Abstract
Formaldehyde (FA) is an economically important industrial chemical to which millions of people worldwide are exposed environmentally and occupationally. Recently, the International Agency for Cancer Research concluded that there is sufficient evidence that FA causes leukemia, particularly myeloid leukemia. To evaluate the biological plausibility of this association, we employed a chromosome-wide aneuploidy study approach, which allows the evaluation of aneuploidy and structural chromosome aberrations (SCAs) of all 24 chromosomes simultaneously, to analyze cultured myeloid progenitor cells from 29 workers exposed to relatively high levels of FA and 23 unexposed controls. We found statistically significant increases in the frequencies of monosomy, trisomy, tetrasomy and SCAs of multiple chromosomes in exposed workers compared with controls, with particularly notable effects for monosomy 1 [P = 6.02E-06, incidence rate ratio (IRR) = 2.31], monosomy 5 (P = 9.01E-06; IRR = 2.24), monosomy 7 (P = 1.57E-05; IRR = 2.17), trisomy 5 (P = 1.98E-05; IRR = 3.40) and SCAs of chromosome 5 (P = 0.024; IRR = 4.15). The detection of increased levels of monosomy 7 and SCAs of chromosome 5 is particularly relevant as they are frequently observed in acute myeloid leukemia. Our findings provide further evidence that leukemia-related cytogenetic changes can occur in the circulating myeloid progenitor cells of healthy workers exposed to FA, which may be a potential mechanism underlying FA-induced leukemogenesis.
Introduction
Formaldehyde (FA) is an economically important industrial chemical to which millions of people worldwide are exposed environmentally and occupationally (1). High levels of FA exposure occur in certain occupational settings (1,2); the United States current permissible occupational exposure limit for FA is 0.75 p.p.m. (parts per million) in air as an 8-h time-weighted average (3). Lower levels of environmental exposure, arising from automobile engines, tobacco smoke and household materials such as furniture made of pressed wood and carpeting, impact a greater number of people (1).
The International Agency for Cancer Research originally classified FA as a human carcinogen (Group 1) based on sufficient evidence for nasopharyngeal cancer (4), and recently reaffirmed it as a carcinogen based on sufficient evidence for nasopharyngeal cancer and leukemia, particularly myeloid leukemia (5). The United States National Toxicology Program also classified FA as a known human carcinogen in its 12th Report on Carcinogens in 2011 (6). Previous studies suggest that aneuploidies, such as monosomy 5, and structural chromosome aberrations (SCAs), such as deletions of 5q and 7q, as well as translocations are commonly found in myeloid leukemia (7–9) and could potentially be involved in FA-induced leukemia. A chromosome-wide aneuploidy study (CWAS) approach allows the simultaneous analysis of aneuploidy of all 24 human chromosomes and SCAs frequently detected in leukemia and lymphoma patients (10–12). Occupational exposure to environmental carcinogens, e.g. benzene, has been shown to effect cytogenetic changes frequently observed in myeloid leukemia, notably aneuploidies and SCAs, particularly monosomy 7 and trisomy 8 (11,13–15). We previously conducted a study of workers exposed to FA that provided evidence for the induction of leukemia-related aneuploidy for chromosomes 7 and 8 in the human myeloid stem/progenitor cell compartment, the potential target cells for leukemia (16). However, the number of individuals analyzed was small (10 exposed and 12 controls) and the study reported cytogenetic results for only monosomy of chromosome 7 and trisomy of chromosome 8. The ability of FA to induce other cytogenetic effects associated with myeloid leukaemia has not been investigated. The present study was designed to address the hypothesis that FA increases the frequency of aneuploidy and SCAs in multiple chromosomes.
We previously reported that the total peripheral white blood cell, granulocyte, platelet, lymphocyte and red blood cell counts were statistically significantly decreased in workers exposed to FA compared to unexposed controls. Further, as noted above, the frequencies of leukemia-related chromosome loss (monosomy 7) and gain (trisomy 8) in myeloid progenitor cells cultured from the peripheral blood of a small subset of individuals were significantly elevated compared with controls (16). Here, we have expanded the number of individuals (29 exposed and 23 controls) to obtain more precise estimates of cytogenetic effects and analyzed the frequency of occurrence of monosomy, trisomy and tetrasomy and total SCAs of all 24 chromosomes in the FA-exposed and unexposed control workers.
Materials and methods
Study subjects and demographics
This cross-sectional study (n = 94) of occupational FA exposure has been described in detail previously (16). We enrolled 43 workers currently exposed to relatively high levels of FA and 51 comparable, unexposed controls. No prior or current exposure to known or suspected leukemogens or hematotoxicants (e.g. benzene, phenol and chlorinated solvents), in excess of exposure levels in the general population, was detected for the exposed and control workers. The study was approved by Institutional Review Boards at the United States National Cancer Institute and the Guangdong Poisoning Control Center; participation was voluntary, and written informed consent was obtained. In the present study, we selected a subset of subjects for CWAS analysis based on scorable metaphases on slides from myeloid progenitor cell cultures, high levels of FA exposures for the exposed workers and availability of demographically comparable controls who also had adequate scorable metaphases. These metaphases were prepared from colony forming unit-granulocyte/macrophage (CFU-GM) progenitor cells as described (16). The 29 exposed subjects analyzed in the current study were young (mean ± SD: 31±5 years), male (90%) and similar to the 23 unexposed controls (Table I). Age, gender, cigarette smoking, alcohol use, current medication use and recent infection were not significantly different between the exposed and control subjects while body mass index approached statistical significance (P = 0.09, Table I).
Table I.
Demographic characteristics of the study subjects from Guangdong, China, analyzed in the current studya
| Demographic characteristics | Control (n = 23) | Exposed (n = 29) | P-value |
|---|---|---|---|
| Age, mean (SD) | 30.0 (5.3) | 30.6 (5.1) | 0.70 |
| BMIb, mean (SD) | 22.5 (2.5) | 21.3 (2.3) | 0.09 |
| Sex, n (%) | 0.84 | ||
| Female | 2 (8.7) | 3 (10.3) | |
| Male | 21 (91.3) | 26 (89.7) | |
| Current smoking, n (%) | 0.83 | ||
| No | 12 (52.2) | 16 (55.2) | |
| Yes | 11 (47.8) | 13 (44.8) | |
| Current alcohol use, n (%) | 0.24 | ||
| No | 14 (60.9) | 22 (75.9) | |
| Yes | 9 (39.1) | 7 (24.1) | |
| Recent infection, n (%) | 0.81 | ||
| No | 15 (65.2) | 18 (62.1) | |
| Yes | 8 (34.8) | 11 (37.9) | |
| Current medicine use, n (%) | 0.63 | ||
| No | 15 (65.2) | 17 (58.6) | |
| Yes | 8 (34.8) | 12 (41.4) | |
| FA air level (p.p.m.), median (10th, 90th percentile) | 0.026 (0.015, 0.026) | 1.38 (0.78, 2.61) |
aThese subjects are a subset of the 94 subjects in the previously described cross-sectional study of occupational FA exposure in factories in Guangdong, China (16).
bBMI, body mass index.
Exposure assessment
Occupational exposure assessment has been described in detail previously (16). In brief, FA exposure was monitored with UMEx 100 diffusion samplers, worn by the workers in the exposed workplaces for a full shift (>240min) on approximately three working days over a 3-week period. The average FA exposure level was calculated by taking the arithmetic mean of each subject’s measurements. The limit of detection was 0.012 p.p.m. Personal exposure to other organic compounds (chloroform, methylene chloride, tetrachloroethylene, trichloroethylene and benzene) was measured at least twice for each FA-exposed worker by 3M organic vapor monitors. Urinary benzene was measured in a subset of enrolled subjects (20 controls and 21 workers from both exposed factories) using gas chromatography–mass spectroscopy methods as described by Kim et al. (17) and benzene was at background levels and comparable between groups (16). The median (10th, 90th percentile) FA air exposure level in the 29 exposed workers analyzed in the current study was 1.38 (0.78, 2.61) p.p.m. as an 8-h time-weighted average while in the controls the level was 0.026 (0.015, 0.026) p.p.m. (Table I).
Biological sampling
Biological samples were collected from the study subjects in June–July 2006 as described (16). Peripheral blood samples were collected from each study subject and delivered to the processing laboratories within ~4 h of collection. Blood from each study subject was drawn into different vacutainer tubes by specially trained nurses and peripheral blood mononuclear cells was separated and used for the culture of circulating myeloid progenitor cells using a methylcellulose-based colony forming assay of CFU-GM (described in detail below). Laboratory personnel involved in sample collection and analysis were blinded with respect to exposure status.
Culturing of myeloid progenitor cells
A fraction of hematopoietic stem and progenitor cells circulate in the bloodstream in dynamic equilibrium with the stem cell pools in the bone marrow. These circulating cells represent a surrogate for bone marrow stem cells that are difficult to access in molecular epidemiology studies. The proliferative potential of these cells can be measured by colony-forming assays, which involves culturing the cells in semisolid medium containing appropriate growth factors (18). The individual colonies can be classified microscopically according to the progenitor cell type. Colonies derived from more committed progenitor cells that give rise to granulocytes and macrophages are called CFU-GM.
In our earlier study, we applied the CFU-GM colony-forming assay to peripheral blood mononuclear cells from all 94 study subjects (16). Hematopoietic progenitor cells from the peripheral blood were cultured in growth factor–containing methylcellulose medium (MethoCult GF H4534, according to the protocol provided by StemCell Technologies,), and the number of CFU-GM colonies formed was scored in 6 Petri dishes after 14 days. Limitations of the field setting and personnel available only allowed for cultures without erythropoietin to be prepared, therefore only CFU-GM colonies could be examined in vivo.
Metaphase preparation from cultured CFU-GM cells
Metaphases from CFU-GM cells were prepared after 14 days of culture by adding colcemid (0.05 μg/ml) to six Petri dishes overnight before harvest. CFU-GM colony cells were harvested, washed and dispersed into a hypotonic solution (0.075M KCI). After 30min, the cells were fixed in methanol/acetic acid (3:1) twice and then dropped onto several slides, air dried and stored in slide boxes at −20°C. High quality metaphase spreads, with a sufficient number of cells available for scoring, were analyzed by the OctoChrome fluorescent in situ hybridization (FISH) procedure.
OctoChrome FISH and scoring procedure
The OctoChrome FISH protocol provided by CytoCell (Banbury, UK) was performed as previously described (10–12). OctoChrome FISH, a chromosomic approach, allows the simultaneous analysis of 24 chromosomes on a single 8-square slide in a single hybridization. A Multiprobe device carries whole chromosome painting probes, on each of 8 squares, for three different chromosomes in three different colored fluorophores, Texas Red, FITC and Coumarin Spectra (red, green and blue), respectively. The chromosomes are arranged on the Multiprobe device in combinations that facilitate the identification of most specific aneuploidy and chromosomal rearrangements related to human leukemia and lymphoma. The fixed cultured CFU-GM metaphases prepared from the blood of FA-exposed workers and unexposed controls are dropped onto the 8-square slides matched to the Multiprobe device. The simultaneous denaturation of the probes and target DNA, and the use of rapid formamide-free stringency wash after overnight hybridization, simplifies the FISH procedure. After the hybridization, post-washing and 4′,6-diamidino-2-phenylindole (DAPI) staining steps, metaphase spreads on each square of the 8-square slides are scanned and localized automatically using Metafer software (MetaSystems, Altlussheim, Germany) and then evaluated on the computer screen. Metaphase cells were selected and scored according to the criteria listed in our previous publications (14,19). At least 150 metaphases per slide were scored for subjects included in this report.
Statistical analysis
Negative binomial (NB) regression was applied in this study because (i) it is commonly used when the outcome variable is a count; (ii) it can naturally adjust for differences in the denominator (total number of cells tested); (iii) it allows for overdispersion; and (iv) it provides interpretable associations between two measures, called incidence rate ratios (IRRs (20). The frequency-matching variables, age and sex, were included in the regression analysis, except for sex in the analysis of X and Y chromosomes. We analyzed aneuploidy effects on the Y chromosome in males, and on the X chromosome in males only, as there were too few female subjects for analysis (2 controls and 3 exposed, Table I). We present results adjusted for the matching variables, age and sex, in the main body of the paper. We ranked the chromosomes based on the P-value from the 24 regressions.
Although there are little data on the impact of lifestyle on chromosomal abnormalities in cultured myeloid progenitor cells, we conducted additional analyses to explore potential confounding by exposures [i.e. current cigarette smoking status (yes/no), current alcohol consumption (yes/no), recent infections (flu or respiratory infections in the previous week), current use of medication (yes/no) and body mass index that might plausibly influence measurements made in hematological cells and included them in models if they were significant at P < 0.05 or if there was evidence of confounding (e.g. greater than a 15% change in the regression coefficient). The analysis of potential confounding of our aneuploidy and structural chromosomal aberration results showed that there were minimal changes in those findings that were statistically significant in models adjusted only for age and sex and conclusions were unchanged (Supplementary Tables S1–S4, available at Carcinogenesis online). Therefore, the primary results presented in this paper are shown adjusted only for age and sex, the matching variables.
We used the above approach to evaluate confounding in our previous report and found that adjustment had a minimal impact on the results (16). Further, excluding the handful of subjects who might have had thalassemia (21) from those analyses also had a negligible impact on the findings (not shown).
Results
Monosomy rates in FA-exposed and control workers
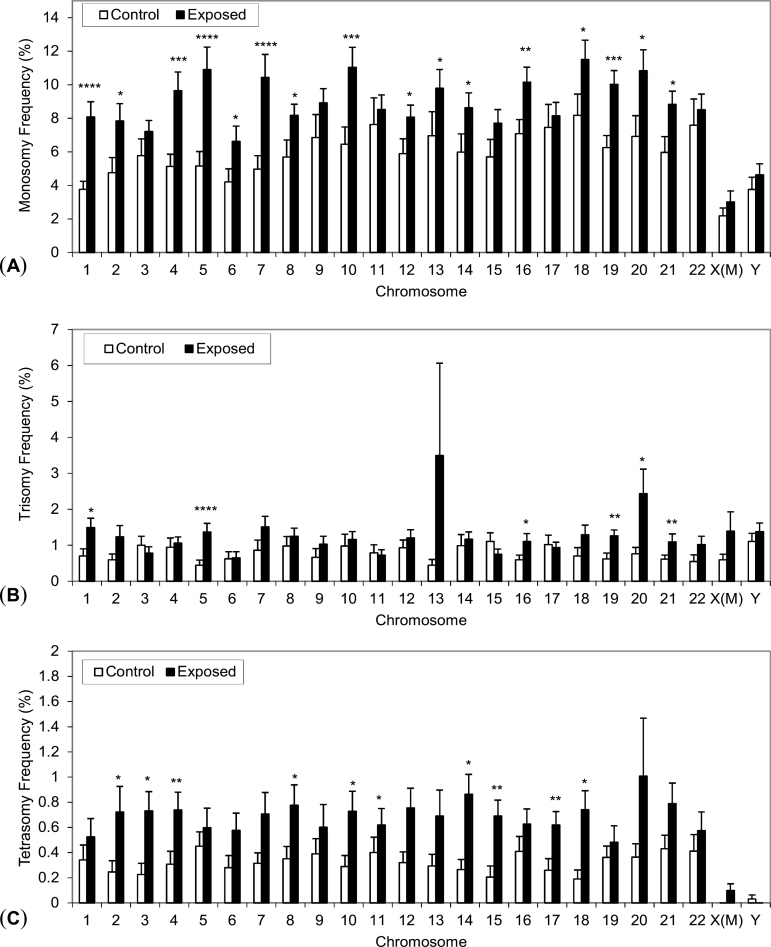
As shown in Table II and Figure 1A, the frequencies of monosomy of chromosomes 1, 5 and 7 were highly significantly elevated in the FA-exposed workers (IRR > 2.0, P = 6.02E-06, 9.01E-06 and 1.57E-05, respectively). The frequencies of monosomy of chromosomes 4, 19, 10 and 16 were significantly elevated at P = 0.0002–0.0075 and the frequencies of chromosomes 21, 2, 8, 18, 12, 20, 13, 6 and 14 were elevated at P = 0.014–0.044. The frequencies of loss of chromosome X and Y were higher in exposed males compared to controls (Figure 1A), but these increases were not significant (Table II). The frequencies of monosomy of the remaining chromosomes were not significantly different between exposed and control workers.
Table II.
Monosomy rates of all 24 chromosomes between formaldehyde-exposed and control groupsa
| Chromosome | IRRb | 95% CIc | P-value |
|---|---|---|---|
| 1 | 2.31 | 1.61–3.31 | 6.02E-06 |
| 5 | 2.24 | 1.57–3.20 | 9.01E-06 |
| 7 | 2.17 | 1.53–3.08 | 1.57E-05 |
| 4 | 2.02 | 1.40–2.90 | 0.00015 |
| 19 | 1.74 | 1.29–2.34 | 0.00026 |
| 10 | 1.86 | 1.30–2.65 | 0.00064 |
| 16 | 1.54 | 1.12–2.12 | 0.0075 |
| 21 | 1.53 | 1.09–2.15 | 0.014 |
| 2 | 1.72 | 1.11–2.67 | 0.015 |
| 8 | 1.51 | 1.06–2.15 | 0.022 |
| 18 | 1.49 | 1.05–2.11 | 0.024 |
| 12 | 1.54 | 1.06–2.25 | 0.024 |
| 20 | 1.58 | 1.06–2.34 | 0.025 |
| 13 | 1.58 | 1.04–2.42 | 0.033 |
| 6 | 1.58 | 1.03–2.42 | 0.036 |
| 14 | 1.47 | 1.01–2.14 | 0.044 |
| 15 | 1.43 | 0.99–2.06 | 0.058 |
| 9 | 1.41 | 0.98–2.03 | 0.068 |
| Y | 1.33 | 0.86–2.06 | 0.20 |
| X (M)d | 1.39 | 0.84–2.30 | 0.20 |
| 3 | 1.24 | 0.88–1.76 | 0.22 |
| 11 | 1.21 | 0.80–1.83 | 0.37 |
| 22 | 1.19 | 0.79–1.79 | 0.41 |
| 17 | 1.13 | 0.80–1.61 | 0.48 |
aData listed according to P-values.
bIRR, adjusted for age and sex, except for sex in the analysis of X and Y chromosomes.
c95% confidence interval.
dX chromosome in males.
Fig. 1.
Mean aneuploidy frequencies of all 24 chromosomes in workers exposed to formaldehyde and unexposed controls. Mean unadjusted frequency and standard errors of (A) monosomy, (B) trisomy and (C) tetrasomy for all 24 chromosomes in unexposed controls (n = 23) and exposed workers (n = 29) are shown. P-values indicating significance of the increase relative to controls, which are from models adjusted for covariates shown in Tables 2–4, respectively, are denoted as ****<0.0001, ***<0.001, **<0.01 and *<0.05. X(M) represents X chromosome in males.
Trisomy rates in FA-exposed and control workers
FA exposure was associated with highly significant increases in trisomy of certain chromosomes (Table III, Figure 1B). Trisomy of chromosome 5 was the most significantly elevated (IRR > 3.0, P = 1.98E-05). Trisomies of chromosomes 19, 21, 1, 20 and 16 were also more frequent in exposed subjects compared with controls (IRR: 1.72–2.49, P = 0.0055–0.031), though somewhat less significantly than chromosome 5. The frequency of gain of chromosome X and Y was higher in exposed males compared to controls (Figure 1B), but these increases were not significant (Table III). The frequencies of trisomy of other chromosomes were not significantly elevated (P ≥ 0.05).
Table III.
Trisomy rates of all 24 chromosomes between formaldehyde-exposed and control groupsa
| Chromosome | IRRb | 95% CIc | P-value |
|---|---|---|---|
| 5 | 3.40 | 1.94–5.97 | 1.98E-05 |
| 19 | 2.07 | 1.24–3.46 | 0.0055 |
| 21 | 2.09 | 1.22–3.57 | 0.0071 |
| 1 | 1.91 | 1.15–3.17 | 0.012 |
| 20 | 2.49 | 1.21–5.14 | 0.013 |
| 16 | 1.72 | 1.05–2.80 | 0.031 |
| 7 | 1.88 | 0.98–3.61 | 0.056 |
| 18 | 1.74 | 0.96–3.15 | 0.067 |
| 2 | 1.88 | 0.96–3.68 | 0.067 |
| 13 | 2.51 | 0.91–6.91 | 0.075 |
| 12 | 1.53 | 0.95–2.47 | 0.083 |
| 22 | 1.92 | 0.90–4.09 | 0.090 |
| 9 | 1.69 | 0.88–3.23 | 0.11 |
| X (M)d | 1.74 | 0.87–3.45 | 0.11 |
| 14 | 1.40 | 0.82–2.40 | 0.22 |
| 8 | 1.37 | 0.82–2.31 | 0.23 |
| Y | 1.29 | 0.80–2.10 | 0.30 |
| 4 | 1.27 | 0.81–1.99 | 0.30 |
| 10 | 1.33 | 0.76–2.34 | 0.32 |
| 6 | 1.34 | 0.61–2.96 | 0.46 |
| 11 | 1.20 | 0.63–2.30 | 0.58 |
| 17 | 1.12 | 0.70–1.81 | 0.63 |
| 15 | 0.89 | 0.52–1.51 | 0.66 |
| 3 | 0.89 | 0.52–1.54 | 0.68 |
aData listed according to P-values.
bIRR, adjusted for age and sex, except for sex in the analysis of X and Y chromosomes.
c95% confidence interval.
dX chromosome in males.
Tetrasomy rates in FA-exposed and control workers
The frequencies of tetrasomy of chromosomes 4, 15 and 17 were significantly increased at P < 0.005 (Table IV and Figure 1C). Tetrasomy of several other chromosomes (14, 3, 18, 8, 12, 2 and 10) was also associated with FA exposure, though less significantly (P = 0.011–0.034). The frequency of tetrasomy of chromosome X (two copies in males) was higher in exposed males compared to controls in which no tetrasomy X was detected (Figure 1C), but the increase was not significant (Table IV). A low frequency of tetrasomy of chromosome Y was detected in controls but the level was essentially zero in the exposed subjects (Figure 1C).
Table IV.
Tetrasomy rates of all 24 chromosomes between formaldehyde-exposed and control groupsa
| Chromosome | IRRb | 95% CIc | P-value |
|---|---|---|---|
| 4 | 1.64 | 1.21–2.21 | 0.0012 |
| 15 | 3.10 | 1.53–6.28 | 0.0017 |
| 17 | 2.40 | 1.33–4.32 | 0.0036 |
| 14 | 2.13 | 1.19–3.81 | 0.011 |
| 3 | 2.40 | 1.22–4.75 | 0.012 |
| 18 | 2.14 | 1.14–4.01 | 0.018 |
| 8 | 1.84 | 1.10–3.07 | 0.020 |
| 12 | 1.56 | 1.06–2.29 | 0.025 |
| 2 | 2.28 | 1.08–4.82 | 0.031 |
| 10 | 2.26 | 1.06–4.82 | 0.034 |
| 6 | 1.87 | 0.99–3.54 | 0.054 |
| 7 | 2.02 | 0.95–4.30 | 0.067 |
| 13 | 2.00 | 0.95–4.21 | 0.069 |
| 16 | 1.72 | 0.96–3.09 | 0.070 |
| 20 | 2.08 | 0.87–4.94 | 0.099 |
| 1 | 1.77 | 0.89–3.53 | 0.10 |
| 21 | 1.45 | 0.89–2.34 | 0.13 |
| 19 | 1.53 | 0.74–3.15 | 0.25 |
| 11 | 1.38 | 0.76–2.52 | 0.29 |
| 5 | 1.41 | 0.68–2.94 | 0.36 |
| 9 | 1.36 | 0.66–2.82 | 0.41 |
| 22 | 1.24 | 0.61–2.53 | 0.55 |
| Y | 0.85 | 0.25–2.88 | 0.79 |
| X (M)d | N/A | N/A | 1.00 |
aData listed according to P-values.
bIRR, adjusted for age and sex, except for sex in the analysis of X and Y chromosomes.
c95% confidence interval.
dX chromosome in males.
Total structural chromosome abnormality rates in FA-exposed and control workers
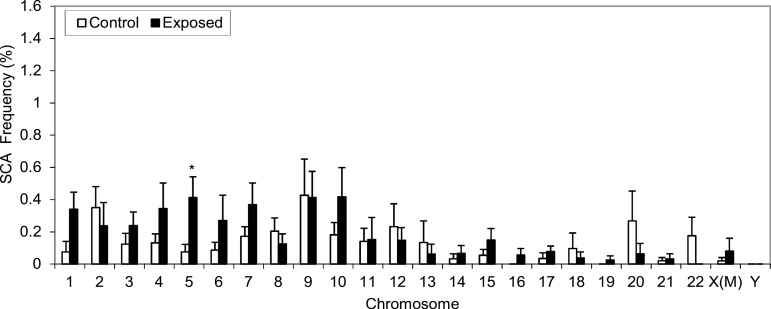
The frequencies of total structural chromosome aberration (SCA) were relatively low in both exposed and control workers, compared with aneuploidies (Figure 2 versus Figure 1), and IRRs had wide confidence intervals in general (Table V). Total SCA, including breaks, deletions and translocations, of chromosome 5 were statistically significantly higher in workers exposed to FA (IRR = 4.15, P = 0.024), Table V, compared to unexposed controls. SCAs were not significantly different for any other chromosome. The frequency of total SCA of chromosome X was higher in exposed males compared to controls (Figure 2), but this increase was not significant, Table V. No SCA were detected in chromosome Y in either controls or exposed subjects.
Fig. 2.
Mean frequencies of total structural chromosome aberration (SCA) for all 24 chromosomes in workers exposed to formaldehyde and unexposed controls. Mean unadjusted frequency and standard errors of total SCA for all 24 chromosomes in unexposed controls (n = 23) and exposed workers (n = 29) are shown. P-values are from models adjusted for covariates shown in table 5. The P-value indicating significance of the increase relative to controls is denoted as *<0.05. X(M) represents X chromosome in males.
Table V.
SCA rates of all 24 chromosomes between formaldehyde-exposed and control groupsa
| Chromosome | IRRb | 95% CIc | P-value |
|---|---|---|---|
| 5 | 4.15 | 1.20–14.35 | 0.024 |
| 1 | 3.68 | 0.83–16.25 | 0.086 |
| 22 | 0.49 | 0.14–1.67 | 0.25 |
| 2 | 0.48 | 0.14–1.71 | 0.26 |
| 15 | 2.16 | 0.54–8.60 | 0.27 |
| 4 | 2.00 | 0.52–7.61 | 0.31 |
| 10 | 1.63 | 0.45–5.92 | 0.46 |
| 8 | 0.70 | 0.26–1.85 | 0.47 |
| 6 | 1.72 | 0.29–10.06 | 0.55 |
| 20 | 0.37 | 0.01–17.06 | 0.61 |
| 7 | 1.29 | 0.45–3.75 | 0.64 |
| 16 | 1.36 | 0.34–5.52 | 0.67 |
| 17 | 1.23 | 0.48–3.19 | 0.67 |
| 3 | 1.26 | 0.34–4.65 | 0.73 |
| 19 | 1.18 | 0.41–3.38 | 0.75 |
| 13 | 1.77 | 0.00–1015 | 0.86 |
| 18 | 0.91 | 0.30–2.76 | 0.87 |
| 11 | 0.86 | 0.08–8.90 | 0.90 |
| X (M)d | 0.94 | 0.28–3.12 | 0.92 |
| 12 | 1.08 | 0.17–6.77 | 0.94 |
| 9 | 0.96 | 0.28–3.35 | 0.95 |
| 14 | 0.92 | 0.07–11.60 | 0.95 |
| 21 | 1.00 | 0.21–4.84 | 1.00 |
| Y | 0.87 | NA | 1.00 |
aData listed according to P-values.
bIRR, adjusted for age and sex, except for sex in the analysis of X and Y chromosomes.
c95% confidence interval.
dX chromosome in males.
Discussion
FA exposure and chromosomal aneuploidy in myeloid progenitor cells
We found statistically significant increases in the frequencies of monosomy, trisomy and tetrasomy of multiple chromosomes and in structural changes (SCA) of chromosome 5 in cultured myeloid progenitor cells from FA-exposed workers compared to unexposed controls. Our previous (16) and current studies are the only reports that show induction of chromosome-specific aneuploidy in cultured myeloid progenitor cells of FA-exposed workers. The most significant earlier finding of FA-associated monosomy 7 was confirmed, and trisomy 8 induction was elevated, but not significantly (16). Here, we examined aneuploidy in all 24 chromosomes simultaneously by CWAS, in CFU-GM from 29 FA-exposed workers (median FA level 1.38 p.p.m. as an 8-h time-weighted average), and 23 occupationally-unexposed control subjects.
Similarity of FA-induced aneuploidies with those seen in AML and following exposure to other known leukemogens
Monosomy, trisomy and total structural aberrations of chromosome 5 were significantly increased in cultured CFU-GM cells among workers with in vivo FA exposure. Loss of whole chromosomes 5 or 7 (−5/−7) or of the long arms (5q−/7q−) of these chromosomes are the most common unbalanced aberrations in therapy-related myelodysplastic syndrome (t-MDS) and acute myeloid leukemia (t-AML (7,8), particularly those resulting from treatment with alkylating drugs, such as melphalan (22,23). We have recently reported that monosomy of chromosome 7 was increased in cultured CFU-GM cells in workers exposed to the established leukemogen benzene (24). Further, through analysis by CWAS, we reported increased monosomy and trisomy of chromosomes 5 and 7 in the peripheral blood lymphocytes of workers exposed to benzene (11).
Trisomy 21, the second most common trisomy in AML and MDS (25), and trisomy 16, which has been reported in myeloid malignancy (26), were also significantly increased in both the CFU-GM of FA-exposed workers in the current study and in the peripheral lymphocytes of benzene-exposed workers (11). Our findings provide further evidence that aneuploidies associated with AML and with exposure to known leukemogens (e.g. benzene and melphalan) might occur in the circulating myeloid progenitor cells of healthy workers exposed to FA, and may be a potential mechanism underlying FA-induced leukemia. Induction of SCA at chromosome 5 in these cells could be another potential mechanism.
Mechanistic relevance of FA-induced chromosomal damage in myeloid progenitor cells
Our previous (16) and current studies are the only ones to demonstrate induction of chromosome-specific aneuploidy in the cultured myeloid progenitor cells of FA-exposed workers. Chromosomal damage, in the form of micronuclei, has previously been demonstrated in the peripheral blood lymphocytes of workers exposed to FA (27–30). Using the cytokinesis-blocked micronucleus assay combined with FISH with a pan-centromeric DNA probe, Orsiere et al. showed that the micronuclei, arising through an aneugenic rather than a clastogenic mechanism, were induced in the peripheral blood lymphocytes of FA-exposed workers (31). More recently, using a cytokinesis-blocked micronucleus assay, Ladiera et al. reported increased micronuclei in the peripheral blood lymphocytes of occupationally exposed histopathology workers compared with non-occupationally exposed administrative staff members (32), and confirmed their findings in a replicate evaluation of the data (33).
The finding of aneuploidy in circulating myeloid progenitor cells may be more mechanistically relevant for myeloid neoplasms than its demonstration in circulating lymphocytes. These circulating myeloid progenitor cells exist in dynamic equilibrium with the corresponding cell pools in the bone marrow (34), and are, together with early hematopoietic stem cells, likely targets for induction of myeloid leukemia. Experiments using genetically marked parabiotic mice, which are surgically conjoined and share a common circulation, revealed that blood-borne hematopoietic stem cells rapidly and constitutively migrate through the blood and play a physiological role in the functional re-engraftment of unconditioned bone marrow (35). Recently, aneuploidy was shown to contribute to genomic instability (36,37) and, as such, could be an initiating event in leukemia. Thus, leukemic stem cells could arise following FA exposure in vivo through the induction of aneuploidy and genomic instability in circulating hematopoietic stem or progenitor cells.
Plausibility of FA-induced bone marrow toxicity
The aneuploidy detected in circulating myeloid progenitors of occupationally exposed workers could have arisen while these cells were circulating in peripheral blood as described above. Alternatively, FA could target these cells in the bone marrow. A lack of evidence that inhaled FA reaches the bone marrow has been one of the major arguments proposed by some authors against the classification of FA as a leukemogen. Application of sensitive methods for detecting specific DNA adducts and distinguishing those arising from endogenous and exogenous FA sources did not detect distant site effects (38–40), including effects on bone marrow and blood, in animal (rat and monkey) studies. However, recent studies show that FA induces DNA-protein crosslinks and oxidative stress in the bone marrow of mice exposed by nose-only inhalation (41,42). The exposed mice also showed reduced blood cell counts in several lineages (42), mirroring our earlier hematotoxicity findings in FA-exposed workers (16), consistent with toxic effects on the bone marrow. These new findings build upon previous data showing induction of cytogenetic damage in the bone marrow of exposed mice and rats, suggesting that FA does target the bone marrow in experimental animals (27,43).
It is uncertain how FA could target the bone marrow. Our current findings confirm our earlier findings that FA exposure was associated with aneuploidy in cultured circulating myeloid progenitor cells in vivo (16). However, aneuploidy was not induced in myeloid progenitor cells exposed to FA in vitro (44,45) and we have recently reported negative or weak effects on monosomy and trisomy of chromosomes 7 and 8 in expanded human erythroid progenitors following in vitro treatment with FA (46). The largely negative in vitro data in myeloid and erythroid progenitors and the positive in vivo data in lymphocytes and myeloid progenitors of exposed workers suggest that FA may interact with a physiological agent to mediate its effects in vivo. Previously, we suggested that FA could travel through the blood to the marrow as methanediol (1), but this hypothesis remains to be fully tested. Another potential explanation is the generation of a stable, but reactive product(s) in the respiratory tract following inflammation produced by FA exposure. We hypothesize that this reactive product(s) could travel through the blood to the marrow producing aneuploidy. Alternatively, cells traveling in the blood could be damaged as they pass through the lung and other portions of the respiratory tract, which receives 25% of the cardiac output. This would explain the generation of aneuploidy in human blood cells in vivo but its relative absence in vitro. It would also explain why endogenously generated FA in the liver and elsewhere does not appear to be genotoxic as it is rapidly metabolized to formate and does not produce inflammation. Further studies are needed to clarify these potential mechanisms of action and related issues.
Potential mechanisms of FA-induced aneuploidy
The mechanism by which FA induces aneuploidy is unknown. Aneuploidy in somatic cells has been associated with defects in mitotic spindle assembly and dynamics, centrosome amplification, cell-cycle regulation, chromatid cohesion and telomere metabolism. These defects may be caused by mutation, epigenetic dysregulation, or altered expression of the genes involved in these processes. FA is genotoxic and mutagenic to mammalian cells in vitro and in vivo (4,47) and it causes DNA-protein crosslinks and DNA adducts (48), which if left unrepaired could potentially lead to mutations in replicating cells. FA also binds to proteins and could disrupt chromosome segregation through interaction with the mitotic apparatus. DNA hypomethylation of subtelomeric, telomeric and pericentric regions has been implicated in aneuploidy induction (49). Recently, long-term, low-dose exposure to FA was shown to induce global genomic hypomethylation in a bronchial epithelial cell line in vitro (50). Oxidative stress overrides spindle checkpoint-dependent arrest (51), representing an indirect mechanism through which FA could induce aneuploidy. Reactive oxygen species-mediated oxidative damage resulting from FA exposure has been detected in distal cells and tissues, including bone marrow (41,42), reproductive tissues (52), liver (53) and brain (54). Each of these potential mechanisms and their relationship to the induction of aneuploidy in hematopoietic cells requires further investigation.
Study strengths and limitations
A strength of the current report, as in our earlier paper (16), is the extent to which we have tried to minimize confounding due to other exposures. Personal exposure to organic compounds was measured at least twice for each FA-exposed worker by organic vapor monitors and no chloroform, methylene chloride, tetrachloroethylene, trichloroethylene, benzene, or hydrocarbons were detected in any of the selected samples and the control population had no occupational exposure to FA or any other hematotoxic or genotoxic chemicals in excess of levels in the general population. In addition, conclusions from our CWAS were unchanged after taking into account age, gender, recent infection, body mass index, and current tobacco, alcohol and medication use (Supplementary Tables S1–S4, available at Carcinogenesis online).
Our study found statistically significant associations for three of our strong a priori hypotheses, i.e. that FA exposure would be associated with higher levels of structural aberrations of chromosome 5 and aneuploidy of chromosomes 5 and 7. We conducted analyses of all other chromosomes in the CWAS, which were more exploratory in nature, and found highly statistically significant elevated aneuploidy frequencies among FA-exposed workers compared to controls for multiple additional chromosomes (i.e. chromosomes 1, 4, 10, 15, 19) that remain statistically significant after applying corrective procedures for multiple comparisons. This indicates that FA is potentially capable of causing anueploidy in multiple chromosomes.
A potential limitation of our study is the possibility that a portion of the chromosomal abnormalities we detected in CFU-GM may have arisen during the 14-day cell in vitro culture period rather than being present in the circulating myeloid progenitor cells while they were still in the study subject (21). These events would therefore reflect a greater tendency for CFU-GM cells from workers exposed to FA to develop chromosomal abnormalities during cell growth compared to control workers who were unexposed to FA. Their significant association with FA exposure clearly shows the potential for FA-related genetic damage or DNA–protein crosslinks to manifest as leukemia-related chromosome changes in subsequent generations of myeloid cells arising from committed and early progenitors. Given the dynamic proliferation of stem cells and progenitor cells in human bone marrow during hematopoiesis, a greater tendency to develop chromosomal abnormalities would also support the leukemogenic potential of FA.
Another limitation of our study was its inability to address exposure-response issues. The study was designed to evaluate mechanistically relevant biomarkers in workers exposed to relatively high levels of FA, and as a consequence there was a relatively narrow range of exposure that precluded assessment of exposure-response relationships (Table I). FA has been reported to produce dose-dependent effects on colony formation from myeloid progenitor cells in vitro (16,45). Further studies of populations exposed to a wider range of FA concentrations are needed to address dose-response in vivo.
Conclusion
In conclusion, our findings strengthen the evidence that leukemia-related aneuploidies and structural changes, especially in chromosomes 5 and 7, can arise in the myeloid progenitor cells of healthy workers exposed to FA, and may be a potential mechanism underlying FA-induced leukemia. Further studies should be conducted to assess the dose-response relationship, to determine whether FA itself or some secondary reactive product is responsible for the chromosome-damaging effect, to determine whether hematopoietic stem cell or progenitors are targeted in the peripheral blood or bone marrow and to elucidate the mechanisms underlying the induction of aneuploidy.
Supplementary material
Supplementary Tables S1–S4 can be found at http://carcin.oxfordjournals.org/
Funding
Intramural funds from the National Cancer Institute and National Institute of Environmental Health Sciences (R01ES017452 to L.Z. and P42ES004705 to M.T.S.).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- CFU-GM
colony forming unit-granulocyte/macrophage
- CWAS
chromosome-wide aneuploidy study
- FA
formaldehyde
- IRR
incidence rate ratio
- SCA
structural chromosome aberration.
References
- 1. Zhang L., et al. (2009). Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat. Res., 681, 150–168. [DOI] [PubMed] [Google Scholar]
- 2. Tang X., et al. (2009). Formaldehyde in China: production, consumption, exposure levels, and health effects. Environ. Int., 35, 1210–1224. [DOI] [PubMed] [Google Scholar]
- 3. OSHA. (1992). Formaldehyde. Washington, DC, US Department of Labor. [Google Scholar]
- 4. IARC. (2006). Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. Monogr. Eval. Carcinog. Risks. Hum., 88, 39–325. [PMC free article] [PubMed] [Google Scholar]
- 5. IARC. (2012). A review of human carcinogens: chemical agents and related occupations: formadehyde. Monogr Eval Carcinog Risks Hum., 100F, 401–435. [PMC free article] [PubMed] [Google Scholar]
- 6. NTP. (2011). 12th Report on Carcinogens. Health and Human Services. http://ntp.niehs.nih.gov/?objectid=03C9AF75-E1BF-FF40- DBA9EC0928DF8B15 (11 January 2013, date last accessed)
- 7. Qian Z, et al. (2009). Cytogenetic and genetic pathways in therapy-related acute myeloid leukemia. Chemico-Biol. Interact., 184, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rowley J.D. (2000). Molecular genetics in acute leukemia. Leukemia, 14, 513–517. [DOI] [PubMed] [Google Scholar]
- 9. Schoch C., et al. (2002). Cytogenetics in acute myeloid leukemia. Curr. Oncol. Rep., 4, 390–397. [DOI] [PubMed] [Google Scholar]
- 10. Ji Z., et al. (2012). Chromosomics: detection of numerical and structural alterations in all 24 human chromosomes simultaneously using a novel OctoChrome FISH assay. J Vis Exp. pii: 3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang L., et al. (2011). Chromosome-wide aneuploidy study (CWAS) in workers exposed to an established leukemogen, benzene. Carcinogenesis, 32, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L., et al. (2005). Use of OctoChrome fluorescence in situ hybridization to detect specific aneuploidy among all 24 chromosomes in benzene-exposed workers. Chem. Biol. Interact., 153-154, 117–122. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L., et al. (1999). Benzene increases aneuploidy in the lymphocytes of exposed workers: a comparison of data obtained by fluorescence in situ hybridization in interphase and metaphase cells. Environ. Mol. Mutagen., 34, 260–268. [PubMed] [Google Scholar]
- 14. Smith M.T., et al. (1998). Increased translocations and aneusomy in chromosomes 8 and 21 among workers exposed to benzene. Cancer Res., 58, 2176–2181. [PubMed] [Google Scholar]
- 15. Zhang L., et al. (1998). Increased aneusomy and long arm deletion of chromosomes 5 and 7 in the lymphocytes of Chinese workers exposed to benzene. Carcinogenesis, 19, 1955–1961. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L., et al. (2010). Occupational exposure to formaldehyde, hematotoxicity, and leukemia-specific chromosome changes in cultured myeloid progenitor cells. Cancer Epidemiol. Biomarkers Prev., 19, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim S., et al. (2006). Modeling human metabolism of benzene following occupational and environmental exposures. Cancer Epidemiol. Biomarkers Prev., 15, 2246–2252. [DOI] [PubMed] [Google Scholar]
- 18. Lan Q., et al. (2004). Hematotoxicity in workers exposed to low levels of benzene. Science, 306, 1774–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L., et al. (1998). Benzene metabolites induce the loss and long arm deletion of chromosomes 5 and 7 in human lymphocytes. Leuk. Res., 22, 105–113. [DOI] [PubMed] [Google Scholar]
- 20. McCullagh P. (1989). Generalized linear models. London, UK, CRC Press. [Google Scholar]
- 21. Gentry P.R., et al. (2013). Formaldehyde exposure and leukemia: critical review and reevaluation of the results from a study that is the focus for evidence of biological plausibility. Crit. Rev. Toxicol., 43, 661–670. [DOI] [PubMed] [Google Scholar]
- 22. Mamuris Z., et al. (1990). Specific chromosomal mutagenesis observed in stimulated lymphocytes from patients with S-ANLL. Int. J. Cancer, 46, 563–568. [DOI] [PubMed] [Google Scholar]
- 23. Mamuris Z., et al. (1989). Chromosomal aberrations in lymphocytes of patients treated with melphalan. Int. J. Cancer, 43, 80–86. [DOI] [PubMed] [Google Scholar]
- 24. Zhang L., et al. (2012). Leukemia-related chromosomal loss detected in hematopoietic progenitor cells of benzene-exposed workers. Leukemia, 26, 2494–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cortes J.E., et al. (1995). Clinical and prognostic significance of trisomy 21 in adult patients with acute myelogenous leukemia and myelodysplastic syndromes. Leukemia, 9, 115–117. [PubMed] [Google Scholar]
- 26. Guillaume B., et al. (2001). Trisomy 16 as the sole anomaly in hematological malignancies. Three new cases and a short review. Cancer Genet. Cytogenet., 128, 168–171. [DOI] [PubMed] [Google Scholar]
- 27. Kitaeva L.V., et al. (1990). [The cytopathic and cytogenetic sequelae of chronic inhalational exposure to formaldehyde on female germ cells and bone marrow cells in rats]. Tsitologiia, 32, 1212–1216. [PubMed] [Google Scholar]
- 28. Ye X., et al. (2005). Cytogenetic analysis of nasal mucosa cells and lymphocytes from high-level long-term formaldehyde exposed workers and low-level short-term exposed waiters. Mutat. Res., 588, 22–27. [DOI] [PubMed] [Google Scholar]
- 29. Yu L.Q., et al. (2005). [Early genetic effects on workers occupationally exposed to formaldehyde]. Zhonghua Yu Fang Yi Xue Za Zhi, 39, 392–395. [PubMed] [Google Scholar]
- 30. Suruda A., et al. (1993). Cytogenetic effects of formaldehyde exposure in students of mortuary science. Cancer Epidemiol. Biomarkers Prev., 2, 453–460. [PubMed] [Google Scholar]
- 31. Orsière T., et al. (2006). Genotoxic risk assessment of pathology and anatomy laboratory workers exposed to formaldehyde by use of personal air sampling and analysis of DNA damage in peripheral lymphocytes. Mutat. Res., 605, 30–41. [DOI] [PubMed] [Google Scholar]
- 32. Ladeira C., et al. (2011). Genotoxicity biomarkers in occupational exposure to formaldehyde–the case of histopathology laboratories. Mutat. Res., 721, 15–20. [DOI] [PubMed] [Google Scholar]
- 33. Speit G., et al. (2012). Re-evaluation of a reported increased micronucleus frequency in lymphocytes of workers occupationally exposed to formaldehyde. Mutat. Res., 744, 161–166. [DOI] [PubMed] [Google Scholar]
- 34. Kreja L., et al. (1999). Stable chromosomal aberrations in haemopoietic stem cells in the blood of radiation accident victims. Int. J. Radiat. Biol., 75, 1241–1250. [DOI] [PubMed] [Google Scholar]
- 35. Wright D.E., et al. (2001). Physiological migration of hematopoietic stem and progenitor cells. Science, 294, 1933–1936. [DOI] [PubMed] [Google Scholar]
- 36. Sheltzer J.M., et al. (2011). Aneuploidy drives genomic instability in yeast. Science, 333, 1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Solomon D.A., et al. (2011). Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science, 333, 1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu K., et al. (2010). Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol. Sci., 116, 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu K., et al. (2011). Molecular dosimetry of N2-hydroxymethyl-dG DNA adducts in rats exposed to formaldehyde. Chem. Res. Toxicol., 24, 159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moeller B.C., et al. (2011). Determination of N2-hydroxymethyl-dG adducts in the nasal epithelium and bone marrow of nonhuman primates following 13CD2-formaldehyde inhalation exposure. Chem. Res. Toxicol., 24, 162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ye X., et al. (2013). Inhaled formaldehyde induces DNA-protein crosslinks and oxidative stress in bone marrow and other distant organs of exposed mice. Environ Mol Mutagen, 54, 705–718. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y., et al. (2013). Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PLoS One, 8, e74974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tao X.Y., et al. (2004). [Study on the genetic damage in mice induced by the volatile organic compounds of decoration materials]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 22, 194–196. [PubMed] [Google Scholar]
- 44. Speit G., et al. (2011). Does formaldehyde induce aneuploidy? Mutagenesis, 26, 805–811. [DOI] [PubMed] [Google Scholar]
- 45. Kuehner S., et al. (2012). Analysis of leukemia-specific aneuploidies in cultured myeloid progenitor cells in the absence and presence of formaldehyde exposure. Toxicol. Sci., 128, 72–78. [DOI] [PubMed] [Google Scholar]
- 46. Ji Z., et al. (2013). Formaldehyde induces micronuclei in mouse erythropoietic cells and suppresses the expansion of human erythroid progenitor cells. Toxicol. Lett., 224, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baan R., et al. ; WHO International Agency for Research on Cancer Monograph Working Group. (2009). A review of human carcinogens–Part F: chemical agents and related occupations. Lancet Oncol., 10, 1143–1144. [DOI] [PubMed] [Google Scholar]
- 48. Zhang L., et al. (2010). Formaldehyde and leukemia: epidemiology, potential mechanisms, and implications for risk assessment. Environ. Mol. Mutagen., 51, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Herrera L.A., et al. (2008). The epigenetic origin of aneuploidy. Curr. Genomics, 9, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Q., et al. (2011). Effects of long-term low-dose formaldehyde exposure on global genomic hypomethylation in 16HBE cells. Toxicol. Lett., 205, 235–240. [DOI] [PubMed] [Google Scholar]
- 51. Limoli C.L., et al. (2003). Induction of chromosomal instability by chronic oxidative stress. Neoplasia, 5, 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duong A., et al. (2011). Reproductive and developmental toxicity of formaldehyde: a systematic review. Mutat. Res., 728, 118–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Im H., et al. (2006). Evaluation of toxicological monitoring markers using proteomic analysis in rats exposed to formaldehyde. J. Proteome Res., 5, 1354–1366. [DOI] [PubMed] [Google Scholar]
- 54. Lu Z., et al. (2008). Effect of inhaled formaldehyde on learning and memory of mice. Indoor Air, 18, 77–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.