | 348 (31.90) | 173 (29.37) | ||
| TP53 Mutation | 516 (45.95) | 277 (49.64) | ||
| MSI Unstable | 185 (15.74) | 14 (2.39) | ||
Overall, the CHIEF pathway was statistically significant for colon cancer (P ARTP = 0.03) and of borderline significance for rectal cancer (P ARTP = 0.06) (Table II). The most significant subpathways for colon cancer were interferons (P ARTP = 0.0253), JAK/STAT/SOCS (P ARTP = 0.0111), telomere (P ARTP = 0.0399) and TGFβ signaling (P ARTP = 0.0043). For rectal cancer, interleukins (P ARTP = 0.0235) and selenoproteins (P ARTP = 0.0047) were statistically significant.
Table II.
Associations between CHIEF pathway and colon and rectal cancer overall and by tumor molecular phenotype
| Sub-Pathway | Colon cancer | Rectal cancer | ||
|---|---|---|---|---|
| Subpathway | Overall pathway | Subpathway | Overall pathway | |
| P value | P value | P value | P value | |
| Angiogenesis | 0.703 | 0.030 | 0.402 | 0.064 |
| Hormone/insulin/growth | 0.077 | 0.790 | ||
| Interferon | 0.025 | 0.379 | ||
| Interleukin | 0.123 | 0.024 | ||
| Jak/Stat/Socs | 0.011 | 0.471 | ||
| Pathway core | 0.183 | 0.165 | ||
| MAPK | 0.164 | 0.368 | ||
| Selenoprotein | 0.856 | 0.0047 | ||
| Telomere | 0.040 | 0.122 | ||
| TGFβ | 0.004 | 0.516 | ||
| TLR | 0.223 | 0.428 | ||
| TNF | 0.151 | 0.204 |
Bold values indicate significant at <0.05 level.
Genes with a P ARTP <0.10 and their related SNPs with a P <0.10 are shown in Table III for colon cancer and in Table IV for rectal cancer. Of the 1246 SNPs assessed, 116 were on genes with a P ARTP <0.1 and were associated with colon cancer at the 0.10 level (5 excluded from score because of LD >0.80: IL6R rs8192284, IL8 rs2227307, JAK2 rs2072593, MTOR rs2295080 and rs718206) and 70 for rectal cancer (15 excluded from score because of high LD: VEGFA rs833069, IL8RA rs4674258, MAPK8 rs10857561, NFKB1 rs3774932, rs1801, rs4648068, PIK3CA rs12509517, rs3755867 and rs1609798, SEPN1 rs4419933, BMPR2 rs6796916). Several significant genes within subpathways were detected, even though the overall subpathway was not significant. For colon cancer, 8 of the 24 genes that were significantly associated with risk at the 0.05 level were in the TGFβ signaling pathway. Three genes in the Jak/Stat/Socs subpathway (STAT3, STAT5A and STAT5B) and three MAPK genes (MAP2K1, MAP3 K3 andMAP3K9) were significantly associated with colon cancer. SLC2A4 (alias GLUT4) and VDR were the only hormone/insulin-related genes associated with colon cancer. MTOR and RPS6KB2 were significantly associated with colon cancer as was TERT. Other genes that showed a significant association with colon cancer were IFGN, IRF3, IL6R, IL8 and TNF. An additional 34 SNPs had SNP P ARTP <0.05 although the gene P ARTP was between 0.05 and 0.10.
Table III.
Associations between subpathway genes and colon cancer for SNPs included in polygenic score (P ARTP < 0.10)
| Subpathway | Gene | Gene P ARTP | SNP | Genotype | SNP P a | OR 95% CIb |
|---|---|---|---|---|---|---|
| Angiogenesis | FLT1 | 0.089 | rs2296189 | AG/GG versus AA | 0.029 | 0.85 (0.74, 0.98) |
| rs3794400 | TT versus CC | 0.012 | 1.36 (0.94, 1.96) | |||
| rs2387632 | TT versus CC/CT | 0.007 | 1.32 (1.08, 1.62) | |||
| rs1324057 | TT versus CC | 0.031 | 1.26 (0.99, 1.60) | |||
| rs7324547 | GA/AA versus GG | 0.033 | 1.16 (1.01, 1.34) | |||
| rs9513088 | GG versus AA | 0.050 | 1.22 (0.99, 1.51) | |||
| rs2296283 | CC versus TT | 0.072 | 1.20 (0.99, 1.45) | |||
| rs12858139 | CA/AA versus CC | 0.010 | 1.21 (1.05, 1.40) | |||
| rs600640 | TC/CC versus TT | 0.041 | 1.15 (1.01, 1.32) | |||
| rs678714 | TA/AA versus TT | 0.020 | 0.82 (0.69, 0.97) | |||
| Hormone/insulin/ growth | SLC2A4 | 0.010 | rs5435 | TT versus CC/CT | 0.001 | 0.73 (0.60, 0.88) |
| TCF7L2 | 0.066 | TCF7L2 | CT/TT versus CC | 0.068 | 1.13 (0.99, 1.30) | |
| VDR | 0.018 | VDR_Fok1 | ff versus FF | 0.005 | 0.75 (0.60, 0.93) | |
| VDR_Poly | SS versus LL | 0.036 | 0.79 (0.65, 0.97) | |||
| Interferons | IFNG | 0.003 | rs2069718 | TT versus CC | 0.070 | 0.84 (0.69, 1.02) |
| rs1861493 | AG/GG versus AA | 0.001 | 0.79 (0.69, 0.91) | |||
| IRF3 | 0.012 | rs2304204 | GG versus AA/AG | 0.004 | 1.43 (1.12, 1.82) | |
| Interleukins | IL15 | 0.061 | rs1519551 | GG versus AA | 0.033 | 0.81 (0.67, 0.99) |
| rs13117878 | TT versus CC | 0.011 | 1.28 (1.06, 1.55) | |||
| IL2RA | 0.095 | rs12244380 | AG/GG versus AA | 0.043 | 1.16 (1.00, 1.34) | |
| rs12722596 | GG versus AA | 0.075 | 1.34 (0.66, 2.69) | |||
| rs12722561 | AA versus GG/GA | 0.004 | 0.49 (0.30, 0.79) | |||
| rs706779 | GG versus AA | 0.062 | 1.19 (0.99, 1.43) | |||
| rs3118470 | TC/CC versus TT | 0.043 | 0.87 (0.76, 1.00) | |||
| IL3 | 0.056 | rs181781 | AA versus GG/GA | 0.025 | 2.42 (1.12, 5.22) | |
| IL6 | 0.095 | rs1800795 | CC versus GG | 0.073 | 0.84 (0.69, 1.03) | |
| rs2069860 | AT/TT versus AA | 0.033 | 0.55 (0.32, 0.95) | |||
| IL6R | 0.033 | rs7549250 | TC/CC versus TT | 0.026 | 0.85 (0.74, 0.98) | |
| rs4845623 | GG versus AA/AG | 0.005 | 1.29 (1.08, 1.53) | |||
| IL8 | 0.027 | rs4073 | AA versus TT | 0.014 | 1.26 (1.04, 1.53) | |
| Jak/Stat/Socs | JAK2 | 0.072 | rs1887429 | GT/TT versus GG | 0.029 | 0.86 (0.75, 0.99) |
| rs3780379 | AA versus GG/GA | 0.007 | 1.68 (1.16, 2.44) | |||
| SOCS2 | 0.057 | rs768775 | TC/CC versus TT | 0.020 | 1.18 (1.03, 1.36) | |
| STAT3 | 0.024 | rs8069645 | AG/GG versus AA | 0.031 | 1.16 (1.01, 1.32) | |
| rs6503695 | CC versus TT | 0.003 | 1.32 (1.07, 1.64) | |||
| rs12949918 | CC versus TT | 0.023 | 1.23 (1.01, 1.49) | |||
| rs1026916 | AA versus GG | 0.064 | 1.20 (0.98, 1.48) | |||
| STAT5A | 0.008 | rs7217728 | CC versus TT | 0.003 | 1.31 (1.04, 1.66) | |
| STAT5B | 0.007 | rs6503691 | TT versus CC | 0.007 | 1.59 (1.00, 2.53) | |
| rs7218653 | GG versus AA | 0.009 | 1.30 (1.03, 1.64) | |||
| STAT6 | 0.095 | rs324015 | GA/AA versus GG | 0.020 | 0.85 (0.74, 0.97) | |
| rs324011 | TT versus CC | 0.087 | 1.20 (0.98, 1.46) | |||
| MAPK | DUSP2 | 0.093 | rs1724120 | AA versus GG/GA | 0.095 | 1.15 (0.98, 1.35) |
| MAP2K1 | 0.043 | rs1432442 | AA versus GG | 0.075 | 0.69 (0.35, 1.38) | |
| rs7181936 | TT versus GG/GT | 0.027 | 1.27 (1.03, 1.58) | |||
| rs8039880 | GG versus AA | 0.007 | 0.57 (0.38, 0.83) | |||
| MAP3K3 | 0.035 | rs11658329 | GC/CC versus GG | 0.035 | 1.16 (1.01, 1.32) | |
| rs3785574 | GG versus AA/AG | 0.031 | 0.79 (0.63, 0.98) | |||
| MAP3K9 | 0.018 | rs11625206 | TT versus CC/CT | 0.002 | 1.41 (1.14, 1.76) | |
| rs11844774 | TC/CC versus TT | 0.008 | 0.83 (0.72, 0.95) | |||
| rs11628333 | CC versus TT/TC | 0.028 | 1.25 (1.02, 1.52) | |||
| rs17176971 | AA versus GG/GA | 0.041 | 1.49 (1.02, 2.20) | |||
| rs11624934 | GG versus AA/AG | 0.002 | 1.42 (1.13, 1.79) | |||
| Pathway core | MTOR | 0.012 | rs1057079 | AG/GG versus AA | 0.006 | 1.21 (1.06, 1.38) |
| rs2024627 | CT/TT versus CC | 0.031 | 1.16 (1.01, 1.33) | |||
| PRKAG2 | 0.072 | rs1029947 | GG versus AA/AG | 0.046 | 1.70 (1.01, 2.87) | |
| rs6464156 | AG/GG versus AA | 0.020 | 0.83 (0.71, 0.97) | |||
| rs953221 | CT/TT versus CC | 0.033 | 0.86 (0.75, 0.99) | |||
| rs9648723 | CC versus AA/AC | 0.013 | 1.51 (1.09, 2.09) | |||
| rs6947064 | GG versus AA | 0.061 | 0.81 (0.66, 1.00) | |||
| rs2374270 | AA versus CC | 0.069 | 0.83 (0.67, 1.03) | |||
| rs1860743 | AA versus GG | 0.018 | 0.65 (0.36, 1.16) | |||
| rs2536068 | AA versus GG | 0.072 | 1.18 (0.84, 1.66) | |||
| rs1104897 | AA versus GG | 0.009 | 1.46 (0.94, 2.26) | |||
| rs6965771 | TT versus CC | 0.090 | 0.89 (0.69, 1.16) | |||
| rs9632641 | CC versus AA | 0.023 | 1.28 (0.92, 1.78) | |||
| rs6464170 | GG versus CC/CG | 0.038 | 0.73 (0.54, 0.98) | |||
| rs9648724 | AA versus GG | 0.022 | 0.74 (0.56, 1.00) | |||
| rs12703162 | CT/TT versus CC | 0.049 | 0.87 (0.75, 1.00) | |||
| RPS6KB2 | 0.010 | rs917570 | CG/GG versus CC | 0.004 | 0.81 (0.70, 0.94) | |
| Telomere | TERT | 0.040 | rs2853668 | AA versus CC | 0.036 | 0.70 (0.54, 0.92) |
| rs2736118 | GG versus AA | 0.048 | 1.31 (1.02, 1.69) | |||
| rs2736100 | GG versus TT/TG | 0.016 | 0.83 (0.71, 0.97) | |||
| TGFβ | BMP2 | 0.002 | rs1979855 | TC/CC versus TT | 0.001 | 1.29 (1.11, 1.48) |
| rs3178250 | TC/CC versus TT | 0.009 | 1.20 (1.05, 1.38) | |||
| rs235770 | TT versus CC | 0.022 | 0.78 (0.63, 0.97) | |||
| BMPR1A | 0.038 | rs6586034 | TG/GG versus TT | 0.010 | 0.82 (0.71, 0.95) | |
| rs7088641 | TC/CC versus TT | 0.006 | 0.83 (0.73, 0.95) | |||
| BMPR1B | 0.017 | rs9307147 | GG versus AA | 0.003 | 0.75 (0.62, 0.91) | |
| rs4490463 | GG versus AA | 0.038 | 0.82 (0.67, 1.00) | |||
| rs17616243 | TT versus CC | 0.006 | 1.45 (0.94, 2.26) | |||
| rs2120834 | CC versus GG/GC | 0.019 | 0.79 (0.65, 0.96) | |||
| rs7662504 | CC versus AA | 0.017 | 0.78 (0.64, 0.96) | |||
| rs7694043 | CT/TT versus CC | 0.095 | 1.12 (0.98, 1.29) | |||
| rs13134042 | AA versus GG | 0.081 | 0.69 (0.49, 0.97) | |||
| rs1863652 | TT versus CC/CT | 0.030 | 0.79 (0.64, 0.98) | |||
| EIF4E | 0.007 | rs12498533 | CC versus AA | 0.013 | 1.25 (1.03, 1.52) | |
| rs11727086 | GG versus AA | 0.004 | 1.33 (0.99, 1.77) | |||
| RUNX1 | 0.090 | rs2834645 | TC/CC versus TT | 0.006 | 0.82 (0.72, 0.95) | |
| rs2834650 | CT/TT versus CC | 0.066 | 0.85 (0.71, 1.01) | |||
| rs2268281 | GG versus AA | 0.076 | 1.40 (0.96, 2.03) | |||
| rs2248720 | AC/CC versus AA | 0.053 | 0.86 (0.74, 1.00) | |||
| rs2252585 | CC versus TT | 0.057 | 1.20 (0.94, 1.53) | |||
| rs8134179 | CC versus TT | 0.060 | 0.71 (0.48, 1.06) | |||
| rs2834670 | GG versus AA | 0.097 | 1.14 (0.85, 1.53) | |||
| rs2242878 | CT/TT versus CC | 0.011 | 1.20 (1.04, 1.38) | |||
| rs7279123 | CT/TT versus CC | 0.015 | 1.18 (1.03, 1.35) | |||
| SMAD2 | 0.044 | rs1787199 | TT versus AA | 0.028 | 1.24 (1.03, 1.51) | |
| rs4940086 | CC versus TT | 0.019 | 1.33 (1.06, 1.66) | |||
| SMAD3 | 0.015 | rs12904944 | AA versus GG | 0.069 | 0.81 (0.64, 1.01) | |
| rs1498506 | CC versus AA | 0.0003 | 0.69 (0.57, 0.84) | |||
| rs12901071 | GG versus AA | 0.004 | 0.67 (0.53, 0.84) | |||
| rs2414937 | CC versus GG/GC | 0.034 | 0.68 (0.47, 0.97) | |||
| SMAD7 | 0.045 | rs4939827 | TC/CC versus TT | 0.028 | 0.84 (0.73, 0.98) | |
| rs12953717 | TT versus CC | 0.003 | 1.36 (1.12, 1.65) | |||
| TGFB1 | 0.000 | rs4803455 | AA versus CC | 0.0003 | 1.43 (1.18, 1.74) | |
| rs1800469 | AA versus GG | 0.001 | 0.66 (0.52, 0.85) | |||
| TGFBR1 | 0.087 | rs6478974 | TA/AA versus TT | 0.037 | 0.85 (0.74, 0.99) | |
| rs1571590 | GG versus AA/AG | 0.049 | 1.41 (1.00, 1.98) | |||
| TNF | TNF | 0.036 | rs1799964 | CC versus TT | 0.055 | 1.29 (0.94, 1.76) |
| rs1800630 | CA/AA versus CC | 0.020 | 1.19 (1.03, 1.38) |
Genes with P ARTP <0.05 are indicated in bold.
a P values based on 1-df Wald chi-square tests.
bModels adjusted for age, study center, race/ethnicity and sex.
Table IV.
Associations between subpathway genes and SNPs with rectal cancer that were used in polygenic score (P ARTP < 0.10)
| Subpathway | Gene | Gene P ARTP | SNP | Genotype | SNP P a | OR 95% CIb |
|---|---|---|---|---|---|---|
| Angiogenesis | MPO | 0.041 | rs2243828 | AG/GG versus AA | 0.039 | 0.81 (0.67, 0.99) |
| VEGFA | 0.057 | rs2010963 | GC/CC versus GG | 0.007 | 1.30 (1.07, 1.58) | |
| Hormone/insulin/ growth | IGF1R | 0.079 | IGF1R | CC versus AA | 0.078 | 1.51 (0.93, 2.43) |
| Interferons | IFNGR1 | 0.052 | rs3799488 | CC versus TT/TC | 0.040 | 2.30 (1.04, 5.08) |
| rs9376267 | CT/TT versus CC | 0.065 | 1.20 (0.99, 1.46) | |||
| IRF2 | 0.072 | rs3775554 | CC versus GG | 0.057 | 1.06 (0.46, 2.44) | |
| rs3775556 | GG versus AA | 0.007 | 1.48 (1.00, 2.20) | |||
| rs10009261 | TT versus CC | 0.038 | 0.77 (0.57, 1.04) | |||
| rs7677486 | TT versus CC | 0.003 | 1.54 (1.16, 2.03) | |||
| rs6827018 | GG versus AA | 0.032 | 1.70 (0.75, 3.83) | |||
| rs3733473 | AA versus GG | 0.078 | 0.97 (0.61, 1.53) | |||
| rs3775574 | AG/GG versus AA | 0.003 | 0.74 (0.60, 0.90) | |||
| Interleukins | IL15 | 0.064 | rs12508866 | TC/CC versus TT | 0.019 | 0.79 (0.65, 0.96) |
| rs17461269 | TA/AA versus TT | 0.025 | 1.25 (1.03, 1.52) | |||
| rs13117878 | TT versus CC | 0.066 | 1.29 (0.98, 1.69) | |||
| IL8RA | 0.006 | rs1008562 | GG versus CC | 0.001 | 1.57 (1.20, 2.05) | |
| IL8RB | 0.002 | rs1126579 | TT versus CC | 0.001 | 1.56 (1.19, 2.05) | |
| Jak/Stat/Socs | STAT3 | 0.083 | rs2293152 | CC versus GG/GC | 0.014 | 0.71 (0.54, 0.93) |
| STAT6 | 0.100 | rs3024979 | TA/AA versus TT | 0.016 | 0.73 (0.56, 0.94) | |
| MAPK | DUSP1 | 0.030 | rs322351 | TT versus CC | 0.013 | 1.43 (1.09, 1.88) |
| DUSP7 | 0.051 | rs9851576 | AG/GG versus AA | 0.054 | 0.81 (0.66, 1.00) | |
| MAPK8 | 0.055 | rs10508901 | AA versus CC/CA | 0.023 | 1.45 (1.05, 1.99) | |
| Pathway core | NFKB1 | 0.017 | rs230510 | TT versus AA | 0.006 | 0.67 (0.50, 0.90) |
| rs3821958 | GG versus AA | 0.043 | 1.35 (1.02, 1.78) | |||
| rs11722146 | AA versus GG | 0.015 | 1.42 (1.02, 1.97) | |||
| PIK3CA | 0.077 | rs2699905 | GA/AA versus GG | 0.054 | 0.82 (0.67, 1.00) | |
| rs7651265 | GG versus AA | 0.063 | 2.38 (1.04, 5.43) | |||
| rs7640662 | GG versus CC | 0.012 | 0.30 (0.11, 0.79) | |||
| PIK3CG | 0.019 | rs4460309 | TT versus CC | 0.005 | 0.66 (0.43, 1.00) | |
| rs11766675 | GG versus AA | 0.003 | 0.57 (0.34, 0.96) | |||
| RPS6KA2 | 0.072 | rs10946164 | CT/TT versus CC | 0.020 | 0.78 (0.64, 0.96) | |
| rs9347128 | GG versus CC/CG | 0.027 | 1.32 (1.03, 1.68) | |||
| rs1040446 | TG/GG versus TT | 0.038 | 0.78 (0.61, 0.99) | |||
| rs6911624 | AA versus GG | 0.002 | 0.55 (0.31, 0.98) | |||
| rs4709127 | TT versus CC | 0.047 | 0.74 (0.52, 1.06) | |||
| rs2345067 | CC versus TT | 0.035 | 0.75 (0.57, 0.98) | |||
| rs1202621 | CC versus AA | 0.073 | 1.25 (0.93, 1.68) | |||
| rs7745781 | AG/GG versus AA | 0.001 | 1.42 (1.15, 1.76) | |||
| RPS6KB2 | 0.057 | rs1638588 | AA versus CC | 0.028 | 0.73 (0.56, 0.96) | |
| TSC1 | 0.043 | rs13295634 | GG versus TT/TG | 0.004 | 0.61 (0.43, 0.86) | |
| Selenoproteins | SEPN1 | 0.002 | rs718391 | GG versus CC | 0.010 | 0.70 (0.53, 0.93) |
| rs2072749 | GG versus AA | 0.003 | 0.53 (0.35, 0.80) | |||
| rs11247735 | AA versus GG/GA | 0.021 | 1.31 (1.04, 1.64) | |||
| rs4659382 | GG versus CC/CG | 0.007 | 0.58 (0.39, 0.86) | |||
| SEPP1 | 0.092 | rs3877899 | AA versus GG | 0.039 | 0.68 (0.43, 1.07) | |
| rs11959466 | GA/AA versus GG | 0.019 | 1.46 (1.06, 2.00) | |||
| rs28919882 | AG/GG versus AA | 0.023 | 0.72 (0.55, 0.96) | |||
| TXNRD3 | 0.004 | rs4679274 | TT versus CC/CT | 0.034 | 0.73 (0.54, 0.98) | |
| rs9637365 | TT versus CC/CT | 0.006 | 0.70 (0.55, 0.90) | |||
| rs11718498 | GA/AA versus GG | 0.001 | 1.42 (1.15, 1.74) | |||
| TGFβ | BMPR2 | 0.026 | rs4675278 | AA versus GG | 0.087 | 1.29 (0.92, 1.81) |
| rs17199235 | AG/GG versus AA | 0.018 | 1.35 (1.05, 1.72) | |||
| rs2228545 | GA/AA versus GG | 0.002 | 1.93 (1.28, 2.91) | |||
| TNF | NFAT5 | 0.030 | rs12447326 | TT versus CC/CT | 0.003 | 0.60 (0.43, 0.84) |
| rs16959025 | GG versus TT/TG | 0.025 | 0.57 (0.35, 0.93) |
Significant genes with P ARTP <0.05 are indicated in bold.
a P values based on 1-df Wald chi-square tests.
bModels adjusted for age, study center, race/ethnicity and sex.
Fewer genes and SNPs were significantly associated with rectal cancer (Table IV). Genes in seven subpathways were statistically significant at the P ARTP <0.05, whereas another 13 genes had P ARTP values between 0.05 and 0.10. However, in this last group of genes, there were 24 SNPs with P values of <0.05. The interleukin and selenoprotein subpathways were more significantly associated with rectal cancer than colon cancer, although TGFβ signaling pathway had less of an impact on rectal cancer.
There were few subpathways associated with specific tumor molecular phenotype (Table V). Interleukins (P ARTP = 0.0456) and MAPK (P ARTP = 0.0392) subpathways were significant for CIMP+ colon tumors, while not significant for overall colon cancers; none of the subpathways were associated with MSI tumors. However, even though the overall subpathways were not statistically significant at the 0.05 level, there were several genes within these subpathways that had P ARTP values <0.05.
Table V.
Associations for subpathway and gene by tumor molecular phenotype
| Subpathway | CIMP | Kras | TP53 | MSI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subpathway P ARTP | Gene | Gene P ARTP | Subpathway P ARTP | Gene | Gene P ARTP | Subpathway P ARTP | Gene | Gene P ARTP | Subpathway P ARTP | Gene | Gene P ARTP | |
| Colon cancera | ||||||||||||
| Angiogenesis | 0.251 | SOD1 | 0.017 | 0.672 | 0.875 | 0.104 | MMP7 | 0.011 | ||||
| SOD1 | 0.016 | |||||||||||
| Hormone/insulin/ growth | 0.380 | 0.169 | EGR2 | 0.019 | 0.069 | PPARG | 0.037 | 0.156 | IRS1 | 0.027 | ||
| VDR | 0.019 | TCF7L2 | 0.003 | PPARG | 0.019 | |||||||
| Interferon | 0.422 | IRF6 | 0.029 | 0.264 | IRF6 | 0.037 | 0.687 | 0.812 | ||||
| Interleukins | 0.046 | IL15 | 0.032 | 0.192 | IL17A | 0.031 | 0.621 | 0.628 | ||||
| IL1A | 0.033 | |||||||||||
| IL6R | 0.027 | |||||||||||
| IL8 | 0.017 | |||||||||||
| Jak/Stat/Socs | 0.234 | SOCS2 | 0.015 | 0.043 | JAK2 | 0.041 | 0.022 | STAT1 | 0.023 | 0.275 | SOCS2 | 0.019 |
| STAT5B | 0.014 | STAT5A | 0.008 | |||||||||
| STAT6 | 0.007 | STAT5B | 0.013 | |||||||||
| Pathway core | 0.514 | PRKAA1 | 0.045 | 0.555 | PRKAA1 | 0.032 | 0.190 | MTOR | 0.016 | 0.192 | MTOR | 0.005 |
| TSC1 | 0.031 | PRKAA1 | 0.009 | |||||||||
| MAPK | 0.039 | DUSP2 | 0.036 | 0.568 | 0.640 | 0.366 | MAP3K9 | 0.017 | ||||
| MAP3K9 | 0.001 | |||||||||||
| Selenoprotein | 0.751 | 0.487 | C11orf31 | 0.048 | 0.845 | 0.680 | ||||||
| Telomere | 0.253 | 0.265 | 0.149 | 0.419 | ||||||||
| TGFβ | 0.181 | BMP4 | 0.019 | 0.027 | BMPR1B | 0.028 | 0.007 | EIF4EBP3 | 0.008 | 0.194 | BMPR2 | 0.039 |
| RUNX2 | 0.042 | RUNX2 | 0.015 | RUNX3 | 0.003 | TGFB1 | 0.017 | |||||
| SMAD2 | 0.031 | TGFB1 | 0.004 | TGFB1 | 0.004 | |||||||
| TLR | 0.371 | 0.780 | 0.509 | 0.163 | TLR4 | 0.041 | ||||||
| TNF | 0.588 | 0.322 | 0.971 | 0.073 | TNFRSF1A | 0.042 | ||||||
| Rectal cancerb | ||||||||||||
| Angiogenesis | 0.478 | 0.849 | 0.216 | MSTN | 0.042 | |||||||
| VEGFA | 0.014 | |||||||||||
| Hormone/insulin/ growth | 0.781 | 0.975 | 0.870 | |||||||||
| Interferon | 0.655 | 0.818 | 0.608 | |||||||||
| Interleukin | 0.902 | 0.282 | IL8RB | 0.028 | 0.024 | IL6 | 0.034 | |||||
| IL8 | 0.050 | |||||||||||
| IL8RA | 0.013 | |||||||||||
| IL8RB | 0.004 | |||||||||||
| Jak/Stat/Socs | 0.305 | 0.456 | 0.561 | |||||||||
| Pathway core | 0.529 | AKT1 | 0.026 | 0.890 | 0.590 | NFKB1 | 0.050 | |||||
| NFKB1 | 0.041 | PTEN | 0.044 | |||||||||
| MAPK | 0.794 | 0.260 | RAF1 | 0.030 | 0.122 | DUSP1 | 0.050 | |||||
| DUSP6 | 0.028 | |||||||||||
| MAPK8 | 0.006 | |||||||||||
| Selenoprotein | 0.801 | 0.066 | SEPN1 | 0.008 | 0.033 | SEP15 | 0.029 | |||||
| SEPX1 | 0.043 | TXNRD2 | 0.016 | |||||||||
| TXNRD3 | 0.015 | |||||||||||
| Telomere | 0.134 | 0.622 | 0.146 | |||||||||
| TGFβ | 0.491 | TGFBR1 | 0.024 | 0.956 | 0.413 | BMP2 | 0.021 | |||||
| TLR | 0.210 | 0.897 | 0.679 | |||||||||
| TNF | 0.259 | 0.763 | 0.678 | |||||||||
aPathway P ARTP values of 0.324 for CIMP, 0.255 for Kras, 0.063 for TP53 and 0.420 for MSI.
bPathway P ARTP values of 0.843 for CIMP, 0.658 for Kras and 0.171 for TP53.
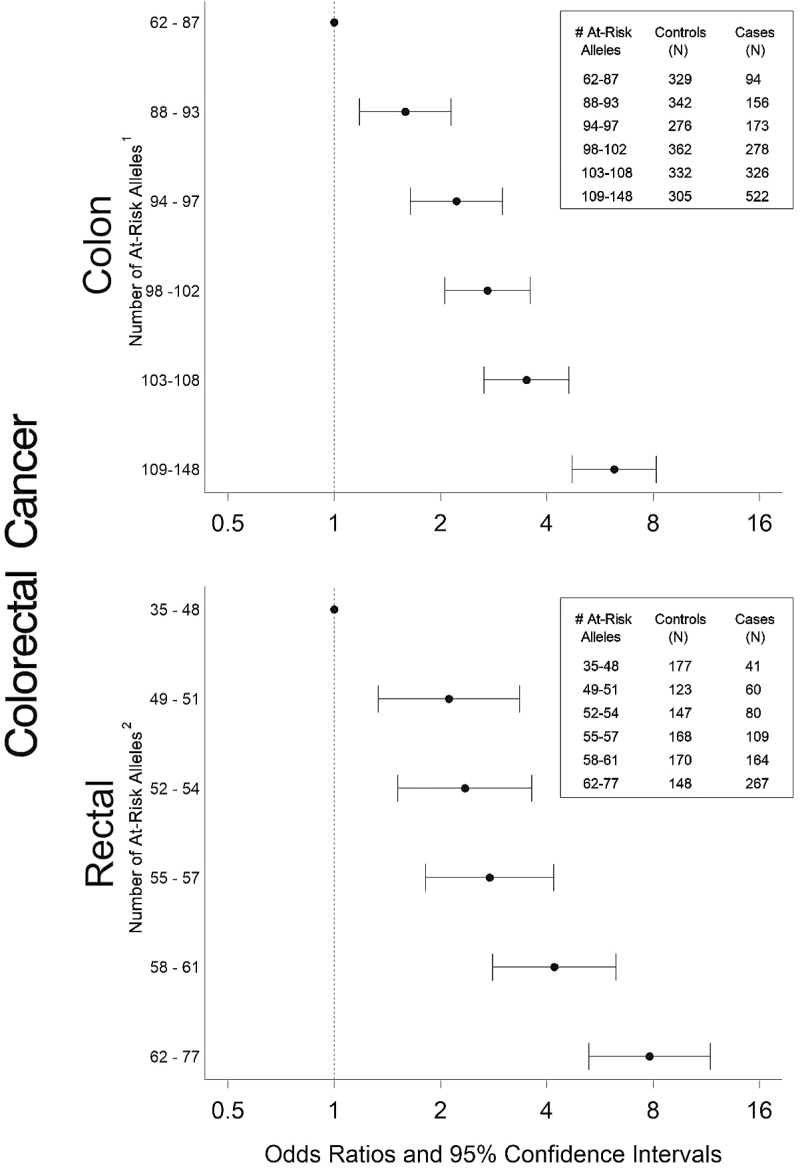
To summarize the risk associated with the CHIEF pathway, we report polygenic summary scores (see Figure 2). With increasing number of at-risk alleles, there was a significant increase in risk of both colon and rectal cancer. The magnitude of risk was slightly greater for rectal cancer (OR: 7.82, 95% CI: 5.26, 11.62) than for colon cancer (OR: 6.21, 95% CI: 4.72, 8.16), despite the fact that more SNPs were included in the risk score for colon cancer than for rectal cancer.
Fig. 2.
Polygenic summary score associated with CHIEF pathway for colon and rectal cancer. Models adjusted for age, study center, race/ethnicity and sex. 1SNPs included in score: BMP2 rs1979855, rs235770, rs3178250, BMPR1A rs6586034, rs7088641, BMPR1B rs13134042, rs17616243, rs1863652, rs2120834, rs4490463, rs7662504, rs7694043, rs9307147, DUSP2 rs1724120, EIF4E rs11727086, rs12498533, FLT1 rs12858139, rs1324057, rs2296189, rs2296283, rs2387632, rs3794400, rs600640, rs678714, rs7324547, rs9513088, MTOR rs1057079, rs2024627, IFNG rs1861493, rs2069718, rs13117878, rs1519551, IL2RA rs12244380, rs12722561, rs12722596, rs3118470, rs706779, IL3 rs181781, IL6R rs4845623, rs7549250, IL6 rs1800795, rs2069860, IL8 rs4073, IRF3 rs2304204, JAK2 rs1887429, rs3780379, MAP2K1 rs1432442, rs7181936, rs8039880, MAP3K3 rs11658329, rs3785574, MAP3K9 rs11624934, rs11625206, rs11628333, rs11844774, 55 rs17176971, PRKAG2 rs1029947, rs1104897, rs12703162, rs1860743, rs2374270, rs2536068, rs6464156, rs6464170, rs6947064, rs6965771, rs953221, rs9632641, rs9648723, rs9648724, RPS6KB2 rs917570, RUNX1 rs2242878, rs2248720, rs2252585, rs2268281, rs2834645, rs2834650, rs2834670, rs7279123, rs8134179, SLC2A4 rs5435, SMAD2 rs1787199, rs4940086, SMAD3 rs12901071, rs12904944, rs1498506, rs2414937, SMAD7 rs12953717, rs4939827, SOCS2 rs768775, STAT3 rs1026916, rs12949918, rs6503695, rs8069645, STAT5A rs7217728, STAT5B rs6503691, rs7218653, STAT6 rs324011, rs324015, TCF7L2, TERT rs2736100, rs2736118, rs2853668, TGFB1 rs1800469, rs4803455, TGFBR1 rs1571590, rs6478974, TNF rs1799964, rs1800630, VDR_Fok1, VDR_Poly; 2SNPs included in score: BMPR2 rs17199235, rs2228545, rs4675278, DUSP1 rs322351, DUSP7 rs9851576, IFNGR1 rs3799488, rs9376267, IGF1R, IL15 rs12508866, rs13117878, rs17461269, IL8RA rs1008562, IL8RB rs1126579, IRF2 rs10009261, rs3733473, rs3775554, rs3775556, rs3775574, rs6827018, rs7677486, MAPK8 rs10508901, MPO rs2243828, NFAT5 rs12447326, rs16959025, NFKB1 rs11722146, rs230510, rs3821958, PIK3CA rs2699905, rs7640662, rs7651265, PIK3CG rs11766675, rs4460309, RPS6KA2 rs1040446, rs10946164, rs1202621, rs2345067, rs4709127, rs6911624, rs7745781, rs9347128, RPS6KB2 rs1638588, SEPN1 rs11247735, rs2072749, rs4659382, rs718391, SEPP1 rs11959466, rs28919882, rs31877899, STAT3 rs2293152, STAT6 rs3024979, TSC1 rs13295634, TXNRD3 rs11718498, rs4679274, rs9637365, VEGFA rs2010963.
Discussion
The physiological structure of the gut and supportive epidemiological and molecular data led us to propose that basal immune activation, a repetitive, mild subclinical inflammation, is the underlying modulator of CRC risk and influences the CRC risk associated with insulin, estrogen and energy-related factors. Overall, the CHIEF pathway was statistically significant for colon cancer and marginally significant for rectal cancer. While subpathways within the overall pathway indicate areas of importance, specific genes within pathway also are important. This is not surprising since these genes work in multiple pathways and could contribute to risk through their involvement in other subpathways than the one in which they were evaluated. Additionally, pathways of significance appear to differ for colon and rectal cancer. The TGFβ signaling pathway was most important for colon cancer, whereas the interleukin and selenoprotein subpathways were important for rectal cancer. Although a greater number of genes and SNPs were associated with colon cancer, the risk associated with rectal cancer was slightly greater than that observed for colon cancer.
We have previously reported associations for SNPs within subpathways taking into account multiple comparisons. Our goal in these analyses was to summarize the significance of the CHIEF pathway, using hierarchical modeling within ARTP to estimate the overall association as well as to summarize the importance of the subpathways and genes. ARTP utilizes a highly efficient permutation algorithm to determine the significance of association of each gene and of all genes combined, and thus allows us to summarize the statistical significance of the pathway. While many of our results from ARTP are similar to those previously reported, there are some differences. Previously, we focused on assessment of SNPs within genes, adjusting for multiple comparisons and identified SNPs in FLT1, CYP19A1, IFNGR1, TSC2, RUNX3, TLR2, TLR3, TLR4 and selenoproteins that were significant for colon cancer but were not identified here as significant (21–27). For rectal cancer, we identified significant findings for FLT1, VEGFA, MAPK8, RUNX1, SMAD3, TLR3, STK11 and PRKAG2 (21,22,24–26,28,29) in our previous work. Differences in association stem from the focus on the analysis. Using ARTP, we focus on genes and pathways and only considered SNPs to be important if the gene was statistically significant (P ARTP < 0.05 or marginally significant P ARTP < 0.10). Previously, we focused on SNPs within genes. While these SNPs may still be important, having only one or two modest associations would not necessarily result in the gene being significant.
However, the major difference in our current and previous findings stems from the inability to incorporate lifestyle exposures and evaluate gene by environment interactions in ARTP. Our previous analyses have shown that multiple lifestyle factors interact with pathway genes, and that the risk associated with genes alone underestimates their influence on CRC risk. Important lifestyle factors that interact with genes in the pathway are dietary components with MAPK (28,30), angiogenesis (31) and Toll-like receptor (TLR) (26) genes; non-steroidal anti-inflammatory drugs use with angiogenesis (31), estrogen-related genes (22), cytokines (32,33), MAPK (28), JAK/STAT (34), TGFβ signaling pathway (29), TLR (26) and selenoproteins (23); cigarette smoking with angiogenesis (31), cytokines (27,33), MAPK (28), JAK/STAT (34), TGFβ (29) and selenoproteins (23) subpathways; body mass index with angiogenesis (31), estrogen-related genes (22), VDR (35) and cytokines (27,33). Thus, to fully estimate the importance of this pathway, methods to evaluate genetic interactions with lifestyle factors are needed.
The polygenic score allowed us to summarize the magnitude of the risk associated with the pathway, rather than merely looking at a P value. We determined significance based on the ARTP method and incorporated genes that had a P value of <0.10 and SNPs within these genes with a P value of <0.10. This was more conservative than including only those genes and SNPs with the smallest P values. Increasing risk was observed with increasing number of ‘at-risk’ genotypes for both colon and rectal cancer.
Based on our findings, several subpathways and groups of genes were significantly associated with colon cancer. The TGFβ signaling pathway appeared to be most important for colon cancer, with BMP2, BMPR1A, BMPR1B, EIF4E, SMAD2, SMAD3, SMAD7 and TGFB1 associated with risk. The TGFβ signaling pathway is an essential regulator of cellular proliferation, differentiation, apoptosis and extracellular matrix remodeling in the cell that is involved in angiogenesis and inflammation (13). It mediates intracellular actions of proinflammatory cytokines, including activation of NF-κB (36,37). BMPs trigger a Smad-signaling cascade that has been linked to reduced cell proliferation and cellular growth (38,39) and may play a key role in regulating tumor initiation. Genome-wide association study have reported that both BMP2 and BMP4 were 2 of the top 10 genes identified as contributing to colon cancer risk (40). BMPR1A and BMPR1B are the two best-characterized type I BMP receptors. Smad proteins are substrates for these receptors and are key intracellular mediators of the transcriptional responses to TGFβ signaling (41). STAT3 has been shown to be a promoter of tumor invasiveness and angiogenesis (42). Activation of STAT5 results in regulation of several genes involved in cell apoptosis, survival and proliferation (43). It has been shown that aspirin, a consistently recognized protective factor for CRC, regulates apoptosis by downregulating the IL6-STAT3 pathway (44).
Along the pathway core, MTOR and RPS6KB2 were significantly associated with colon cancer. MTOR represses anabolic processes (ATP utilization) and enhances catabolic processes (ATP generation), restoring the system toward normal energy homeostasis, whereas RPS6KB is involved in a signaling pathway that involves angiotensin II activation of NF-κB (45). The importance of cytokines, including interferons, interleukins and TNF and the MAPK and STATs that are involved in multiple subpathways, appeared to be more important than those genes directly included with hormones, growth factors and insulin-related factors. However, SLC2A4, also known as GLUT4, was associated with colon cancer. Studies have shown that the cytokine TNF decreases GLUT4 expression in adipocytes resulting in impaired insulin action (46). VDR has been associated with colon cancer risk in numerous studies (47,48). Studies have shown that VDR interacts with PPARG to alter rectal cancer risk (49) and is involved in TGFβ/Smad3 signaling (50). Interleukins are a type of cytokine that control growth and differentiation, cell migration and inflammatory and anti-inflammatory responses by the immune system. TNF, a proinflammatory cytokine, stimulates cell proliferation and induces cell differentiation and is thought to be one of the most important promoters of inflammation. TNF mediates cell survival and apoptosis through TNF receptors by activating at least two major signaling pathways, NF-κB and the p38 MAPK pathway.
A different set of genes was identified for rectal cancer than for colon cancer. Although some of the same subpathways were identified as containing significant genes, those identified as associated with rectal cancer risk were, for the most part, different than those identified for colon cancer. Only four genes, IL15, STAT3, STAT6 and RPS6KB2, were associated with both colon and rectal cancer and only IL15 rs13117878 was associated with both cancer sites with the same magnitude of association. For instance, along the pathway core, NFKB1, PIK3CG and TSC1 were associated with rectal cancer compared with MTOR and RPS6KB2 that were identified for colon cancer; only BMPR2 was associated with rectal cancer, whereas eight genes in the TGFβ signaling pathway were associated with colon cancer; IL8 receptors were associated with rectal cancer, whereas IL6R and IL8 were associated with colon cancer. Selenoproteins appeared to be important for rectal cancer. While differences were observed between colon and rectal cancer, it is important to note that the sample size was considerably less for rectal cancer, and failure to detect associations observed for colon cancer could be from the smaller sample size. Despite few significant genes being associated with rectal cancer, the polygenic score indicated slighter greater risk for rectal cancer. This could indicate that the associations were generally stronger for SNPs that were associated or that the combined effect was greater since the SNPs associated contributed independently. It is possible that having additional at-risk SNPs may not contribute additional risk, given the presence of other SNPs in the pathway.
Evaluation of tumor molecular phenotype showed most similarities between TP53 and overall colon cancer, which would be expected, given that TP53 is the most common tumor molecular phenotype in CRCs and therefore of interest. The power to detect associations was weaker for the less common tumor molecular phenotypes such as MSI and CIMP. However, SOD1, SOCS1 and MAP3K9 were only associated with risk for MSI and CIMP+ tumors, whereas cytokines appeared to be more uniformly associated with CIMP+ tumors. These data illustrate the importance of incorporating tumor molecular phenotype when evaluating risk, given that unique pathways and genes may be associated with specific tumor molecular phenotypes that would be missed if separate analyses were not conducted. Many strengths and limitations have been discussed by Ogino and colleagues (51). Strengths of our study is nearly complete ascertainment of all diagnosed cases of CRC in the target areas (97% for Utah and 85% for Kaiser) which decreases selection bias and increases generalizability to other populations. Likewise, our sample size is large which enables a more powerful assessment of specific tumor molecular phenotype. However, there are some limitations when doing such analysis, including varying sample sizes for the various tumor phenotypes, thus ability to detect associations is not uniform across tumor phenotypes. Additionally, in more traditional analysis, penalties for multiple comparisons could be applied. However, our statistical approach is powerful in that incorporates adjustments as part of the statistical computation. Additionally, error in lab measurements are possible; however, because we did sequencing of TP53 rather than immunohistochemistry, we have more accurate measurements.
The study had several other strengths and limitations. The pathway approach was novel and an attempt to summarize the statistical significance as well as the related risk with a pathway. ARTP allows us to adaptively combine single SNP P values using the rank truncated product statistic and assess significance via permutations at multiple levels, including the gene, subpathway and overall pathway level. However, our results could be from chance and therefore need replication. While we selected genes that we believed were most important to the pathway, there are many other genes and SNPs involved in this pathway that could be important and contribute to colon and rectal cancer risk. Because of our using a customized platform, we were unable to include all potentially relevant genes along the pathway. One of the major limitations is our inability to evaluate the risk associated with these genes from their interaction with lifestyle factors. Our previous analyses suggest that interaction is important, however, at this time, we are only able to determine the significance of these genes based on their main effect and not that component of risk that comes from interaction.
In summary, the CHIEF pathway was significantly associated with colon cancer overall and marginally associated with rectal cancer. However, increasing numbers of at-risk alleles increased risk of both colon and rectal cancer. The TGFβ signaling pathway was the most important subpathway for colon cancer. Other important subpathways were those that included cytokines, JAK/STAT/SOC and MAPK.
Supplementary material
Supplementary Tables 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute (NCI) (CA48998); The Utah Cancer Registry—funded by contract #N01-PC-67000 from the National Cancer Institute—with additional support: State of Utah Department of Health; Northern California Cancer Registry; Sacramento Tumor Registry.
Supplementary Material
Acknowledgements
We would like to acknowledge the contributions of Dr Bette Caan, Judy Morse, Donna Schaffer and the Kaiser Permanente Medical Research Program; Sandra Edwards and Jennifer Herrick at the University of Utah and Dr Kristin Anderson and Dr John Potter for data management and collection at the University of Minnesota. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ARTP
Adaptive Rank Truncation Product
- CHIEF
convergence of hormones, inflammation and energy-related factors
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- CRC
colorectal cancer
- KPMCP
Kaiser Permanente Medical Care Program of Northern California
- LD
linkage disequilibrium
- MAPK
mitogen-activated kinase
- MSI
microsatellite instability
- MTOR
mammalian target of rapamycin
- NF-κB
nuclear factor kappa B
- OR
odds ratio
- SNP
single nucleotide polymorphism
- STAT
signal transduction and activation of transcription
- TGFβ
transforming growth factor β
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor.
References
- 1. Slattery M.L., et al. (2009). Convergence of hormones, inflammation, and energy-related factors: a novel pathway of cancer etiology. Cancer Prev. Res. (Phila)., 2, 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carling D. (2004). Ampk. Curr. Biol., 14, R220. [DOI] [PubMed] [Google Scholar]
- 3. Hardie D.G. (2003). Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology, 144, 5179–5183. [DOI] [PubMed] [Google Scholar]
- 4. Carling D. (2004). The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem. Sci., 29, 18–24. [DOI] [PubMed] [Google Scholar]
- 5. Viollet B., et al. (2003). Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem. Soc. Trans., 31, 216–219. [DOI] [PubMed] [Google Scholar]
- 6. Watson C.J., et al. (2000). Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine, 12, 1232–1235. [DOI] [PubMed] [Google Scholar]
- 7. Waldner M.J., et al. (2010). VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J. Exp. Med., 207, 2855–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nilsson B.O. (2007). Modulation of the inflammatory response by estrogens with focus on the endothelium and its interactions with leukocytes. Inflamm. Res., 56, 269–273. [DOI] [PubMed] [Google Scholar]
- 9. McKay L.I., et al. (1999). Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr. Rev., 20, 435–459. [DOI] [PubMed] [Google Scholar]
- 10. De Bosscher K., et al. (2006). Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene, 25, 6868–6886. [DOI] [PubMed] [Google Scholar]
- 11. Chadwick C.C., et al. (2005). Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc. Natl Acad. Sci. USA., 102, 2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ray P., et al. (1997). Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett., 409, 79–85. [DOI] [PubMed] [Google Scholar]
- 13. Gordon K.J., et al. (2008). Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta., 1782, 197–228. [DOI] [PubMed] [Google Scholar]
- 14. Ono M. (2008). Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci., 99, 1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slattery M.L., et al. (1997). Energy balance and colon cancer–beyond physical activity. Cancer Res., 57, 75–80. [PubMed] [Google Scholar]
- 16. Slattery M.L., et al. (2003). Physical activity and colorectal cancer. Am. J. Epidemiol., 158, 214–224. [DOI] [PubMed] [Google Scholar]
- 17. Yu K., et al. (2009). Pathway analysis by adaptive combination of P-values. Genet. Epidemiol., 33, 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samowitz W.S., et al. (2005). Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology, 129, 837–845. [DOI] [PubMed] [Google Scholar]
- 19. Slattery M.L., et al. (2000). Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J. Natl. Cancer Inst., 92, 1831–1836. [DOI] [PubMed] [Google Scholar]
- 20. Slattery M.L., et al. (2009). A comparison of colon and rectal somatic DNA alterations. Dis. Colon Rectum, 52, 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slattery M.L., et al. (2011). Associations between genetic variation in RUNX1, RUNX2, RUNX3, MAPK1 and eIF4E and risk of colon and rectal cancer: additional support for a TGF-β-signaling pathway. Carcinogenesis, 32, 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slattery M.L., et al. (2011). Variation in the CYP19A1 gene and risk of colon and rectal cancer. Cancer Causes Control, 22, 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slattery M.L., et al. (2012). Genetic variation in selenoprotein genes, lifestyle, and risk of colon and rectal cancer. PLoS One, 7, e37312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slattery M.L., et al. (2014). VEGFA, FLT1, KDR and colorectal cancer: assessment of disease risk, tumor molecular phenotype, and survival. Mol. Carcinog., 53 (suppl. 1), E140–E150. [DOI] [PubMed] [Google Scholar]
- 25. Slattery M.L., et al. (2010). Genetic variation in a metabolic signaling pathway and colon and rectal cancer risk: mTOR, PTEN, STK11, RPKAA1, PRKAG2, TSC1, TSC2, PI3K and Akt1. Carcinogenesis, 31, 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slattery M.L., et al. (2012). Toll-like receptor genes and their association with colon and rectal cancer development and prognosis. Int. J. Cancer, 130, 2974–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slattery M.L., et al. (2011). Interferon-signaling pathway: associations with colon and rectal cancer risk and subsequent survival. Carcinogenesis, 32, 1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slattery M.L., et al. (2012). MAP kinase genes and colon and rectal cancer. Carcinogenesis, 33, 2398–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slattery M.L., et al. (2012). Genetic variation in the transforming growth factor-β-signaling pathway, lifestyle factors, and risk of colon or rectal cancer. Dis. Colon Rectum, 55, 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slattery M.L., et al. (2013). Dietary influence on MAPK-signaling pathways and risk of colon and rectal cancer. Nutr. Cancer, 65, 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slattery M.L., et al. (2012). Oxidative balance and colon and rectal cancer: interaction of lifestyle factors and genes. Mutat. Res., 734, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slattery M.L., et al. (2011). Tumor necrosis factor-related genes and colon and rectal cancer. Int. J. Mol. Epidemiol. Genet., 2, 328–338. [PMC free article] [PubMed] [Google Scholar]
- 33. Bondurant K.L., et al. (2013). Interleukin genes and associations with colon and rectal cancer risk and overall survival. Int. J. Cancer, 132, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slattery M.L., et al. (2013). JAK/STAT/SOCS-signaling pathway and colon and rectal cancer. Mol. Carcinog., 52, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slattery M.L., et al. (2004). Associations between BMI, energy intake, energy expenditure, VDR genotype and colon and rectal cancers (United States). Cancer Causes Control, 15, 863–872. [DOI] [PubMed] [Google Scholar]
- 36. Hong S., et al. (2007). Smad7 sensitizes tumor necrosis factor induced apoptosis through the inhibition of antiapoptotic gene expression by suppressing activation of the nuclear factor-kappaB pathway. Cancer Res., 67, 9577–9583. [DOI] [PubMed] [Google Scholar]
- 37. Halder S.K., et al. (2005). Smad7 induces tumorigenicity by blocking TGF-beta-induced growth inhibition and apoptosis. Exp. Cell Res., 307, 231–246. [DOI] [PubMed] [Google Scholar]
- 38. Piccirillo S.G., et al. (2006). Bone morphogenetic proteins regulate tumorigenicity in human glioblastoma stem cells. Ernst Schering Found. Symp. Proc., 5, 59–81. [DOI] [PubMed] [Google Scholar]
- 39. Piccirillo S.G., et al. (2006). Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature, 444, 761–765. [DOI] [PubMed] [Google Scholar]
- 40. Peters U., et al. (2012). Meta-analysis of new genome-wide association studies of colorectal cancer risk. Hum. Genet., 131, 217–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang G., et al. (2010). Smad4-mediated TGF-beta signaling in tumorigenesis. Int. J. Biol. Sci., 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haricharan S., et al. (2014). STAT signaling in mammary gland differentiation, cell survival and tumorigenesis. Mol. Cell. Endocrinol., 382, 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wei L., et al. (2008). New insights into the roles of Stat5a/b and Stat3 in T cell development and differentiation. Semin. Cell Dev. Biol., 19, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim S.R., et al. (2009). Aspirin induces apoptosis through the blockade of IL-6-STAT3 signaling pathway in human glioblastoma A172 cells. Biochem. Biophys. Res. Commun., 387, 342–347. [DOI] [PubMed] [Google Scholar]
- 45. Zhang L., et al. (2005). A new cellular signaling mechanism for angiotensin II activation of NF-kappaB: an IkappaB-independent, RSK-mediated phosphorylation of p65. Arterioscler. Thromb. Vasc. Biol., 25, 1148–1153. [DOI] [PubMed] [Google Scholar]
- 46. Tesz G.J., et al. (2007). Tumor necrosis factor alpha (TNFalpha) stimulates Map4k4 expression through TNFalpha receptor 1 signaling to c-Jun and activating transcription factor 2. J. Biol. Chem., 282, 19302–19312. [DOI] [PubMed] [Google Scholar]
- 47. Murtaugh M.A., et al. (2006). Vitamin D receptor gene polymorphisms, dietary promotion of insulin resistance, and colon and rectal cancer. Nutr. Cancer, 55, 35–43. [DOI] [PubMed] [Google Scholar]
- 48. Peters U., et al. ; Prostate, Lung, Colorectal and Ovarian Cancer Screening Project Team. (2004). Circulating vitamin D metabolites, polymorphism in vitamin D receptor, and colorectal adenoma risk. Cancer Epidemiol. Biomarkers Prev., 13, 546–552. [PubMed] [Google Scholar]
- 49. Slattery M.L., et al. (2006). PPARgamma and colon and rectal cancer: associations with specific tumor mutations, aspirin, ibuprofen and insulin-related genes (United States). Cancer Causes Control, 17, 239–249. [DOI] [PubMed] [Google Scholar]
- 50. Daniel C., et al. (2007). The TGFbeta/Smad 3-signaling pathway is involved in butyrate-mediated vitamin D receptor (VDR)-expression. J. Cell. Biochem., 102, 1420–1431. [DOI] [PubMed] [Google Scholar]
- 51. Ogino S., et al. (2011). Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut, 60, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.